Abstract
Background
Retinopathy of prematurity is one of the leading causes of childhood blindness worldwide, with vessel growth cessation and vessel loss in phase I followed by neovascularization in phase II. Ischemia contributes to its pathogenesis and lutein protects against ischemia-induced retinal damages. We aimed to investigate the effects of lutein on a murine model of oxygen-induced retinopathy.
Methods
Mouse pups were exposed to 75% oxygen for five days and returned to room air for another five days. Vascular obliteration, neovascularization and blood vessel leakage were examined. Immunohistochemistry for glial cells and microglia were performed.
Results
Compared with vehicle controls, mouse pups receiving lutein treatment displayed smaller central vaso-obliterated area and reduced blood vessel leakage. No significant difference in neovascular area was found between lutein and vehicle controls. Lutein promoted endothelial tip cell formation and maintained the astrocytic template in the avascular area in oxygen-induced retinopathy. No significant changes in Müller cell gliosis and microglial activation in the central avascular area were found in lutein-treated pups.
Conclusions
Our observations indicated that lutein significantly promoted normal retinal vascular regrowth in the central avascular area, possibly through promoting endothelial tip cell formation and preserving astrocytic template. Our results indicated that lutein might be considered as a supplement for the treatment of proliferative retinopathy of prematurity due to its role in facilitating the revascularization of normal vasculature.
Keywords: retinopathy of prematurity, oxygen-induced retinopathy, vascular development
Introduction
Retinopathy of prematurity (ROP), with vessel growth cessation and vessel loss followed by vision-threatening pathological vessel proliferation,1 is a common eye disorder in preterm babies. With advances in neonatal care and increased survival rate of extremely smaller preterm infants, the incidence of ROP increases.2
The pathogenesis of ROP is multi-factorial and the main risk factors are a gestational age of 32 weeks or less, low birth weight and oxygen use.1,3 Relative hypoxia develops after oxygen therapy, triggering pathologic neovessel growth in response to increased metabolic demand of developing retina. Therefore, ischemia plays a crucial role in the development of ROP.2 Lutein is a member of xanthophyll family of carotenoids and contained in dark green vegetables, such as spinach and kale.4-7 Lutein protects against retinal ischemia/reperfusion injury in vivo and directly protects retinal neuronal cells from H2O2-induced oxidative stress and cobalt chloride (CoCl2)-induced hypoxia in vitro.5,8 We hypothesized that lutein might protect against ischemia-induced ROP.
Three recent randomized controlled trial studies of lutein in ROP patients reported that lutein appeared to be ineffective in preventing proliferative ROP in preterm infants.9-11 However, a decreasing trend of the progression rate from early ROP stages to threshold ROP (defined as beyond which there is 50% likelihood of the incidence of unfavorable outcomes) was shown.9,12 Therefore, the effect of lutein in ROP needs to be further assessed. In addition, as preterm infants are fragile with incomplete development, the efficacy of lutein treatment in ROP needs to be evaluated thoroughly in pre-clinical animal models. In this study, mouse oxygen-induced retinopathy (OIR) modeling human ROP was used to investigate the effect of lutein on retinal vasculature.13
Methods
Animals
All the experimental and animal handling procedures were in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The use of animals was conducted according to the requirements of the Cap. 340 Animals (Control of Experiments) Ordinance and Regulations, and all relevant legislation and Codes of Practice in Hong Kong, and was approved by the Faulty Committee on the Use of Live Animals in Teaching and Research in The University of Hong Kong (CULATR 2423-11).
Mouse model of OIR
OIR was induced in C57BL/6 mice. Neonatal mice and their nursing dams were exposed to 75% oxygen (PRO-OX110 chamber controller; Biospherix Ltd., New York, NY) from postnatal day seven (P7) to P12.13-15 From P12 to P17, littermate mouse pups were daily intraperitoneally injected with lutein (0.2mg/kg, Sigma-Aldrich Co, St. Louis, MO)5 or vehicle 10% dimethyl sulfoxide (DMSO). On P17, samples were collected and only pups with weight ≥6g were used.16 In the other animal group, 0.2mg/kg lutein or vehicle was injected from P7 to P11 and the samples were collected on P12. During hyperoxic exposure, soda lime served as CO2 quencher.16 Mouse pups were limited to 7 to 8 per litter to reduce the runty phenotype.16
Central Vaso-obliteration and Neovascularization
The retinal flat-mounts were permeabilized in 70% ice-cold ethanol for twenty minutes followed by 1% Triton X-100 PBS for thirty minutes. The retinae were then stained with Alexa Fluor-488 conjugated isolectin GS-IB4 from Griffonia simplicifolia (0.2mg/ml; Invitrogen, Carlsbad, CA) overnight at 4°C15. The retinal flat-mounts were imaged with an upright microscope (Eclipse 80i, Nikon, Tokyo, Japan) under 40× magnification. The vaso-obliterated and total retinal areas were measured using Sigma Scan Pro software (SPSS, Chicago, IL). The percentage of obliterated area over total retinal area was calculated.
Neovascular area was quantified with the tuft area selected by hand in Photoshop CS5 and measured in Image J as reported.15,17 The percentage of neovascular area over total retinal area was calculated. In addition, the neovascular tufts invading into the vitreous were also counted on retinal cross sections.13 Eight sagittal sections (each 30μm apart) without optic nerve head, four on each side of the optic nerve, were stained with hematoxylin and eosin. Images were taken under 400× magnification.
Vascular leakage: IgG extravasation
The retinal sections with optic nerve head were de-paraffinized and incubated with proteinase K. The sections were then reacted with Mouse-On-Mouse biotinylated anti-mouse IgG secondary antibody. IgG immunoreactivity was visualized by incubation with avidin-biotin-peroxidase complex (Vector Laboratories Inc., Burlingame, CA) and the substrate, diaminobenzidine (Invitrogen, Carlsbad, CA). The sections were counterstained with hematoxylin. IgG staining outside blood vessel lumen indicated vascular leakage.15,17,18 Images were taken under 600× magnification.
Assessment of endothelial cell tip cells
Endothelial cell tip cells were visualized on isolectin-stained retinal flat-mounts.19 Images of the boundary between avascular and vascular areas were collected using an upright microscope. The number of endothelial cell tip cells in seven to eight selected 200× fields of view was determined.
Assessment of astrocytic template
The retinal flat-mounts were permeabilized, blocked with 10% goat serum, incubated with rabbit anti-GFAP (1:200, Dako, Denmark) and isolectin GS-IB4 at 4°C overnight. After three rinses in PBS, the retinal flat-mounts were incubated with Alexa Fluor 568 goat anti-rabbit IgG (1:500; Molecular Probes, Invitrogen, Carlsbad, CA). Six to eight fields were selected in the central avascular zone with similar distance from the optic nerve head. Images were taken with a confocal laser scanning microscope under 200× magnification (LSM700, Carl Zeiss, Thornwood, NY). The extent of astrocyte persistence in the avascular areas was scored on a 1-6 scale as reported.20 Value 1-2 indicated no or few astrocytes; value 3-4 indicated some astrocytes but these astrocytes lacking standard normal astrocytic template; value 5-6 indicated fairly or completely normal astrocytic template.
Immunohistochemistry for glial cells
The de-paraffinized retinal sections with optic nerve head were blocked with serum and incubated with primary rabbit anti-glial fibrillary acidic protein (GFAP 1:500, Dako, Glostrup, Denmark) at 4°C overnight. The retinal sections were then incubated with Alexa Fluor 568 goat anti-rabbit IgG (1:500; Molecular Probes, Invitrogen, Carlsbad, CA) to visualize the signal. Photomicrographs were captured under 400× magnification.
Assessment of retinal microglial responses
The retinal flat-mounts were permeabilized and blocked with 10% goat serum, followed by rabbit anti-Iba-1 (1:800, Wako Chemicals USA, Richmond) and isolectin GS-IB4 for 3 days at 4°C. The retinal flat-mounts were incubated with Alexa Fluor 568 goat anti-rabbit IgG (1:500; Molecular Probes, Invitrogen, Carlsbad, CA). Microglia cells were counted in eight selected 200× fields of confocal images from the avascular area15.
Statistical Analysis
Data was presented as mean ± SEM. The immunohistochemical investigations were performed in one single experiment. Unpaired-t-test, one-way ANOVA and Bonferroni's Multiple comparison test were used (Prism v5.0, GraphPad Software Inc., San Diego, CA). Statistically significant difference was set at p<0.05.
Results
Lutein promoted physiological retinal revascularization
After hyperoxia, vaso-obliteration occurred in vehicle- and lutein-treated retinae (Figure 1A-B), which became gradually smaller starting from P14 until P17. The avascular area was significantly smaller in the lutein- than vehicle-treated retinae (Fig 1C, n = 6 to 9 mice for each group). Neovasuclar area in flat-mounted retinae (Fig 1D-E) was measured and intravitreal neovascular vessels on hematoxylin-and-eosin-stained retinal sections (arrows, Fig 1G-H) were quantified. No significant difference in retinal neovascularization was observed between vehicle- and lutein- treated OIR mice (Fig 1F, 1I, n = 6 to 9 mice for each group).
Figure 1.
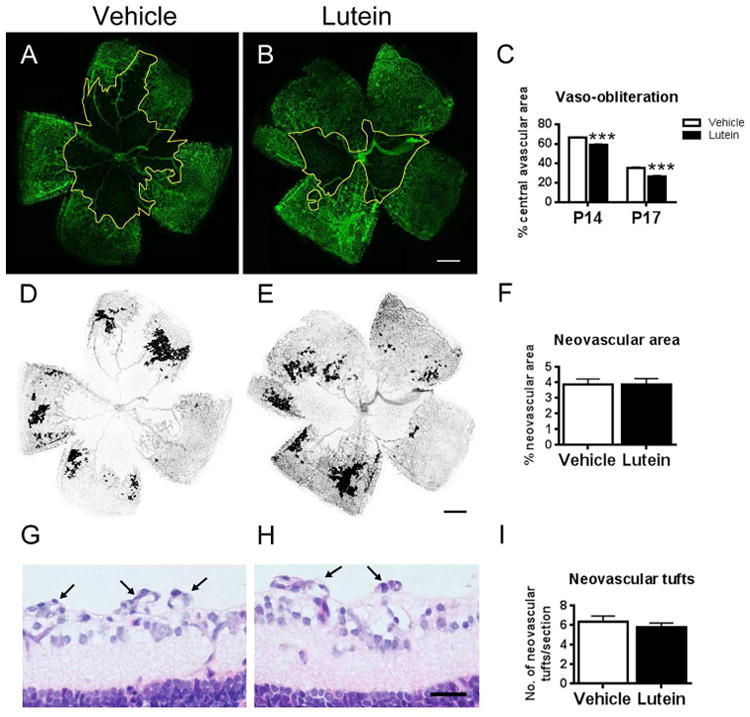
Lutein promoted the normal vessel regrowth in the central avascular area in oxygen-induced retinopathy (OIR). Flat-mounted retinae were stained with isolectin GS-IB4 for blood vessels (green, A-B). After OIR, a central avascular area was observed (outlined in yellow). (C) Percentage of the central avascular area over the total retinal area at different postnatal days was estimated. Significantly reduced avascular area was observed in the lutein- versus vehicle-treated retinae on P14 and P17. In addition, bucketed flat-mounted retinae (D-E) and hematoxyline-and-eosin-stained retinal sagittal sections (G-H) were used to analyze neovascularization on P17. No significant difference in neovascular area and number of neovascular tufts was shown between vehicle- and lutein- treated OIR retinae (F, I). n = 6 to 9 for each group. ***p<0.001, Unpaired t test. Scale bar, 500μm (B, E); 25μm (H).
Lutein reduced retinal blood vascular leakage
The number of leaky blood vessels, which was presented as IgG immunostaining detected outside the blood vessel lumen,15,17 was counted in the ganglion cell layer (GCL) and outer plexiform layer (OPL) (Fig 2A-D). Significantly reduced leaky vessels were observed in GCL in lutein-treated versus vehicle-treated OIR retinae (Fig 2E-F, n = 5 to 8 mice for each group).
Figure 2.
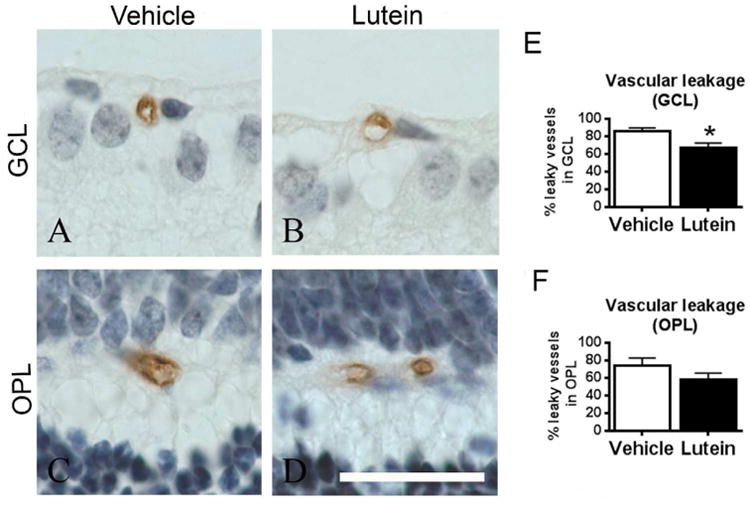
Lutein reduced the retinal vascular leakage along ganglion cell layer (GCL) in OIR. Representative images of IgG-stained retinal sagittal sections in GCL (A-B) and outer plexiform layer (OPL) (C-D) on P17. After OIR, intact vessel lumen and IgG staining outside the lumen were observed (A-D). The number of leaky vessels was quantified and expressed as a percentage of total number of retinal vessels (E-F). Significantly reduced retinal leaky vessels in GCL and a trend of decrease in OPL were found in lutein- versus vehicle-treated mice. The sections were counterstained with hematoxylin (blue) for nucleus. n = 5 to 8 for each group. *p<0.05, Unpaired t test. Scale bar, 10μm.
Lutein facilitated endothelial cell tip cell formation in avascular retinae
The facilitated revascularization by lutein in OIR was also investigated by analyzing the formation of endothelial cell tip cells, whose filopodia extended from the exiting endothelial cells to the vaso-obliterated area on P14 (Fig 3A-D). When comparing the lutein-treated and vehicle-treated OIR mice, significantly increased endothelial cell tip cells were observed in retinae with lutein treatment on P14 (Fig 3E, n = 7 to 12 for each group).
Figure 3.
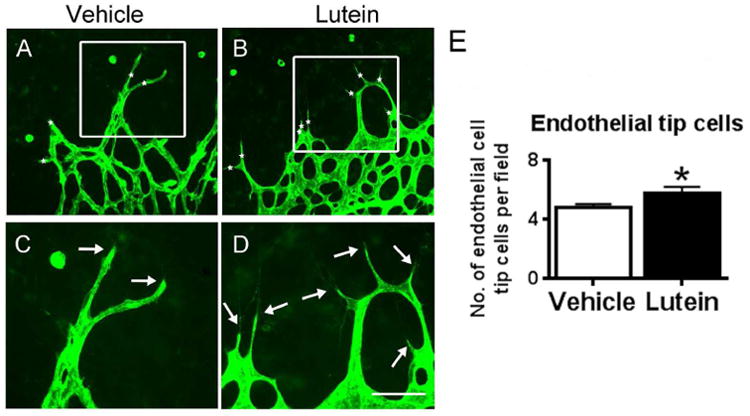
Lutein increased the endothelial cell tip formation in the retinal avascular area at P14. Flat-mounted retinae were stained with isolectin GS-IB4 for blood vessels (green, A-H). Representative images of retinal endothelial cell tip cells (asterisks, A-B) and filopodia (arrows, C-D) were shown. The number of the endothelial cell tip cells was quantified and compared between vehicle- and lutein- treated groups at P14. Significantly increased endothelial tip cells were observed with lutein treatment (E). n = 7 to 12 for each group. *p<0.05, Unpaired t test. Scale bar, 50μm.
Lutein preserved astrocytic template in avascular retinae
Astrocytes showed stellate/dendritic morphology and were tightly correlated with the normal retinal vasculature (Fig 4A-B). In OIR, the astrocytes lost their normal structure and network (Fig 4C-D). In the avascular retinal area, few astrocytes were present in the vehicle-treated retinae (Fig 4C) while more astrocytes were observed in lutein-treated retinae (Fig 4D). The status of astrocytic template was scored and an increased score was observed with lutein treament (Fig 4E, n = 7 to 9 for each group.).
Figure 4.
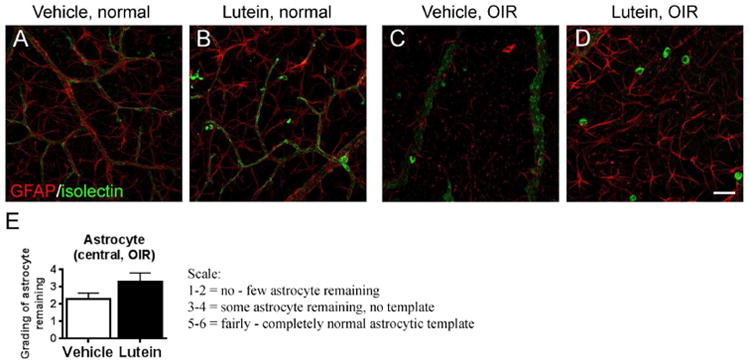
Lutein maintained the astrocytic template in the retinal avascular area in OIR. Immunohistochemical staining of glial cells with GFAP antibody were conducted in retinal flat-mounts on P17 (A-D). In the normal retinae, GFAP-positive astrocytes (red) showed stellate/dendritic morphology and were tightly correlated with the superficial layer of retinal vasculature (isolectin GS-IB4, green). In OIR, the astrocytes lost their normal structure and network in avascular areas. Few astrocytes were present in the vehicle-treated retinae while more astrocytes were observed in lutein-treated retinae. n = 7 to 9 for each group. Scale bar, 50μm.
Lutein did not influence glial and microglial cell responses in OIR retinae
GFAP staining was localized in astrocytes around the blood vessels along inner limiting membrane (ILM) in normal retina (Fig 5A-B). In OIR, stronger GFAP immunoreactivity was observed in the astrocytes along ILM and in Müller cell processes across IPL in lutein- and vehicle-treated groups (Fig 5C-D).
Figure 5.
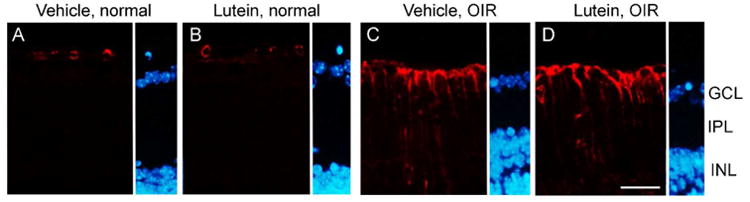
No significant differences were found in Müller cell gliosis between vehicle- and lutein- treated OIR retinas. GFAP immunoreactivity (red) was observed in astrocytes around the blood vessel lumen in room-air controls (A-B). Increased GFAP immunoreactivity along inner limiting membrane (ILM) and across inner plexiform layer (IPL) was observed in both OIR groups compared with their room-air controls respectively (C-D). The sections were counterstained with DAPI (blue) for nucleus. INL, inner nuclear layer. n = 7 to 9 for each group. Scale bar, 25μm.
Microglia undergoes morphological change upon activation. Activated ameboid microglia showed large cell bodies and also expressed isolectin GS-IB421-23. The non-activated ramified microglia showed small cell bodies with long processes and did not express isolectin GS-IB421-23. In this study, the microglia status was evaluated in the central retinal area nearby the optic nerve. In the normal retina, inactivated ramified microglia was the major type (arrow, Fig 6A-B). In OIR, activated ameboid microglia dominated in the central avascular area (star, Fig 6C-D). Ameboid-form microglia was also immuno-positive for isolectin, indicative of activation (star, Fig 6A-D). The number of ameboid- and ramified- form microglia was quantified in the central avascular area. Increased ameboid-form microglia was found in OIR retinae versus normal retinae in both vehicle- and lutein-treated groups (Fig 6E, n = 5 to 9 for each group). However, there was no significant difference between vehicle-treated and lutein-treated OIR retinae (Fig 6E, n = 5 to 9 for each group). No significant changes in the number of inactivated ramified-form microglia were observed between OIR and normal retinae.
Figure 6.
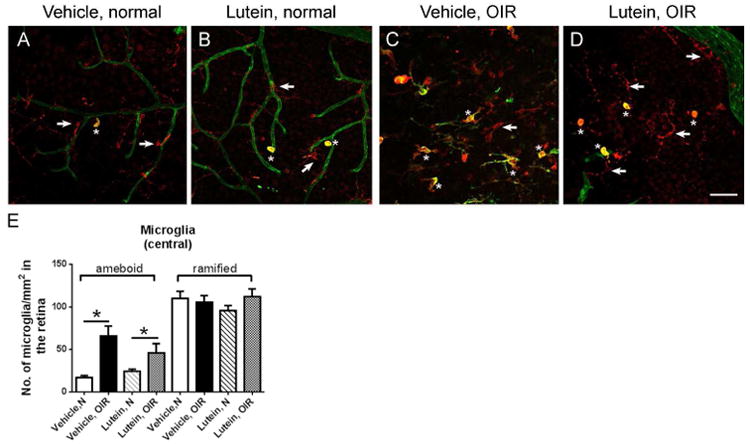
No significant differences were found in microglial activation between vehicle- and lutein- treated OIR retinas. Representative images of retinal areas stained with Iba-1 antibody (red) and isolectin GS-IB4 (green) on P17. Generally, ramified microglia dominated (arrow, A-B) while occasionally ameboid microglia (asterisk, A-B) were observed in room-air retinae. In OIR, positive staining with both Iba-1 and isolectin GS-IB4 indicated activated microglia. In the retinal avascular area, ameboid microglia (asterisk, C-D) was significantly increased in superficial vascular layers (E). n = 5 to 9 for each group. *p<0.05, one-way ANOVA followed by Bonferroni's Multiple Comparison Test. Scale bar, 50μm.
Lutein did not prevent hyperoxia-induced retinal vessel loss
The littermate mice were treated with either vehicle or lutein daily from P7 to P11 and the retinas were collected immediately after hyperoxic exposure at P12 (Fig 7A-B). No significant differences in central vaso-obliterated area were found between vehicle-treated and lutein-treated groups (Fig. 7C, n = 7 to 8 mice for each group)
Figure 7.
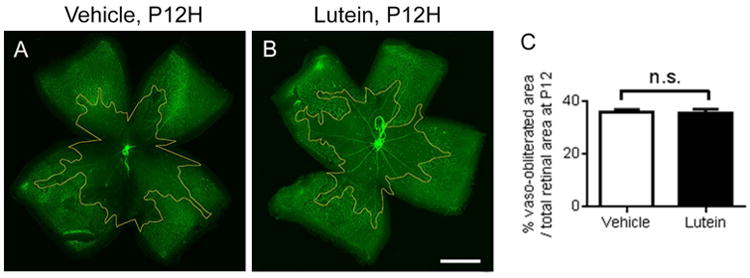
No significant differences were found in hyperoxia-induced vessel loss at P12. Representative images of retinal whole mount stained with isolectin GS-IB4 (green) on P12. The vaso-obliterated area was outlined in yellow. n = 7 to 8 for each group. Unpaired t test. Scale bar, 1mm.
Discussion
In the present study, central vaso-obliteration, neovascularization, blood vessel leakage and revascularization were observed in the mouse model of OIR as previously reported.13,15 We found that lutein in part protected the OIR retinae by facilitating normal retinal vascular regrowth and reducing blood vessel leakage.
Endothelial cell tip cells are essential for the development of new capillaries. Their specialized apical filopodia attach to the astrocytes, which in turn provide the principal cues for guidance of endothelial tip cells and their filopodia.19,24 Previously, reduction in endothelial cell tip cells was shown to reduce neovascularization without interfering with intra-retinal revascularization in a rat model of ROP.25 In the mouse model of OIR, revascularization occurs from P12 immediately after hyperoxia.16 Neovascularization starts from P14 to P17.16 We here demonstrated that lutein increased endothelial cell tip cells on P14, in line with facilitated intra-retinal revascularization.
In addition, astrocytic changes and Müller cell activation are observed in the animal models of OIR.13,15 Astrocytes and Müller cells are two main groups of glial cells in the retina. Retinal astrocytes are involved in the retinal vascular development, forming a template in the superficial vascular layer to facilitate the extension of endothelial cell tip cells.26 In OIR, astrocytes are lost under hypoxia20,27 and maintenance of the astrocytic template normalizes revascularization in OIR.20 In the present study, lutein treatment in OIR appeared to preserve astrocytic template in the avascular area, corresponding to the facilitated revascularization. Müller cells are specialized glial cells stretching through the entire retina. When activated, Müller cells undergo reactive gliosis, characterized by GFAP up-regulation.13,15,28 Müller cell gliosis contributes to retinal neovascularization and blood-retinal-barrier breakdown.29,30 Lutein reduces the Müller cell response in ischemic retina.31 Here, we found that there were no significant changes in Müller cell gliosis comparing vehicle-treated and lutein-treated OIR mice, corresponding to no change in retinal neovascularization.
Furthermore, microglia, which are resident macrophages to maintain retinal integrity, play an important role in OIR.15,22,32 Upon activation, microglia undergo morphological change from ramified to ameboid form.15,22 Promotion of vascular repair by microglia was possibly through hypoxia-inducible factor-1-alpha in ischemic retinae.33 A certain degree of microglial activation in the central avascular retinal area was reported to facilitate revascularization better.15 Yet, there was no significant difference in the number of activated microglia in avascular between lutein- and vehicle- treated retinas, indicating that protective effects of lutein on promoting revascularization may not be through microglial modulation.
Taken together, lutein did not have significant impact on the status of Müller cell and microglia in OIR, possibly explaining partially why there was no significant changes in neovascularization as Müller cell gliosis34 and microglial activation35,36 have been suggested to contribute to retinal neovascularization in OIR. The potential mechanism behind the positive effects of lutein on revascularization during the hypoxic phase might be partly due to the increased endothelial cell tip cell formation and the preservation of astrocytic template in central avascular area, which might provide guidance signaling for the normal vessel growth. However, further exploration is needed to determine if lutein has more direct effects on retinal astrocytic preservation and endothelial cell function as there are still limited studies in this field. In addition, photoreceptors are the most metabolically active cell in the body and very susceptible to metabolic derangement and oxidative stress.37,38 Dysregulated photoreceptor metabolism triggers pathologic retinal angiogenesis39 and the loss of photoreceptors also diminished the mouse oxygen-induced proliferative retinopathy.40 These observations seemed to indicate that photoreceptor might play a determining role in regulating pathologic retinal vessel growth. Lutein is a strong antioxidant that accumulates in the macula41 and protects the retinal neurons against oxidative stress-induced apoptosis in vivo and in vitro.5,42 We speculate that photoreceptors might also be one of the potential targets in lutein's modulation of retinal vasculature.
As lutein promoted normal vessel growth under hypoxia, we further examined if lutein protected against loss of retinal blood vessels during hyperoxic phase and no significant differences were founded. In human ROP, in addition to the oxygen, the loss of nutrients and maternal growth factors due to the premature birth also contributes to the pathogenesis of the disease progression.43 Therefore, further studies with respect to the other risk factors are needed before drawing the final conclusion of lutein's efficacy in Phase I ROP. For example, hyperglycemia is recently observed as a novel risk factor for the development of ROP44-51 and hyperglycemic induction delayed retinal vascular development in neonatal rats.52 Examining the effects of lutein in the neonatal rodent model of hyperglycemia-inhibited retinal angiogenesis will help further explore the role of lutein in Phase I ROP.
Lutein is one of the carotenoids that can be selectively absorbed by the intestines and accumulated in the macula.41 Upon ingestion, carotenoids undergo emulsification and micellization for enterocyte absorption. In the enterocyte, carotenoids are packaged into chylomicrons, transported to the liver, repackaged into lipoproteins and transported through the blood.53 The difference between the routes of IP and oral administration is in the absorption phase before entering into the circulation. The drugs are absorbed in the gastrointestinal tract by oral administration and diffused across the peritoneal membrane which is lined with capillary bed by IP injection. Emulsification and micellization of lutein are largely affected by the dietary components; for example, high fat diets increase the bioavailability of lutein in the plasma.54 Therefore, it would also be worthwhile to consider increasing the bioavailability of lutein through oral administration with supplementation of other lipids like omage-3 long-chain polyunsaturated fatty acids, which are essential in retinal development and inhibits neovascularization in OIR.55,56
Clinical trials have reported an ineffective role of lutein in preventing proliferative ROP while there was a decreasing trend in ROP progression from early ROP to later vision-threatening ROP.9-11 The present study demonstrated an effective role of lutein in accelerating revascularization but ineffective in inhibiting the neovascularization in mouse oxygen-induced retinopathy, corresponding to these clinical trial outcomes. In general, our observations indicated that lutein might be considered as a supplement with other ROP treatments through facilitating the normal vascular regrowth, which may help stabilize the functional vasculature in hypoxic retinae and in turn reduce the frequency of treatments for pathologic neovessels.
Acknowledgments
Funding sources: ACYL was supported by the Seed Funding Programme for Basic Research from The University of Hong Kong (201411159087) as well as the Germany/Hong Kong Joint Research Scheme 2009/2010 (RGC Project No.: G HK029/09). LEHS was supported by NIH EY024864, EY017017.
Footnotes
Conflict of interest: None
References
- 1.Terry TL. Fibroblastic Overgrowth of Persistent Tunica Vasculosa Lentis in Infants Born Prematurely: II. Report of Cases-Clinical Aspects. Transactions of the American Ophthalmological Society. 1942;40:262–84. [PMC free article] [PubMed] [Google Scholar]
- 2.Li SY, Fu ZJ, Lo AC. Hypoxia-induced oxidative stress in ischemic retinopathy. Oxidative medicine and cellular longevity. 2012;2012:426769. doi: 10.1155/2012/426769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terry TL. Retrolental Fibroplasia in the Premature Infant: V. Further Studies on Fibroplastic Overgrowth of the Persistent Tunica Vasculosa Lentis. Transactions of the American Ophthalmological Society. 1944;42:383–96. [PMC free article] [PubMed] [Google Scholar]
- 4.Kijlstra A, Tian Y, Kelly ER, Berendschot TT. Lutein: more than just a filter for blue light. Progress in retinal and eye research. 2012;31:303–15. doi: 10.1016/j.preteyeres.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Li SY, Fu ZJ, Ma H, Jang WC, So KF, Wong D, Lo AC. Effect of lutein on retinal neurons and oxidative stress in a model of acute retinal ischemia/reperfusion. Investigative ophthalmology & visual science. 2009;50:836–43. doi: 10.1167/iovs.08-2310. [DOI] [PubMed] [Google Scholar]
- 6.Sommerburg O, Keunen JE, Bird AC, van Kuijk FJ. Fruits and vegetables that are sources for lutein and zeaxanthin: the macular pigment in human eyes. The British journal of ophthalmology. 1998;82:907–10. doi: 10.1136/bjo.82.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribaya-Mercado JD, Blumberg JB. Lutein and zeaxanthin and their potential roles in disease prevention. Journal of the American College of Nutrition. 2004;23:567S–87S. doi: 10.1080/07315724.2004.10719427. [DOI] [PubMed] [Google Scholar]
- 8.Li SY, Lo AC. Lutein Protects RGC-5 Cells Against Hypoxia and Oxidative Stress. International journal of molecular sciences. 2010;11:2109–17. doi: 10.3390/ijms11052109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manzoni P, Guardione R, Bonetti P, Priolo C, Maestri A, Mansoldo C, Mostert M, Anselmetti G, Sardei D, Bellettato M, Biban P, Farina D. Lutein and Zeaxanthin Supplementation in Preterm Very Low-Birth-Weight Neonates in Neonatal Intensive Care Units: A Multicenter Randomized Controlled Trial. American journal of perinatology. 2012 doi: 10.1055/s-0032-1321494. [DOI] [PubMed] [Google Scholar]
- 10.Romagnoli C, Giannantonio C, Cota F, Papacci P, Vento G, Valente E, Purcaro V, Costa S. A prospective, randomized, double blind study comparing lutein to placebo for reducing occurrence and severity of retinopathy of prematurity. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians. 2011;24(1):147–50. doi: 10.3109/14767058.2011.607618. [DOI] [PubMed] [Google Scholar]
- 11.Dani C, Lori I, Favelli F, Frosini S, Messner H, Wanker P, De Marini S, Oretti C, Boldrini A, Massimiliano C, Bragetti P, Germini C. Lutein and zeaxanthin supplementation in preterm infants to prevent retinopathy of prematurity: a randomized controlled study. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians. 2012;25:523–7. doi: 10.3109/14767058.2011.629252. [DOI] [PubMed] [Google Scholar]
- 12.Fierson WM. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131:189–95. doi: 10.1542/peds.2012-2996. [DOI] [PubMed] [Google Scholar]
- 13.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen-induced retinopathy in the mouse. Investigative ophthalmology & visual science. 1994;35:101–11. [PubMed] [Google Scholar]
- 14.Fu Z, Nian S, Li SY, Wong D, Chung SK, Lo AC. Deficiency of aldose reductase attenuates inner retinal neuronal changes in a mouse model of retinopathy of prematurity. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2015;253:1503–13. doi: 10.1007/s00417-015-3024-0. [DOI] [PubMed] [Google Scholar]
- 15.Fu ZJ, Li SY, Kociok N, Wong D, Chung SK, Lo AC. Aldose reductase deficiency reduced vascular changes in neonatal mouse retina in oxygen-induced retinopathy. Investigative ophthalmology & visual science. 2012;53:5698–712. doi: 10.1167/iovs.12-10122. [DOI] [PubMed] [Google Scholar]
- 16.Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, Sapieha P, Stahl A, Willett KL, Smith LE. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nature protocols. 2009;4:1565–73. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung AK, Fung MK, Lo AC, Lam TT, So KF, Chung SS, Chung SK. Aldose reductase deficiency prevents diabetes-induced blood-retinal barrier breakdown, apoptosis, and glial reactivation in the retina of db/db mice. Diabetes. 2005;54:3119–25. doi: 10.2337/diabetes.54.11.3119. [DOI] [PubMed] [Google Scholar]
- 18.Haley MJ, Lawrence CB. The blood-brain barrier after stroke: Structural studies and the role of transcytotic vesicles. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2016 doi: 10.1177/0271678X16629976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. The Journal of cell biology. 2003;161:1163–77. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorrell MI, Aguilar E, Jacobson R, Trauger SA, Friedlander J, Siuzdak G, Friedlander M. Maintaining retinal astrocytes normalizes revascularization and prevents vascular pathology associated with oxygen-induced retinopathy. Glia. 2010;58:43–54. doi: 10.1002/glia.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–34. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 22.Vessey KA, Wilkinson-Berka JL, Fletcher EL. Characterization of retinal function and glial cell response in a mouse model of oxygen-induced retinopathy. The Journal of comparative neurology. 2011;519:506–27. doi: 10.1002/cne.22530. [DOI] [PubMed] [Google Scholar]
- 23.Langmann T. Microglia activation in retinal degeneration. Journal of leukocyte biology. 2007;81:1345–51. doi: 10.1189/jlb.0207114. [DOI] [PubMed] [Google Scholar]
- 24.Siemerink MJ, Klaassen I, Van Noorden CJ, Schlingemann RO. Endothelial tip cells in ocular angiogenesis: potential target for anti-angiogenesis therapy. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2013;61:101–15. doi: 10.1369/0022155412467635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Budd S, Byfield G, Martiniuk D, Geisen P, Hartnett ME. Reduction in endothelial tip cell filopodia corresponds to reduced intravitreous but not intraretinal vascularization in a model of ROP. Experimental eye research. 2009;89:718–27. doi: 10.1016/j.exer.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubota Y, Suda T. Feedback mechanism between blood vessels and astrocytes in retinal vascular development. Trends in cardiovascular medicine. 2009;19:38–43. doi: 10.1016/j.tcm.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Downie LE, Pianta MJ, Vingrys AJ, Wilkinson-Berka JL, Fletcher EL. AT1 receptor inhibition prevents astrocyte degeneration and restores vascular growth in oxygen-induced retinopathy. Glia. 2008;56:1076–90. doi: 10.1002/glia.20680. [DOI] [PubMed] [Google Scholar]
- 28.Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Muller cells in the healthy and diseased retina. Progress in retinal and eye research. 2006;25:397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Lundkvist A, Reichenbach A, Betsholtz C, Carmeliet P, Wolburg H, Pekny M. Under stress, the absence of intermediate filaments from Muller cells in the retina has structural and functional consequences. Journal of cell science. 2004;117:3481–8. doi: 10.1242/jcs.01221. [DOI] [PubMed] [Google Scholar]
- 30.Bai Y, Ma JX, Guo J, Wang J, Zhu M, Chen Y, Le YZ. Muller cell-derived VEGF is a significant contributor to retinal neovascularization. The Journal of pathology. 2009;219:446–54. doi: 10.1002/path.2611. [DOI] [PubMed] [Google Scholar]
- 31.Li SY, Fung FK, Fu ZJ, Wong D, Chan HH, Lo AC. Anti-inflammatory effects of lutein in retinal ischemic/hypoxic injury: in vivo and in vitro studies. Investigative ophthalmology & visual science. 2012;53:5976–84. doi: 10.1167/iovs.12-10007. [DOI] [PubMed] [Google Scholar]
- 32.Fischer F, Martin G, Agostini HT. Activation of retinal microglia rather than microglial cell density correlates with retinal neovascularization in the mouse model of oxygen-induced retinopathy. Journal of neuroinflammation. 2011;8:120. doi: 10.1186/1742-2094-8-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ritter MR, Banin E, Moreno SK, Aguilar E, Dorrell MI, Friedlander M. Myeloid progenitors differentiate into microglia and promote vascular repair in a model of ischemic retinopathy. The Journal of clinical investigation. 2006;116:3266–76. doi: 10.1172/JCI29683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bai Y, Ma JX, Guo J, Wang J, Zhu M, Chen Y, Le YZ. Muller cell-derived VEGF is a significant contributor to retinal neovascularization. The Journal of pathology. 2009;219:446–54. doi: 10.1002/path.2611. [DOI] [PubMed] [Google Scholar]
- 35.Fischer F, Martin G, Agostini HT. Activation of retinal microglia rather than microglial cell density correlates with retinal neovascularization in the mouse model of oxygen-induced retinopathy. Journal of neuroinflammation. 2011;8:120. doi: 10.1186/1742-2094-8-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies MH, Eubanks JP, Powers MR. Microglia and macrophages are increased in response to ischemia-induced retinopathy in the mouse retina. Molecular vision. 2006;12:467–77. [PubMed] [Google Scholar]
- 37.Wong-Riley MT. Energy metabolism of the visual system. Eye and brain. 2010;2:99–116. doi: 10.2147/EB.S9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okawa H, Sampath AP, Laughlin SB, Fain GL. ATP consumption by mammalian rod photoreceptors in darkness and in light. Current biology : CB. 2008;18:1917–21. doi: 10.1016/j.cub.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joyal JS, Sun Y, Gantner ML, Shao Z, Evans LP, Saba N, Fredrick T, Burnim S, Kim JS, Patel G, Juan AM, Hurst CG, Hatton CJ, Cui Z, Pierce KA, Bherer P, Aguilar E, Powner MB, Vevis K, Boisvert M, Fu Z, Levy E, Fruttiger M, Packard A, Rezende FA, Maranda B, Sapieha P, Chen J, Friedlander M, Clish CB, Smith LE. Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nature medicine. 2016;22:439–45. doi: 10.1038/nm.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lahdenranta J, Pasqualini R, Schlingemann RO, Hagedorn M, Stallcup WB, Bucana CD, Sidman RL, Arap W. An anti-angiogenic state in mice and humans with retinal photoreceptor cell degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:10368–73. doi: 10.1073/pnas.181329198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kotake-Nara E, Nagao A. Absorption and metabolism of xanthophylls. Marine drugs. 2011;9:1024–37. doi: 10.3390/md9061024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chucair AJ, Rotstein NP, Sangiovanni JP, During A, Chew EY, Politi LE. Lutein and zeaxanthin protect photoreceptors from apoptosis induced by oxidative stress: relation with docosahexaenoic acid. Investigative ophthalmology & visual science. 2007;48:5168–77. doi: 10.1167/iovs.07-0037. [DOI] [PubMed] [Google Scholar]
- 43.Hellstrom A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet. 2013;382:1445–57. doi: 10.1016/S0140-6736(13)60178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Au SC, Tang SM, Rong SS, Chen LJ, Yam JC. Association between hyperglycemia and retinopathy of prematurity: a systemic review and meta-analysis. Scientific reports. 2015;5:9091. doi: 10.1038/srep09091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmadpour-Kacho M, Motlagh AJ, Rasoulinejad SA, Jahangir T, Bijani A, Pasha YZ. Correlation between hyperglycemia and retinopathy of prematurity. Pediatrics international : official journal of the Japan Pediatric Society. 2014;56:726–30. doi: 10.1111/ped.12371. [DOI] [PubMed] [Google Scholar]
- 46.Garg R, Agthe AG, Donohue PK, Lehmann CU. Hyperglycemia and retinopathy of prematurity in very low birth weight infants. Journal of perinatology : official journal of the California Perinatal Association. 2003;23:186–94. doi: 10.1038/sj.jp.7210879. [DOI] [PubMed] [Google Scholar]
- 47.Mohamed S, Murray JC, Dagle JM, Colaizy T. Hyperglycemia as a risk factor for the development of retinopathy of prematurity. BMC pediatrics. 2013;13:78. doi: 10.1186/1471-2431-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaempf JW, Kaempf AJ, Wu Y, Stawarz M, Niemeyer J, Grunkemeier G. Hyperglycemia, insulin and slower growth velocity may increase the risk of retinopathy of prematurity. Journal of perinatology : official journal of the California Perinatal Association. 2011;31:251–7. doi: 10.1038/jp.2010.152. [DOI] [PubMed] [Google Scholar]
- 49.Mohsen L, Abou-Alam M, El-Dib M, Labib M, Elsada M, Aly H. A prospective study on hyperglycemia and retinopathy of prematurity. Journal of perinatology : official journal of the California Perinatal Association. 2014;34:453–7. doi: 10.1038/jp.2014.49. [DOI] [PubMed] [Google Scholar]
- 50.Ertl T, Gyarmati J, Gaal V, Szabo I. Relationship between hyperglycemia and retinopathy of prematurity in very low birth weight infants. Biology of the neonate. 2006;89:56–9. doi: 10.1159/000088199. [DOI] [PubMed] [Google Scholar]
- 51.Chavez-Valdez R, McGowan J, Cannon E, Lehmann CU. Contribution of early glycemic status in the development of severe retinopathy of prematurity in a cohort of ELBW infants. Journal of perinatology : official journal of the California Perinatal Association. 2011;31:749–56. doi: 10.1038/jp.2011.19. [DOI] [PubMed] [Google Scholar]
- 52.Kermorvant-Duchemin E, Pinel AC, Lavalette S, Lenne D, Raoul W, Calippe B, Behar-Cohen F, Sahel JA, Guillonneau X, Sennlaub F. Neonatal hyperglycemia inhibits angiogenesis and induces inflammation and neuronal degeneration in the retina. PloS one. 2013;8:e79545. doi: 10.1371/journal.pone.0079545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yonekura L, Nagao A. Intestinal absorption of dietary carotenoids. Molecular nutrition & food research. 2007;51:107–15. doi: 10.1002/mnfr.200600145. [DOI] [PubMed] [Google Scholar]
- 54.Roodenburg AJ, Leenen R, van het Hof KH, Weststrate JA, Tijburg LB. Amount of fat in the diet affects bioavailability of lutein esters but not of alpha-carotene, beta-carotene, and vitamin E in humans. The American journal of clinical nutrition. 2000;71:1187–93. doi: 10.1093/ajcn/71.5.1187. [DOI] [PubMed] [Google Scholar]
- 55.Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, Hellstrom A, Kang JX, Chew EY, Salem N, Jr, Serhan CN, Smith LE. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nature medicine. 2007;13:868–73. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fu Z, Lofqvist CA, Shao Z, Sun Y, Joyal JS, Hurst CG, Cui RZ, Evans LP, Tian K, SanGiovanni JP, Chen J, Ley D, Hansen Pupp I, Hellstrom A, Smith LE. Dietary omega-3 polyunsaturated fatty acids decrease retinal neovascularization by adipose-endoplasmic reticulum stress reduction to increase adiponectin. The American journal of clinical nutrition. 2015;101:879–88. doi: 10.3945/ajcn.114.099291. [DOI] [PMC free article] [PubMed] [Google Scholar]


