Key Points
Question
Does an intervention to train surgeons to use the Best Case/Worst Case framework change surgeon communication and promote shared decision making for high-stakes surgical decisions?
Findings
In this pre- and postintervention study that included 32 frail older inpatients with acute surgical problems, objective measures of shared decision making improved postintervention. Surgeons who used Best Case/Worst Case emphasized a treatment choice, described outcomes rather than discrete procedural risks, and involved patients and families in deliberation.
Meaning
Use of the Best Case/Worst Case framework can promote shared decision making, and this intervention may help surgeons structure challenging treatment conversations to support patients and families.
Abstract
Importance
Although many older adults prefer to avoid burdensome interventions with limited ability to preserve their functional status, aggressive treatments, including surgery, are common near the end of life. Shared decision making is critical to achieve value-concordant treatment decisions and minimize unwanted care. However, communication in the acute inpatient setting is challenging.
Objective
To evaluate the proof of concept of an intervention to teach surgeons to use the Best Case/Worst Case framework as a strategy to change surgeon communication and promote shared decision making during high-stakes surgical decisions.
Design, Setting, and Participants
Our prospective pre-post study was conducted from June 2014 to August 2015, and data were analyzed using a mixed methods approach. The data were drawn from decision-making conversations between 32 older inpatients with an acute nonemergent surgical problem, 30 family members, and 25 surgeons at 1 tertiary care hospital in Madison, Wisconsin.
Interventions
A 2-hour training session to teach each study-enrolled surgeon to use the Best Case/Worst Case communication framework.
Main Outcomes and Measures
We scored conversation transcripts using OPTION 5, an observer measure of shared decision making, and used qualitative content analysis to characterize patterns in conversation structure, description of outcomes, and deliberation over treatment alternatives.
Results
The study participants were patients aged 68 to 95 years (n = 32), 44% of whom had 5 or more comorbid conditions; family members of patients (n = 30); and surgeons (n = 17). The median OPTION 5 score improved from 41 preintervention (interquartile range, 26-66) to 74 after Best Case/Worst Case training (interquartile range, 60-81). Before training, surgeons described the patient’s problem in conjunction with an operative solution, directed deliberation over options, listed discrete procedural risks, and did not integrate preferences into a treatment recommendation. After training, surgeons using Best Case/Worst Case clearly presented a choice between treatments, described a range of postoperative trajectories including functional decline, and involved patients and families in deliberation.
Conclusions and Relevance
Using the Best Case/Worst Case framework changed surgeon communication by shifting the focus of decision-making conversations from an isolated surgical problem to a discussion about treatment alternatives and outcomes. This intervention can help surgeons structure challenging conversations to promote shared decision making in the acute setting.
This pre- and postintervention study evaluates the association of the Best Case/Worst Case framework with surgical communication when communicating to older patients about treament options in high-stakes surgical decisions.
Introduction
For frail older adults, acute surgical problems often have life-altering effects. Serious complications are common; 20% of patients aged older than 65 years who undergo urgent or emergent abdominal surgery die within 30 days, and those who survive often lose their independence. Despite this grim trajectory, nearly one-third of Medicare beneficiaries have an operation during their last year of life. These procedures may be inconsistent with patients’ long-term goals, as most Americans prefer to avoid onerous treatments with limited capacity to preserve their functional status.
Best-practice guidelines endorse shared decision making (SDM) in the context of serious illness to present options, engage patients in deliberation about treatment outcomes, and integrate patient preferences into a recommendation. However, describing a complex and often uncertain prognosis is a formidable task. In accordance with informed consent, surgeons traditionally rely on disclosure of discrete procedural complications aided by robust risk calculators. Nonetheless, enumerating a 20% chance of stroke or a 25% risk of renal failure does not allow patients to consider how they might experience adverse outcomes or encourage deliberation to ensure decisions align with individual preferences. Furthermore, efforts to assist patients and families are hindered by the acute nature of surgical illness and lack of preexisting patient-doctor relationships.
Scenario planning is a strategy to facilitate decision making in the setting of uncertainty. A well-constructed scenario encourages people to comprehend a new, previously unimaginable reality and prepare for major shifts in a way simple forecasting cannot. This approach may be useful for older patients because acute surgical conditions portend a major health change compounded by prognostic uncertainty.
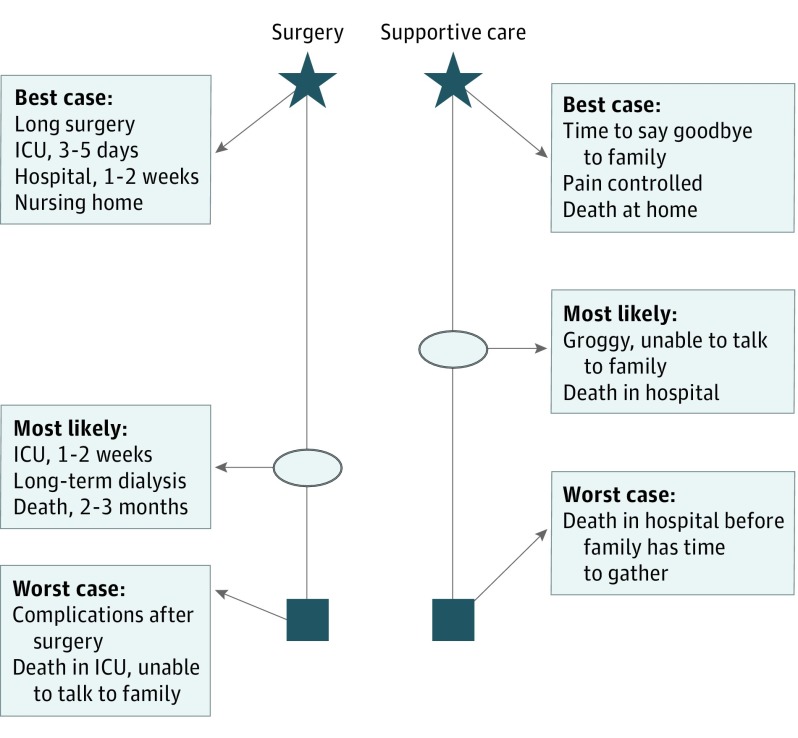
Building on the practice of scenario planning and a conceptual model of SDM, we designed the Best Case/Worst Case (BC/WC) framework as a strategy to change how surgeons communicate with patients about serious illness. Best Case/Worst Case combines narrative description and a handwritten graphic aid to illustrate choice between treatments and engage patients and families. Surgeons use stories to describe how patients might experience a range of possible outcomes in the best case, worst case, and most likely scenarios (Figure 1). We hypothesize that training surgeons to use BC/WC will promote SDM during preoperative communication in high-stakes surgical decisions.
Figure 1. Best Case/Worst Case Graphic Aid.
Example of a Best Case/Worst Case graphic aid that the surgeon would create and use during a decision-making discussion for an older patient with a serious surgical problem. The box represents the worst case scenario, the star represents the best case scenario, and the oval indicates the most likely outcome. ICU indicates intensive care unit.
Methods
From July 2014 until August 2015, we performed a prospective, pre-post pilot study to evaluate the proof of concept of an intervention training surgeons to use the BC/WC framework at a tertiary care hospital in Madison, Wisconsin. The University of Wisconsin instutional review board approved this study and participants gave written informed consent. Surgeons were compensated $245 for completing the training session, and all other study participants were not compensated.
Participants
Study staff screened inpatient rosters to identify patients aged 65 years and older with acute, nonemergent surgical problems and confirmed the surgeon would offer surgery and an alternative treatment. Eligible patients met 1 of the following criteria: a Porock frailty score of 21 or more, a more than 40% risk for serious complication or more than 8% risk for perioperative mortality using the American College of Surgeons risk calculator, or indication from the surgeon that comorbidities would affect long-term outcomes. We also recruited 1 family member present during the decision-making conversation. Patients without decision-making capacity were enrolled with consent from their surrogate. We excluded deaf or non-English speaking individuals and patients with an emergent indication for surgery—such as ruptured aneurysm or perforated viscus—as these patients are typically rushed to the operating room with little time for shared decision making.
Intervention
Excepting the senior author, we invited all 30 surgeons at the University of Wisconsin Hospital who practice cardiothoracic, vascular, or acute care surgery to participate. Surgeons completed a 2-hour training session to learn the BC/WC framework using simulation with standardized patients and 1-on-1 coaching with an expert in palliative care and education (S.K.J., A.Z., and T.C.C.). Postintervention, surgeons used BC/WC with study-enrolled inpatients. Details of surgeon training are reported elsewhere and training materials are available online (http://www.hipxchange.org/BCWC).
Data Collection
We recorded demographics, presenting diagnosis, operations performed, intensive care admissions, palliative care consultations, discharge disposition, and death within 30 days. We audio-recorded and transcribed verbatim the primary surgeon-patient decision-making conversation for each patient enrolled pre- and postintervention and archived copies of the graphic aid for patients enrolled after the surgeon completed BC/WC training.
Data Analysis
OPTION 5 is an observer measure of shared decision making based on a 100 point scale. Originally developed for primary care consultations, this validated instrument permits quantitative measurement of the physician’s effort to include patients in decision making. We calibrated this measure to the surgical setting within 5 domains: presentation of treatment options, surgeon-patient partnership, description of treatments, elicitation of preferences, and integration of preferences with a recommendation (eAppendix in the Supplement). Four investigators (L.J.T., J.L.T., K.J.B., and M.L.S.) independently scored each transcript. We summarized quantitative data using descriptive statistics and calculated intraclass correlation (ICC) between the 4 raters using the “psych” package in R version 3.2.1 (R Foundation for Statistical Computing), assuming raters were a random sample from the population of raters (ICC: 2, k). We also computed ICC to quantify the reliability expected in a new set of 2 raters.
After OPTION 5 scoring, investigators independently analyzed all preintervention and postintervention transcripts using qualitative content analysis. We used an inductive coding strategy to generate and attach codes to the data to catalog themes, constructs and occurrences, and a group process with code adjudication as a gateway to higher-level analysis. We also employed a deductive strategy to compare pre- and postintervention transcripts by examining conversation content within the domains of OPTION 5. This approach served as an additional opportunity to ensure the rigor of our inductive analysis and pinpoint disconfirming data. We drafted construct tables to ensure that the themes were accurately represented in the data and used qualitative research software, NVivo 10 (QSR International), to organize codes and support theme comparison.
Results
Twenty-five surgeons completed the BC/WC training; 1 declined participation and 4 were unable to attend a session after multiple scheduling attempts. Seventeen of these trained surgeons led a decision-making conversation with study-enrolled patients. We approached 53 patients; 32 patients and 30 family members enrolled. Surgical problems ranged from intestinal obstruction to critical limb ischemia (Table 1). Alternative treatments included antibiotics, less-invasive procedures including feeding tubes or drain placement, or simply “no surgery.” Postintervention, all surgeons offered at least 2 options and used BC/WC to present best and worst case scenarios; 1 did not construct the graphic aid and 1 failed to describe the most likely scenarios. We lost the data for 1 conversation because of a technical failure.
Table 1. Description of Patient Characteristics.
| Characteristic | Control (n=12) |
Intervention (n=20) |
|---|---|---|
| Age, median (range) | 78.5 (68-88) | 86.5 (67-95) |
| Male, No. (%) | 9 (75) | 7 (35) |
| White, No. (%) | 12 (100) | 19 (95) |
| Comorbid conditions, No. (%) | ||
| 0 to ≤2 | 2 (17) | 7 (35) |
| >2 to ≤4 | 1 (8) | 8 (40) |
| ≥5 | 9 (75) | 5 (25) |
| Patients without decision making capacity, No. (%) | 0 | 5 (20) |
| Education, No. (%) | ||
| Some high school or less | 0 (0) | 2 (10) |
| High school diploma or GED | 4 (33) | 7 (35) |
| Vocational degree or some college | 1 (8) | 2 (10) |
| College degree | 5 (42) | 1 (5) |
| Graduate degree or higher | 1 (8) | 2 (10) |
| Unknown | 1 (8) | 6 (30) |
| Proposed surgical treatment, No. (%) | ||
| General surgery | 5 (42) | 16 (80) |
| Bowel resection | 4 | 11 |
| Cholecystectomy | 1 | 2 |
| Nonemergent surgery for traumaa | 0 | 2 |
| Paraesophageal hernia repair | 0 | 1 |
| Cardiothoracic | 5 (42) | 1 (5) |
| Pleurodesis | 1 | 0 |
| Esophagectomy | 1 | 0 |
| Cardiac valve replacement/repair | 2 | 1 |
| Coronary artery bypass grafting | 1 | 0 |
| Vascular | 2 (16) | 3 (15) |
| Vascular bypass | 2 | 2 |
| Amputation | 0 | 1 |
| Received proposed surgery, No. (%) | 5 (42) | 10 (50) |
| ICU admission within 30 d, No. (%) | 2 (16) | 3 (15) |
| Palliative care consult or hospice admission within 30 d, No. (%) | 4 (33) | 6 (30) |
| Discharge disposition, No. (%) | ||
| Home | 6 (50) | 4 (20) |
| Assisted living | 0 (0) | 1 (5) |
| Skilled nursing facility | 3 (25) | 9 (45) |
| Hospice | 2 (17) | 1 (5) |
| Death in hospital within 30 d of treatment | 1 (8) | 5 (25) |
Abbreviations: GED, general education development; ICU, intensive care unit.
aHip hemiarthroplasty and tracheostomy for patients admitted to the trauma service following a fall and motor vehicle collision, respectively.
Assessment of Shared Decision Making
The median OPTION 5 score improved from 41 preintervention (interquartile range, 26-66) to 74 (interquartile range, 60-81) after training (Figure 2). The intraclass correlation was 0.80 (95% CI, 0.64-0.90) for the mean score across 4 raters. Assuming scores were generated as the average of 2 raters, the estimated ICC was 0.67 (95% CI, 0.48-0.81).
Figure 2. OPTION 5 Scores.
Box plots depicting OPTION 5 scores for patients in the control and intervention arms. Dot indicates mean score within each treatment arm.
Our qualitative analysis reinforced these findings. Our deductive analysis demonstrated a postintervention difference in communication content for each OPTION 5 domain (Table 2), and our inductive analysis revealed a shift in how surgeons structured conversations according to 3 primary elements: presentation of treatment options, description of treatments, and deliberation over alternatives (Figure 3). Before the surgeons underwent training, conversations with patients began with an explanation of the problem and an operative solution followed by a surgeon-led deliberation about the patient’s candidacy for surgery. Postintervention, the discussion focused on making a treatment decision within the context of the patient’s overall health. Surgeons described outcomes rather than risks, and sought to clarify patient values and goals, using this information during deliberation to revise treatment options to match preferences.
Table 2. Results of Deductive Coding Analysis Demonstrating the Contrast Between Content of Pre- and Postintervention Conversations Based on the Domains of OPTION 5.
| OPTION 5 Domain | Preintervention Observations | Representative Quotations | Postintervention Observations | Representative Quotations |
|---|---|---|---|---|
| Presentation of options | • Surgeon described the acute problem with a surgical solution • Surgeon described the nonoperative alternative as secondary |
“To get that opened up, we need surgery.” “If we do not do the procedure and the focus becomes on comfortable care, then obviously that …will not take care of your heart [valve] problem.” |
• Surgeon called attention to a clear treatment choice • Surgeon presented the nonoperative option as a valid choice |
“We have a choice to make…” “The other option is to take him home and make him comfortable and not to have the surgical procedure.” |
| Surgeon-patient partnership | • Surgeon supported patient as decision maker • Surgeon provided information to assist decision making but decision left up to the patient |
“It’s your decision, so it’s the right decision.” “… to give you the most information that you can have to make the decision for yourself.” |
• Surgeon provided explicit support and offered guidance to the patient and family in deliberation | “…[I’m] try[ing] to relate to what your choices are…but also I think to guide you.” |
| Treatment description | • Surgeon described isolated risks using probabilities to convey likelihood of adverse events • Surgeon described death as a risk of surgery |
“Those risks are bleeding, um, infection in the area we operate, damaging the liver…damaging the intestines around in that area.” “Expected mortality rate is in between 5 and 10 percent” |
• Surgeon used stories to describe treatment outcomes • Surgeon incorporated the patient’s chronic health problems and frailty • Surgeon described death as an outcome of treatment rather than a risk |
“Under the best of circumstances, that would involve being in the hospital for probably a week maybe two...because of your age and the heart problems…that might involve being in the intensive care unit.” “Worst case scenario we pull the breathing tube…you’re struggling with coughing and secretions that you have, you, your ribs hurt…and you pass away fairly quickly.” |
| Preference elicitation | • Surgeon assessed for understanding • Surgeon requested a treatment decision • Surgeon queried if patient was willing to tolerate the burdens of treatment |
“Do you have any questions?” “What do you think you’d be interested in?” “The decision you have to make is…what you’re willing to do given what this is.” |
• Surgeon solicited information about the patient’s appraisal of specific outcomes | “How are you thinking about the difference between walking and not walking, because to me that was the big difference between these 2 [choices]…And so I wonder if you could tell us how you think about that?” |
| Preference integration | • Surgeon encouraged the patient to choose • Surgeon made a recommendation based on operative risk or disease characteristics |
“The choice is up to you.” “After looking at the foot I’m inclined not to be doing more angioplasty… I’m leaning to you choosing the antibiotics and getting him off his foot right now.” |
• Surgeon made an effort to match patient preferences with treatment decisions | “But to really recover from this, and really have a reasonable outcome, you’d have to be aggressive, and not everyone wants that, and I’m not sure that you would want that.” |
Figure 3. Conversation Structure.
Results of inductive coding analysis demonstrating the differences in communication patterns preintervention (A) and postintervention (B).
Presentation of Options: Preintervention
Before training, surgeons universally initiated conversations with detailed explanations of the disease process, linking the acute illnesses to a surgical solution. Surgeons introduced illness as something that required action, for example, “The problem is a mechanical problem, so now something needs to be done here to solve the problem.” To illustrate how surgery could remedy an abnormality, surgeons explained the disease using language like “blockage” or “narrowing” coupled with an intervention to “bypass” or “widen.”
While all surgeons offered a choice, framing diseases as deviations from normal undermined the value of nonoperative treatment because surgery was initially described as the solution. One surgeon explained, “the choice to help you fix that and avoid that outcome [death], it’s obviously another surgery” without conceding that surgery could also result in death. To describe alternatives, surgeons favored “no surgery” or “medical management” without explicitly offering palliative care or hospice. Some surgeons did not offer any nonoperative treatment, asking patients to choose between surgery now or surgery in the future. A few emphasized valid alternatives and aimed to promote value-concordant decisions, for example, “I wanted to come and talk to you guys about different options because I think neither of them is wrong…we need to know what you think would be best for him.”
Description of Treatments: Preintervention
Surgeons candidly disclosed discrete procedural risks like “a risk of stroke to the brain and also pneumonia because we have to put the [breathing] tube in,” noting that some complications necessitated further procedures, for example, “water may accumulate in the chest, then we may need to put the needle and remove the water.” Some used percentages to quantify the likelihood of adverse events such as “the risk of re-intubation is…7 to 8 percent” and referenced patient age, comorbidities, and past operations to support their overall risk assessment.
Surgeons acknowledged treatment impact on quality of life, noting that “complications…may keep him in the hospital for a while and have significant impact on his life.” However, they did not integrate comorbidities or functional status within a description of how patients might experience adverse outcomes, whether patients could live independently or enjoy specific activities. Rather than describing how death might occur, some used overt statements like “there is risk of death with esophagectomy,” while others favored using euphemism to suggest postoperative mortality, for example, “Chances of having something come up that we can’t get over and get you out of the hospital are… fairly possible.”
Deliberation: Preintervention
Surgeons cited physical examination findings and physiologic signs like leukocytosis or tachycardia as cause to reject nonoperative options. One surgeon explained, “If your abdominal exam gets much worse…we’re finished. We go to the operating room.” Surgeons rationalized decisions based on patient eligibility, discussing comorbidities, overall functional status, and preoperative testing to justify specific treatments. Others described surgery as a “big deal,” placing onus on the patient with questions like “the decision you have to make is…what you’re willing to go through to sort of get better or not” to evaluate whether the patient had the “mental drive and the willingness to live” to tolerate the burdens of surgery and postoperative care.
Few surgeons engaged in more explicit discussions of goals and values, favoring generalized statements like “Some people tell me they don’t want an operation regardless” and queries like “Does that make sense?” to evaluate understanding. They did not personalize these assertions with elicitation and integration of the patient’s goals with a course of action. After presenting options, surgeons noted, “it’s up to you [to decide];” only 1 surgeon clearly reinforced partnership, referencing “the decision that you and I make.”
Presentation of Options: Postintervention
After their training, surgeons abbreviated description of the disease process and treatment and explicitly demonstrated a choice between surgery and a valid alternative, using the graphic aid to augment discussion. One surgeon stated, “We have a choice to make…I want to use this little diagram to…go through the choices.” By presenting the decision as preference-sensitive, surgeons highlighted the importance of patient and family input because “either choice is reasonable given your sense of where this problem has hit you in your life.” Surgeons integrated description of the proposed operation into their narrative about how patients might experience best and worst case scenarios, for example, “even under the best of circumstances that would be a big enough operation for you that even if you did great, you’d be in the hospital for another week, and it’d be a couple of months probably to get over this…it’s certainly not going to make you any stronger than you were a month ago.”
Description of Treatments: Postintervention
Instead of discrete risks, surgeons discussed the expected hospital course, incorporated patients’ unique comorbidities, and described anticipated functional decline. Surgeons explained complications by illustrating the worst case scenario involving a constellation of setbacks and burdensome interventions, for example, “Your breathing would get worse, you’d stay in the ICU with a breathing tube, we’d have to talk about feeding tubes…you’d still have that pain, and you still wouldn’t be able to move around.” Similarly, surgeons provided clear descriptions about how death might occur, for example, “You’d have complications from the surgery that wouldn’t allow you to really get better. And you’d die in the intensive care unit or somewhere in the hospital…that wouldn’t occur right away, but it might occur in a few weeks.”
To convey prognostic uncertainty, surgeons positioned the most likely scenario between the boundaries of the best and worst case. Rather than using statistics, surgeons incorporated phrases like “I think it’s more likely that we can get you through the thing than not, but it’s kind of more in the middle than you might want” and referenced the graphic aid to illustrate the location of the most likely outcome. An example of this was, “If you look at where we are between best case and worst case, with nonsurgical treatment we’re here. And with surgical treatment, we’re somewhere in here.”
Deliberation: Postintervention
Surgeons involved patients and families by explicitly asking them to evaluate plausible health trajectories because “different people in this moment could feel differently…how do you feel about that?” Surgeons requested input about specific outcomes, asking, for example, “if we do surgery you’re likely going to a nursing facility, is that something you’d be ok with?” However, the ability to incorporate patient inclinations within a recommendation was mixed. Some revised options based on patient input, adjusting “no surgery” to hospice after sensing interest in a palliative strategy and aimed to match treatment choice with patient preferences, noting “…[I’m] try[ing] to relate to what your choices are…but also I think to guide you.” Others provided less assistance, asking patients to decide independently. A few clearly integrated patient preferences within their recommendation, for example, “This is what I know about her…she didn’t want a lot of these interventions…we’re gonna do a maximum amount of those things if we decide to go for surgery…so…surgery where she ends up in a nursing home, with complications from surgery, is not something that she ever wanted.”
Discussion
We trained surgeons to use the BC/WC framework to discuss treatment options with frail older inpatients facing high-stakes surgical decisions. This intervention promoted SDM as measured by a combination of OPTION 5 and qualitative analysis whereby we observed a pronounced shift in conversation structure and content postintervention in 3 primary areas: presentation of options, description of treatments, and deliberation over alternatives. Surgeons who used BC/WC emphasized a difficult decision and presented 2 authentic options. Rather than disclosing isolated procedural risks, trained surgeons described how patients might experience treatments and asked them to evaluate outcomes based on personal goals. Nonetheless, surgeons’ ability to integrate patient preferences into a recommendation varied.
The aim of the BC/WC framework is to clarify the limits of what is possible so patients and families can manage uncertainty and prepare for poor outcomes. Similar to corporate decision making, simple forecasting and risk prediction are only helpful in times of relative stability, when decision makers can assume that tomorrow will be similar to today. Akin to conditions of economic volatility, assumptions of stability fail frail older patients and their families in the setting of acute illness. Thus, a well-designed scenario does not seek to predict the future. Rather, the goal is to explore a set of plausible futures and describe a path from the present to a longer-term outcome. Scenarios improve decisions by allowing people to understand the interplay between elements—an acute surgical problem and underlying frailty—and develop a new mental model. Within this new reality, patients can think strategically and make decisions based on what is most important to them. These observations have important implications for surgeons, patients, and families.
For surgeons, BC/WC provides a framework to promote SDM and clarify outcomes. Despite efforts to improve prognostication, studies suggest that surgeons’ risk estimates are highly variable and physicians are overly optimistic in communicating prognosis. In part, this inconsistency stems from a lack of confidence in prognostic accuracy, particularly for long-term outcomes, and a desire to preserve hope. Presenting a range of plausible scenarios within the boundaries of a best and worst case may mitigate concerns about delivering an inaccurate prediction and help define the limits of what is possible with surgery. Best Case/Worst Case allows surgeons to set expectations so patients can maintain hope for the best and prepare for the worst. Furthermore, incorporating descriptions of the effect of surgery on overall quality of life can help surgeons preoperatively identify patients for whom even the best case surgical outcome is unacceptable.
For patients and families, BC/WC promotes a comparison of treatment outcomes and structures conversations so surgeons can learn what outcomes matter to them. While traditional models suggest desire for decision-making responsibility varies by individual and clinical scenario, newer theories posit that most patients prefer to be involved but are unsure how to engage. Best Case/Worst Case can help surgeons encourage patients to consider how they might value postoperative outcomes and avoid the perception that surgery is imperative. By presenting multiple scenarios, BC/WC supports the Lynn and DeGrazia “outcomes model” of medical decision making in which the physician avoids the need to fix the physiologic abnormality and elevates the validity of nonoperative alternatives. Furthermore, visualizing scenarios may clarify important misunderstandings, for example, the perception of the worst case as a painless death in the operating room or assumptions about postoperative quality of life.
Limitations
This study has strengths and limitations. Taken together, our mixed-methods approach suggests teaching surgeons to use BC/WC improves observer-rated SDM in the acute setting, but our small, single-center study was not powered to observe differences in OPTION 5 as a stand-alone measure. Because of significant challenges recruiting seriously ill older patients, we were unable to gather data from a postintervention conversation with all of the trained surgeons. Given space constraints, the formal analysis of our training program is reported elsewhere. Although we demonstrated that our intervention can distinctly change how surgeons communicate in high-stakes discussions, we were unable to identify a measurable health outcome that would allow us to test whether this intervention improves clinical outcomes beyond shared decision making. All patients in this study were old and frail, yet significant heterogeneity in patient preferences, surgical indication, and postoperative consequences makes defining the “right treatment choice” and the “good outcome” a formidable methodological challenge for this and future studies.
Conclusions
Training surgeons to use the BC/WC framework promotes SDM for frail older patients with acute surgical problems. This intervention helps surgeons present treatment outcomes and engage patients and families in a conversation closer to best practice guidelines. With this proof of concept, this intervention can be used to change surgeon behavior to support patients and families in difficult treatment decisions.
eAppendix. OPTION 5 Scale Calibrated to a Surgical Setting
References
- 1.Finlayson E, Fan Z, Birkmeyer JD. Outcomes in octogenarians undergoing high-risk cancer operation: a national study. J Am Coll Surg. 2007;205(6):729-734. [DOI] [PubMed] [Google Scholar]
- 2.Saxton A, Velanovich V. Preoperative frailty and quality of life as predictors of postoperative complications. Ann Surg. 2011;253(6):1223-1229. [DOI] [PubMed] [Google Scholar]
- 3.Louis DJ, Hsu A, Brand MI, Saclarides TJ. Morbidity and mortality in octogenarians and older undergoing major intestinal surgery. Dis Colon Rectum. 2009;52(1):59-63. [DOI] [PubMed] [Google Scholar]
- 4.Massarweh NN, Legner VJ, Symons RG, McCormick WC, Flum DR. Impact of advancing age on abdominal surgical outcomes. Arch Surg. 2009;144(12):1108-1114. [DOI] [PubMed] [Google Scholar]
- 5.Cooper Z, Mitchell SL, Gorges RJ, Rosenthal RA, Lipsitz SR, Kelley AS. Predictors of mortality up to 1 year after emergency major abdominal surgery in older adults. J Am Geriatr Soc. 2015;63(12):2572-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson TN, Wu DS, Stiegmann GV, Moss M. Frailty predicts increased hospital and six-month healthcare cost following colorectal surgery in older adults. Am J Surg. 2011;202(5):511-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farhat JS, Velanovich V, Falvo AJ, et al. . Are the frail destined to fail? frailty index as predictor of surgical morbidity and mortality in the elderly. J Trauma Acute Care Surg. 2012;72(6):1526-1530. [DOI] [PubMed] [Google Scholar]
- 8.Kwok AC, Semel ME, Lipsitz SR, et al. . The intensity and variation of surgical care at the end of life: a retrospective cohort study. Lancet. 2011;378(9800):1408-1413. [DOI] [PubMed] [Google Scholar]
- 9.Fried TR, Van Ness PH, Byers AL, Towle VR, O’Leary JR, Dubin JA. Changes in preferences for life-sustaining treatment among older persons with advanced illness. J Gen Intern Med. 2007;22(4):495-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Somogyi-Zalud E, Zhong Z, Hamel MB, Lynn J. The use of life-sustaining treatments in hospitalized persons aged 80 and older. J Am Geriatr Soc. 2002;50(5):930-934. [DOI] [PubMed] [Google Scholar]
- 11.Kon AA, Davidson JE, Morrison W, Danis M, White DB. Shared decision-making in intensive care units. executive summary of the American College of Critical Care Medicine and American Thoracic Society policy statement. Am J Respir Crit Care Med. 2016;193(12):1334-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper Z, Koritsanszky LA, Cauley CE, et al. . Recommendations for best communication practices to facilitate goal-concordant care for seriously ill older patients with emergency surgical conditions. Ann Surg. 2016;263(1):1-6. [DOI] [PubMed] [Google Scholar]
- 13.Bernacki RE, Block SD; American College of Physicians High Value Care Task Force . Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174(12):1994-2003. [DOI] [PubMed] [Google Scholar]
- 14.Neuman MD, Bosk CL. What we talk about when we talk about risk: refining surgery’s hazards in medical thought. Milbank Q. 2012;90(1):135-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilimoria KY, Liu Y, Paruch JL, et al. . Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833-842.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen ME, Ko CY, Bilimoria KY, et al. . Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg. 2013;217(2):336-346.e1. [DOI] [PubMed] [Google Scholar]
- 17.D’Amico TA, Krasna MJ, Krasna DM, Sade RM. No heroic measures: how soon is too soon to stop? Ann Thorac Surg. 2009;87(1):11-18. [DOI] [PubMed] [Google Scholar]
- 18.Lidz CW, Meisel A, Osterweis M, Holden JL, Marx JH, Munetz MR. Barriers to informed consent. Ann Intern Med. 1983;99(4):539-543. [DOI] [PubMed] [Google Scholar]
- 19.Wack P. Scenarios: uncharted waters ahead. Harv Bus Rev. 1985;63(5)73-89. https://hbr.org/1985/09/scenarios-uncharted-waters-ahead. Accessed February 26, 2016. [Google Scholar]
- 20.Wack P. Scenarios: shooting the rapids. Harv Bus Rev. 1985;63(6)139-150. https://hbr.org/1985/11/scenarios-shooting-the-rapids. Accessed February 26, 2016. [Google Scholar]
- 21.Schwartz P. The Art of the Long View: Planning for the Future in an Uncertain World. New York, NY: Doubleday/Currency; 1991. [Google Scholar]
- 22.Elwyn G, Frosch D, Volandes AE, Edwards A, Montori VM. Investing in deliberation: a definition and classification of decision support interventions for people facing difficult health decisions. Med Decis Making. 2010;30(6):701-711. [DOI] [PubMed] [Google Scholar]
- 23.Elwyn G, Frosch D, Thomson R, et al. . Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruser JM, Nabozny MJ, Steffens NM, et al. . “Best Case/Worst Case”: qualitative evaluation of a novel communication tool for difficult in-the-moment surgical decisions. J Am Geriatr Soc. 2015;63(9):1805-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarze ML, Kehler JM, Campbell TC. Navigating high risk procedures with more than just a street map. J Palliat Med. 2013;16(10):1169-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Best Case/Worst Case. Best Case/ Worst Case (BC/WC) communication tool–whiteboard video. https://www.youtube.com/watch?v=FnS3K44sbu0. Accessed March 29, 2016.
- 27.Porock D, Parker-Oliver D, Petroski GF, Rantz M. The MDS Mortality Risk Index: The evolution of a method for predicting 6-month mortality in nursing home residents. BMC Res Notes. 2010;3:200-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American College of Surgeons National Surgical Quality Improvement Program ACS NSQIP surgical risk calculator. http://riskcalculator.facs.org/. Accessed August 28, 2015.
- 29.Kruser JM, Taylor LJ, Nabozny MJ, et al. . Best case/worst case: training surgeons to use a novel communication tool for high-risk acute surgical problems [published January 3, 2017]. J Pain Symptom Manage. doi: 10.1016/j.jpainsymman.2016.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elwyn G, Edwards A, Wensing M, Hood K, Atwell C, Grol R. Shared decision making: developing the OPTION scale for measuring patient involvement. Qual Saf Health Care. 2003;12(2):93-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420-428. [DOI] [PubMed] [Google Scholar]
- 32.McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods. 1996;1(1):30-46. doi: 10.1037/1082-989X.1.130 [DOI] [Google Scholar]
- 33.Schwab P, Cerutti F, Hélène von Reibnitz U. Foresight–using scenarios to shape the future of agricultural research. Foresight. 2003;5(1):55-61. doi:dx.doi.org/ 10.1108/14636680310471299 [DOI] [Google Scholar]
- 34.Amer M, Daim TU, Jetter A. A review of scenario planning. Futures. 2013;46:23-40. doi:dx.doi.org/ 10.1016/j.futures.2012.10.003 [DOI] [Google Scholar]
- 35.Cauley CE, Block SD, Koritsanszky LA, et al. . Surgeons’ perspectives on avoiding nonbeneficial treatments in seriously ill older patients with surgical emergencies: a qualitative study. J Palliat Med. 2016;19(5):529-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sacks GD, Dawes AJ, Ettner SL, et al. . Surgeon perception of risk and benefit in the decision to operate. Ann Surg. 2016;264(6):896-903. [DOI] [PubMed] [Google Scholar]
- 37.Lamont EB, Christakis NA. Prognostic disclosure to patients with cancer near the end of life. Ann Intern Med. 2001;134(12):1096-1105. [DOI] [PubMed] [Google Scholar]
- 38.Glare P, Virik K, Jones M, et al. . A systematic review of physicians’ survival predictions in terminally ill cancer patients. BMJ. 2003;327(7408):195-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stone PC, Lund S. Predicting prognosis in patients with advanced cancer. Ann Oncol. 2007;18(6):971-976. [DOI] [PubMed] [Google Scholar]
- 40.Back AL, Arnold RM, Quill TE. Hope for the best, and prepare for the worst. Ann Intern Med. 2003;138(5):439-443. [DOI] [PubMed] [Google Scholar]
- 41.Singh JA, Sloan JA, Atherton PJ, et al. . Preferred roles in treatment decision making among patients with cancer: a pooled analysis of studies using the Control Preferences Scale. Am J Manag Care. 2010;16(9):688-696. [PMC free article] [PubMed] [Google Scholar]
- 42.Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. Can J Nurs Res. 1997;29(3):21-43. [PubMed] [Google Scholar]
- 43.Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision making. Patient Educ Couns. 2014;94(3):291-309. [DOI] [PubMed] [Google Scholar]
- 44.Lynn J, DeGrazia D. An outcomes model of medical decision making. Theor Med. 1991;12(4):325-343. [DOI] [PubMed] [Google Scholar]
- 45.Neuman MD. Surgeons’ decisions and the financial and human costs of medical care. N Engl J Med. 2010;363(25):2382-2383. [DOI] [PubMed] [Google Scholar]
- 46.Silverman WA. Medical decisions: an appeal for reasonableness. Pediatrics. 1996;98(6, pt 1):1182-1184. [PubMed] [Google Scholar]
- 47.Nabozny MJ, Kruser JM, Steffens NM, et al. . Constructing high-stakes surgical decisions: it’s better to die trying. Ann Surg. 2016;263(1):64-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. OPTION 5 Scale Calibrated to a Surgical Setting