Abstract
Purpose
Traditional germline sequencing and deletion/duplication analysis does not detect Lynch syndrome-causing mutations in all individuals whose colorectal or endometrial tumors demonstrate mismatch repair (MMR) deficiency. Unique inversions and other rearrangements of the MMR genes have been reported in families with Lynch syndrome. In 2014, a recurrent inversion of MSH2 exons 1-7 was identified in five families suspected to have Lynch syndrome. We aimed to describe our clinical experience in identifying families with this specific inversion.
Methods
Four probands whose Lynch syndrome-associated tumors demonstrated absence of MSH2/MSH6 staining and who had negative MMR germline testing were evaluated for the MSH2 inversion of exons 1-7, offered during initial genetic workup or upon routine clinical follow-up.
Results
All four probands tested positive for the MSH2 inversion. Proband cancer diagnoses included colon and endometrial adenocarcinoma and sebaceous adenoma. A variety of Lynch syndrome-associated cancers were reported in the family histories, although only one family met Amsterdam II criteria. Thirteen at-risk relatives underwent predictive testing.
Conclusion
MSH2 inversion of exons 1-7 was found in four probands previously suspected to have Lynch syndrome based on family history and tumor testing. This testing should be offered routinely to patients with tumors demonstrating loss of MSH2/MSH6 staining.
Keywords: Lynch syndrome, mismatch repair, MSH2 inversion
Introduction
Lynch syndrome (LS) is the most common hereditary predisposition to colorectal cancer (CRC) and is associated with mutations in the mismatch repair (MMR) genes MLH1, MSH2, MSH6, PMS2, and EPCAM (OMIM 120435) [1, 2]. Immunohistochemistry (IHC) for the MMR proteins in CRC or endometrial cancer is often used to drive genetic testing for the gene corresponding with the pattern of protein loss. However, not all individuals with MMR-deficient tumors will harbor a germline MMR mutation. This group of patients is known as mutation-negative Lynch syndrome or Lynch-like syndrome [3, 4] and many are due to biallelic somatic mutations in the MMR genes [5–7]. However, germline mutations undetectable by traditional sequencing or MLPA deletion/duplication analyses are also a potential cause. Deletions involving the EPCAM gene upstream of MSH2 gene were described in 2009, providing an explanation for some such cases [8]. Inversions and other rearrangements involving MMR genes have also been implicated [9–11].
More recently, a set of patients with MSH2/MSH6-deficient tumors were identified to have the MSH2 inversion of exons 1-7, not detectable through traditional testing methods[12]. This testing methodology is now available at several commercial genetic testing laboratories; however, this testing has not yet been described in a clinical setting. Here, we aim to describe four families identified to have this MSH2 inversion via clinical genetic workup and testing.
Patients and methods
All probands were evaluated by genetic counselors at our institution; tumor-based immunohistochemistry and microsatellite instability testing were performed at our institutional pathology laboratory. Germline genetic testing was performed at CLIA-approved laboratories. Informed consent was obtained from all individual participants included in the study.
Proband 1 is a male who presented to our institution at age 46 for treatment recommendations for a recently diagnosed colon carcinoma. He had a colonoscopy performed for intermittent abdominal pain and a large ulcerated mass was found in the ascending colon, which was revealed to be an invasive moderately differentiated adenocarcinoma with a mucinous component and loss of MSH2/MSH6 in the tumor cells. The patient’s family history is positive for a father diagnosed with glioblastoma multiforme at 26, and two of his father’s half-siblings and paternal grandfather with brain cancer. There is no history of CRC in the family. Due to loss of MSH2/MSH6 staining, MSH2/EPCAM germline testing was ordered and no mutations were identified. Subsequent MSH2 inversion testing identified inversion of exons 1-7.
Proband 2 is a female who presented at age 55 with a previous history of endometrial cancer at age 41 (status post-hysterectomy) and a recently diagnosed adenocarcinoma of the ascending colon for which she underwent right hemicolectomy. The patient’s family history is significant for a maternal grandmother diagnosed with CRC, uterine cancer, and breast cancer in her 50s as well as a maternal aunt with breast and colon cancer. Tumor testing revealed loss of MSH2/MSH6 protein expression with high levels of microsatellite instability (MSI) with 6 of 7 alleles shifted. Comprehensive germline testing was performed and did not reveal any mutations in MLH1, MSH2/EPCAM, MSH6, or PMS2. One year later she was diagnosed with adenocarcinoma of the transverse colon arising from a tubulovillous adenoma with high-grade dysplasia. Given her history, she underwent subtotal colectomy with ileorectal anastomosis. This new diagnosis prompted follow-up genetics evaluation and MSH2 inversion testing, which was positive.
Proband 3 is a male who presented at age 37 for screening regarding a family history consistent with Lynch syndrome. At presentation the patient had no personal history of cancer and had not yet undergone colonoscopy. His family history was significant for his father with two primary colon cancers at age 48 and ileal adenocarcinoma at age 64 who was also evaluated in the institution by the genetics program; IHC performed on the ileal adenocarcinoma revealed loss of MSH2/MSH6 protein expression, but subsequent germline testing was negative for mutations. Other family history included the patient’s paternal grandmother with CRC at age 54, paternal aunt with CRC at age 41, and this aunt’s son diagnosed with synchronous CRCs at age 39 whose tumors also showed loss of MSH2/MSH6 protein expression but tested negative for germline mutations. The patient underwent colonoscopy based on the family history; colonoscopy revealed an obstructing large polypoid mass in the transverse colon. The patient underwent a subtotal colectomy and pathology revealed a well differentiated mucinous adenocarcinoma with loss of MSH2/MSH6 protein expression. No germline testing was pursued due to the previous uninformative testing in other relatives. He was seen with routine clinical follow-up and offered MSH2 inversion testing; he tested positive.
Proband 4 is a male who was referred to this institution at age 54 to be evaluated for a suspicion of Lynch syndrome, Muir-Torre variant, given a history of two lesions in the left axilla and left upper lip diagnosed as sebaceous adenomas. He had no prior personal cancer history and had undergone colonoscopy in the past with no abnormalities. Family history was significant for father with an unknown primary cancer with liver metastasis and paternal grandfather with CRC diagnosed at age 43. Tumor-based testing was performed on one sebaceous adenoma from the proband; this demonstrated absence of MSH2/MSH6 staining. MSI analysis was not performed due to insufficient tissue. MSH2 comprehensive testing was performed and included inversion analysis; he tested positive.
Results
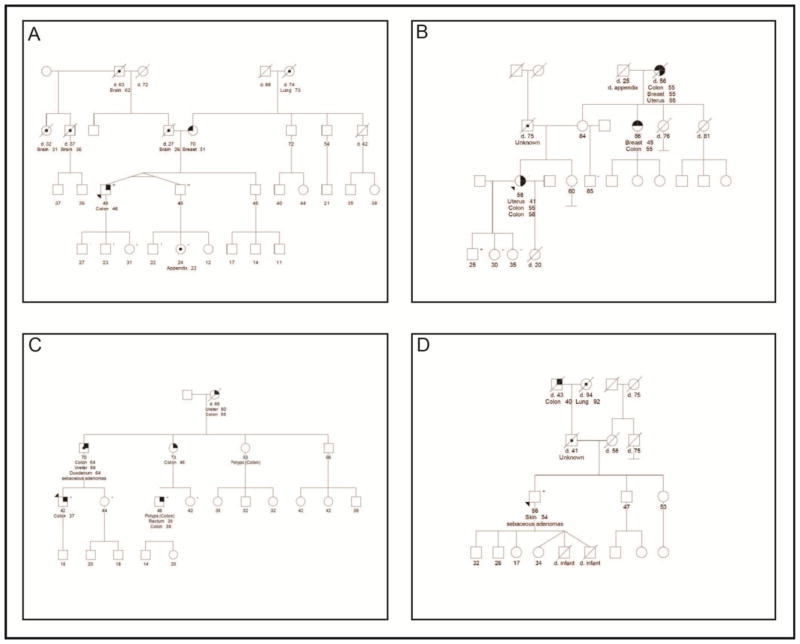
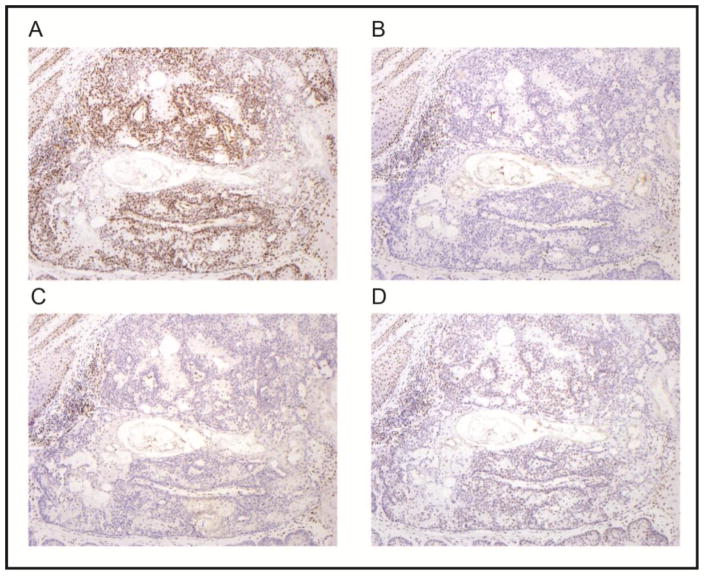
This report describes four probands and their families, with clinical histories summarized in Table 1 and pedigrees in Figure 1. They had varied clinical presentations and only one of the families met Amsterdam I or II criteria. To the best of our knowledge based on analysis of three-generation pedigrees, the probands were unrelated to each other and were also unrelated to the families previously reported with this inversion [12]. All probands’ tumors had absence of MSH2/MSH6 protein expression with intact staining of MLH1/PMS2 on tumor-based testing immunohistochemistry (Figure 2). The single proband tested for microsatellite instability was MSI-high. The average age at onset of the first Lynch-syndrome associated cancer in the combined report was 45 years.
Table 1.
Clinical and pathologic characteristics of probands positive for the MSH2 inversion
| Sex | Cancer diagnosis | Age at diagnosis (y) | MSI analysis | IHC | Amsterdam II criteria | |
|---|---|---|---|---|---|---|
| 1 | Male | Ascending colon adenocarcinoma | 46 | ND | Absent MSH2/MSH6 |
No |
|
| ||||||
| 2 | Female | Endometrial cancer | 41 | MSI-H | Absent | No |
| Ascending colon adenocarcinoma | 55 | MSH2/MSH6 | ||||
| Transverse colon adenocarcinoma | 56 | |||||
|
| ||||||
| 3 | Male | Transverse colon adenocarcinoma | 37 | ND | Absent MSH2/MSH6 |
Yes |
|
| ||||||
| 4 | Male | Sebaceous Adenoma | 54 | ND | Absent MSH2/MSH6 |
No |
MSI microsatellite instability; ND not done; MSI-H microsatellite instability-high; IHC immunohistochemistry
Fig. 1.
Pedigrees demonstrating family history for proband 1 (A), proband 2 (B), proband 3 (C), and proband 4 (D)
Fig. 2.
Immunohistochemical staining results for sebaceous adenoma in proband 4 (A, MLH1; B, MSH2; C, MSH6; and D, PMS2). There is loss of expression of MSH2 and MSH6 in the neoplastic cells. The tumor cells retain nuclear expression of MLH1 and PMS2; although staining intensity is stronger for MLH1 Normal tissue (hyperplastic squamous epithelium and lymphocytes in upper left and lower right corners) shows preserved nuclear expression of all four proteins (internal positive control). (MLH1 Cell Marque G168-728 clone, dilution 1:300; MSH2 Calbiochem clone FE11, dilution 1:100; MSH6 BD Biosciences clone 44, dilution 1:300; PMS2 BD Biosciences clone A16-4, dilution 1:125; magnification x100)
All probands underwent testing for inversion of exons 1-7 in MSH2 on a clinical basis after standard testing did not identify a causative mutation. Probands 1 and 4 were tested during initial genetic workup and were found to be positive for this inversion. Probands 2 and 3 whose germline testing resulted negative 9 months and 4.5 years before, respectively, were seen with clinical follow-up to offer this new testing method and they both had positive results for inversion of exons 1-7 in MSH2 gene, confirming our suspicions of Lynch syndrome. Additionally, a total of 13 family members were tested. Seven of them were found to be positive for the inversion and 6 of them had negative results (Figure 1).
Discussion
This case series represents, to the best of our knowledge, the first description of families identified to have the recently-described inversion of exons 1-7 in MSH2 via clinical genetic testing after the initial description of this new inversion [12]. Four unrelated probands who presented with a diverse personal and family history of cancer but whose tumors all demonstrated absence of MSH2 and MSH6 protein staining tested positive for this MSH2 inversion. The variable clinical presentations and family histories are similar to the cancers seen in families with previously-reported MSH2 pathogenic mutations and are consistent with the wide variety of cancers reported in the patients previously identified to have this specific inversion [12].
The identification of this mutation in our families provided confirmation of the high clinical suspicion of LS and importantly, allowed for predictive genetic testing in at-risk family members and initiation of surveillance in those individuals who tested positive. At the time of publication, 7 relatives tested positive and were recommended to undergo LS-directed surveillance, while 6 others tested true negative and were recommended to have population-level surveillance. Family 3 had been suspected to have LS for many years and at-risk relatives were previously undergoing LS-based surveillance without informative genetic testing; one of these relatives tested true negative for the inversion and ended years of surveillance.
Our series represents two probands whose MSH2 inversion was identified via initial genetic workup and two whose inversion was identified 9 months to 4.5 years after initial genetic workup. This highlights the importance of including inversion analysis in clinical genetic testing of patients being evaluated for LS, especially those whose tumors demonstrate absence of MSH2 and MSH6 protein staining. In addition, the latter two probands demonstrate the importance of recontacting patients suspected to have LS whose germline genetic testing was uninformative, especially with MSH2/MSH6-deficient tumors. A systematic research effort at the population level is needed to determine the prevalence of this inversion, for example if it is similar to EPCAM deletions, and to confirm that the tumor spectrum and cancer risks are similar to other described MSH2 mutations. Finally, this inversion has only been described in populations from the state of Texas and study in other populations is warranted.
Acknowledgments
This work was supported by the Feinberg Family Fund (to E.V).
Footnotes
The authors disclose no potential conflicts of interest.
References
- 1.Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clinical genetics. 2009;76(1):1–18. doi: 10.1111/j.1399-0004.2009.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moller P, Seppala T, Bernstein I, et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut. 2015 doi: 10.1136/gutjnl-2015-309675. [DOI] [PMC free article] [PubMed]
- 3.You YN, Vilar E. Classifying MMR variants: time for revised nomenclature in Lynch syndrome. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19(9):2280–2. doi: 10.1158/1078-0432.CCR-13-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Soler M, Perez-Carbonell L, Guarinos C, et al. Risk of cancer in cases of suspected lynch syndrome without germline mutation. Gastroenterology. 2013;144(5):926–32. e1. doi: 10.1053/j.gastro.2013.01.044. quiz e13-4. [DOI] [PubMed] [Google Scholar]
- 5.Haraldsdottir S, Hampel H, Tomsic J, et al. Colon and endometrial cancers with mismatch repair deficiency can arise from somatic, rather than germline, mutations. Gastroenterology. 2014;147(6):1308–16. e1. doi: 10.1053/j.gastro.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geurts-Giele WR, Leenen CH, Dubbink HJ, et al. Somatic aberrations of mismatch repair genes as a cause of microsatellite-unstable cancers. J Pathol. 2014;234(4):548–59. doi: 10.1002/path.4419. [DOI] [PubMed] [Google Scholar]
- 7.Mensenkamp AR, Vogelaar IP, van Zelst-Stams WA, et al. Somatic mutations in MLH1 and MSH2 are a frequent cause of mismatch-repair deficiency in Lynch syndrome-like tumors. Gastroenterology. 2014;146(3):643–6. e8. doi: 10.1053/j.gastro.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Ligtenberg MJ, Kuiper RP, Chan TL, et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3′ exons of TACSTD1. Nature genetics. 2009;41(1):112–7. doi: 10.1038/ng.283. [DOI] [PubMed] [Google Scholar]
- 9.Chen JM. The 10-Mb paracentric inversion of chromosome arm 2p in activating MSH2 and causing hereditary nonpolyposis colorectal cancer: re-annotation and mutational mechanisms. Genes Chromosomes Cancer. 2008;47(6):543–5. doi: 10.1002/gcc.20556. [DOI] [PubMed] [Google Scholar]
- 10.Morak M, Koehler U, Schackert HK, et al. Biallelic MLH1 SNP cDNA expression or constitutional promoter methylation can hide genomic rearrangements causing Lynch syndrome. J Med Genet. 2011;48(8):513–9. doi: 10.1136/jmedgenet-2011-100050. [DOI] [PubMed] [Google Scholar]
- 11.Liu Q, Hesson LB, Nunez AC, et al. A cryptic paracentric inversion of MSH2 exons 2-6 causes Lynch syndrome. Carcinogenesis. 2016;37(1):10–7. doi: 10.1093/carcin/bgv154. [DOI] [PubMed] [Google Scholar]
- 12.Rhees J, Arnold M, Boland CR. Inversion of exons 1-7 of the MSH2 gene is a frequent cause of unexplained Lynch syndrome in one local population. Fam Cancer. 2014;13(2):219–25. doi: 10.1007/s10689-013-9688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]