Abstract
Acute systemic toxicity testing provides the basis for hazard labeling and risk management of chemicals. A number of international efforts have been directed at identifying non-animal alternatives for in vivo acute systemic toxicity tests. A September 2015 workshop, Alternative Approaches for Identifying Acute Systemic Toxicity: Moving from Research to Regulatory Testing, reviewed the state-of-the-science of non-animal alternatives for this testing and explored ways to facilitate implementation of alternatives. Workshop attendees included representatives from international regulatory agencies, academia, nongovernmental organizations, and industry. Resources identified as necessary for meaningful progress in implementing alternatives included compiling and making available high-quality reference data, training on use and interpretation of in vitro and in silico approaches, and global harmonization of testing requirements. Attendees particularly noted the need to characterize variability in reference data to evaluate new approaches. They also noted the importance of understanding the mechanisms of acute toxicity, which could be facilitated by the development of adverse outcome pathways. Workshop breakout groups explored different approaches to reducing or replacing animal use for acute toxicity testing, with each group crafting a roadmap and strategy to accomplish near-term progress. The workshop steering committee has organized efforts to implement the recommendations of the workshop participants.
Keywords: Acute systemic toxicity, alternatives, LD50, oral, dermal, inhalation, 3Rs, in vitro, in silico
1. Introduction
All substances are potentially toxic at sufficiently high doses. Regulatory authorities often require testing of substances to characterize their toxicity. Testing information is then used to assign substances to toxicity categories, which determine the content of product labels to indicate precautions to be taken while handling. Acute systemic toxicity tests are conducted for these purposes via three routes of exposure: oral, dermal, and/or inhalation.
The traditional in vivo tests commonly included in regulatory test guidelines generate an LD50, which is the dose that produces lethality in 50% of the animals tested. However, substantial progress has been made in recent decades in reducing and refining animal use for these tests. The first acute oral toxicity test accepted as a test guideline by the Organisation for Economic Co-Operation and Development (OECD) in 1987 (Test Guideline 401) was deleted in 2002 in favor of versions designed to use fewer animals (Test Guidelines 420, 423, and 425; (OECD 2002; OECD 2002; OECD 2008). Two acute inhalation OECD test guidelines exist, with Test Guideline 436 being a reduction alternative to Test Guideline 403; in addition, a draft Test Guideline 433 is currently being developed as a reduction and refinement alternative to Test Guideline 403 (OECD 2009; OECD 2009; OECD 2015). OECD Test Guideline 402 for acute dermal toxicity testing is generally used only following confirmation of dermal penetration by Test Guideline 428 or other method (OECD 1987; OECD 2004). In March 2016, EPA published a draft policy to waive all acute lethality dermal studies for formulated pesticide products and registrants can submit waiver requests through existing processes (www.epa.gov/pesticides/new-epa-guidance-testing-pesticides-will-reduce-animal-testing). Additionally, in 2016, the OECD published a guidance document on considerations for waiving or bridging of mammalian acute toxicity tests that is applicable to chemical pesticides and other substances (OECD 2016).
More recent international efforts have been directed at identifying non-animal alternatives for in vivo acute systemic toxicity tests. The multiple mechanisms associated with acute systemic toxicity preclude a single cell- or biochemical-based assay serving as a full replacement, but integrating data from multiple assays with other physiologically relevant information (e.g., water solubility, molecular weight) about the test substance or a structurally similar substance could provide sufficient information to predict toxicity. Computational models to integrate these data and information, built using machine learning approaches that leverage existing data, physicochemical properties, and other information to predict a substance’s toxicity, are becoming widely used in toxicology.
With the goal of identifying approaches that could potentially replace animal use for required acute systemic toxicity testing, an international group of experts convened in September 2015 to discuss progress and challenges associated with the development, validation, and implementation of alternatives. The workshop on Alternative Approaches for Identifying Acute Systemic Toxicity: Moving from Research to Regulatory Testing included more than 60 representatives from regulatory agencies, academia, nongovernmental organizations, and industry.
The objectives of the workshop were to:
review the regulatory landscape in order to define when and how acute systemic toxicity data are used,
review the state-of-the-science for alternative approaches to identifying acute systemic toxicity, including mechanism-based models, in vitro and in silico approaches, lower vertebrate and invertebrate models, and adverse outcome pathway (AOP)-based approaches,
identify mechanisms of acute systemic toxicity and relevant AOP data and data gaps in order to promote development of AOPs for acute toxicity testing applications, and
set a defined plan to reach the workshop goal.
In the months leading up to the workshop, the organizing committee convened a series of webinars in order to provide necessary background to inform the workshop discussions (Table 1). The webinars provided specifics on current strategies and approaches to acute systemic toxicity testing.
Table 1.
List of webinar presentations held prior to the workshop.
| Speaker | Affiliation | Title | |
|---|---|---|---|
| Webinar 1 | Dr. Pilar Prieto | European Union Reference Laboratory for Alternatives to Animal Testing |
EURL ECVAM’s strategy to replace, reduce, and refine the use of animals in the assessment of acute mammalian systemic toxicity and an industry perspective |
| Dr. Lawrence Milchak |
3M Company | ||
| Webinar 2 | Dr. Stefan Schulz |
Helmholtz Center for Environmental Research |
High-throughput models and zebrafish embryos as alternatives to assess acute systemic toxicity |
| Dr. Daniel Wilson |
The Dow Chemical Company |
||
| Webinar 3 | Dr. Michael Bolger |
Simulations Plus | Estimation of acute toxicity using in vitro to in vivo extrapolation; acute oral toxicity modeling |
| Dr. Ann Detroyer |
L’Oréal | Acute oral toxicity modeling accounting for mechanism and toxicological mode of action |
|
| Webinar 4 | Dr. Anna Forsby | Stockholm University and Swedish Toxicology Sciences Research Center (Swetox) |
Neuronal specific modes of action of acute systemic toxicity |
| Webinar 5 | Dr. Stephen Ferguson |
National Institute of Environmental Health Sciences |
Tox21 phase III: in vitro and in silico approaches to predict xenobiotic metabolism and toxicity potential |
| Dr. Harvey Clewell |
The Hamner Institutes for Health Sciences |
Extrapolation of in vitro toxicity assay results to provide information regarding acute in vitro exposures |
|
| Webinar 6 | Mr. John Redden | U.S. EPA | How the U.S. EPA Pesticide Program uses inhalation toxicity studies |
| Dr. Patrick Hayden | MatTek Corporation | Use of EpiAirway™ organotypic human airway models and assay methods for in vitro inhalation toxicology screening |
|
| Dr. Samuel Constant | Epithelix Sàrl | The use of human airway tissue-based assays (MucilAir™) for acute toxicity assessment |
The workshop was composed of five sessions followed by breakout groups and a final main group discussion and wrap-up; a detailed agenda and speaker presentations are available on the National Toxicology Program website (http://ntp.niehs.nih.gov/go/atwksp-2015). This report summarizes the proceedings and outcome of the workshop.
2. The regulatory landscape: When is acute toxicity data required and how is it used?
A critical first step in identifying acceptable alternative approaches for acute toxicity testing is a survey of the national and international regulatory requirements for such testing. While there are similarities across regulatory authorities, regional and interagency differences exist that impact the utility of proposed alternatives. In addition, it is important to understand both the formal legislative requirements for acute toxicity testing and the manner in which the respective regulatory authorities evaluate and apply testing results. Examples of requirements for and uses of acute toxicity testing data for three regulatory agencies were presented at the workshop: The U.S. Consumer Product Safety Commission, the U.S. Environmental Protection Agency, and Health Canada.
2.1 U.S. Consumer Product Safety Commission
Requirements for acute systemic toxicity data at the U.S. Consumer Product Safety Commission (CPSC) are associated with specific regulatory legislation (Table 2). The Federal Hazardous Substances Act (FHSA) and Poison Prevention Packaging Act (PPPA) provide specific examples to illustrate CPSC requirements. The FHSA takes into account exposure and requires a case-by-case hazard assessment. Toys and other articles intended for children and containing a hazardous substance are banned.1 Products not specifically intended for children may require precautionary labeling.2 The PPPA requires child resistant packaging to protect children from serious personal injury or serious illness resulting from handling, using, or ingesting hazardous household substances. The responsibility for testing is placed on the manufacturer and involvement by the CPSC occurs only when there is a perceived problem with a product. In general, CPSC tends to focus on susceptible populations with children being a primary focus.
Table 2.
Regulatory statutes governing systemic toxicity data at the U.S. Consumer Product
| Statute | Regulatory Legislation |
|---|---|
| Consumer Product Safety Act | 15 U.S.C. § 2051–2084 |
| Federal Hazardous Substances Act (FHSA) | 15 U.S.C. § 1261–1278 |
| Labeling of Hazardous Art Materials Act (LHAMA) | 15 U.S.C. § 1261–1278 |
| Poison Prevention Packaging Act (PPPA) | 15 U.S.C. § 1471–1477 |
| Toys and other articles intended for children and containing a hazardous substance are banned |
15 U.S.C. § 1261(q)(1) |
| Products not specifically intended for children may require precautionary labeling |
15 U.S.C. § 1261(p) |
CPSC defines acute systemic toxicity as toxicity occurring from a single event or short-term exposure. CPSC’s assessment of acute toxicity attempts to identify hazard and relies on exposure assessment, a dose-response evaluation, and risk characterization, with preference for available human data. Potential exposure to the product is evaluated relative to a derived exposure limit. A product may be a “hazardous substance” if the estimated exposure exceeds the acceptable daily intake based on either a no-observed-adverse-effect level or a lowest-observed-adverse-effect level. Establishment of an acceptable daily intake includes uncertainty factors for extrapolation from animal data to human, protection for sensitive populations, and accounting for cases where a no-observed-adverse-effect level is not established. While the FHSA in particular does not require manufacturers to perform any specific battery of tests to assess the potential risk of hazards, the manufacturer is required to label appropriately and in accordance with FHSA requirements. For the last five years, CPSC staff have used existing data in assessing acute hazards.
The CPSC updated its animal testing policy in 2013. The FHSA does not require that any animal tests be performed to determine whether a hazard exists. Therefore, the CPSC and its staff strongly encourage the use of scientifically valid alternatives to animal testing, noting that methods that have not been formally validated may be acceptable (CPSC 2012). For each of three exposure routes (oral, dermal, and inhalation), the FHSA distinguishes two levels of acute toxicity, highly toxic and toxic, from substances that are not toxic and therefore do not require labeling. These terms are defined in 16 CFR § 1500.3, along with a description of the traditional method for determining the acute toxicity endpoint, the LD50 or the LC50. Currently, there are no CPSC-approved non-animal methods for inhalation and dermal toxicity testing. However, CPSC staff recommends the revised oral up-and-down procedure for determining acute oral toxicity for the purpose of classification and labeling under the FHSA. CPSC staff have also determined that in vitro basal cytotoxicity tests are appropriate for determining a starting dose for the oral LD50 test. The test guidelines are referenced in CPSC staff’s response to the Interagency Coordinating Committee for the Validation of Alternative Methods on the use of in vitro basal cytotoxicity test methods for estimating starting doses for acute oral systemic toxicity testing (ICCVAM 2006), as well as on the CPSC animal testing policy webpage (www.cpsc.gov/en/Business--Manufacturing/Testing-Certification/Recommended-Procedures-Regarding-the-CPSCs-Policy-on-Animal-Testing/).
2.2 U.S. Environmental Protection Agency
2.2.1 Office of Pesticide Programs
Under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), the U.S. Environmental Protection Agency (EPA) Office of Pesticide Programs (OPP) regulates and has acute toxicity testing requirements for both antimicrobials and conventional pesticides. Acute systemic toxicity data are required for both the technical grade active ingredient and the end-use product. Registration of these substances requires data from the so-called “six pack” test battery, which may include some or all of the following: acute oral toxicity in rats, acute dermal toxicity in rats or rabbits, acute inhalation toxicity in rats, primary ocular and dermal irritation in rabbits, and dermal sensitization in guinea pigs or mice. The six pack data or related assessments are used to label formulations for personal protective equipment requirements (see www.epa.gov/sites/production/files/2015-09/documents/ghscriteria-summary.pdf). The dermal LD50 data for technical grade active ingredients are also often used in ecological risk assessments.
OPP accepts data from alternative test methods for eye irritation, skin irritation, and skin sensitization, and has opportunities for bridging or waiving acute systemic toxicity testing (U.S. EPA OPP 2012; U.S. EPA OPP 2015 (revised)). Registrants may cite existing data for substantially similar substances, or seek a waiver for substances that are, for example, corrosive. In 2016, OPP issued final guidance titled “Process for Evaluating and Implementing Alternative Approaches to In Vivo Acute Toxicity Studies for FIFRA Regulatory Use” (www.epa.gov/sites/production/files/2016-03/documents/final_alternative_test_method_guidance_2-4-16.pdf), and released for public comment draft guidance on waving acute dermal toxicity tests for pesticide formulations (www.epa.gov/pesticides/new-epa-guidance-testing-pesticides-will-reduce-animal-testing).
2.2.2 Office of Pollution Prevention and Toxics
A company intending to manufacture or import a chemical that is not currently in the U.S. marketplace is required under the Toxic Substances Control Act (TSCA) to submit available information about the characteristics, production, and use of the chemical to the EPA Office of Pollution Prevention and Toxics (OPPT). OPPT then has 90 days to review the chemical information, which it uses to identify possible concerns and develop safe handling recommendations. TSCA requires EPA to determine that new chemicals are not likely to present unreasonable risk, and to facilitate the use of alternative methods. OPPT commonly uses non-testing approaches such as predictive models and professional judgment to assess potential risks. The OPPT Structure Activity Team identifies information for the test chemical and structural analogs from the submission, as well as internal and public databases. For human health hazard evaluation, the Structure Activity Team determines the likelihood of absorption (by route), identifies possible effects and concerns based on all information sources including submitted data, data with analogs, and professional judgment. The information is then used for hazard identification for use in screening-level risk assessment. In terms of acute toxicity information, the primary concern of OPPT in human health risk assessment is developing information for a Safety Data Sheet to ensure worker safety and appropriate personal protective equipment. EPA currently reviews data on approximately 1,000 new chemicals per year. By comparison, the TSCA inventory of chemicals includes over 84,000 chemicals with approximately 22,400 chemicals having been added since the initial TSCA inventory in 1979. Of the new chemicals added to the TSCA inventory, approximately 10–15% have testing data, typically consisting of acute systemic toxicity data, data on skin and eye irritation, and data on genotoxicity collected using the Ames assay.
2.3 Health Canada
Health Canada’s Pest Management Regulatory Agency (PMRA) regulates pesticides under the Pest Control Products Act, which requires submission of acute toxicity information for the technical grade active ingredient, integrated system product, or end-use product. The required tests are the same as those listed above for EPA OPP, with testing conducted according to OECD test guidelines under good laboratory practice standards. Acute toxicity data is used to determine pesticide labeling for hazard, personal protective equipment, and first aid according to the PMRA classification and labeling system.
Canada is adopting the United Nations Globally Harmonized System of Classification and Labelling (GHS) in order to align with its trading partners. Health Canada implemented GHS for the Workplace Hazardous Material Information System in February 2015 and GHS now applies to workplace chemicals with phased implementation to 2018. For pesticides, implementation will begin with Safety Data Sheets. Health Canada will develop guidelines on Safety Data Sheets for commercial, manufacturing, and restricted class products in consultation with industry.
3. State-of-the-science for acute toxicity testing alternatives
3.1 In vitro methods
The development and use of in vitro alternatives for acute toxicity testing is growing rapidly. Goh et al. catalogued the number of in vitro tests carried out by seven pharmaceutical companies, noting that very few in vitro tests were conducted through the mid-1990’s (Goh, Weaver et al. 2015). However, the first decade of the 21st century marked a period of explosive growth in in vitro testing for genotoxicity, safety pharmacology, and absorption-distribution-metabolism-excretion (ADME), eclipsing 130,000 individual tests per year by 2010. In addition, there have been a number of large collaborative efforts to identify effective non-animal approaches to predict acute toxicity potential, particularly in the European Union. These collaborations include the Multicenter Evaluation of In Vitro Cytotoxicity (MEIC) program (Ekwall, Barile et al. 1998) and the ACuteTox project. The European Union Reference Laboratory for alternatives to animal testing (EURL ECVAM) database service on Alternative Methods to animal experimentation (DB-ALM, http://ecvam-dbalm.jrc.ec.europa.eu), now lists 25 protocols for alternative test methods that are relevant to acute systemic toxicity testing.
Much research on alternatives to acute systemic toxicity testing has centered on the use of in vitro cytotoxicity assays, leading to the development of the 2014 EURL ECVAM strategy document on replacing, reducing, and refining animal use in acute systemic toxicity testing (EURL ECVAM 2014). Aim 1 as identified in the document is reduction and replacement of animal testing, and the 3T3 neutral red uptake (NRU) toxicity test is an important underpinning of that effort. Currently EURL ECVAM is exploring the use of quantitative structure-activity relationship (QSAR) modelling to increase confidence in the 3T3 NRU and to account for the lack of metabolism in 3T3 cells.
Acute systemic toxicity often involves a target organ including brain, liver, kidney, or lung. Attempts are being made to complement 3T3 NRU results with mechanistically relevant, target organ information. This work is ongoing, with 115 toxic substances mapped to up to eight target organs. In short, the 3T3 NRU method could form a valuable part of an integrated testing strategy to identify non-classified substances (acute oral LD50>2000mg/kg b.w.). Work is also ongoing at EURL ECVAM to improve 3T3 NRU test method accuracy for acutely toxic substances with a range of physico-chemical properties using QSAR modelling of metabolism and incorporation of mechanistic data on specific target organs. In addition, EURL ECVAM has proposed an approach to identify non-toxic substances (LD50> 2,000mg/kg) using information from 28-day repeated dose toxicity studies, and thereby avoiding acute systemic toxicity testing (Bulgheroni, Kinsner-Ovaskainen et al. 2009; Graepel, Asturiol et al. 2016).
Additional studies should focus on determining if bioavailability data could be used to improve predictivity of the 3T3 NRU assay for all categories. Inclusion of other mechanistic data might improve the predictive capabilities of the method. Findings from the European Union funded ACuteTox project (www.acutetox.eu) suggest that additional in vitro-derived data regarding organ specific toxicity will improve the predictivity further (Cerrato, Valeri et al. 2009; Kinsner-Ovaskainen, Prieto et al. 2013; Prieto, Kinsner-Ovaskainen et al. 2013; Zurich, Stanzel et al. 2013).
3.2 Embryo-larval fish models
Embryo-larval fish are used with increasing frequency to screen the toxicity of compounds including use as a high throughput screen in the ToxCast battery of assays (Padilla, Corum et al. 2012). Unlike testing in traditional cell-based in vitro assays, screening of compounds in the fish permits evaluation of the toxic responses of an intake vertebrate model. Zebrafish, Danio rerio, and other small aquarium fish species, offer the additional advantages of rapid development, high fecundity, externally visible organs, and low maintenance costs when compared with traditional in vivo models. From a 3R’s perspective, prior to hatching, zebrafish embryos are not defined as a vertebrate animal by current NIH guidance (https://oacu.oir.nih.gov/sites/default/files/uploads/arac-guidelines/zebrafish.pdf). Lower vertebrates are also considered to have reduced welfare concerns compared with higher order vertebrates.
Test guidelines for determining acute toxicity of compounds using zebrafish embryos have been validated (OECD TG 236). While the purpose of the guideline is determination of acute toxicity in fish species, the conservation of biological pathways between fish and other vertebrate suggests utility of this model in acute toxicity testing. As stated in the guideline, the model has been successfully applied to a wide range of substances exhibiting diverse modes of action, solubility, volatility, and hydrophobicity (OECD TG 236). Data generated in zebrafish are also being used to model acute toxicity. Ducharme et al collected data from published studies using zebrafish embryos and correlated endpoints measured in the zebrafish to acute toxicity measured in rodent or rabbit (Ducharme, Reif et al. 2015). Results of their study show promise in the use of zebrafish embryo toxicity measures as a surrogate for traditional in vivo acute toxicity testing.
3.3 Quantitative structure-activity relationship models
Quantitative structure-activity relationships (QSARs) are statistically established correlations relating quantitative parameter(s) (e.g., physico-chemical properties) to a quantitative measure of biological activity. In silico methods such as QSAR models provide a promising approach to developing mechanistic models to predict human toxicity. Successful application of QSAR requires availability of a large database of substances that have relevant structural and target toxicity information to generate chemical descriptors such as PubChem (http://pubchem.ncbi.nlm.nih.gov/). QSAR allows association of chemical descriptors with toxicity for the substances in the database to generate a pattern matrix. The applicability domain of a QSAR model is directly dependent upon the training set of chemicals used to define the model. Building a model with a large, diverse training set helps ensure a broad applicability domain.
However, QSAR models can be poor at correctly predicting the toxicities of very similar substances in a biological system due to the steep drop-off in activity that can occur at a certain dose in in vivo systems. For example, in some cases QSAR models based on chemical descriptors did not correctly predict the human liver toxicity profile of similar substances compared to in vitro PubChem assays (Kim, Huang et al. 2016). The results of the study define a basic workflow including profiling biological responses from high throughput screening data, incorporating QSAR models to fill data gaps, and evaluating in vitro to in vivo correlations. The analysis identified toxicophores and assays that can be used to assess liver damage induced by oxidative stress. This type of workflow can be adapted to model other complex in vivo endpoints (Zhang, Hsieh et al. 2014; Zhu, Zhang et al. 2014). High throughput screening hits in an assay for the antioxidant response element (ARE) were retrieved from PubChem, matched with data from FDA liver toxicants, and combined with assays related to ARE activation and liver damage. Useful associations were defined by filtering the retrieved assays based on an association with both ARE and liver damage, by containing a positive association that occurs at a frequency greater than would be expected if it were random, by the presence of an in vitro assay representing the response, and finally by literature supporting the association.
3.4 Incorporating biokinetics and metabolism into alternative methods for acute toxicity testing
Toxicity is determined by the concentration and time of exposure to the critical substance, whether toxicity is attributable to the parent compound or a metabolite, at the critical site of action (Blaauboer, Boekelheide et al. 2012). Accordingly, using in vitro cytotoxicity data to estimate in vivo acute toxicity makes several assumptions, including (1) that basal cytotoxicity is a good predictor of acute toxicity in vivo; (2) that cytotoxic concentrations in vitro mirror blood plasma concentrations; and (3) that blood plasma concentrations mirror target tissue concentrations (Kopp-Schneider, Prieto et al. 2013). Application of these assumptions is complicated by the kinetic behavior or toxicity a substance may have towards a specific cell type or organ (Blaauboer, Hermens et al. 2005; Clemedson, Blaauboer et al. 2005). It is also important to consider that substances with high basal cytotoxicity would be expected to produce effects at the application site: skin, lungs, or gastrointestinal tract, depending on the route of exposure. In contrast, systemic toxicity would only manifest if the process of toxicity is less rapid, if other cell types are more sensitive, or if the ultimate toxicant is a biotransformation product that acts at the site of metabolism. Furthermore, in vitro cytotoxicity results can be expected to predict systemic effects well only if the quality of in vitro experimental data and in vivo reference data are good and the concentrations producing effects in vitro are relevant to in vivo target tissue concentration.
Predicting toxicity from in vitro data requires an accurate assessment of the active substance concentration at the target site required to produce the toxic effect. Target site concentration is in turn determined by the biokinetic behavior of the substance within the organism. If in vivo absorption is limited, in vitro toxic concentrations will overestimate in vivo toxicity. This hypothesis is realized in the validation data for the 3T3 NRU cytotoxicity assay, which has good overall accuracy but a rather high false positive rate (Prieto, Cole et al. 2013). In vivo distribution also often leads to higher or lower concentrations at the target site based upon the substance’s properties. For example, lipophilic substances partition into adipose tissue (where they are less bioavailable) and the central nervous system due to its high lipid content. In a similar fashion, bioavailability is decreased by chemical binding to plasma proteins. Estimating the unbound fraction of a chemical improves dose-response calculations (Groothuis, Heringa et al. 2015). Additionally, biokinetic activity is influenced by metabolism; rapid metabolism results in rapid elimination or generation of toxic metabolites and rapid excretion or exhalation will quickly reduce in vivo concentrations (Coecke, Ahr et al. 2006).
Researchers should use in vitro biokinetics to correlate in vitro effective concentrations to a dose in vivo to improve the applicability of in vitro data to risk assessment (Groothuis, Heringa et al. 2015).
4. Applications and case studies
The following examples describe how the approaches described in the preceding sections have or could be used in an overall program to predict acute toxicity.
4.1 Development of a program to predict acute toxicity: Recommendations to the Department of Defense
The U.S. Department of Defense (DoD) needs to identify the substances that are most likely to be harmful to enlisted personnel, and is exploring the use of non-animal approaches to do this in a more efficient manner. The DoD recently commissioned the National Research Council report on Application of Modern Toxicology Approaches for Predicting Acute Toxicity for Chemical Defense (National Research Council 2015). This report was written by a committee of 10 experts assembled from academia, industry, and government, who were tasked with considering modern approaches for predicting toxicity and suggesting an overall conceptual approach for using such information to predict acute toxicity.
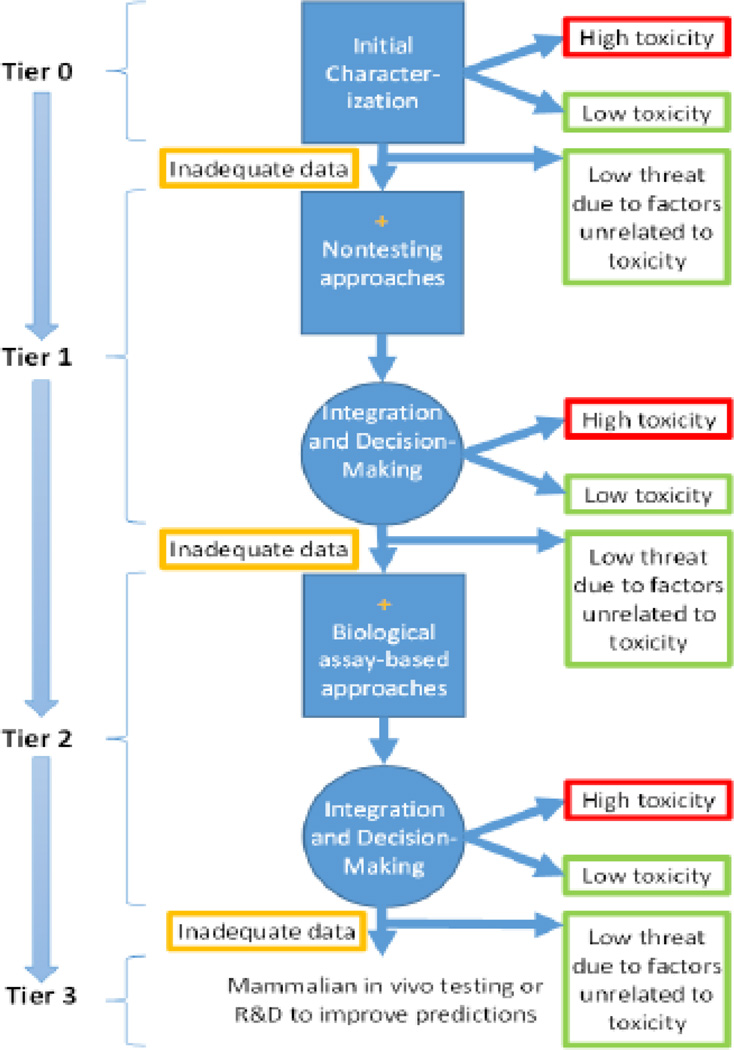
The conceptual framework described in the report is a proposed tiered testing strategy with multiple integration and decision points to allow chemical prioritization based on potential toxicity (Figure 1). A decision to move on to the next tier is not made before interpreting the existing information. The framework involves (1) collecting data on chemical structure, physicochemical properties, biochemical properties, and activity in cells or lower organisms, and (2) examining empirical correlations, toxicokinetics, and mechanistic pathways to draw conclusions about organ-system or whole-organism toxicity in mammals. This framework aims to predict whole-animal toxicity by using information from lower levels of complexity. Such an approach requires utilization of existing and experimental data on chemical structure, physicochemical properties, and biological activity. This information is analyzed using read-across, QSAR, concentration-response, toxicokinetic, and integrated models to provide toxicity estimate outputs. Outputs can be mechanism-specific, organ-specific, or nonspecific toxicity measures including acute systemic toxicity. However, curated databases needed to make such predictions are not currently available. The framework also considers other information such as the purpose of the testing and the level of confidence needed. Substances can be deselected from further testing at any tier due to low toxicity or low threat due to factors unrelated to toxicity.
Figure 1.
Proposed prioritization strategy for testing of chemicals for acute toxicity adapted from (National Research Council 2015)
The current state-of-the-science suggests that developing a predictive acute toxicity program will require extensive DoD investment in computational modeling approaches, assay development, methods for extrapolation of in vitro results to in vivo conditions, and data-integration methods. Accordingly, the report recommended that DoD should initiate pilot studies to evaluate chemical classes of highest concern with well-characterized reference substances. Such studies would allow DoD to (1) develop the novel assays and tools needed to predict acute toxicity efficiently and accurately; (2) evaluate the rate of false negatives and false positives; (3) examine how generalizable the results of various assays and tools are from one chemical class to another; and (4) begin to address the size of the chemical space needed to make predictions about unknown substances. The committee emphasized that DoD should leverage ongoing efforts with other activities within the U.S. government, such as the EPA ToxCast™ program, which would allow DoD to complete pilot studies more rapidly and maximize the return on its investment.
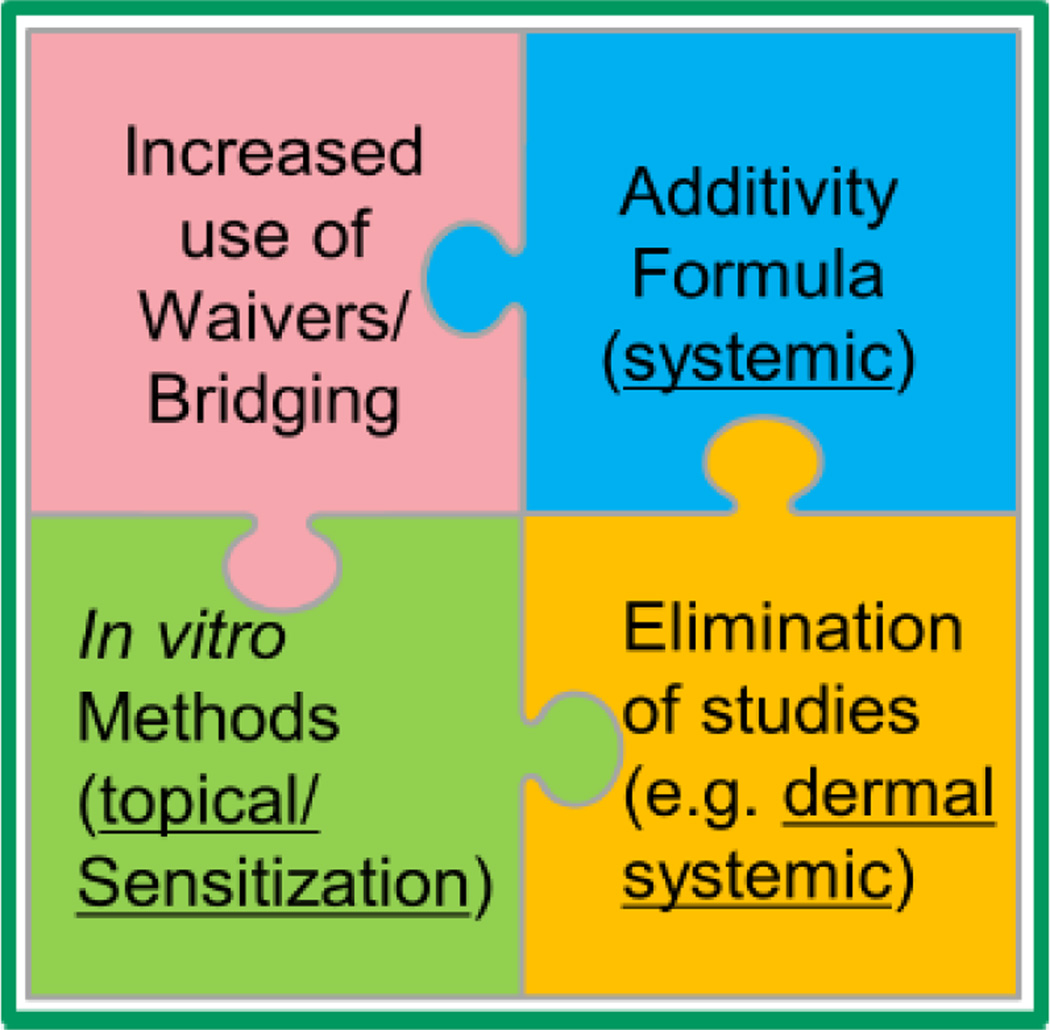
4.2 A proposed, multifaceted approach to animal-free acute toxicity assessments
Moving away from traditional animal tests will require incorporating multiple non-animal approaches, including integrating available data on individual formulation ingredients, waivers and bridging, and in vitro methods (Figure 2). Successful implementation of such approaches requires a global, coordinated effort amongst industry and regulators. As noted in the preceding sections describing EPA and Health Canada regulatory data requirements, drivers for conducting acute systemic toxicity testing for pesticides include hazard identification; classification and labeling for transport and worker and consumer protection; and risk assessment. Most regulatory authorities require data from the tests included in the EPA six pack for each active ingredient and also for each formulation; each six pack typically uses approximately 60 animals. Some countries may have additional requirements for testing active ingredients and agrochemical formulations. There are often multiple agrochemical formulations for each pesticide active ingredient that range in quantitative composition and physical state (i.e., liquid or dry formulations). Accordingly, alternative approaches applied specifically to formulations could significantly decrease the number animals used for evaluating the acute systemic toxicity of pesticides overall.
Figure 2.
Schematic representing the use of alternatives to move away from animal tests. Putting the pieces together involves multiple approaches and requires increased harmonization and cooperation among regulatory agencies and industry.
EPA OPP and Health Canada’s PMRA currently have guidance documents on waiving or bridging acute toxicity studies (U.S. EPA OPP 2012). For example, waivers may be based on physicochemical properties (e.g., volatility and extreme pH), exposure, and the technical feasibility of a toxicity test, while bridging and read-across may be based on existing data for similar formulations. Additionally, the GHS additivity formula can be used to classify mixtures based on the toxicity and concentration of its individual components (United Nations 2015). A recent retrospective analysis compared classifications predicted using historical in vivo data to classifications predicted based on the GHS additivity method for 225 agrochemical mixtures (Corvaro et al., submitted manuscript). For acute oral, dermal, and inhalation toxicity, accuracy for determining negatives versus positives (not classified/classified in 4 different classification systems) was considered sufficient to replace acute systemic toxicity testing with the GHS additivity formula (Corvaro et al., submitted manuscript). This approach has been applied to determine the classification for a novel formulation using read-across and the GHS additivity formula. Further work is ongoing to expand the dataset to make it more representative of the universe of agrochemical formulations, including herbicides, insecticides, and fungicides of varying toxicity and to make this large, chemically diverse database publically available.
Several studies have found that classification for acute toxicity is rarely driven by the results of acute systemic dermal studies, suggesting this requirement could simply be removed (Creton, Dewhurst et al. 2010; Seidle, Prieto et al. 2011; Moore, Andrew et al. 2013). The limited utility of the acute dermal systemic toxicity test suggests that it should not be a default pesticide registration requirement. Recent EPA guidance waiving this requirement for pesticide formulations is based on a database comparing the results of oral and dermal systemic toxicity studies (U.S. EPA OPP 2016). Similarly, for plant protection product active ingredients in Europe, dermal studies can be waived if the oral LD50 >2000mg/kg (European Union 2013).
4.3 Using computational tools to predict acute toxicity hazard in the absence of experimental data
Non-testing approaches, such as read-across and other in silico predictions, have been used for determining the acute toxicity of a variety of substances (including organic fragrances, nitriles, bisphenol A in thermal paper, alternative flame retardants, polymers, inorganics, and organometallics) or for identifying issues of concern (e.g., metabolite formation). Computational tools and approaches are now available for predicting acute toxicity hazard in the absence of experimental data.
Predictions of human health hazard can be developed using analogs (data available on closely related substances), read-across (applying available data to structural analogs), chemical class, metabolites, and reaction products (e.g., hydrolysis). It is also important to consider physicochemical properties and to have a mechanistic understanding of functional group(s), mechanism of action, metabolites, electronic effects, steric demands, initiating event of an adverse outcome pathway, and mixtures. Importantly, physicochemical properties such as solubility can have important effects that may not be detected with structural similarity. Experience and expert judgment has, over time, led to structural alerts and other rules that continually improve non-testing approaches. These rules can be expanded with further knowledge of toxicity mechanisms.
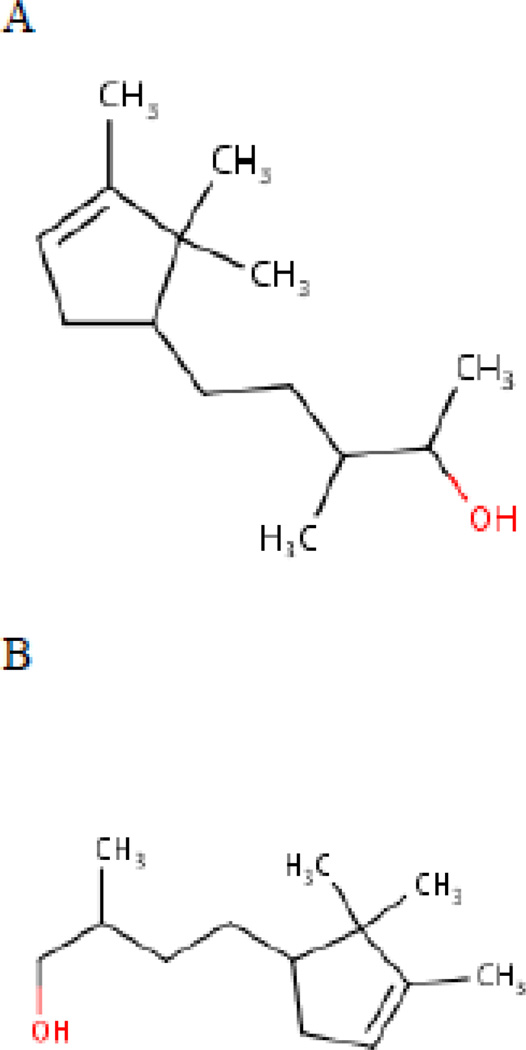
Caution should be taken when considering “similarity” assessments; toxicity will depend on more than structural similarity, and expert judgment is needed. For example, in an analysis of the synthetic fragrance Sandalore, an analog (3-cyclopentene-1-butanol, beta,2,2,3-tetramethyl-) was identified that had similarity score of 91% (Figure 3). The only significant difference was that one substance had a primary hydroxyl group, while the analog was secondary. However, unlike Sandalore, the analog can be oxidized to a carboxylic acid, resulting in significant differences in rates of metabolism, distribution, removal, and toxicity. Therefore, even though these two substances had a very high similarity score, they are actually poor analogs for one another.
Figure 3.
A: Sandalore; B: 3-cylopentene-l-butanol, beta,2,2,3-tetramethyl-
To understand the chemical descriptors that influence toxicity, a number of tools have been developed to help predict acute toxicity. These include the Analog Identification Methodology, which is designed to help a user identify data and analogs for a target substance, and the Chemical Assessment Clustering Engine, which clusters substances from a user-supplied list based on common fragments and defines clusters where members are analogs of each other. Both tools, as well as information about training opportunities, are available for free from the EPA’s Sustainable Futures website: http://www.epa.gov/sustainable-futures.
4.4 Prediction of toxicity using embryo-larval fish models
Although ethical concerns and species extrapolation issues require consideration, the embryo-larval zebrafish model provides information on an intact organism while integrating molecular, cellular, and tissue-levels effects. Embryo-larval zebrafish models are increasingly being used in toxicity testing. This model provides an intact organism to interrogate putative AOPs leading to acute lethality. It may also provide an in vivo model system with which to follow up in vitro and/or in silico predictions that do not address one or more of the key event in the AOP. As such, it can be considered a valuable component of an integrated strategy for identifying acutely lethal compounds more rapidly and efficiently than in vivo mammalian models.
One example of using the zebrafish model in predicting acute toxicity associated can be shown with the adverse outcome pathway (AOP) associated with acute organophosphorus poisoning is complex, involving several key events including acetylcholinesterase (AChE) inhibition, acetylcholine accumulation, nicotinic and muscarinic receptor activation, mitochondrial respiration, oxidative stress, disruption of calcium signaling, inflammatory response, immune response, and cell death (Faria, Garcia-Reyero et al. 2015). These key events are collectively referred to as the cholinergic toxidrome. The AOP for AChE inhibition leading to acute lethality has been published and was developed using OECD guidance (Russom et al. 2014. Development of an adverse outcome pathway for acetylcholinesterase inhibition leading to acute mortality. Environmental Toxicology and Chemistry. 33:2157-2169, doi: 10.1002/etc.2662).
A larval zebrafish model of the cholinergic toxidrome has been developed that is suitable for in vivo medium and high throughput screening to identify better antidotes. Zebrafish recapitulate most of the pathophysiological mechanisms underlying human acute organophosphorus poisoning, including AChE inhibition. Therefore, this model is well-suited for interrogating the adverse effects of potentially neurotoxic compounds by encompassing several of the key events in the organophosphorus-AChE inhibition leading to acute lethality AOP. Chlorpyrifos-oxon was used as a prototypic organophosphorus compound to develop the model, which includes a variety of biochemical, morphological, and behavioral endpoints to grade severity of organophosphorus poisoning. Characteristics of mild, moderate, and severe organophosphorus poisoning have been codified in zebrafish larvae exposed to chlorpyrifos-oxon for 24 hours at seven days post-fertilization (Faria, Garcia-Reyero et al. 2015).
5. Using mechanisms and adverse outcome pathways to integrate high throughput data
5.1 Organizing toxicity mechanisms into adverse outcome pathways
There are many mechanisms associated with acute systemic toxicity, and understanding these mechanisms is critical to the successful development of non-animal testing approaches. Understanding the mechanism of toxicity can help determine whether effects observed in animals are relevant to humans, possibly providing a rationale for development of a non-animal test. Understanding the mechanism of toxicity also better enables read-across, the design of in vitro assays, and the building of QSAR models. While in vitro and QSAR models are available that can predict relatively nontoxic substances, which make up the bulk of chemical space (Bulgheroni, Kinsner-Ovaskainen et al. 2009; Luechtefeld, Maertens et al. 2016), most currently available QSAR tools do not assess the acute toxicity of substances acting through highly specific mechanisms (e.g., AChE inhibition). More research is needed to focus on identifying substances of high inherent or specific toxicity and identifying mechanisms associated with acute toxicity.
Studying acute toxicity mechanisms is often difficult due to a lack of available mechanistic data. Acute systemic toxicity tests are typically limited to those needed to determine LD50/LC50 values for classification and labeling purposes. Because the study endpoint is lethality (independent of cause), resources are rarely devoted to determining the specific mechanism(s) of toxicity, and thus mechanistic data is absent in typical acute toxicity databases. Work is ongoing to determine relevant mechanisms of action for acute systemic toxicity, including an effort to build a large, publicly available database that includes relevant mechanistic information. The initial results of this work are shown in Table 3, which illustrates how this information can be used to develop adverse outcome pathways (AOPs).
Table 3.
Selected mechanisms of acute toxicity.1
| MIE or Upstream Key Event | Example Stressor | Relevant AOP |
|---|---|---|
| GABA receptor inhibition | Fipronil | Binding to the picrotoxin site of ionotropic GABA receptors leading to epileptic seizuresa |
| Sodium channel inhibition | Pyrethroids | Axonal sodium channel modulation leading to acute mortalityb |
| Protein synthesis inhibition | Ricin | |
| Sodium-potassium ATPase inhibition | Digoxin | |
| Mitochondrial inhibition | 2-Buten-1-ol, 1-thenyl-4,4,4- trifluoro-3-trifluoromethyl- |
|
| Binding of benzodiazepine sites on GABA receptor |
Tetrazepam | |
| Acetylcholinesterase inhibition | 4-(Methylamino)-3,5-xylyl methylcarbamate |
Acetylcholinesterase inhibition leading to acute mortalityc |
| GSH depletion followed by covalent binding of reactive metabolite to cellular proteins |
Acetaminophen | |
| Michael acceptor reaction | Acrolein | |
| Voltage-gated sodium channel inhibition |
Sodium valproate | |
| NMDA receptor antagonism | Methadone | |
| Anticoagulation | Coumadin | |
| Dopaminergic D2 receptor antagonism |
Thioridazine hydrochloride |
This table provides an outline of the some of the known mechanisms involved in acute systemic toxicity along with prototypical initiators. In some cases, the exact molecular initiating event (MIE) isn’t known. Examples of adverse outcome pathways (AOPs) under development in the OECD AOP Wiki are noted and can be found on the web:
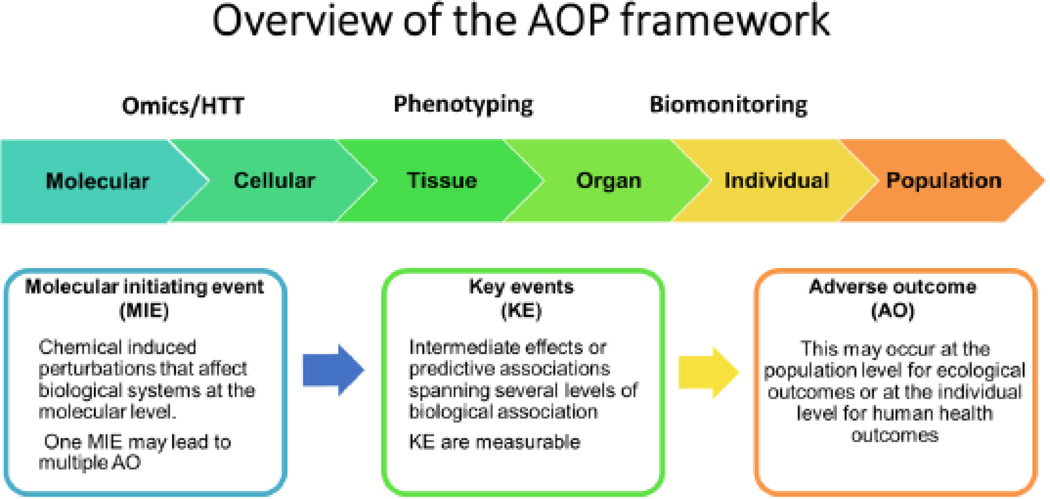
AOPs are conceptual models that facilitate the organization of information about biological interactions and toxicity mechanisms into models of toxicity. When fully developed, an AOP reflects the initial interaction with the system, the molecular initiating event (MIE), through a series of measurable key events that lead to a specific adverse outcome (Figure 4) (Ankley, Bennett et al. 2010).
Figure 4.
Overview of the AOP framework (adapted from (Oki, Nelms et al. 2016).
The AOP framework can help to identify in vitro assays relevant to the toxicity pathway of interest. In some cases, it may be informative to determine the MIE caused by particular substances; if this event is shared by many substances, a useful QSAR or in vitro assay could be built. However, it is likely that the diversity of actual MIEs for acute systemic toxicity will make it necessary to group MIEs by downstream key events, including phenotypic readouts (e.g., cytotoxicity, apoptosis, cell membrane integrity, and mitochondrial toxicity) or cell signaling (e.g., stress or immune response). Examples of other ways to determine novel acute mechanisms include:
3-D crystalline protein structure mapping
Gene expression data-mining
Identifying protein targets using wet-lab binding interactions
Examining pathology and clinical pathology data
Examining the relationship of acute toxicity to high throughput screening (HTS) data
Whether it is necessary to define an AOP for each MIE or adverse outcome relevant to acute systemic toxicity may depend on whether the mechanism is nonspecific (e.g., reactivity) or a specific, target-activated (e.g., anticoagulation) mechanism. A suite of high-throughput screens may be most useful for covering nonspecific mechanisms, as some substances create responses in many assays, and are therefore acting against multiple pathways. High-throughput assays are also suitable for bringing together common key events based on downstream specific mechanisms, for example, altered signal transduction pathways or ion homeostasis that cover a wide array of MIEs.
The usefulness of an AOP for a particular application is dependent on the type and strength of information in the AOP. Quantitative, data-rich AOPs will likely be needed to develop a quantitative risk assessment of a novel substance, but the manual data curation and building of such AOPs is resource-intensive (Edwards, Tan et al. 2016). Any linkage of one or more key events with an adverse outcome can be useful when applied in the appropriate regulatory context, especially as the diverse chemical space involved in acute systemic toxicity is mapped.
The OECD is leading an international effort to collect AOP information, facilitate collaboration on developing AOPs, and ultimately enable AOPs to be used in regulatory applications. OECD and collaborators have developed the AOP Knowledge Base (https://aopkb.org), a set of web-based tools that includes the AOP Wiki, Effectopedia, and AOP XPlorer. These tools offer platforms for recording qualitative AOP information, visualizing quantitative AOP linkages, and exploring AOP networks.
5.2 Computationally predicted AOPs
The manual development of a network of semi- or fully quantitative AOPs is time-consuming. Using computational predictions to build relational networks may be central to the successful use of AOPs for building the predictive networks that will be necessary for the replacement of animal testing for acute systemic toxicity.
Computational and informatics tools permit integration of data from multiple sources and mining of large datasets. These tools permit movement from building AOPs upon years of empirically derived knowledge of key events towards rapid, computationally-predicted adverse outcome pathways (cpAOPs) assembled from computationally driven associations between key events. CpAOPs can be used to transparently translate data into population effects. Network-based and cpAOP approaches can accelerate the rate of AOP development by providing a scaffold for development. A cpAOP network is created with data from different sources, such as phenotypic outcomes or HTS data. Annotated associations can then be built using data integration methods and supplemented with HTS data. The resulting network is a cpAOP network relating a perturbation from a substance to the biological pathway and phenotypic changes that lead from that initial perturbation to the adverse outcome (Bell, Angrish et al. 2016). These cpAOPs can then be manually verified and expanded with biological knowledge in the scientific literature or, if necessary, new experimental evidence.
5.3 Applying AOPs to high throughput data
Tox21 is a U.S. federal interagency collaboration among the National Institutes of Health (National Center for Advancing Translational Sciences [NCATS] and the National Toxicology Program at the National Institute of Environmental Health Sciences [NIEHS]), the EPA, and the U.S. Food and Drug Administration (FDA). Tox21 uses HTS assays to quickly and efficiently test chemicals for activity across a battery of assays that target cellular processes. The results of these tests can then be used to provide insight on potential human health effects.
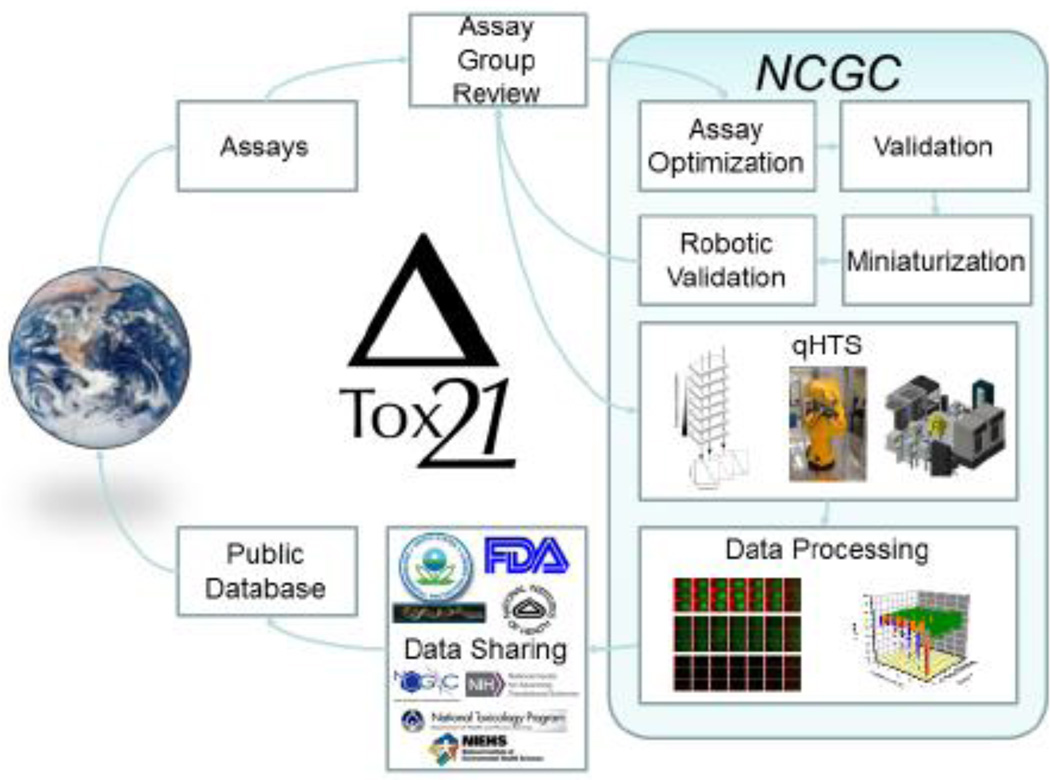
Figure 5 provides details of the Tox21 screening process. The NCATS Chemical Genomics Center is responsible for assay and robotic optimization, validation, and miniaturization of the in vitro assays in the Tox21 robotic platform (Attene-Ramos, Miller et al. 2013). Substances are tested at multiple concentrations in order to understand the effect of dose on toxicity. EPA, NIEHS/National Toxicology Program (NTP), NIH, and FDA collaborate to identify substances and assays for inclusion in the program, to develop data evaluation approaches, and to identify appropriate applications for the data. Tox21 and similar initiatives such as the EPA’s HTS program, ToxCast™ (Judson, Houck et al. 2010), provide data to better understand mechanisms of acute toxicity. This, in turn, can inform design of additional in vitro assays targeted to these mechanisms that can be used to more accurately predict the acute toxicity of novel substances. An example of a Tox21 assay relevant to acute toxicity is a mitochondrial membrane potential (MMP) assay, which screened substances for their ability to decrease MMP in HepG2 cells (Sakamuru, Li et al. 2012). The assay identified many substances that induce mitochondrial dysfunction, demonstrating its ability to reproducibly and rapidly detect substances that decrease mitochondrial membrane-potential (Attene-Ramos, Huang et al. 2015). The data generated by this assay also correlated well with the existing fish, daphnia, and rat (intravenous) acute toxicity results (Bhhatarai, Wilson et al. 2015), suggesting that a MMP assay could serve as an alternative for acute toxicity testing.
Figure 5.
Tox21 involved the testing of more than 10.000 substances using the automated robotic screening system housed at NCATS. During Phase II of testing, the assay is optimized, validated, and miniaturized into a 1536-well plate format, followed by the robotic validation Robotic qHTS against the Tox21 10K library is then run, followed by data processing The screening results are first shared by government partners, and then are made publicly accessible. Figure reproduced from (Attene-Ramos, Miller et al. 2013).
A large quantity of Tox21 data have been made publicly available (Tice, Austin et al. 2013; Huang, Xia et al. 2016). These datasets have been used by the scientific community to further the development of predictive models for human health assessment. Using additional models of higher biological relevance can help to confirm suspected mechanisms derived from HTS assays; an AOP framework can help to identify appropriate models or provide context for multiple data streams. While molecular mechanisms such as MMP disruption may manifest as cytotoxicity in a more comprehensive assay like the 3T3 NRU-based cytotoxicity assay, the detection of early events still has advantages. An understanding of specific MIEs could be used to build QSARs or support a read-across prediction, and mechanistic assays may be faster to conduct. A detailed mechanistic explanation of every pathway relevant to acute toxicity is not needed, but a comprehensive understanding of relevant phenotypes will be essential to progress. Ultimately, AOPs will provide a framework for organization of HTS data that will enable use of these data to predict acute toxicity for regulatory applications.
6. Conclusions and recommendations
A key objective of the workshop was to set a defined plan to reach the workshop goal of identifying approaches that could potentially replace animal use for regulatory-required acute systemic toxicity testing. The following section outlines that plan and identifies some of relevant challenges to be addressed and resources to be utilized. It is essential that momentum is maintained to ensure that the workshop goals are achieved. A multi-pronged approach that incorporates a diverse group of stakeholders will be needed to replace the use of animals for acute systemic toxicity testing.
6.1. General recommendations
6.1.1 Limitations of in vivo reference data
The historic use of animal tests as the “gold standard” for human safety assessment has resulted in acute toxicity regulatory categories being defined in terms of animal test outcomes. Although human reference data would be the most appropriate comparator, alternative methods are developed to predict animal outcomes as the regulatory benchmark because of the available database produced by years of in vivo testing.
New methods are limited by their comparison to animal tests, which themselves have never been validated to predict human toxicity. Accordingly, the performance and associated variability of the in vivo tests should be factored in when determining the necessary performance threshold for an alternative method to be considered sufficient for regulatory use. The in vivo data generate a point estimate, but data describing the variability around that estimate are often lacking. Comparison of the alternative methods to animal results places an unfair burden on alternative methods because of the inherent variability associated with much of the animal data. In some cases, even animals cannot replicate data from other animal studies, and there is considerable variation in LD50 values depending on the strain and sex of the animals and the laboratory used for testing.
6.1.2. Mechanistic research
Alternative methods for acute systemic toxicity could be advanced by developing a better understanding of the mechanisms underlying the adverse effects to be predicted. Table 3 outlines an initial list of potential mechanisms which could be expanded to ensure coverage of the entire chemical space. Better understanding of mechanisms of acute systemic toxicity can be obtained through the construction of AOPs. Assays and approaches that assess the key events within AOPs could then be developed and used. Even if single MIEs for each substance are not identified, assays for common key events among pathways may be developed to cover multiple MIEs.
A list of assays and approaches applicable to acute systemic toxicity that are nearest to implementation for regulatory testing should be created by searching a database such as the EURL ECVAM Database on Alternative Methods to Animal Experimentation (DB-ALM). This list should include the current usefulness and limitations of existing methods, as well as needs for further optimization and/or development for more advanced applications.
6.1.3. ADME considerations
Additional research needs include a focus on improving the available in vitro methods so that ADME are also predicted and applied to assessments of acute systemic toxicity. ADME data are crucial for the extrapolation of in vitro concentrations of substances that produce adverse effects to in vivo blood levels, and how they relate to actual concentrations of exposure. The recently published EURL ECVAM strategy for using toxicokinetics in systemic toxicity determinations in order to reduce, refine, and replace animals in toxicity testing provides an excellent resource for any recommended research activities (EURL ECVAM 2015).
ADME needs for acute systemic toxicity assessments should be fit for a specific purpose. Computational kinetics and dynamics or population-wide metabolism may be needed for some uses (e.g., emergency response), but less detailed information may be needed for another regulatory decision or purpose (i.e., to determine whether toxicity information for a certain route maybe waived because there is little to no absorption by that route).
Efforts to define additional ADME needs should start by developing a survey of available ADME methods and approaches that would build off recommendations of the European Partnership for Alternative Approaches to Animal Testing (EPAA) workshop on in vitro and in silico approaches for ADME in safety testing (Schroeder, Bremm et al. 2011). Structure-activity relationships may provide adequate information for determining absorption. Using such information to determine whether a particular exposure route is relevant to human exposure could allow waiving of toxicity studies for an irrelevant route. The pharmaceutical industry may have assays and approaches that would assist in determining absorption via the relevant exposure routes. There are currently alternative methods to estimate dermal penetration (e.g., OECD TG 428), but for inhalation, there is a lack of understanding regarding the relationship of exposed dose to the dose that reaches target site. This relationship is complicated by the interaction of the test substance with the portal of entry. For example, nose-only and whole body exposures can produce different effects based on the difference in dose that reaches the target site and this fact has implications for the comparison of new approaches to available in vivo data.
6.1.4 Development of integrated approaches to testing and assessment
The integration of relevant information and test method results (e.g., physicochemical information, ADME and pharmacokinetics, mechanistic information, and in vitro and in silico methods) are generally referred to as integrated approaches to testing and assessment (IATA). While IATA will likely be needed to address many questions related to the assessment of systemic acute toxicity potential, the inhalation route of exposure could especially benefit from the IATA concept due to the special considerations mentioned above. Consideration of a substances’ physicochemical properties, physical states, portal-of-entry effects, potential for distribution to specific areas of the lung, and potential for absorption into the systemic circulation could be used to waive in vivo acute inhalation testing or in combination with other in vitro or in vivo methods used to assess oral acute toxicity, to fill inhalation data needs. Methods to assess some or all of these for certain substances may already be available. A working group has spun off of this workshop to specifically address alternative approaches for inhalation toxicity (www.piscltd.org.uk/acute-inhalation-toxicity/).
6.1.5 Education and training
Barriers to the adoption of new methods include a general resistance to change. The adoption of alternatives will not occur without leadership willing to take steps toward making that switch. For example, the EPA Office of Pesticide Programs recently released an open letter to stakeholders announcing their immediate goal of reducing animal use in acute toxicity testing and their associated progress on objectives leading towards that goal (www.epa.gov/pesticides/new-epa-guidance-testing-pesticides-will-reduce-animal-testing). Education and communication coupled with cooperation and engagement among regulators and industry are key to this process. Thus, there is a critical need for training in the use of alternative methods, recognizing that training for users and regulatory reviewers on available alternative methods and approaches and their potential applications could facilitate use and regulatory acceptance of these methods and approaches for acute systemic toxicity testing. As development and use of alternative approaches often requires a diverse team with an integrated skill set and cross-disciplinary knowledgebase, training opportunities across various sectors, including academia, industry, and regulatory, will be important. An emphasis should be placed on training graduate students and other scientists at an early career stage. In addition to regulatory scientists, potential targets for education are diverse and may include user communities such as contract research organizations, labeling and transportation professionals, field soldiers, emergency responders, and consumers. There are several groups that determine industrial and emergency exposure limits that could be important outreach targets (Table 4).
Table 4.
Acute systemic toxicity data are often used to set a variety of reference values relevant to workers, consumers, or emergency response professionals.
| Organization | Limit set |
|---|---|
| American Conference of Governmental Industrial Hygienists |
Threshold Limit Values |
| National Institute of Occupational Safety and Health |
Immediately Dangerous to Life and Health values |
| U.S. Occupational Safety and Health Administration |
Permissible Exposure Limits |
| American Industrial Hygiene Association | Workplace Environmental Exposure Limits |
| U.S. Environmental Protection Agency | Acute Exposure Guideline Levels |
Training events could take the form of conference sessions, focused seminars, webinars, and workshops. One example of an effective training program is the EPA’s Sustainable Future Program, which has training available upon request. The European Union also provides training to support read-across, including the European Chemicals Agency read-across assessment framework (http://echa.europa.eu/view-article/-/journal_content/title/assessing-read-across-how-echa-does-it).
Development and presentation of case studies illustrating the successful application of alternative approaches to regulatory submissions will increase the understanding and acceptance of the approaches. These cases could be expanded beyond those discussed in this report, and should include varied product sectors and regulatory purposes (e.g., prioritization, hazard assessment, and risk assessment) to demonstrate how alternatives could be used in place of the standard LD50/LC50 values. Importantly, regulators should be involved in creating these cases so that regulatory need and applicability remain a central focus.
The National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM) and EURL ECVAM should continue to provide complete and current information on alternative methods on their websites. The Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) should also be engaged to promote alternatives to individuals and groups actively involved in animal research. The National Library of Medicine is another potential site for promotion of alternatives. Individuals can be made aware of developments with alternatives through the NICEATM email list, the Society of Toxicology In Vitro and Alternative Methods Specialty Section, the American Society for Cellular and Computational Toxicology, and AltTox.org, for example.
6.1.6 Regulatory requirements
The data that are needed and accepted for acute systemic toxicity can vary greatly across regulatory authorities in different global regions, and within a region (e.g., variations among the U.S. regulatory agencies). For example, the acute toxicity data required for transportation of hazardous substances is largely driven by international authorities (i.e., United Nations) while the data requirements for pesticides are regional. One way to understand local and regional regulatory requirements for acute systemic toxicity data would be a standardized survey that includes the needs of regulatory agencies in various states and countries, how such data are used, and the path to the acceptance of new methods. This survey could then be published in the form of a white paper on the U.S. regulatory landscape that addresses:
Which agencies use acute systemic toxicity data?
How is acute systemic toxicity data used by agencies?
What information is needed from a replacement to fulfill these needs?
What flexibility currently exists for the use of alternative test methods?
What is the process for acceptance of new methods and approaches?
What regulatory standards are used for acute systemic toxicity testing? (e.g., ISO, OECD, or ASTM)
How many acute systemic toxicity tests are being conducted for each agency - to know which area is the greatest use of animals?
Once a U.S.-focused white paper is complete (Strickland et al; manuscript in prep), efforts should be directed towards a global review of regulatory requirements in order to address considerations for international harmonization (see Section 6.1.7).
To reduce animal usage in the short-term, regulatory agencies can adopt existing methods that reduce testing. Acceptance of the GHS additivity formula, waivers for dermal acute studies, and read-across of data from a pesticide active ingredient to a formulation could all help to reduce current testing. Another way to decrease the use of animals for acute systemic toxicity testing is to create guidance for requesting waivers of acute toxicity data requirements. The guidance could suggest the types of substances and arguments for which data waivers may be supported and successful (OECD 2016). Such policy guidance can be implemented faster than changes in regulations. A retrospective analysis of waiver applications, highlighting successful strategies, could be performed. The process for accomplishing any policy changes at the international level should be well documented with successful case studies. Such documentation can serve as guidance to facilitate future attempts at changing policy.
Policies should ensure that alternatives under consideration are evaluated with respect to whether they are fit for the purpose intended. For example, chemical warfare agents are highly predicted by alternative test methods because they are so toxic. However, this is very different from other chemical sectors, such as industrial or consumer products, that are not designed to be toxic. One suggestion could be to have two “levels” of testing: a simpler, high-throughput test(s) to provide a rapid “toxic or non-toxic” assessment, and a second more complex alternative test(s) to provide a more detailed classification when needed.
6.1.7 International harmonization: challenges and opportunities
A lack of international harmonization of regulatory testing requirements remains one of the biggest challenges for successful and widespread adoption of non-animal alternatives. In a global marketplace, stakeholders will follow the testing requirements that will afford them acceptance in all regions, which typically means that an animal test is still performed. For example, a pesticide manufacturer may willingly conduct an alternative method that is accepted for regulatory classification and labeling by one country, but will also conduct the animal test for countries that import the pesticide and have yet to adopt the alternative method. Thus, acceptance of the alternative has no impact on animal use.
There are some currently available assays and approaches that are ready or almost ready for use in regulatory decision-making. Accordingly, rather than focus on novel approaches, significant opportunities to replace, reduce, or refine animal use for acute systemic toxicity testing exist through global adoption of alternatives that have already been adopted by some regulatory bodies. These include:
As noted above, use of the GHS additivity formula for pesticide mixtures. Currently pesticide active ingredients and the various formulations (manufactured for different applications as well as preparation for sale in different markets) are tested for acute toxicity. Use of the GHS additivity formula for formulations could eliminate the testing for pesticide formulations when information on the toxicity of their components are available. This approach is accepted in the European Union, but not in other regions (European Union 2013).
Eliminate inhalation testing as a default requirement. Inhalation testing in the European Union is a triggered requirement based on exposure potential but is a default requirement in the United States. Global adoption of the exposure potential trigger for inhalation studies would eliminate testing of substances where inhalation is not a likely route of exposure (http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013R0284&from=EN).
Reduce testing requirements for non-toxic substances. The European Chemicals Agency is developing guidance on a weight of evidence approach (see http://echa.europa.eu/support/guidance/consultation-procedure/ongoing-reach). For substances with little to no signs of toxicity, it may be possible to develop a schema to waive acute systemic toxicity testing requirements.
Revise the requirement for acute systemic toxicity testing of medical device extracts. Acute systemic toxicity testing of medical device extracts is required by the FDA Center for Devices and Radiologic Health according to ISO-10993; however, the results of these tests are almost always negative. FDA could engage industry and interested stakeholders in a discussion of why these data are required and whether the testing may be avoided (Prieto, Kinsner-Ovaskainen et al. 2013).
Globally harmonize the top limit dose for acute systemic toxicity testing at 2,000 mg/kg (Seidle, Robinson et al. 2010). Currently some countries require testing of up to 5,000 mg/kg (e.g., Australia, U.S. EPA, U.S. DOT) while others set a top limit of 2,000 mg/kg. Uniformity in test design would eliminate duplicative testing based on different dose ranges. A first step in addressing this discrepancy is an analysis of whether any value is added by testing up to 5,000 mg/kg versus 2,000 mg/kg.
6.1.8 Data curation and sharing
Interested parties should take advantage of opportunities for collaboration. There are a number of federal agencies that require or use acute toxicity data, and many are funding programs to develop non-animal methods to generate these data. Stakeholder investment in research to further the current understanding of human physiology to improve the predictivity of human outcomes is needed. To this end, the work of federal agencies and private organizations could be coordinated to maximize effective use of resources and avoid duplication of effort.
For time and resource efficiency, extensive data sharing is needed to expedite research efforts for alternative methods and approaches to acute toxicity. A collaborative mechanism is needed so that test developers and companies with data can share their activities and experience in a manner that is faster than publishing in the peer-reviewed scientific literature. Databases of in vivo and in vitro data could be established using a neutral data curator that is acceptable to data donors. This effort should also include regular discussions with regulators to ensure that methods that meet regulatory needs are being developed.
Paramount to advancing computational approaches is a high-quality, well-curated library of reference data. The challenges of obtaining such data for acute toxicity are primarily associated with a lack of available study results with which to calculate a point estimate in some cases (e.g., a range of LD50 values is reported) and the relevance of rodent data to human outcomes. A feasibility study could define what data are needed to build accurate models and also determine how best to obtain access to available data. Such an effort would begin with the “low-hanging fruit” by focusing on the most toxic mechanisms of action associated with acute toxicity that also have clinical data. These data could be used to develop a model that would identify the “worst actors”; once successfully developed, efforts could be made towards identifying a broader range of toxicity.
Data for definitive negatives are required in order to effectively characterize model sensitivity and specificity. Given that negative data are not typically published or reported, obtaining these data will require effective engagement of stakeholders that possess such study results. There are numerous ongoing efforts within individual groups that could benefit from collaborative discussions and sharing of resources. One possible approach could be a call for developers to provide data that would be made publicly available. This would necessarily lead to defining where the data would be housed (e.g., in a centralized database) along with the responsible party and allocation of resources for infrastructure and personnel needed to maintain such a database. Another recognized need that would greatly increase efficiency and communication is a glossary of the existing acute toxicity databases. This resource would identify not only the content of these databases, but also the database owner and associated accessibility information.
It is important to ensure that any curation efforts include proper consideration of the team involved in the curation in order to maximize efficiency. Data extraction often seems superficially simple, but experience dictates that engaging staff familiar with the study types/endpoints involved greatly increases efficiency and streamlines quality control efforts.
Invariably, there will be data gaps identified that need to be filled. To most efficiently address this issue, high throughput screening in vitro data can be used as a surrogate to cover a wide range of mechanisms and chemical space.
NICEATM is working with the EPA to create a database of in vivo acute oral, dermal, and inhalation systemic toxicity results that will ultimately be publicly available. Industry groups also have internal databases representing multiple species that could be valuable resources if made available. Engagement with industry trade groups and individual companies to determine their willingness to contribute data from their archives is needed. Finally, raw data from such sources as the European Union ACuteTox and Multicenter Evaluation of In Vitro Cytotoxicity projects should be reviewed and compiled; some data have already been made available (Kinsner-Ovaskainen, Prieto et al. 2013; Prieto, Kinsner-Ovaskainen et al. 2013).
Maintaining momentum is critical to ensuring that the goals outlined above are achieved. Successful implementation of non-animal approaches for acute systemic toxicity testing will require input and engagement from an international group of stakeholders. Careful coordination with global regulatory scientists will maximize the likelihood of developing approaches that adequately address the many regulatory needs for these type of data, and that continue to protect public health.
Highlights.
Summarizes the state-of-the-science of alternatives for acute systemic toxicity testing
Explores ways to facilitate implementing such alternatives
Identifies necessary resources: high-quality reference data, training, global harmonization
Emphasizes the need to understand the mechanisms of acute toxicity
Provides a roadmap and strategy to accomplish near-term progress
Acknowledgments
This workshop was cosponsored by NICEATM, the PETA International Science Consortium Ltd., and the Physicians Committee for Responsible Medicine. Special thanks to the workshop organizing committee: David Allen, Warren Casey, Amy Clippinger, Evisabel Craig, Kelly Coleman, Jose Gayoso, Rabea Graepel, Jon Hamm, Anna Lowit, Julia Melia, Larry Milchak, William Polk, Pilar Prieto, Elissa Reaves, John Redden, Louis (“Gino”) Scarano, PV Shah, Judy Strickland, Kristie Sullivan, Jay Tunkel, Garland Waleko, Dan Wilson, and Hao Zhu. With thanks to the webinar and workshop presenters, and those who assisted during the planning and hosting of the workshop, Aryenish Birdie, Hayley Haynes, Catherine Sprankle, Sharon Barbour, and Shannon Bell. Thanks also to Pilar Prieto (EURL-ECVAM) for her careful technical review and editing of this report. This project was funded in part with federal funds from the NIEHS, NIH under Contract No. HHSN273201500010C to ILS in support of NICEATM.
Footnotes
15 U.S.C. § 1261(q)(1)
15 US.C. § 1261(p)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ankley GT, Bennett RS, et al. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 2010;29(3):730–741. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- Attene-Ramos MS, Huang R, et al. Profiling of the Tox21 chemical collection for mitochondrial function to identify compounds that acutely decrease mitochondrial membrane potential. Environ Health Perspect. 2015;123(1):49–56. doi: 10.1289/ehp.1408642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attene-Ramos MS, Miller N, et al. The Tox21 robotic platform for the assessment of environmental chemicals--from vision to reality. Drug Discov Today. 2013;18(15–16):716–723. doi: 10.1016/j.drudis.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SM, Angrish MM, et al. Integrating Publicly Available Data to Generate Computationally Predicted Adverse Outcome Pathways for Fatty Liver. Toxicol Sci. 2016;150(2):510–520. doi: 10.1093/toxsci/kfw017. [DOI] [PubMed] [Google Scholar]
- Bhhatarai B, Wilson DM, et al. Acute Toxicity Prediction in Multiple Species by Leveraging Mechanistic ToxCast Mitochondrial Inhibition Data and Simulation of Oral Bioavailability. Toxicol Sci. 2015;147(2):386–396. doi: 10.1093/toxsci/kfv135. [DOI] [PubMed] [Google Scholar]
- Blaauboer B, Hermens J, et al. Estimating Acute Toxicity Based on In Vitro Cytotoxicity: Role of Biokinetic Modelling. ALTEX. 2005;22(Special Issue 2):250–253. [Google Scholar]
- Blaauboer BJ, Boekelheide K, et al. The use of biomarkers of toxicity for integrating in vitro hazard estimates into risk assessment for humans. ALTEX. 2012;29(4):411–425. doi: 10.14573/altex.2012.4.411. [DOI] [PubMed] [Google Scholar]
- Bulgheroni A, Kinsner-Ovaskainen A, et al. Estimation of acute oral toxicity using the No Observed Adverse Effect Level (NOAEL) from the 28 day repeated dose toxicity studies in rats. Regul Toxicol Pharmacol. 2009;53:16–19. doi: 10.1016/j.yrtph.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Cerrato L, Valeri A, et al. In vitro sensitivity of granulo-monocytic progenitors as a new toxicological cell system and endpoint in the ACuteTox Project. Toxicol Appl Pharmacol. 2009;238(2):111–119. doi: 10.1016/j.taap.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Clemedson C, Blaauboer B, et al. ACuteTox-Optimisation and pre-validation of an in vitro test strategy for predicting human acute toxicity. ALTEX. 2005;22(Special Issue 2):254–258. [Google Scholar]
- Coecke S, Ahr H, et al. Metabolism: a bottleneck in in vitro toxicological test development. The report and recommendations of ECVAM workshop 54. Altern Lab Anim. 2006;34(1):49–84. doi: 10.1177/026119290603400113. [DOI] [PubMed] [Google Scholar]
- CPSC. Codification of animal testing policy (16 CFR part 1500) Federal Register. 2012;77 (No..237) [Google Scholar]
- Creton S, Dewhurst IC, et al. Acute toxicity testing of chemicals-Opportunities to avoid redundant testing and use alternative approaches. Crit Rev Toxicol. 2010;40(1):50–83. doi: 10.3109/10408440903401511. [DOI] [PubMed] [Google Scholar]
- Ducharme NA, Reif DM, et al. Comparison of toxicity values across zebrafish early life stages and mammalian studies: Implications for chemical testing. Reprod Toxicol. 2015;55:3–10. doi: 10.1016/j.reprotox.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SW, Tan YM, et al. Adverse Outcome Pathways-Organizing Toxicological Information to Improve Decision Making. J Pharmacol Exp Ther. 2016;356(1):170–181. doi: 10.1124/jpet.115.228239. [DOI] [PubMed] [Google Scholar]
- Ekwall B, Barile FA, et al. MEIC Evaluation of Acute Systemic Toxicity: Part VI. The Prediction of Human Toxicity by Rodent LD50 Values and Results From 61 In Vitro Methods. Altern Lab Anim. 1998;(26 Suppl 2):617–658. [PubMed] [Google Scholar]
- EURL ECVAM. EURL ECVAM strategy to replace, reduce and refine the use of animals in the assessment of acute mammalian systemic toxicity. 2014 from https://eurl-ecvam.jrc.ec.europa.eu/news/acute-mammalian-systemic-toxicity-testing-eurl-ecvam-releases-its-strategy.
- EURL ECVAM. EURL ECVAM Strategy for Achieving 3Rs Impact in the Assessment of Toxicokinetics and Systemic Toxicity. 2015 from https://ec.europa.eu/jrc/en/publication/eur-scientific-and-technical-research-reports/eurl-ecvam-strategy-achieving-3rs-impact-assessment-toxicokinetics-and-systemic-toxicity.
- European Union. Regulation (EU) No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. Official Journal of the European Union. 2013;L93:1–84. [Google Scholar]
- European Union. Regulation (EU) No 284/2013 of 1 March 2013 setting out the data requirements for plant protection products, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. Official Journal of the European Union. 2013;L93:85–152. [Google Scholar]
- Goh J-Y, Weaver RJ, et al. Development and use of in vitro alternatives to animal testing by the pharmaceutical industry 1980–2013. Toxicology Research. 2015;4:1297–1307. [Google Scholar]
- Graepel R, Asturiol D, et al. Exploring Waiving Opportunities for Mammalian Acute Systemic Toxicity Tests. ATLA. 2016;44(3) doi: 10.1177/026119291604400306. [DOI] [PubMed] [Google Scholar]
- Groothuis FA, Heringa MB, et al. Dose metric considerations in in vitro assays to improve quantitative in vitro-in vivo dose extrapolations. Toxicology. 2015;332:30–40. doi: 10.1016/j.tox.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Huang R, Xia M, et al. Modelling the Tox21 10 K chemical profiles for in vivo toxicity prediction and mechanism characterization. Nat Commun. 2016;7:10425. doi: 10.1038/ncomms10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICCVAM. ICCVAM test method evaluation report: in vitro cytotoxicity test methods for estimating starting doses for acute oral systemic toxicity tests. 2006. NIH Publication No: 07-4519. [Google Scholar]
- Judson RS, Houck KA, et al. In vitro screening of environmental chemicals for targeted testing prioritization: the ToxCast project. Environ Health Perspect. 2010;118(4):485–492. doi: 10.1289/ehp.0901392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MT, Huang R, et al. Mechanism Profiling of Hepatotoxicity Caused by Oxidative Stress Using the Antioxidant Response Element Reporter Gene Assay Models and Big Data. Environ Health Perspect. 2016 doi: 10.1289/ehp.1509763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsner-Ovaskainen A, Prieto P, et al. Selection of test methods to be included in a testing strategy to predict acute oral toxicity: an approach based on statistical analysis of data collected in phase 1 of the ACuteTox project. Toxicol In Vitro. 2013;27(4):1377–1394. doi: 10.1016/j.tiv.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Kopp-Schneider A, Prieto P, et al. Design of a testing strategy using non-animal based test methods: lessons learnt from the ACuteTox project. Toxicol In Vitro. 2013;27(4):1395–1401. doi: 10.1016/j.tiv.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Luechtefeld T, Maertens A, et al. Global analysis of publicly available safety data for 9,801 substances registered under REACH from 2008–2014. ALTEX. 2016;33(2):95–109. doi: 10.14573/altex.1510052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore NP, Andrew DJ, et al. Can acute dermal systemic toxicity tests be replaced with oral tests? A comparison of route-specific systemic toxicity and hazard classifications under the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) Regul Toxicol Pharmacol. 2013;66(1):30–37. doi: 10.1016/j.yrtph.2013.02.005. [DOI] [PubMed] [Google Scholar]
- National Research Council. Application of Modern Toxicology Approaches for Predicting Acute Toxicity for Chemical Defense. 2015 from www.nap.edu/catalog/21775/application-of-modern-toxicology-approaches-for-predicting-acute-toxicity-for-chemical-defense. [PubMed]
- OECD. Test No. 402: Acute Dermal Toxicity. OECD Guidelines for the Testing of Chemicals, Section 4. 1987 from http://www.oecd-ilibrary.org/environment/test-no-402-acute-dermal-toxicity_9789264070585-en.
- OECD. Test No. 420: Acute Oral Toxicity - Fixed Dose Procedure. OECD Guidelines for the Testing of Chemicals, Section 4. 2002 from http://www.oecd-ilibrary.org/environment/test-no-420-acute-oral-toxicity-fixed-dose-procedure_9789264070943-en.
- OECD. Test No. 423: Acute Oral toxicity - Acute Toxic Class Method. OECD Guidelines for the Testing of Chemicals, Section 4. 2002 from http://www.oecd-ilibrary.org/environment/test-no-423-acute-oral-toxicity-acute-toxic-class-method_9789264071001-en.
- OECD. Test Guideline 428: Skin Absorption: In Vitro Method, OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing; 2004. [Google Scholar]
- OECD. Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure. OECD Guidelines for the Testing of Chemicals, Section 4. 2008 from http://www.oecd-ilibrary.org/environment/test-no-425-acute-oral-toxicity-up-and-down-procedure_9789264071049-en.
- OECD. Test No. 403: Acute Inhalation Toxicity. OECD Guidelines for the Testing of Chemicals, Section 4. 2009 from http://www.oecd-ilibrary.org/environment/test-no-403-acute-inhalation-toxicity_9789264070608-en.
- OECD. Test No. 436: Acute Inhalation Toxicity – Acute Toxic Class Method. OECD Guidelines for the Testing of Chemicals, Section 4. 2009 from http://www.oecd-ilibrary.org/environment/test-no-436-acute-inhalation-toxicity-acute-toxic-class-method_9789264076037-en.
- OECD. Draft TG 433: Acute Inhalation Toxicity - Fixed Concentration Procedure. OECD Guidelines for the Testing of Chemicals, Section 4. 2015 from http://www.oecd.org/env/ehs/testing/section4-health-effects.htm.
- OECD. No. 237: Guidance Document on Considerations for Waiving or Bridging of Mammalian Acute Toxicity Tests Series on Testing & Assessment. 2016 [Google Scholar]
- Oki NO, Nelms MD, et al. Accelerating Adverse Outcome Pathway Development Using Publicly Available Data Sources. Curr Environ Health Rep. 2016;3(1):53–63. doi: 10.1007/s40572-016-0079-y. [DOI] [PubMed] [Google Scholar]
- Padilla S, Corum D, et al. Zebrafish developmental screening of the ToxCast Phase I chemical library. Reprod Toxicol. 2012;33(2):174–187. doi: 10.1016/j.reprotox.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Prieto P, Cole T, et al. Assessment of the predictive capacity of the 3T3 Neutral Red Uptake cytotoxicity test method to identify substances not classified for acute oral toxicity (LD50>2000 mg/kg): results of an ECVAM validation study. Regul Toxicol Pharmacol. 2013;65(3):344–365. doi: 10.1016/j.yrtph.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Prieto P, Kinsner-Ovaskainen A, et al. The value of selected in vitro and in silico methods to predict acute oral toxicity in a regulatory context: results from the European Project ACuteTox. Toxicol In Vitro. 2013;27(4):1357–1376. doi: 10.1016/j.tiv.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Sakamuru S, Li X, et al. Application of a homogenous membrane potential assay to assess mitochondrial function. Physiol Genomics. 2012;44(9):495–503. doi: 10.1152/physiolgenomics.00161.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder K, Bremm KD, et al. Report from the EPAA workshop: in vitro ADME in safety testing used by EPAA industry sectors. Toxicol In Vitro. 2011;25(3):589–604. doi: 10.1016/j.tiv.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Seidle T, Prieto P, et al. Examining the regulatory value of multi-route mammalian acute systemic toxicity studies. ALTEX. 2011;28(2):95–102. doi: 10.14573/altex.2011.2.095. [DOI] [PubMed] [Google Scholar]
- Seidle T, Robinson S, et al. Cross-sector review of drivers and available 3Rs approaches for acute systemic toxicity testing. Toxicol Sci. 2010;116(2):382–396. doi: 10.1093/toxsci/kfq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tice RR, Austin CP, et al. Improving the human hazard characterization of chemicals: a Tox21 update. Environ Health Perspect. 2013;121(7):756–765. doi: 10.1289/ehp.1205784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA OPP. Guidance for Waiving or Bridging of Mammalian Acute Toxicity Tests for Pesticides and Pesticide Products (Acute Oral, Acute Dermal, Acute Inhalation, Primary Eye, Primary Dermal, and Dermal Sensitization) 2012 from http://www.epa.gov/sites/production/files/documents/acute-data-waiver-guidance.pdf.
- U.S. EPA OPP. Use of an alternative testing framework for classification of eye irritation potential of EPA pesticide products. 2015 revised from http://www2.epa.gov/sites/production/files/2015-05/documents/eye_policy2015update.pdf.
- U.S. EPA OPP. New EPA Guidance for Testing Pesticides Will Reduce Animal Testing. 2016 from https://www.epa.gov/pesticides/new-epa-guidance-testing-pesticides-will-reduce-animal-testing.
- Zhang J, Hsieh JH, et al. Profiling animal toxicants by automatically mining public bioassay data: a big data approach for computational toxicology. PLoS One. 2014;9(6):e99863. doi: 10.1371/journal.pone.0099863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Zhang J, et al. Big data in chemical toxicity research: the use of high-throughput screening assays to identify potential toxicants. Chem Res Toxicol. 2014;27(10):1643–1651. doi: 10.1021/tx500145h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurich MG, Stanzel S, et al. Evaluation of aggregating brain cell cultures for the detection of acute organ-specific toxicity. Toxicol In Vitro. 2013;27(4):1416–1424. doi: 10.1016/j.tiv.2012.06.018. [DOI] [PubMed] [Google Scholar]