Abstract
Light sheet microscopy is a powerful technique for rapid, three-dimensional fluorescence imaging of large specimen such as drosophila and zebrafish embryos. Yet, beam divergence results in a loss of axial resolution at the periphery of the light sheet. Here, we demonstrate how an electrically tunable lens can be utilized to maintain the minimal, diffraction-limited thickness of the light sheet over a wide field of view (>600 μm) at high frame rates (40 fps). This mode of operation is necessary for the application of fluorescence fluctuation spectroscopy in images.
Keywords: Fluorescence microscopy, light sheet microscopy, fluorescence fluctuation spectroscopy
INTRODUCTION
With its high spatiotemporal resolution, axial sectioning capability and low light exposure levels, light sheet microscopy is the ideal tool for in vivo fluorescence imaging of biological specimen up to one millimeter in size. In its original conception, a cylindrical lens is used to generate a thin sheet of light that is injected from the side to illuminate the focal plane of the detection lens (Voie, 1993; Huisken, 2004). Due to this confinement of the illumination, out-of-focus background is minimized. The propagation length over which the minimal thickness is maintained is called the Rayleigh length. It defines the distance,
| (1) |
at which the cross section of a Gaussian beam has doubled. The beam waist at the focal point, ω0, can be approximated by
| (2) |
where λ is the light wavelength and NA the numerical aperture of the focusing lens. Consequently, while the axial resolution increases only linearly with increasing NA, the field of view (FOV) with optimal axial resolution decreases with the square of the NA. This relation results in a fundamental design problem, i.e., a large FOV is not compatible with high axial resolution. For example, with 488-nm excitation light, a Rayleigh length of 500 μm restricts the sheet thickness to ~9 μm (NA 0.02) while a 1 μm thickness (NA 0.3) results in a Rayleigh length of only 6.4 μm. To overcome this limitation, various ways to minimize beam divergence have been proposed. Sample illumination with non-Gaussian beams such as Bessel and Airy beams has lead to substantial improvements in the ratio of axial resolution to FOV (Fahrbach and Rohrbach, 2010; Planchon, 2011; Vettenburg 2014). However, those beams comprise side lobes that illuminate neighboring planes. To avoid image artifacts, either non-linear, multi-photon excitation or structured illumination and/or image deconvolution must be used. Another approach to maintain high axial resolution was derived from confocal slit scanning microscopy, in which the sample is scanned with a light sheet propagating along the optical axis of the detection lens. To suppress out-of-focus fluorescence, this line is reimaged on a slit and can be detected by subsequent scanning across a camera chip. Many modern sCMOS cameras feature a rolling shutter that can be configured as a virtual slit which simplifies the optical setup. In light sheet microscopy, where sample illumination occurs perpendicular to detection, this ‘light sheet mode’ has been successfully applied to remove out-of-focus light (Baumgart and Kubitscheck, 2012). Then again, the slit can also be scanned in the propagation direction of the light sheet. Hence, by moving the focus of a Gaussian sheet with the slit, the minimum sheet thickness can be maintained over the entire FOV independent of the Rayleigh length. Until now, remote focusing of the light sheet was achieved either via a third objective lens imaging the sheet onto a mirror moved with a piezoelectric stage (Dean, 2015) or with a tunable acoustic gradient (TAG) lens (Zong, 2015; Dean and Fiolka, 2014). Piezo stages are relatively slow and/or limited in range of motion. This results in maximum frame rates of ~10 fps and a field of view restricted to <300 μm. Also, the required optical and mechanical elements might be difficult to include in an existing setup. TAG lenses, on the other hand, typically have to be driven with a sine wave at frequencies of >100 kHz, which is not appropriate for synchronization with a camera. This lack of synchronization again leads to a side lobe structure generating out-of-focus blur that has to be rejected by either using multi-photon excitation or a confocal detection scheme with linear deconvolution. Instead, we demonstrate here how an inexpensive electrically tunable lens (ETL) can be used to create a uniform light sheet of minimal, diffraction-limited thickness at high frame rates (~40 fps) over a wide field of view (>600 μm) without the need for multi-photon excitation or image deconvolution.
MATERIALS AND METHODS
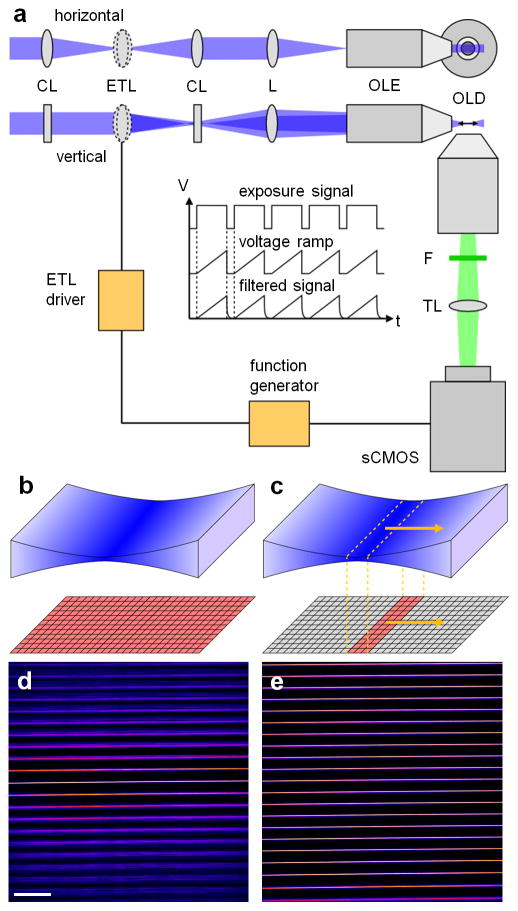
In most selective plane illumination microscopy (SPIM) setups, a cylindrical lens is used to generate the light sheet which is then reimaged into the sample via an objective lens. With an electrically tunable cylindrical lens, the position of the focus could be rapidly adjusted. Unfortunately, electrically tunable cylindrical lenses are currently not commercially available. Yet, a regular tunable lens combined with a few conventional optical elements can be used to achieve the desired effect. In our setup, as depicted in Fig. 1(a), the expanded, parallel excitation beam (488 nm, ISS, Champaign, IL, USA) first passed a telescope consisting of two identical cylindrical lenses, CL (f = 75 mm, LJ1703RM, Thorlabs, Newton, NJ, USA). The electrically tunable lens, ETL (EL-10-30-VIS-LD, Edmund Optics, Barrington, NJ, USA), was positioned in the center of the telescope and, hence, illuminated with a line profile. Consequently, the effective numerical aperture of the tunable lens was close to zero along the focusing, horizontal axis of the cylindrical lens pair while the full numerical aperture was utilized along the non-focusing, vertical axis. The beam exiting the telescope was parallel along the horizontal axis independent of the focal length of the tunable lens while the focal point along the vertical axis could be modulated. Since focusing of the beam into the back aperture of the excitation lens, OLE (CFI Plan Fluor 10XW, NA 0.3, Nikon, Melville, NY, USA), flipped the light sheet axis, a forth relay lens, L (f = 75 mm, AC254-075-A, Thorlabs), was required to maintain the correct modulation axis. Fluorescence was picked up by the detection lens, OLD (XLUMPLFLN 20XW, NA 1, Olympus, Center Valley, PA, USA), and imaged onto the chip of a sCMOS camera (Zyla 4.2, Andor, Belfast, Ireland) via a tube lens, TL (#47-740, Edmund Optics), after filtering with a long pass, F (LP500, Semrock, Rochester, NY, USA). Whole camera chip exposure with a static light sheet is illustrated in Fig. 1(b). To realize the desired confocal slit detection depicted in Fig. 1(c), the rolling shutter of the camera was operated in ‘light sheet mode’ while the focus of the light sheet was modulated with the ETL. To synchronize the ETL with the virtual slit detection, a function generator (DS345, SRS, Sunnyvale, CA, USA) generating the voltage ramp to drive the lens was triggered by the camera exposure signal, see inset in Fig. 1(a). A 5 ms pause between frames was introduced to allow the tunable lens to return to the initial focal position. In addition, the signal from the function generator was filtered with a home-built first order low pass with a cutoff frequency of 210 Hz to suppress oscillations of the lens (refer to datasheet for EL-10-30-VIS-LD available at www.optotune.com). These electronics as well as a voltage follower to eliminate loading effects on the function generator were included in a home-built ETL driver. Typical ETL driving amplitudes were 0.36 V with an offset voltage of 0.5 V. Samples were positioned and imaged as previously described (Hedde and Gratton, 2015; Hedde, 2015). To measure the thickness of the light sheet, we imaged the reflection off a microscope cover slip after removal of the detection filter. Figure 1(d,e) shows a montage of 21 light sheet images acquired at different y positions using a static and a modulated light sheet. Without modulation, the strong divergence of the Gaussian beam caused a rapid loss in axial resolution with increasing distance from the focus of the sheet. However, by moving the focus in sync with the virtual slit of the camera, the minimum width of the sheet could be maintained over the entire FOV.
Fig. 1.
Schematic of the light sheet microscopy setup, refer to text for details. (b,c) Illustration of whole camera chip exposure with a static light sheet as opposed to using an axially scanned light sheet with virtual slit detection. (d,e) Montage of 21 light sheet images acquired at different y positions with a static and an axially scanned light sheet. Scale bar, 100 μm.
RESULTS
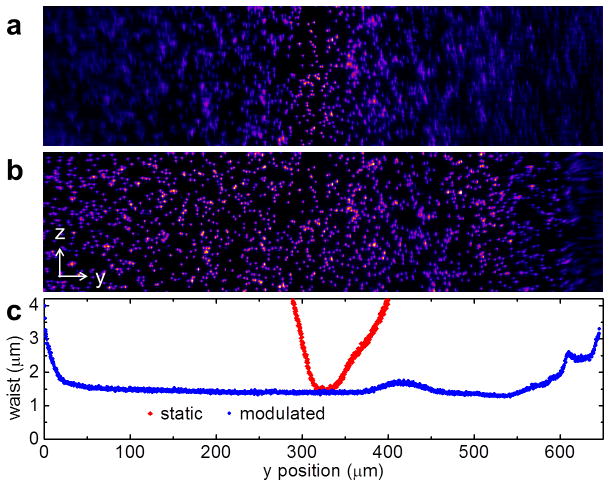
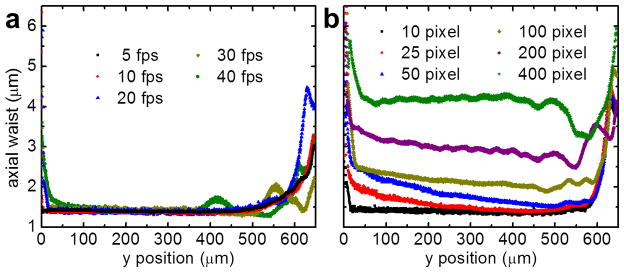
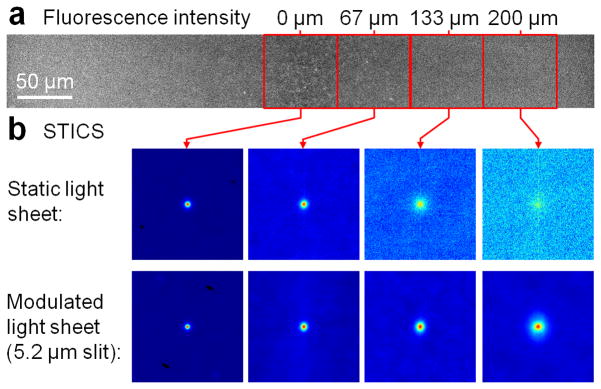
In order to evaluate the performance of our approach, we prepared a tissue phantom consisting of 1% low melting agarose (16520-050, Invitrogen, Carlsbad, CA, USA) doped with 1 μm yellow-green fluorescent microspheres (F-8823, Invitrogen) and subjected the sample to SPIM microscopy with a static as well as a modulated light sheet with synchronized virtual slit detection. Projections of the resulting image stacks along the x axis are shown in Fig. 2(a,b). The laser power was 0.28 W/cm2 at the sample plane. By fluorescence excitation with a static light sheet, the beads are clearly resolved only close to the focal point of the light sheet. The loss of axial resolution with increasing distance from the center is accompanied by increased background from out-of-focus fluorescence. By light sheet modulation with synchronized readout, the beads can be clearly resolved over more than 95% of the FOV. Only a ~30 μm wide disturbance is visible at the right edge of the image due to retracing of the lens. We then removed the fluorescence filter from the detection path and continuously imaged the reflection of the light sheet off a microscope cover slip while changing the distance with respect to the focal plane of the detection lens. By fitting of a Gaussian to the line profiles along the y axis of those images, the width of the sheet was quantified and is plotted in Fig. 2(c). It can be seen that with the modulated, synchronized light sheet the waist (1/e2 radius) can be maintained at 1.5 μm over more than 90% of the FOV (650 μm) using an excitation NA of 0.3. On the contrary, with a static, Gaussian light sheet, the y range of maximum axial resolution is limited to ~30 μm corresponding to only ~5% of the FOV. The data shown in Fig. 2 was acquired at 40 fps and a slit width of 10 camera pixels (3.2 μm). The total time per frame of 25 ms consisted of 20 ms of camera exposure followed by a 5 ms retracing pause resulting in an 80% duty cycle. The retracing pause allowed the focus of the ETL to return and settle at the initial position. The thickness of the light sheet at different frame rates is shown in Fig. 3(a) and can be generally considered flat over >90% of the field of view. Note that, with our camera, the minimum readout time of a full frame in ‘light sheet mode’ was 20 ms (50 fps), limiting the effective frame rate to 40 fps. With a faster camera, higher frame rates would be possible at the expense of a reduced duty cycle. Besides the NA of the excitation lens, the width of the virtual slit also determines the axial resolution, as plotted in Fig 3(b). Besides being able to achieve high frame rates, a good axial sectioning capability is essential for the application of fluorescence fluctuation spectroscopy-based approaches in thick specimen (>1 μm). As an example, we prepared a solution of 20 nm beads, the fluorescence intensity image of a 2,048 pixel long and 256 pixel wide strip along the propagation direction of the light sheet is shown in Fig. 4(a). An image time series of the same field of view was acquired with and without light sheet modulation with synchronized readout at a frame rate of 40 fps. The spatiotemporal image correlation was then calculated within regions of 256 by 256 pixels at increasing distances relative to the center of the field of view, as shown in Fig. 4(b). Without light sheet modulation, the lack of axial resolution towards the periphery of the sheet resulted in a rapid loss of the correlation amplitude. With modulation and synchronized readout, on the other hand, the correlation amplitude was much better maintained. However, it has to be said that an increase of the axial resolution by reducing the camera slit size decreases light throughput. Therefore, the slit size has to be optimized with respect to the signal available.
Fig. 2.
Fluorescence images of 1 μm yellow-green beads embedded in a 1% agarose gel without (a) and with axial light sheet modulation (b). (c) Plot of the corresponding light sheet waist over y position without (red diamonds) and with axial light sheet modulation (blue circles).
Fig. 3.
Plot of the light sheet waist over y position at different frame rates (a) and different virtual slit sizes (b). One pixel, 315 nm.
Fig. 4.
STICS analysis of 20 nm beads diffusing in water solution. (a) Fluorescence intensity image of a 256 by 2,048 px2 strip. (b) Spatiotemporal image correlation of 256 by 256 px2 regions of interest at four different positions along the light sheet propagation axis indicated by red boxes in (a) without (top row) and with (bottom row) light sheet modulation (lag time 25 ms).
DISCUSSION
In this work, we have demonstrated that an inexpensive electrically tunable lens can be used to modulate the focal position of the light sheet in selective plane illumination microscopy. Previously, this remote focusing ability has been used to improve the speed of volume imaging (Fahrbach, 2013; Nakai, 2015). Instead, we combined remote focusing via an electrically tunable lens with synchronized, virtual slit detection to maintain the maximum, diffraction-limited axial resolution independently of the size of the field of view. In order to maintain a 1.5 μm width over 600 μm we used less than 10% of the available tuning range (0.36 V modulation amplitude compared to a 4 V maximum rating of the electrically tunable lens). With different combinations of excitation/detection lenses this vast tuning range could be further exploited to increase resolution and/or field of view. At the same time, current sCMOS camera frame rates (~50 fps in ‘light sheet mode’) are supported due to the rapid response of an ETL compared to, e.g., a piezo stage (Annibale, 2015). Even faster lenses are available today and could handle speeds of >100 fps at a high duty cycle. Thus, this direct, deconvolution-free method is compatible with camera-based fluorescence fluctuation spectroscopy methods to probe spatiotemporal dynamics of biomolecules (Hedde, 2014) where the high axial resolution is crucial for the detection of the fluctuations due to single molecules. Compared to spatial light modulators or remote focusing objectives, an ETL is also easy to integrate into existing SPIM setups. Many sCMOS cameras already feature a rolling shutter that can act as a virtual slit or can be upgraded on site with new firmware.
Acknowledgments
We thank Rachel Cinco for helpful discussion. This work was supported by National Institutes of Health (NIH) P41 GM103540 and P50 GM076516 grants. The authors declare no conflict of interest.
References
- Annibale P, Dvornikov A, Gratton E. Electrically tunable lens speeds up 3D orbital tracking. Biomed Opt Express. 2015;6:2181–2190. doi: 10.1364/BOE.6.002181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart E, Kubitscheck U. Scanned light sheet microscopy with confocal slit detection. Opt Express. 2012;20:21805–21814. doi: 10.1364/OE.20.021805. [DOI] [PubMed] [Google Scholar]
- Dean KM, Fiolka R. Uniform and scalable light-sheets generated by extended focusing. Opt Express. 2014;22:26141–26152. doi: 10.1364/OE.22.026141. [DOI] [PubMed] [Google Scholar]
- Dean KM, Roudot P, Welf ES, Danuser G, Fiolka R. Deconvolution-free subcellular imaging with axially swept light sheet microscopy. Biophys J. 2015;108:2807–2815. doi: 10.1016/j.bpj.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrbach FO, Rohrbach A. A line scanned light-sheet microscope with phase shaped self-reconstructing beams. Opt Express. 2010;18:24229–24244. doi: 10.1364/OE.18.024229. [DOI] [PubMed] [Google Scholar]
- Fahrbach FO, Voigt FF, Schmid B, Helmchen F, Huisken J. Rapid 3D light-sheet microscopy with a tunable lens. Opt Express. 2013;21:21010–21026. doi: 10.1364/OE.21.021010. [DOI] [PubMed] [Google Scholar]
- Hedde PN, Stakic M, Gratton E. Rapid measurement of molecular transport and interaction inside living cells using single plane illumination. Sci Rep. 2014;4:7048. doi: 10.1038/srep07048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedde PN, Gratton E. Active focus stabilization for upright selective plane illumination microscopy. Opt Express. 2015;23:14707–14714. doi: 10.1364/OE.23.014707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedde PN, Ranjit S, Gratton E. 3D fluorescence anisotropy imaging using selective plane illumination microscopy. Opt Express. 2015;23:22308–22317. doi: 10.1364/OE.23.022308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisken J, Swoger J, Del Bene F, Wittbrodt J, Stelzer EHK. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science. 2004;13:1007–1009. doi: 10.1126/science.1100035. [DOI] [PubMed] [Google Scholar]
- Nakai Y, Ozeki M, Hiraiwa T, Tanimoto R, Funahashi A, Hiroi N, Taniguchi A, Nonaka S, Boilot V, Shrestha R, Clark J, Tamura N, Draviam VM, Oku H. High-speed microscopy with an electrically tunable lens to image the dynamics of in vivo molecular complexes. Rev Sci Instrum. 2015;86:013707. doi: 10.1063/1.4905330. [DOI] [PubMed] [Google Scholar]
- Planchon TA, Gao L, Milkie DE, Davidson MW, Galbraith JA, Galbraith CG, Betzig E. Rapid three-dimensional isotropic imaging of living cells using Bessel beam plane illumination. Nat Methods. 2011;8:417–423. doi: 10.1038/nmeth.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vettenburg T, Dalgarno HIC, Nylk J, Coll-Lladó C, Ferrier DEK, Čižmár T, Gunn-Moore FJ, Dholakia K. Light-sheet microscopy using an Airy beam. Nat Methods. 2014;11:541–544. doi: 10.1038/nmeth.2922. [DOI] [PubMed] [Google Scholar]
- Voie H, Burns DH, Spelman FA. Orthogonal-plane fluorescence optical sectioning: three dimensional imaging of macroscopic biological specimens. J Microsc. 1993;170:229–236. doi: 10.1111/j.1365-2818.1993.tb03346.x. [DOI] [PubMed] [Google Scholar]
- Zong W, Zhao J, Chen X, Lin Y, Ren H, Zhang Y, Fan M, Zhou Z, Cheng H, Sun Y, Chen L. Large-field high-resolution two-photon digital scanned light-sheet microscopy. Cell Research. 2015;25:254–257. doi: 10.1038/cr.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]