Abstract
The etiology of rotator cuff tendon overuse injuries is still not well understood. Furthermore, how this overuse injury impacts other components of the glenohumeral joint, including nearby articular cartilage, is also unclear. Therefore, this study sought to better understand the time course of tendon protease activity in a rat model of supraspinatus overuse, as well as determine effects of 10 weeks of overuse on humeral head articular cartilage. For these studies, multiplex gelatin zymography was used to characterize protease activity profiles in tendon and cartilage, while histological scoring/mechanical testing and micro-computed tomography (μCT) imaging were used to quantify structural damage in the supraspinatus tendon insertion and humeral articular cartilage, respectively. Histological scoring of supraspinatus tendon insertions revealed tendinopathic cellular and collagen fiber changes after 10 weeks of overuse when compared to controls, while mechanical testing revealed no significant differences between tensile moduli (overuse: 24.5 ± 11.5 MPa; control: 16.3 ± 8.7 MPa). EPIC-μCT imaging on humeral articular cartilage demonstrated significant cartilage thinning (overuse: 119.6 ± 6.34 μm; control: 195.4 ± 13.4 μm), decreased proteoglycan content (overuse: 2.1 ± 0.18 cm−1; control: 1.65 ± 0.14 cm−1), and increased subchondral bone thickness (overuse: 216.2 ± 10.9 μm; control: 192 ± 17.8 μm) in the overuse animals. Zymography results showed no significant upregulation of cathepsins or matrix metalloproteinases in tendon or cartilage at 2 or 10 weeks of overuse compared to controls. These results have further elucidated timing of protease activity over 10 weeks and suggest that damage occurs to other tissues in addition to the supraspinatus tendon in this overuse injury model.
Keywords: overuse injury, supraspinatus tendon, cartilage, micro-computed tomography, zymography
Graphical abstract
After 10 weeks of downhill treadmill running in a rat model ofrotator cuff overuse, tendon histology indicated structuraldamage near the tendon insertion, although no changes were observed in mechanical modulus or proteolytic (cathepsin or matrix metalloproteinase) activity. Contrast-enhanced microcomputed tomography (EPIC-μCT) on corresponding humeral head cartilage demonstrated decreased cartilage thickness and GAG content, with no changes in proteolytic activity. Results suggest rotator cuff tendon overuse affects matrix integrity of both supraspinatus tendon and nearby humeral articular cartilage.
Rotator cuff tendon overuse or tendinopathy, is a common degenerative disorder that can result in pain and limited range of motion.1,2 This injury particularly affects athletes and laborers using a repetitive overhead motion, as well as older adults.1,2 Tendinopathy is characterized clinically by changes in tenocyte phenotype, disorganization and misalignment of collagen fibers, and decreased mechanical properties.1,3 If left untreated, overuse injuries can predispose tissue to full or partial-thickness tears, requiring surgical intervention.4 Thus, elucidating underlying mechanisms of early tendon injury is important to develop new interventions to prevent tendon rupture.
Though the etiology of overuse is multifactorial, accepted intrinsic factors of tendinopathy include both excessive mechanical loading that leads to microtears and decreased loading and morphological changes of resident tenocytes, as well as imbalances in proteases that degrade extracellular matrix (ECM) components.4–6 Although proteolytic imbalances have been implicated in rotator cuff tendon ECM degradation, how proteases cause deterioration in early tendon damage is not well understood.7,8 In previous work, our laboratory demonstrated that after 4 and 8 weeks of rat supraspinatus tendon overuse, cysteine proteases cathepsins K and L were upregulated at the tendon insertion region (17% of length from the osseotendinous junction) when compared to their age-matched controls.9 This finding of cathepsin upregulation only near the insertion region motivated the current study to focus on protease activity in tissues near the humeral head, notably the tendon insertion, and the humeral articular cartilage. We investigated tissue degeneration in these areas using a well-established rat model of supraspinatus tendon overuse that displays similar bony anatomy to the human rotator cuff.3,5,9–12 Because our previous experiments focused on tendon damage at 4 and 8 weeks of overuse, showing cathepsins K and L upregulated at 4 weeks and only cathepsin L upregulated at 8 weeks, we have expanded this time course to investigate damage at earlier (2 weeks) and later (10 weeks) time points of tendon overuse in order to identify the time of greatest tissue damage and peak proteolytic activity.
Several studies have implicated cathepsin K, the most powerful mammalian collagenase13 in several tissue destructive diseases, including cancer, osteoporosis, and osteoarthritis (OA).14–16 Human cathepsin V is the most potent mammalian elastase identified, but also possesses collagenolytic activity and is upregulated in cancer and autoimmune disorders.17–19 Its ortholog, murine cathepsin L,20 is upregulated in diabetic mice at mRNA and protein expression levels and contributes to cardiovascular disease.19,21 As demonstrated in our previous work,9 cysteine cathepsins K and L are active and may result in collagen degradation in tendon overuse injury. In particular, it is known that cathepsin K can cleave collagen at both telopeptide ends, and can also cleave intrahelically, making collagen more susceptible to further degradation by other proteases.13
Moreover, cathepsins are also capable of cleaving and activating matrix metalloproteinases (MMPs).22 Thus, examining the proteolytic contributions of these two protease families in tandem could provide valuable mechanistic information about ECM degradation in tendon. MMPs are a family of zinc-dependent endopeptidases that can degrade and remodel tendon ECM.4,23 A prior study demonstrated upregulation of MMP-1 activity and down regulation of MMP-2 and -3 activity in ruptured human supraspinatus tendon,24 while another found upregulation of MMP-2 mRNA and protein activity and MMP-14 mRNA in a rat model of overuse,25 similar to the model used in the current research. These findings confirm that MMPs are present during injury and may be contributing to tendon degeneration in addition to cathepsins.
Rotator cuff tendons provide stability to the glenohumeral joint, but tears can alter joint loading and can induce cartilage degeneration.4,8,26 Several studies in rats have found evidence of osteoarthritic-like changes in cartilage after tendon transection (modeling full tendon tears).11,27,28 However, it remains unclear how tendon overuse injury affects nearby articular cartilage, both in terms of structural damage and proteolytic activity. Cathepsin K has been found to be upregulated in osteoarthritic cartilage and synovial tissues.16 Additionally, MMP-2, -9, and -13 have also been implicated in human and rat cartilage degeneration.29,30 Thus, we analyzed articular cartilage on the humeral head using safranin-O staining and Equilibrium Partitioning of Ionic Contrast agent via micro-computed tomography (EPIC-μCT)31 after 10 weeks of treadmill running to determine if supraspinatus tendon overuse would result in cartilage degeneration, as well as examined protease activity in this tissue.
Overall, the objective of this study was to characterize the level of tissue damage and proteolytic activity in supraspinatus tendon insertion tissues after 2 and 10 weeks of rat supraspinatus overuse, and also to determine if this model yielded tissue damage in humeral articular cartilage and subchondral bone, or proteolytic upregulation in humeral articular cartilage. Based on previous results, as well as their ability to activate MMPs, it was hypothesized that the greatest cathepsin upregulation in the supraspinatus tendon insertion tissues would occur at the earliest point of overuse. It was also hypothesized that both supraspinatus tendon insertion and cartilage tissues would show evidence of damage and proteolytic upregulation at 10 weeks of overuse.
Methods
Rat Overuse Model
All animal procedures in this study were approved by the Georgia Institute of Technology Institutional Animal Care and Use Committee. Sixty-eight male Dahl Salt-Resistant rats (∼350 g initial weight, 14–15 weeks initial age) were obtained (Harlan Labs, Indianapolis, IN), chosen because they are an inbred strain derived from the outbred Sprague–Dawley strain used to develop the injury model and have previously been used in work published by our group.9 Animals were separated into 2- or 10-week control and experimental groups. Experimental rats were subjected to a downhill treadmill rotator cuff overuse regimen as described previously.3 Thirty-four animals were trained for 2 weeks, running at a 10° decline for 1 h/day for 5 days/week increasing speeds up to a final 17m/min. Rats were then subjected to overuse for 2 or 10 weeks (n = 13/group/time point). Age-matched control rats were allowed normal cage activity (n = 13/group/time point). At each time point, control and overuse animals were sacrificed and supraspinatus tendons were harvested from animals designated for tendon histological and biochemical analysis. For biochemical analysis, the insertion region of the tendon (first 17% of the length from the osseotendinous junction) was isolated prior to analysis. Animals designated for tendon mechanical testing and cartilage biochemical analysis were sacrificed at 10 weeks and prepared for further processing.
Tendon Histology and Scoring
For histological evaluation, tendons were embedded in optimum cutting temperature (OCT) and frozen in a liquid nitrogen-chilled ethanol slurry. Tendon tissues were sectioned into 10 μm longitudinal sections using a cryostat (CryoStar NX70, Thermo Fisher Scientific, Waltham, MA). Slides were stained with hematoxylin and eosin (H&E) (VWR, Radnor, PA) and the insertion area of each tendon was imaged with a Nikon TE2000. H&E stained sections were histologically graded using a semi-quantitative scale, similar to the Bonar and Movin scales.32 Within each time point, the control and experimental groups were compared and scored in each of the following categories: regional variations in cellularity, cell shape, collagen fiber organization, and vascularization. A 4-point scoring system was used, where 0 indicated a normal tendon appearance and 3 a markedly abnormal appearance. Five graders, blinded from experimental group and time point, (TR, LT, JL, MO, JK) scored four images from each tendon (n = 8–9/group/time point). Scores were ranked and analyzed as individual events for statistical analysis (see “Statistical Analysis” section).
Tendon Mechanical Testing
Following a well-established protocol for mechanical testing,3,33 supraspinatus tendons (n = 10/group/time point) were cleaned of extraneous tissue and the humeral bones were mounted into custom-designed acrylic rings using Ortho-Jet BCA acrylic resin (Lang Dental, Wheeling, IL) and left in phosphate buffered saline (PBS) overnight to polymerize. Subsequently, tendon width and thickness were measured at the approximate middle point along the length of the tissue using calipers. Prior to testing, the free end of each tendons was clamped with fine grit sandpaper and marked every 2 mm, beginning at the insertion, using India Ink. Tendons were submerged in a 37°C PBS bath and preconditioned for 10 cycles. Samples were loaded to failure using a 100 N load cell at a constant 24 μm/s rate on a MTS Mini Bionix II system. Testing was captured using a Manta G504B ASG digital Camera (Graftek Imaging, Austin, TX) and recorded using LabView. Measurements for determining insertion moduli were taken by analyzing displacement between the two lines closest to the insertion region in ImageJ (NIH).
Cartilage Micro-Computed Tomography (μCT) and Equilibrium Partitioning of Ionic Contrast Agent via μCT (EPIC-μCT)
For microcomputed tomography, samples (n = 8/group/time point) were analyzed by μCT and EPIC-μCT based on established techniques.31 Following sacrifice, shoulder joints were scanned using viva CT (Scanco Medical, Brüttisellen, Switzerland) at 55kVp, 142 μA, and a 200 ms integration. Four distinct points along the humeral head were chosen for analysis based on three subdivided cartilage regions analyzed previously.27 Joint gap thickness was measured at these four points for both control and overuse animals. Subsequently, humeral heads from the same shoulders were detached from remaining humeral bone and tendons at the insertion region and submerged in a 10% solution of Hexab-rix 320 ionic contrast agent (Covidien, Dublin, Ireland) at 37°C for 30 min. Humeral heads, oriented based on the location of the removed tissue, were scanned in the sagittal plane with the μCT50 (Scanco Medical) at 45kVp, 200 mA, and a 600 ms integration time. The Hexabrix attenuation in cartilage at equilibration is inversely correlated to glycosaminoglycan (GAG) content, which is lost in cartilage degeneration.34 Following segmentation and reconstruction of the 3D images, cartilage thickness, cartilage attenuation, and subchondral bone thickness and attenuation were measured at four points along the humeral head for both control and overuse animals using μCT50 software.
Cartilage Histology
Scanned humeral heads (n = 8/group/time point) were decalcified by submerging them in Cal-Ex II (Thermo Fisher Scientific) for 1 week, exchanging solution every other day. After complete decalcification was achieved (as verified by a single X-ray scan using the viva CT), humeral heads were rinsed with PBS and embedded in OCT and frozen in a slurry of liquid nitrogen-chilled ethanol. The tissue was sectioned to 10 μm using a cryostat (CryoStat NX70). Slides were stained with 0.5% safranin-O and 0.2% fast green stains (Sigma–Aldrich, St. Louis, MO) and imaged using a Nikon TE2000 to observe sulfated GAG content.
Multiplex Gelatin Zymography
In preparation for zymography, the insertion region of each supraspinatus tendon (n = 6/group/time point) was isolated, and humeral articular cartilage (n = 7/group/time point) was carved from the humeral head. Tissues were homogenized in a zymography lysis buffer with 0.1 mM leupeptin, and the supernatant was collected. Multiplex zymography for cathepsins and MMPs was performed as previously described.14,35 Protein concentration in each lysate sample was determined by a micro BCA kit (Thermo Fisher Scientific). A total of 11 μg of protein from each sample was loaded into 12.5% (cathepsin) and 10% (MMP) SDS–polyacrylamide gels embedded with a 0.2% gelatin substrate. Using electrophoresis, enzymes separated by molecular weight. Then the gels were washed in renaturing buffer to remove the SDS and allow the enzymes to return to their active confirmation. Gels were then incubated in pH4 cathepsin assay buffer or pH7.4 MMP assay buffer for 18 h at 37°C to allow the proteases to degrade the gelatin. Subsequently, gels were stained with Coomassie Blue and destained to reveal white bands indicative of active proteases. Gels were then imaged using the ImageQuant LAS 4000 (GE Healthcare, Little Chalfont, United Kingdom).
Densitometry analysis was performed on the acquired images using ImageJ. Bands in zymography gels comparing control and overuse supraspinatus insertion samples at each time point were normalized to 1 ng of recombinant mouse cathepsin L (R&D Systems, Minneapolis, MN) and 0.01 ng recombinant human MMP-2 (ENZO, Farmingdale, NY). Zymography bands from cartilage samples were normalized to the control averages for each gel.
Statistical Analysis
The sample sizes were chosen based on power analysis performed on data from our previously published work.9 The power analysis revealed a minimum n = 5 for tendon and cartilage zymography with α = 0.05 and β = 0.15. The power analysis showed a required n = 8 with α = 0.05 and β = 0.2 for tendon histology and n = 5 with α = 0.05 and β = 0.2 for tendon mechanical testing. The power analysis revealed a minimum n = 8 for cartilage μCT imaging with α = 0.05 and β = 0.05. Histological scoring of tendon insertions was evaluated by the non-parametric Mann–Whitney test using Minitab. Data from the Mann–Whitney analysis was presented as rankings between 0 and 2 based on the frequency of each score value. Statistical significance for μCT data was determined by a Student's t-test in Minitab. Statistical significance for tendon zymography data was determined using a two-way ANOVA between normalized control and overuse data in GraphPad. Statistical significance for all other data was determined by a Student's t-test between normalized control and overuse data with a p<0.05 using Microsoft Excel.
Results
Supraspinatus Tendon Insertion Histology
Histological staining of 2-week control supraspinatus tendon insertions showed minimal damage indicated by aligned collagen fiber structure with elongated, spindle-shaped cells (Fig. 1A). Similarly, the 2-week overuse tissues demonstrated very little evidence of damage with minimal fiber disruption and elongated cells (Fig. 1B). At 10 weeks, control tissues maintained similar levels of tissue structure with aligned, tightly packed fibers, and a slight mix of elongated and rounded cells (Fig. 1C). Only in the 10-week overuse tissues, however, was there evidence of cellular rounding, collagen fiber misalignment, and fiber thinning, consistent with tendinopathic histological changes (Fig. 1D). Analysis of histological scoring reported decreased variation in cellularity at 2 weeks in the overuse compared to the controls, while there were no significant differences in scores for cell shape, collagen fiber organization, or vascularity at that time point. Additionally, only in 10-week overuse animals did tendons receive significantly higher scores for cell shape, indicating cell rounding, and in collagen fiber organization, indicating a disruption in the collagen fiber arrangement. Neither regional variation of cellularity nor vascularization received scores that were statistically different at 10 weeks in either experimental group (Table 1).
Figure 1.
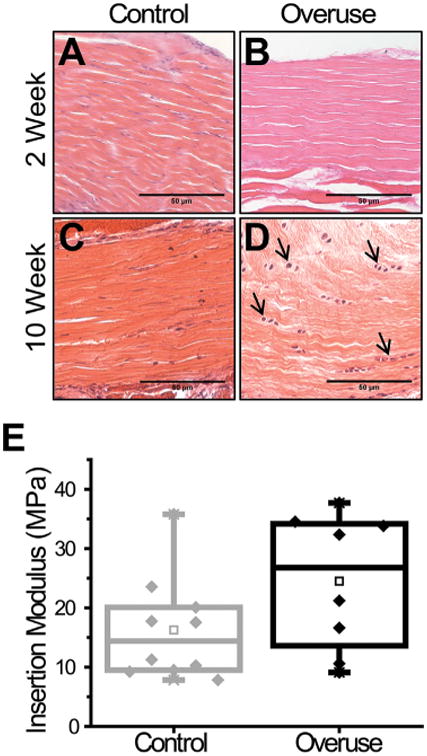
Histological and mechanical assessment of supraspinatus damage at 2 and 10 weeks of overuse. Collagen fiber disorganization and cell rounding were seen in the supraspinatus insertion by 10 weeks of overuse. The insertion region for 2-week (A) and 10-week control (C), animals are contrasted with the insertion region of 2-week (B) and 10-week overuse (D) tendons (n = 8–9). Arrows indicated rounded tendon cells. Tensile testing of the supraspinatus insertion regions of both control and overuse animals showed no significant difference (E) (n = 10 ± SD).
Table 1. Histological Scoring Analysis of Control and Overused Supraspinatus Tendon Insertion Regions.
| Category | 2-week | 10-week | ||
|---|---|---|---|---|
|
|
|
|||
| Control | Overuse | Control | Overuse | |
| Regional variation of cellularity | 1a | 0 | 0 | 0 |
| Cell shape | 0 | 0 | 0 | 1a |
| Collagen fiber organization | 1 | 1 | 0 | 1a |
| Vascularization | 0 | 0 | 0 | 0 |
Denotes significant difference (n = 8–9).
Supraspinatus Tendon Insertion Mechanical Testing
No significant difference was found in cross-sectional area between tendons from overuse animals (0.66± 0.14 mm0) versus control (0.77 ± 0.16 mm2). Similarly, analysis of mechanical testing results showed no significant differences in the tensile moduli between overused (24.5 ± 11.5 MPa) and control (16.3 ± 8.7 MPa) tissues (Fig. 1E).
EPIC-μCT and Histological Analysis of Humeral Articular Cartilage and Subchondral Bone
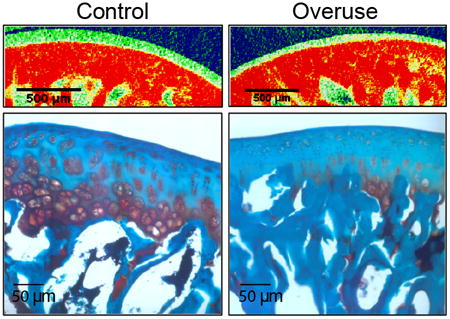
Joint gap distances determined from reconstructed models of the rat glenohumeral joint were significantly reduced in running animals (527.3 ± 16.3 μm; 264.3 ± 8.9 μm; 291.9 ± 40.7 μm; 263.8 ± 29.4 μm) in comparison to their age-matched controls (616.8 ± 35.9 μm; 366.4± 32.7 μm; 395.1 ± 68.8 μm; 336 ± 41.9 μm) along the four points on the humeral head (Fig. 2). Measurements from EPIC-μCT scans revealed that cartilage thickness was decreased with experimental animals (119.6 ± 6.34 μm; 93.2 ± 10.8 μm; 74.1 ± 10.4 μm; 56.9 ± 6.43 μm) compared to their age-matched controls (195.4 ± 13.4 μm; 141.6 ± 7.68 μm; 111.1 ± 11.9 μm; 90.1 ± 9.63 μm) at each of the four points analyzed (Fig. 3A–C). EPIC-μCT also revealed decreased GAG content, as indicated by the significant increase in contrast dye attenuation (overuse: 2.1 ± 0.18 cm−1; control: 1.65 ± 0.14cm−1) (Fig. 3B, C, and F), which was confirmed with safranin-O staining of the decalcified humeral heads (Fig. 3D and E). Subchondral bone thickness was higher in overuse animals (216.2 ± 10.9 μm) compared to control animals (192 ± 17.8 μm) (Fig. 3G). However, there was no difference in mineral density of the subchondral bone between the two groups (overuse: 4.79 ± 0.06 cm−1; control: 4.78 ± 0.09 cm−1) as demonstrated by quantification of subchondral bone attenuation (Fig. 3H).
Figure 2.
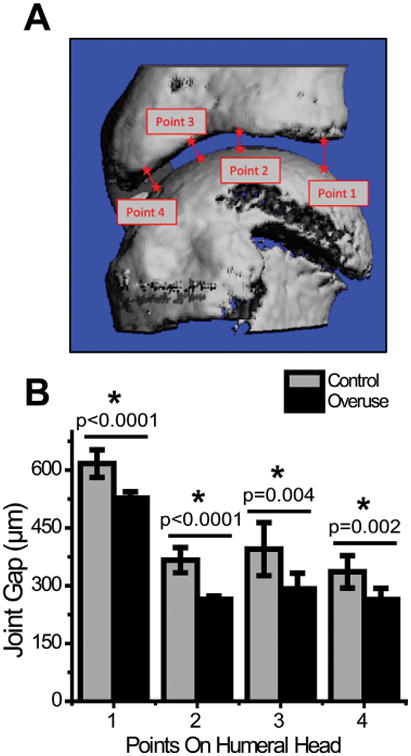
μCT measurements of glenohumeral joint gap. Joint gap thickness as measured by μCT along four different points of the glenohumeral joint (A) showed decreased joint space in overuse animals as compared to age-matched controls (B). *Denotes significant difference, p values as stated (n = 8 ± SD).
Figure 3.
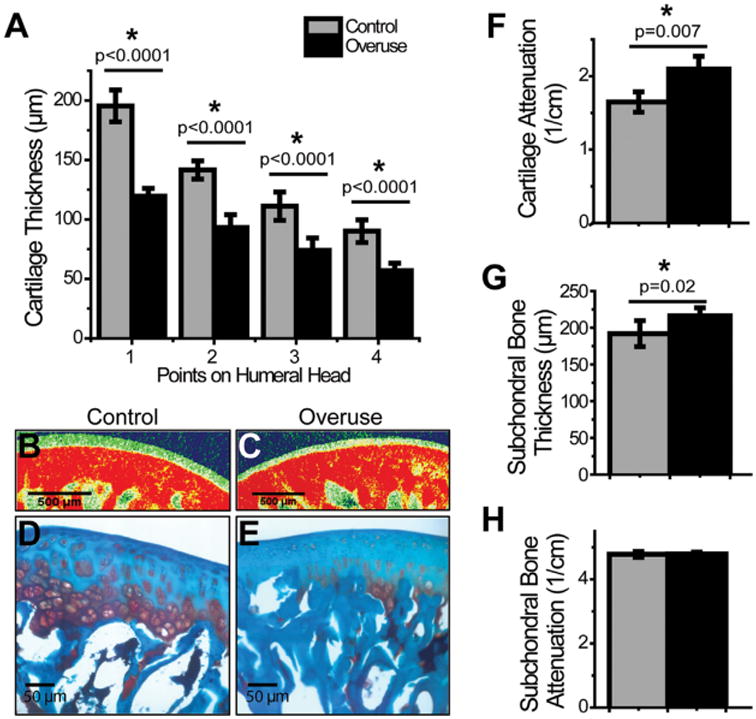
EPIC-μCT measurements of humeral articular cartilage and subchondral bone. Humeral heads demonstrated a reduced cartilage thickness in overuse animals in comparison to their age-matched controls (A). Humeral cartilage also displayed decreased GAG content by EPIC-μCT in overuse animals in comparison to controls (B, C, F) (SD: control: ± 0.14cm−1; overuse: ± 0.18 cm−1), which was confirmed with safranin-O staining of the decalcified humeral head (D and E). While subchondral bone thickness was higher in overuse animals as compared to controls (G), there was no difference in mineral attenuation in the subchondral bone (H). *Denotes significant difference, p values as stated (n = 8 ± SD).
Cathepsin and MMP Activity in Supraspinatus Tendon Insertion
After 2 and 10 weeks of overuse, active cathepsin L was detected in the pH 4 cathepsin zymograms between 25 and 35 kD, while active pro- and mature MMP-2 were detected in pH 7.4 MMP zymograms around 68 and 72 kD, respectively, in rat supraspinatus insertions of both overuse and control animals. Normalized densitometric analysis revealed no significant differences in the amounts of either active cathepsin L or MMP-2 between after 2 or 10 weeks of overuse (Fig. 4A and B).
Figure 4.
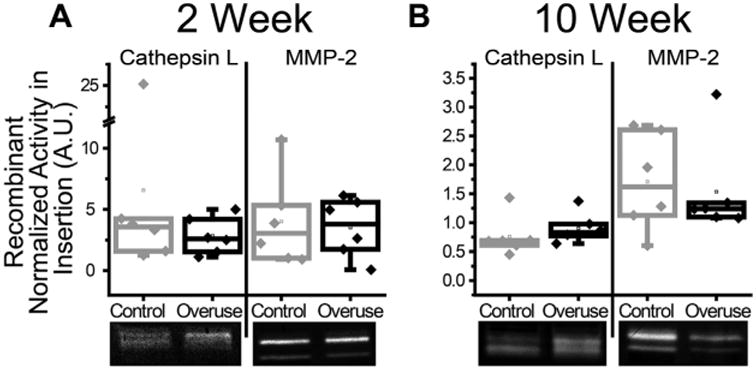
Cathepsin L and MMP-2 activity in supraspinatus tendon insertion. No significant differences in active rat cathepsin L or MMP-2 were measured by zymography between animals subjected to overuse or age-matched controls of rat supraspinatus tendon insertion after 2 weeks (A) or 10 weeks (B) of overuse. Values were normalized to recombinant mouse cathepsin L and human MMP-2 in cathepsin and MMP gels, respectively. Zymography bands below all plots are representative of the corresponding protease, time, and condition (n = 6 ± SD).
Cathepsin and MMP Activity in Humeral Articular Cartilage
Cartilage tissue harvested from 10-week control and overuse rat humeral heads assayed by multiplex cathepsin zymography at pH4 exhibited unidentified active bands at 100 and 75kD (Fig. 5A). MMP zymography on same tissues exhibited active pro- and mature MMP-2 bands at around 72 and 68kD, respectively (Fig. 5C). Zymograms from both proteolytic families exhibited varying amounts of active proteases between individual animals; however, neither cathepsin nor MMP activity demonstrated any significant difference between the control and overuse animals as quantified by densitometry (Fig. 5B and D).
Figure 5.
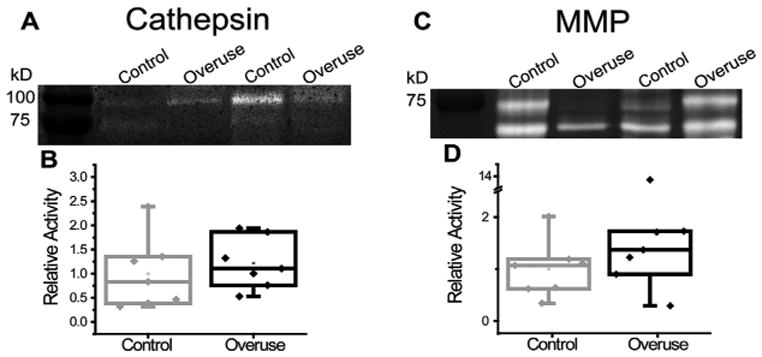
Cathepsin and MMP activity in humeral cartilage. Cathepsins appeared active in humeral head cartilage as visualized by zymography, with bands at 100 and 75 kD (A), but neither band demonstrated significant differences between control and overuse cartilage tissues (B) MMPs also appeared active by zymography, with bands around 75 and 65 kD, indicative of the pro-form and mature form of MMP-2, respectively (C). However, neither band demonstrated significance between the control and overuse cartilage tissues (D) (n = 7 ± SD).
Discussion
In addition to tendon degeneration, damage to glenohumeral articular cartilage can occur due to rotator cuff tendon injuries.36,37 Studies have found decreased glenoid cartilage thickness, decreased compressive modulus, and diminished histological properties after full supraspinatus and infraspinatus rotator cuff tears.27,28 However, to our knowledge, no studies have investigated the effect of overuse injury alone on the surrounding tissues. It has been shown that cytokines and proteases are upregulated in the synovial fluid after tendon tears, and that these synovial factors are positively correlated with the development of early osteoarthritic degeneration.27,38 This suggests that proteases active due to supraspinatus overuse could be contributing to cartilage degeneration via the synovial fluid. Given this information coupled with our previous observation that damage occurred only at the tendon insertion region, we hypothesized concomitant degenerative effects of supraspinatus tendon overuse on tendon and humeral articular cartilage at 10 weeks.
Supporting this hypothesis, results demonstrated structural changes in rat supraspinatus tendon insertion and humeral articular cartilage after 10 weeks of supraspinatus tendon overuse. Histological scoring at the 2-week time point indicated significant variation in regional cellularity in the control supraspinatus insertions compared to the overused tissue (Table 1 and Fig. 1A). Although unexpected, this may be a result of the natural level of variation in locations of cells within the tissues of both the control and overuse groups at 2 weeks. Furthermore, no other category showed any significant differences at this time point, indicating no overall histological evidence of pathology over 2 weeks. At 10 weeks, however, cellular shape and fiber organization, two of the main histopathological changes found in injured tendon1 received significantly greater scores in the overuse samples (Table 1 and Fig. 1D), which is reflective of the changes in regional cellularity and shape previously observed after 4 and 8 weeks of overuse.9 Taken together, these data suggest that tendon tissue damage becomes histologically evident at 4 weeks, continues to degenerate by 8 weeks, and persists at 10 weeks, which is consistent with findings from other studies.3,9,32 Mechanical testing demonstrated no difference in insertion tensile modulus (Fig. 1E) or overall tendon modulus (data not shown) between overuse and control animals, indicating that not enough damage has occurred to significantly change the overall modulus of the entire insertion region. However, the tendon insertion histological scoring and the observed humeral cartilage degeneration (discussed below) suggest that damage to the joint overall occurs as a result of the overuse injury.
To assess damage to articular cartilage in early stages of glenohumeral joint injury, we employed contrast-enhanced μCT (EPIC-μCT).28,31,39 The decreases observed in joint space distance, cartilage thickness, proteoglycan content, and increased subchondral bone thickness (Fig. 3) are all indications of osteoarthritic-like changes in the humeral head cartilage.16,31 The articular cartilage degeneration observed may result from altered mechanical loading of the glenohumeral joint and/or proteolytic imbalances in the synovial fluid.8,16,28,40,41 Such results are similar to those found in other rat models of traumatic shoulder injury involving tendon transection. In one study, cartilage thinning and a significant decrease in elastic modulus was observed at several regions across the glenoid in rats, 4 weeks after supraspinatus and infraspinatus transection compared to their contralateral tissues.28 Another study used a modified Mankin scoring system to quantify damage in glenoid cartilage tissue sections 12 weeks after either a supraspinatus and infraspinatus tenotomy or a suprascapular nerve transection. Significant damage was found as indicated by cellularity, proteoglycan content, structure, and tidemark integrity in both injury models when compared to controls.27
Increased protease activity has been found previously in damaged tendon and cartilage tissue. Cathepsin K protein expression was found upregulated in a rabbit model of flexor tendon injury42 and mRNA levels were elevated in human calcific tendinopathy.43 Cathepsin K was also upregulated in human osteoarthritic cartilage44 and in articular chondrocytes of an osteoarthritic mouse model.45 MMPs have shown increased expression and proteolytic activity, both in human ruptured supraspinatus tendons as well as overuse tissues in a rat model after 2 and 4 weeks of activity.24,25 Increased MMP-2, -9, and -13 activity as indicated by zymography and fluorescent enzymatic assays was found in human osteoarthritic chondro-cytes.29 In our study, in order to understand the proteolytic contribution to tendon damage progression observed in this model of overuse injury, we used multiplex gelatin zymography to quantify active proteases.14,46 From cathepsin and MMP zymography on supraspinatus insertion tissues, we observed no significant upregulation of active forms for either of the protease families between control and overuse animals for either 2 or 10 weeks of injury (Fig. 4A and B). However, the tendon histology data present indications of degeneration by 10 weeks. Our previous findings showed upregulation of cathepsins K and L at 4 weeks and cathepsin L at 8 weeks overuse.9 Along with current data, it suggests that the proteolytic activity of both cathepsins and MMPs may be subject to multiphasic temporal regulation over the course of overuse injury, and in this particular model, are likely upregulated before 10 weeks and induce damage to the ECM as observed histologically as early as 4 weeks in our previous study.9
The cartilage tissue cathepsin zymography presented high molecular weight active bands at 75 and 100kD (Fig. 5). These bands are thought to be caused by cathepsins bound to ECM, as they were seen in supraspinatus tendon tissue and endometriosis lesions.9,47 These cathepsins are likely bound to insoluble collagen and proteoglycans, and may become proteolytically active upon release from resident cells.18,48 These data could provide a mechanism of collagen cleavage in a tendon overuse context for future studies. Although neither cathepsins nor MMPs were upregulated in cartilage by 10 weeks of injury, it is possible that, similar to the tendon insertion, proteolytic upregulation in the humeral articular cartilage would occur before the 10-week point. Further work is required to investigate the time course of cartilage injury due to tendon overuse to better identify when therapeutics might be best introduced to reduce damage to both tissues.
This study included several limitations. The rat was chosen as a model of overuse due to coracoacromial arch similarities to the human shoulder. However, it is important to note that because rats are quadruped animals, their shoulders are not loaded in the same manner as in humans, and therefore do not completely recapitulate the mode of clinical overuse. While no known human studies are available that examine cartilage changes during tendon overuse, this study may motivate collection of more clinical data in this area. This study was also unable to distinguish whether cartilage or tendon degeneration initiates damage to other tissue in the joint, or if these occur concurrently.
In summary, this work has exhibited degeneration in multiple tissues adjacent to the humeral head as a result of an overuse protocol, as quantified by histological scoring of rat supraspinatus tendon tissues as well as cartilage thickness and dye attenuation as measured by EPIC-μCT. In tendon tissues, active cathepsin L and MMP-2 were not upregulated in overused tissues compared to their age-matched controls. Similarly, after 10 weeks of tendon overuse, humeral articular cartilage also showed no significant difference between control and overuse tissues in terms of proteolytic activity. This work suggests a necessity to treat both the tendon and nearby cartilage to slow or reverse tissue damage during glenohumeral overuse injuries. Taken together with our previous data that indicates upregulation in cathepsins in tendon overuse injury, these results also highlight the importance of elucidating temporal regulation of proteolytic activity to best determine timing of any future therapeutic strategies employing protease inhibitors.
Acknowledgments
The authors would like to thank Torri Rinker, Liane Tellier, Jennifer Lei, Molly Ogle, and Jack Krieger for serving as graders for tendon image scoring. The authors would also like to thank Erin Radcliffe for her assistance in processing samples. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Grant sponsor: National Institute of Arthritis and Musculoskeletal; Grant sponsor: Skin Diseases of the National Institutes of Health; Grant number: R01AR063692; Grant sponsor: National Institutes of Health Cell and Tissue Engineering Training; Grant number: T32GM008433.
Footnotes
Authors' Contributions: ANP contributed to the research design, acquired, analyzed, and interpreted the zymography data, and wrote the paper. JMF acquired, analyzed, and interpreted all micro-computed tomography (μCT) data and histological data, as well as contributed to drafting the paper. AC assisted with acquiring and analyzing the histological scoring data. EPY assisted with acquiring and analyzing zymography data. REG helped develop design, acquisition, and analysis of the μCT and contributed to critical revision of the paper. MOP and JST developed all research designs, analysis, and interpretation of all data, and contributed to critical revisions of the paper. All authors have read and approved the final submitted manuscript.
References
- 1.Xu Y, Murrell GAC. The basic science of tendinopathy. Clin Orthop Relat Res. 2008;466:1528–1538. doi: 10.1007/s11999-008-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maganaris CN, Narici MV, Almekinders LC, et al. Biomechanics and pathophysiology of overuse tendon injuries: ideas on insertional tendinopathy. Sport Med. 2004;34:1005–1017. doi: 10.2165/00007256-200434140-00005. [DOI] [PubMed] [Google Scholar]
- 3.Soslowsky LJ, Thomopoulos S, Tun S, et al. Neer award 1999 Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elb Surg. 2000;9:79–84. [PubMed] [Google Scholar]
- 4.Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Jt Surg. 2005;87:187. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- 5.Soslowsky LJ, Carpenter JE, DeBano CM. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elb Surg. 1996;5:383–392. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 6.Galloway MT, Lalley AL, Shearn JT. The role of mechanical loading in tendon development, maintenance, injury, and repair. J Bone Joint Surg Am. 2013;95:1620–1628. doi: 10.2106/JBJS.L.01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomopoulos S, Parks WC, Rifkin DB, et al. Mechanisms of tendon injury and repair. J Orthop Res. 2015;33:832–839. doi: 10.1002/jor.22806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huegel J, Williams AA, Soslowsky LJ. Rotator cuff biology and biomechanics: a review of normal and pathological conditions. Curr Rheumatol Rep. 2015;17:476. doi: 10.1007/s11926-014-0476-x. [DOI] [PubMed] [Google Scholar]
- 9.Seto S, Parks A, Qiu Y, et al. Cathepsins in rotator cuff tendinopathy: identification in human chronic tears and temporal induction in a rat model. Ann Biomed Eng. 2015;43:1–11. doi: 10.1007/s10439-014-1245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hast MW, Zuskov A, Soslowsky LJ. The role of animal models in tendon research. Bone Joint Res. 2014;3:193–202. doi: 10.1302/2046-3758.36.2000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reuther KE, Thomas S, Sarver J, et al. Effect of return to overuse activity following an isolated supraspinatus tendon tear on adjacent intact tendons and glenoid cartilage in a rat model. J Orthop Res. 2013;31:710–715. doi: 10.1002/jor.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reuther KE, Thomas S, Tucker J, et al. Disruption of the anterior-posterior rotator cuff force balance alters joint function and leads to joint damage in a rat model. J Orthop Res. 2014;32:638–644. doi: 10.1002/jor.22586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garnero P, Borel O, Byrjalsen I, et al. The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J Biol Chem. 1998;273:32347–32352. doi: 10.1074/jbc.273.48.32347. [DOI] [PubMed] [Google Scholar]
- 14.Wilder CL, Park KY, Keegan PM, et al. Manipulating substrate and pH in zymography protocols selectively distinguishes cathepsins K, L, S, and V activity in cells and tissues. Arch Biochem Biophys. 2011;516:52–57. doi: 10.1016/j.abb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deaton DN, Tavares FX. Design of cathepsin K inhibitors for osteoporosis. Curr Top Med Chem. 2005;5:1639–1675. doi: 10.2174/156802605775009676. [DOI] [PubMed] [Google Scholar]
- 16.Kozawa E, Cheng X, Urakawa H, et al. Increased expression and activation of cathepsin K in human osteoarthritic cartilage and synovial tissues. J Orthop Res. 2016;34:127–134. doi: 10.1002/jor.23005. [DOI] [PubMed] [Google Scholar]
- 17.Reiser J, Adair B, Reinheckel T. Specialized roles for cysteine cathepsins in health and disease. J Clin Invest. 2010;120:3421–3431. doi: 10.1172/JCI42918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z, Yasuda W, Li W, et al. Regulation of collagenase activities of human cathepsins by glycosaminoglycans. J Biol Chem. 2004;279:5470–5479. doi: 10.1074/jbc.M310349200. [DOI] [PubMed] [Google Scholar]
- 19.Keegan PM, Wilder CL, Platt MO. Tumor necrosis factor alpha stimulates cathepsin K and V activity via juxtacrine monocyte-endothelial cell signaling and JNK activation. Mol Cell Biochem. 2012;367:65–72. doi: 10.1007/s11010-012-1320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bromme D, Li Z, Barnes M, et al. Human cathepsin V functional expression, tissue distribution, electrostatic surface potential, enzymatic characterization, and chromosomal localization. Biochemistry. 1999;38:2377–2385. doi: 10.1021/bi982175f. [DOI] [PubMed] [Google Scholar]
- 21.Yang M, Zhang Y, Pan J, et al. Cathepsin L activity controls adipogenesis and glucose tolerance. Nat Cell Biol. 2007;9:970–977. doi: 10.1038/ncb1623. [DOI] [PubMed] [Google Scholar]
- 22.Christensen J, Shastri VP. Matrix-metalloproteinase-9 is cleaved and activated by cathepsin K. BMC Res Notes. 2015;8:322. doi: 10.1186/s13104-015-1284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garofalo R, Cesari E, Vinci E, et al. Role of metalloproteinases in rotator cuff tear. Sports Med Arthrosc. 2011;19:207–212. doi: 10.1097/JSA.0b013e318227b07b. [DOI] [PubMed] [Google Scholar]
- 24.Riley GP, Curry V, DeGroot J, et al. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. 2002;21:185–195. doi: 10.1016/s0945-053x(01)00196-2. [DOI] [PubMed] [Google Scholar]
- 25.Attia M, Huet E, Gossard C, et al. Early events of overused supraspinatus tendons involve matrix metalloproteinases and EMMPRIN/CD147 in the absence of inflammation. Am J Sports Med. 2013;41:908–917. doi: 10.1177/0363546512473817. [DOI] [PubMed] [Google Scholar]
- 26.Pastoureau PC, Hunziker EB, Pelletier JP. Cartilage, bone and synovial histomorphometry in animal models of osteoarthritis. Osteoarthr Cartil. 2010;18:S106–S112. doi: 10.1016/j.joca.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Kramer EJ, Bodendorfer B, Laron D, et al. Evaluation of cartilage degeneration in a rat model of rotator cuff tear arthropathy. Arthritis Care Res. 2013;22:1702–1709. doi: 10.1016/j.jse.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reuther KE, Sarver J, Schultz S, et al. Glenoid cartilage mechanical properties decrease after rotator cuff tears in a rat model. J Orthop Res. 2012;30:1435–1439. doi: 10.1002/jor.22100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson MT, Moradi B, Smith M, et al. Activation of matrix metalloproteinases 2, 9, and 13 by activated protein C in human osteoarthritic cartilage chondrocytes. Arthritis Rheumatol. 2014;66:1525–1536. doi: 10.1002/art.38401. [DOI] [PubMed] [Google Scholar]
- 30.Beckett J, Jin W, Schultz M, et al. Excessive running induces cartilage degeneration in knee joints and alters gait of rats. J Orthop Res. 2012;30:1604–1610. doi: 10.1002/jor.22124. [DOI] [PubMed] [Google Scholar]
- 31.Thote T, Lin A, Raji Y, et al. Localized 3D analysis of cartilage composition and morphology in small animal models of joint degeneration. Osteoarthr Cartil. 2013;21:1132–1141. doi: 10.1016/j.joca.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Maffulli N, Longo UG, Franceschi F, et al. Movin and Bonar scores assess the same characteristics of tendon histology. Clin Orthop Relat Res. 2008;466:1605–1611. doi: 10.1007/s11999-008-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang TF, Perry SM, Soslowsky LJ. The effect of overuse activity on Achilles tendon in an animal model: a biomechanical study. Ann Biomed Eng. 2004;32:336–341. doi: 10.1023/b:abme.0000017537.26426.76. [DOI] [PubMed] [Google Scholar]
- 34.Palmer AW, Guldberg RE, Levenston ME. Analysis of cartilage matrix fixed charge density and three-dimensional morphology via contrast-enhanced microcomputed tomography. Proc Natl Acad Sci USA. 2006;103:19255–19260. doi: 10.1073/pnas.0606406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kupai K, Szucs G, Cseh S, et al. Matrix metalloproteinase activity assays: importance of zymography. J Pharmacol Toxicol Methods. 2010;61:205–209. doi: 10.1016/j.vascn.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Neer C, Craig E, Fukuda H. Cuff tear arthropathy. J Bone Jt Surg. 1983;65:1232–1244. [PubMed] [Google Scholar]
- 37.Hsu HC, Luo ZP, Stone JJ, et al. Correlation between rotator cuff tear and glenohumeral degeneration. Acta Orthop Scand. 2003;74:89–94. doi: 10.1080/00016470310013725. [DOI] [PubMed] [Google Scholar]
- 38.Yoshihara Y, Hamada K, Nakajima T. Biochemical markers in the synovial fluid of glenohumeral joints from patients with rotator cuff tear. J Orthop Res. 2001;19:573–579. doi: 10.1016/S0736-0266(00)00063-2. [DOI] [PubMed] [Google Scholar]
- 39.Baragi VM, Becher G, Bendele A, et al. A new class of potent matrix metalloproteinase 13 inhibitors for potential treatment of osteoarthritis: evidence of histologic and clinical efficacy without musculoskeletal toxicity in rat models. Arthritis Rheum. 2009;60:2008–2018. doi: 10.1002/art.24629. [DOI] [PubMed] [Google Scholar]
- 40.Andriacchi TP, Mündermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr Opin Rheumatol. 2006;18:514–518. doi: 10.1097/01.bor.0000240365.16842.4e. [DOI] [PubMed] [Google Scholar]
- 41.Pozgan U, Caglic D, Rozman B, et al. Epression and activity profiling of selective cysteine cathepsins and matrix metalloproteinases in synovial fluids from patients with rheumatoid arthritis and osteoarthritis. Biol Chem. 2010;391:571–579. doi: 10.1515/BC.2010.035. [DOI] [PubMed] [Google Scholar]
- 42.Berglund ME, Hart DA, Reno C, et al. Growth factor and protease expression during different phases of healing after rabbit deep flexor tendon repair. J Orthop Res. 2011;29:886–892. doi: 10.1002/jor.21330. [DOI] [PubMed] [Google Scholar]
- 43.Oliva F, Barisani D, Grasso A, et al. Gene expression analysis in calcific tendinopathy of the rotator cuff. Eur Cell Mater. 2011;21:548–557. doi: 10.22203/ecm.v021a41. [DOI] [PubMed] [Google Scholar]
- 44.Dejica VM, Mort J, Laverty S, et al. Cleavage of type II collagen by cathepsin K in human osteoarthritic cartilage. Am J Pathol. 2008;173:161–169. doi: 10.2353/ajpath.2008.070494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morko JP, Söderström M, Säämänen AMK, et al. Up regulation of cathepsin K expression in articular chondrocytes in a transgenic mouse model for osteoarthritis. Ann Rheum Dis. 2004;63:649–655. doi: 10.1136/ard.2002.004671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li WA, Barry Z, Cohen J, et al. Detection of femtomole quantities of mature cathepsin K with zymography. Anal Biochem. 2010;401:91–98. doi: 10.1016/j.ab.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 47.Porter KM, Wieser FA, Wilder CL, et al. Cathepsin protease inhibition reduces endometriosis lesion establishment. Reprod Sci. 2015;23:1–7. doi: 10.1177/1933719115611752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aguda AH, Panwar P, Du X, et al. Structural basis of collagen fiber degradation by cathepsin K. Proc Natl Acad Sci USA. 2014;111:17474–17479. doi: 10.1073/pnas.1414126111. [DOI] [PMC free article] [PubMed] [Google Scholar]


