Abstract
Background
Early temporal artery biopsy is recommended in all patients with suspected cranial GCA (Giant Cell Arteritis) by the BSR (British Society of Rheumatology) and BHPR (British Health Professionals in Rheumatology) guidelines. This should be performed within one week ideally.
Aim
To assess ACR (American College of Rheumatology) score at presentation and whether temporal artery biopsy result affects clinical management of the clinically suspected GCA patient.
Method
Case records of all temporal artery biopsies performed within January 2012 until December 2014 were analysed for size and result of biopsy and this was correlated to clinical management following result.
Results
129 temporal arteries were biopsied with a total of 17 positive biopsy results. 10 biopsy samples were insufficient to confirm or refute GCA. 8 patients within the biopsies negative for GCA had their prednisolone therapy stopped. 5 patients had unknown follow up, with the remainder (89, 87.3%) of the patients continued prednisolone management for treatment of GCA for at least 6 weeks.
Conclusion
Overall 13.2% of our biopsies were positive for GCA and 87.3% of biopsy negative patients continued prednisolone therapy on clinical grounds. In the face of new diagnostic tests (high resolution MRI (Magnetic Resonance Imaging), colour duplex USS (Ultra Sound Scan) and PET (Positive Emission Topography) can we justify invasive surgery to all patients on histological grounds when the results may not alter management? Further investigation is needed directly comparing newer imaging modalities to histology.
Highlights
-
•
Of the 129 temporal artery biopsies performed 17 (13.2%) were positive for Giant Cell Arteritis.
-
•
83% of patients had an American College of Rheumatology score of 3 or more and would not be biopsied in America.
-
•
Colour Duplex Ultrasound scan now offers a greater sensitivity and specificity for the diagnosis of GCA.
1. Introduction
Giant cell arteritis (GCA) is an inflammatory vasculopathy affecting medium- and large-sized arteries. Also referred to as temporal arteritis, it characteristically affects branches of the carotid artery. While the superficial temporal branch of the carotid artery is particularly susceptible, arteries at any site can be affected. Temporal arteritis is defined by a granulomatous panarteritis with mononuclear cell infiltrates and giant cell formation within the vessel wall [1].
It is among the common causes of acute blindness and is a medical emergency. Visual loss occurs in up to one-fifth of patients [2]. Guidelines from the British Society of Rheumatology (BSR), British Health Professionals in Rheumatology (BHPR) [2] and the European League Against Rheumatism (EULAR) [3] recommend initiating treatment immediately if giant cell arteritis is suspected. High-dose prednisolone has been shown, during decades of clinical practice, to be a very effective treatment [4].
Early temporal artery biopsy (TAB) is recommended in all patients with suspected cranial GCA by the BSR and BHPR guidelines 2010, which is reflected in the NICE guidance 2014 (National Institute of Clinical Excellence). This should be performed within one week ideally; however the guidelines and evidence suggest TAB may remain positive for 2–6 weeks following initiation of glucocorticosteroids [2].
If biopsy negative the guidelines recommend continuing treatment if: “there is a typical clinical and laboratory picture and response to glucocorticosteroids, or typical findings on ultrasound, or ischaemic complications typical of GCA (such as anterior ischaemic optic neuritis).” [2].
Table 1 shows the American College of Rheumatology's (ACR) classification criteria for GCA. Presence of three of the criteria are said to be diagnostic of GCA in America with a sensitivity of 93.5% and specificity of 91.2%. Note only one point is designated to TAB and that this differs to UK guidance.
Table 1.
The American College of Rheumatology (ACR) classification criteria for GCA.
|
For purposes of classification, a patient shall be said to have GCA (TA) if at least three of these five criteria are present. The presence of any three or more criteria yields a sensitivity of 93.5% and a specificity of 91.2%.
Recent evidence suggests that Colour Duplex Ultrasound (CDS) and possibly other imaging modalities may have a significant role to play in the diagnosis of GCA and its associated pathologies. This combined with evidence that TAB in a small proportion of patients may not change the management of GCA may lead to a greater role for imaging modalities in combination with scoring systems such as the ACR classification for GCA [5].
2. Aim
The aim of this study was to assess the impact of TAB histology on the clinical management of the patient with suspected GCA and the ACR score at presentation. Our hypothesis was three fold
-
1)
The majority of patients at time of biopsy would have an ACR score of > or = 3
-
2)
That given the serious nature of temporal arteritis and the possibility of visual loss the majority of patients would remain on prednisolone despite a negative TAB; on clinical grounds.
-
3)
Identifying a smaller cohort for potential targeting for TAB
3. Method
In designing this cohort study we followed the (Strengthening the Reporting of Observational Studies in Epidemiology) STROBE guidelines [6] and the study was registered on research registry under UIN 897.
A prospectively maintained database was queried for all TABs performed between 01/2012 until 03/2014 at a local District General Hospital. All patients' records were analysed for demographical data and histology. Discharge summaries for subsequent admissions and GP records were queried for the treatment instigated and whether the management was altered 6 weeks post biopsy. Statistical analyses were performed using SPSS (IBM©), descriptive statistics used are indicated within the tables they are quoted. Hospital episode database, discharge summaries subsequently were analysed for the presence of prednisolone on discharge summaries. If records were negative for prednisolone 6 weeks after TAB, GP records were consulted.
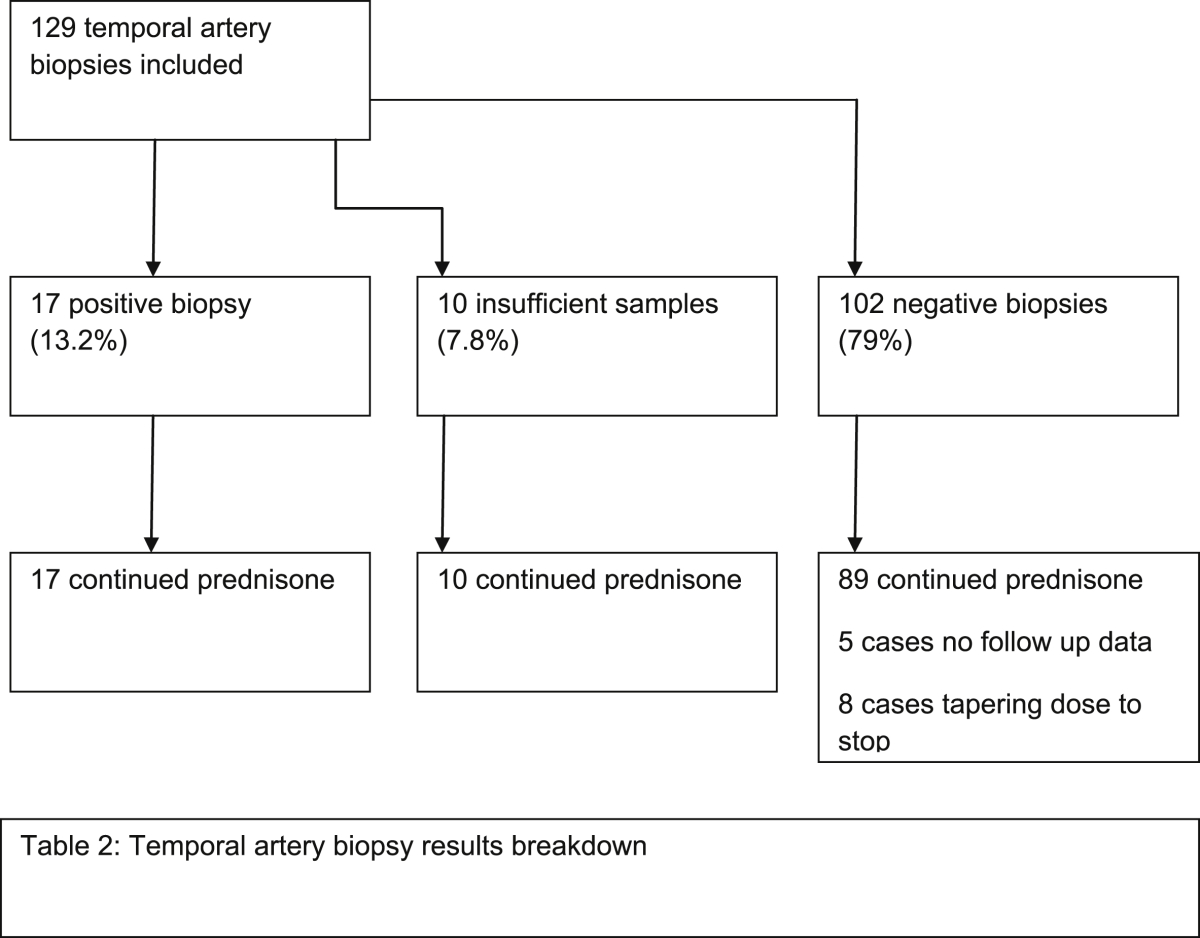
Patient inclusion criteria were any patient regardless of demographical data undergoing a TAB between 01/2012 to 03/2014; there were no exclusion criteria. Patient breakdown including lost to follow up in Table 2.
Table 2.
Temporal artery biopsy results breakdown.
Outcome data were demographical data, date of referral and biopsy, biopsy result, ACR score at time of biopsy and status of steroid therapy at 6 weeks post biopsy.
4. Results
A total of 129 Temporal arteries were biopsied between 01/2012 and 03/2014 with a total of 17 positive biopsy results. 10 biopsy samples were insufficient to confirm or refute GCA. Demographical data are shown in Table 3 including the average length of biopsies and time taken from referral to biopsy (see Table 4).
Table 3.
Demographical data of TAB results; values are mean (s.d.); statistical analyses: € X2 test, βANOVA and ¥Kruskal –Wallis test.
| TAB+ve (n = 17) | TAB-ve (n = 102) | TAB insufficient (n = 10) | P | |
|---|---|---|---|---|
| Age (years) | 78 [8] | 73 [11] | 75 [12] | 0.972β |
| Sex | ||||
| Sex ratio (F:M) | 15:2 | 74:28 | 8:2 | 0.005€ |
| Side | 0.538€ | |||
| Right (n) | 8 | 55 | 5 | |
| Left (n) | 9 | 47 | 5 | |
| Average length (mm) of TAB | 10.8 (3.0) | 10.1 (3.7) | 5.6 (2.0) | 0.461¥ |
| Time to biopsy (days) | 5.2 (2.9) | 6.1 (4.2) | 7.6 (4.9) | 0.432¥ |
Table 4.
TAB grouped by time of referral to biopsy (≤7 days vs > 7 days); € X2 test.
| TAB <7 days | TAB >7days | P | ||
|---|---|---|---|---|
| no evidence | 80 (78.4%) | no evidence | 22 (81.5%) | |
| temporal arteritis | 16 (15.7%) | temporal arteritis | 1 (3.7%) | |
| insufficient | 6 | insufficient | 4 | |
| average size (mm) | 10.2 | average size (mm) | 9.7 | |
| total | 102 | total | 27 | 0.261€ |
Of the biopsies performed within a week from referral 16 were positive (out of 102; 15.7%), with 80 being negative (78.4%) and 6 insufficient. This compared to 27 samples which were taken more than 7 days after presentation of which 22 were negative out of 26 (84.6%) with only 1 positive biopsy result and 4 insufficient samples.
There was no statistical difference between length of biopsies in both groups with the average length of biopsy being 9.7 mm in the >7day biopsy group and 10.2 mm in the under 7 day group.
In Table 5 the ACR score given to patients at the time of TAB is shown (ACR criteria; Table 1). 83% of patients in our cohort had a ACR ≥3 at presentation.
Table 5.
ACR criteria scores in our study [n (%)].
| ACR criteria score | n (%) |
|---|---|
| ACR < 1 | 2 (1.5%) |
| ACR = 2 | 20 (15.5%) |
| ACR ≥ 3 | 107 (83%) |
| total | 129 |
Table 6 shows the breakdown of steroid management at 6 weeks post TAB. No histological evidence of GCA was shown in 102 patients. Of these 89 patients continued on their dose of glucocorticosteroid at 6 weeks post biopsy; 5 cases had no records on databases queried or at their GP of steroid duration. Eight cases were placed onto a tapering dose to stop based on poor clinical histories with negative biopsies. The 17 cases which were identified as positive continued their treatment as did the 10 cases that had insufficient biopsy's taken and continued the current prednisolone management on clinical grounds. No statistical difference was demonstrated in the steroid management between biopsy result groups (p = 0.236).
Table 6.
TAB result showing management outcome at 6 weeks; € X2 test; TAB results with the number scoring ACR ≥ 3 for GCA classification.
| Management | TAB +ve | ACR ≥ 3 | TAB -ve | ACR ≥ 3 | TAB indeterminate | P value |
|---|---|---|---|---|---|---|
| Continued course of prednisolone | 17 (100%) | 17 | 89 (87.3%) | 84 | 10 (100%) | 4 |
| Tapered prednisolone | 0 | 0 | 8 (7.8%) | 0 | ||
| Unknown | 0 | 0 | 5 (4.9%) | 2 | 0 | |
| Total | 17 | 102 | 10 | 0.236€ |
5. Discussion
Time from referral to biopsy was performed within 7 days in 102 biopsies (79%). Overall the number of positive biopsies remains low with a maximum of 13.2%. At our unit we have a daycase pathway for temporal artery biopsy. This allows rapid referral to treatment times by one of two general surgeons lists twice weekly. Here the patient is assessed, consented and operated on within the same session. Demographical data (Table 2) was statistically insignificant between groups except for sex which was significant. This is to be expected as female sex is a well established risk factor for GCA.
After 7 days (Table 3) only one positive biopsy result was found. However 81.4% of all biopsies performed at our institution are done so within 7 days. As such there is not enough evidence within our study to support whether TAB positive results will remain positive for a time beyond seven days as previous evidence suggests.
Currently our unit follows the BSR and NICE guidelines on TAB being performed in all patients suspected of having GCA. However Table 5 shows that nearly 83% of patients by time of presentation will have an ACR score of 3 or more and by American (ACR) guidelines would not warrant a biopsy; having sufficient clinical grounds to diagnose GCA (Sensitivity 93.5% and specificity 91.2%).
In our study nearly 8 out of 10 patients had a negative biopsy result and only 7.8% of these cases had their management altered subsequently (Table 6). The average biopsy length recommended by the BSR guidelines is 1 cm, of which are average is above in this cohort, however there were a number of samples that fell below this minimum recommended size of 1–2 cm. Interestingly in the TAB negative group 84/89 patients had an ACR ≥3; this is highly indicative that the reason steroids were continued after 6 weeks was on clinical grounds.
We make the readers aware of the following limitations of our data:
-
1)
Patients on steroids at 6 weeks does not confirm how long these patients remained on steroid treatment for; although we picked 6 weeks as we felt this was ample time for patients to be placed on a tapering dose following a negative result.
-
2)
We do not make the distinction between ophthalmic and non ophthalmic GCA. Patients with ophthalmic GCA will remain on high dose steroid for two weeks and will then taper their dose to a maintenance level.
-
3)
Our TAB length meet the average minimum length for the guideline of >1 cm but many samples do not. This is important as skip lesions can be missed on short segments thereby resulting in a biopsy that is negative for GCA but in a patient whom has GCA.
Further study and discussion are needed on the appropriateness of TAB as a diagnostic test given the small number whose management is altered based on the result. In patients with a classical clinical presentation the diagnosis of GCA is straightforward with TAB providing histological confirmation only. Many argue that this confirmation is needed with the average GCA patient receiving 1 year's treatment of prednisolone with a 50% risk of relapse [7].
By contrast, the diagnosis becomes challenging with nonspecific symptoms, which may present in the wide range of GCA manifestations. Our study concurs with other literature which has found similar rates of biopsy results to our own with low sensitivity, high false negative rates (7%, 1.8%, 18%, 16%, 31% and 34%.) [5], [8], [9], [10], [11] and therefore the risk of under detection. There are many reasons for a low pickup rate including skip lesions and steroid therapy duration prior to biopsy. Within our study we have tried to eliminate the latter with the short time to biopsy from referral; but this relies on the patient presenting within a short time of symptom onset and the referring clinician starting treatment in conjunction with referral.
It is clear that a temporal artery biopsy comprises only 1 point from a possible 5 points in order to make the diagnosis of temporal arteritis (Table 1). It has been shown in a study of 111 temporal artery biopsies that 75 (67.5%) of these cases already had an American College of Rheumatology score of 3 or greater before a biopsy was performed and so the biopsy should not have affected management in this subset of patients [5], [8].The result of a temporal artery biopsy is not always rapidly available. Given the nature of complications of giant cell arteritis, treatment is instituted or discontinued before biopsy results are available in 60–86% of cases [11].
Temporal artery biopsies are not without complications and difficulties. These have included unintended biopsies of veins and nerves, postoperative haematoma, scalp necrosis, wound infection, damage to the facial nerve, and drooping of the eyebrow [12]. As well as cosmetic consequences of incisional alopecia, widening of the scar and foreign body reaction to entrapped hairs [1]. However it is a safe procedure, performed frequently with excellent outcomes in most units around the country.
GCA is now being recognised as a part of a larger process of vasculitis in many patients. The involvement of the thoracic aorta or its main branches in more than 45% of GCA patients is also associated with a 50% negative TAB [13], [14] New imaging modalities such as Positron Emission Topography (PET), Magnetic Resonance Imaging (MRI) and Colour Duplex Ultrasound (CDS) are demonstrating a much wider role in GCA with an ever increasing evidence base without the risks of surgery.
Positron Emission Topography (PET) imaging has become an established tool in oncology but it has also shown a promising role in the field of inflammatory diseases [15]. One of the main limitations of 18F-FDG PET/CT to become a reliable diagnostic tool is the lack of a standardized definition of vascular inflammation based on the intensity of the glucose analogue uptake.
High resolution MRI with contrast enhancement has shown signs of mural inflammation even in patients who received corticosteroids for >2 days before MRI imaging. A sensitivity of 0.80 and a specificity of 0.80 for the detection of GCA by using this imaging protocol were comparable with recent studies also with reported values of sensitivities between 80% and 85% and specificities ranging from 71% to 95% [15], [16].
Colour Duplex Ultrasound (CDS) was the first imaging modality to show signs of temporal arteritis. Since then the resolution has increased dramatically and is now the highest resolution imaging of all the modalities used for diagnosis of vasculitis [17]. Typical findings are from oedema in the arterial wall often referred to as ‘the halo’ sign. The temporal artery in one study showed in biopsy confirmed temporal arteritis a characteristic ‘halo’ sign in 72% of patients; which persisted a mean of 16 days after initiation of prednisolone therapy. In the biopsy negative group this sign was not seen at all.
Three meta-analyses have been published on the use of CDS in GCA diagnosis [17], [18] [19]. The sensitivity of temporal artery duplex ultrasound was 87% with regard to clinical diagnosis, and specificity was 96% in one of the metaanalyses [17]. The presence of a bilateral halo seems to increase the specificity [19]. Some centres in the USA have reported reliable results with ultrasound examination and replaced first line temporal artery biopsy in cases with definitive clinical and ultrasound findings [20], [21], [22].
In CDS the occipital arteries can also be included, which may be exclusively involved in some patients, particularly if they are presenting with retroauricular pain [23]. As can the facial arteries with jaw claudication. An experienced sonographer can perform a standardized ultrasound examination of the temporal and axillary arteries in 10 min [17].To date evidence has failed to show whether CDS guided TAB can improve sensitivity [24].
6. Conclusion
Many new modalities which are non-invasive are demonstrating a growing evidence base in the diagnosis of GCA. It is now becoming evident that GCA is a much more complex disease with associations including polymyalgia rheumatica, aneurysm formation and extracranial vessel involvement. Many units report low biopsy positive rates but these do vary; this concurs with our own results in this study. In our study we have shown that 83% of our patient would have not undergone biopsy if following ACR guidelines.
Temporal artery biopsy is an invasive surgery that carries risk. It is the authors opinion that temporal artery biopsy needs direct comparison with newer modalities such as CDS, PET or MRI that have in many studies to date shown comparable high specificity and sensitivity. New data emerging suggests that CDS can achieve similar specificity with a better sensitivity [25].
As a less totalitarian approach as to whether to perform TAB we would argue a more targeted approach with biopsying those patients who have a clinical GCA diagnosis that does not fulfil the American College of Rheumatology diagnostic criteria with the investigation in the future as to whether diagnostic imaging could further reduce the number we biopsy. However this would have to be offered in a timely fashion to ensure that biopsy is not delayed therefore decreasing the likelihood of achieving a positive result.
In the face of non-invasive investigations that are offering potentially comparable results it is the author's opinion that the TAB is reduced to a smaller subset of patients where:
-
1)
The diagnostic criteria similar to the American College of Rheumatology criteria are not met
-
2)
In the future negative imaging or non diagnostic imaging once the evidence base has grown for these modalities
Given the emergence of better potential results with CDS the management of temporal arteritis needs further investigation in the coming future with particular interest in the direct comparison of CDS and other imaging modalities to TAB.
The diagnosis of GCA can be difficult with both undertreatment and treatment carrying significant risks. We must offer clinicians pathways and investigations that support the clinician in making the difficult decisions regarding starting or cessating longterm steroid therapy; whilst most importantly limiting risk to the patient.
Ethical approval
Audit Department South Devon Healthcare.
Sources of funding
None.
Author contribution
All authors (Mr Bowling, Atkinson, Rait and Srinivas) contributed equally to study design, data collection, data analysis and writing.
All contributors have been mentioned at the end of the article.
Conflicts of interest
None.
Guarantor
Mr Kirk Bowling.
Authorship
All persons designated as authors have participated sufficiently in the substantial contributions to conception and design or analysis and interpretation of data, drafting of the manuscript or revising it for important intellectual content and, final approval of the version to be published. And agree that the manuscript is not under consideration elsewhere. That the work is original and all authors have agreed to submission to The Annals of medicine and surgery.
Acknowledgements
The authors acknowledge and thank all members of the daycase surgery unit team and the nurse practitioners. The authors declare no conflict of interest.
References
- 1.Temporal Artery Biopsy. MedScape; 11 01, 2016. http://emedicine.medscape.com/article/1520091-overview#a03 [Online] [Google Scholar]
- 2.Dasgupta Audit working group et. BSR and BHPR Guidelines for the management of giant cell arteritis. Rheumatol. Oxf. 2010;49(8):1594–1597. doi: 10.1093/rheumatology/keq039a. [DOI] [PubMed] [Google Scholar]
- 3.Mukhtyar C., Guillevin L., Cid M.C. EULAR Recommendations for the management of large vessel vasculitis. Ann. Rheumatic Dis. 2009;68(3):318–323. doi: 10.1136/ard.2008.088351. [DOI] [PubMed] [Google Scholar]
- 4.NICE . NICE; 2014. NICE CKS.https://cks.nice.org.uk/giant-cell-arteritis#!topicsummary [Online] [Google Scholar]
- 5.Cristaudo Adam, Mizumoto Ryo, Hendahewa Rasika. The impact of temporal artery biopsy on surgical practice. Ann. Med. Surg. 2016;11:47–51. doi: 10.1016/j.amsu.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.group, STROBE . Jan 2016. STROBE Statement.http://strobe-statement.org/index.php?id=available-checklists [Online] [Google Scholar]
- 7.Miguel A. González-Gay. Trinitario Pina and Ricardo Blanco. The search for improvement in the sensitivity of temporal artery biopsy in giant cell arteritis. Rheumatology. 2015;54(379):380. doi: 10.1093/rheumatology/keu312. [DOI] [PubMed] [Google Scholar]
- 8.Davies C., Frost B., Eshan O., McLain A.D., Shandall A. Temporal artery biopsy. Who needs one? Postgrad. Med. J. 2006 Jul;82(969):476–478. doi: 10.1136/pgmj.2005.043646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quinn E.M., Kearney D.E., Kelly J., Keohane C., Redmond H.P. Temporal artery biopsy is not required in all cases of suspected giant cell arteritis. Ann. Vasc. Surg. 2012 Jul;26(5):649–654. doi: 10.1016/j.avsg.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Chong E.W., Robertson A.J. Is temporal artery biopsy a worth-while procedure? ANZ J. Surg. 2005;75:388–391. doi: 10.1111/j.1445-2197.2005.03399.x. [DOI] [PubMed] [Google Scholar]
- 11.Lenton J., Donnelly R., Nash J.R. Does temporal artery biopsy influence the management of temporal arteritis? QJM. 2006 Jan;99(1):33–36. doi: 10.1093/qjmed/hci141. [DOI] [PubMed] [Google Scholar]
- 12.Guffey Johnson J., Grossniklaus H.E., Margo C.E., Foulis P. Frequency of unintended vein and peripheral nerve biopsy with temporal artery biopsy. Arch. Ophthalmol. 2009;127:703. doi: 10.1001/archophthalmol.2009.77. [DOI] [PubMed] [Google Scholar]
- 13.Besson F.L., Parienti J., Bienvenu B. Diagnostic per- formance of 18F-fluorodeoxyglucose positron emission tomog- raphy in giant cell arteritis: a systematic review and meta- analysis. Eur. J. Nucl. Med. Mol. Imaging. 2011;38(9):1764–1772. doi: 10.1007/s00259-011-1830-0. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt W.A., Seifert A., Gromnica-ihle E., Krause A., Natusch A. Ultrasound of proximal upper extremity arteries to increase the diagnostic yield in large-vessel giant cell arteritis. Rheumatology. 2008;47(1):96–101. doi: 10.1093/rheumatology/kem322. [DOI] [PubMed] [Google Scholar]
- 15.Cristina Puppo. Giant cell arteritis: a systematic review of the qualitative and semiquantitative methods to assess vasculitis with 18F-fluorodeoxyglucose positron emission tomography. Biomed. Res. Int. 2014;574248 doi: 10.1155/2014/574248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehmann P., Buchtala S., Achajew N. 18F-FDG PET as a diagnostic procedure in large vessel vasculitis-a controlled, blinded re-examination of routine PET scans. Clin. Rheumatol. 2011;30(1):37–42. doi: 10.1007/s10067-010-1598-9. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt W.A. Role of ultrasound in the understanding and management of vasculitis. Ther. Adv. Musculoskel Dis. 2014;6(2):39–47. doi: 10.1177/1759720X13512256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karassa F., Matsagas M., Schmidt W., Ioannidis J. Meta-analysis: Test performance of ultrasonography for giant-cell arteritis. Ann. InternMed. 2005;142:359–369. doi: 10.7326/0003-4819-142-5-200503010-00011. [DOI] [PubMed] [Google Scholar]
- 19.Arida A., Kyprianou M., Kanakis M., Sfikakis P. The diagnostic value of ultrasonographyderived edema of the temporal artery wall in giant cell arteritis: a second meta-analysis. BMC Musculoskelet Disord. 2010;11 doi: 10.1186/1471-2474-11-44. 44–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schirmer M., Duftner C., Schmidt W., Dejaco C. Ultrasonography in inflammatory rheumatic disease: an overview. Nat. Rev. Rheumatol. 2011;7:479–488. doi: 10.1038/nrrheum.2011.95. [DOI] [PubMed] [Google Scholar]
- 21.Porta F., Gargani L., Kaloudi O., Schmidt W., Picano E., Damjanov N. The new frontiers of ultrasound in the complex world of vasculitides and scleroderma. Rheumatol. Oxf. 2012;51(Suppl. 7):26–30. doi: 10.1093/rheumatology/kes336. [DOI] [PubMed] [Google Scholar]
- 22.Alberts M. Temporal arteritis: improving patient evaluation with a new protocol. Perm. J. 2013;17:56–62. doi: 10.7812/TPP/12-067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfadenhauer K., Weber H. Duplex sonography of the temporal and occipital artery in the diagnosis of temporal arteritis. A prospective study. J. Rheumatol. 2003;30:2177–2181. [PubMed] [Google Scholar]
- 24.Germano G., Muratore F., Cimino L. Is colour duplex sonography-guided temporal artery biopsy useful in the diagnosis of giant cell arteritis? A randomized study. Rheumatology. 2015;54:4004. doi: 10.1093/rheumatology/keu241. [DOI] [PubMed] [Google Scholar]
- 25.Croft A., Thompson N., Duddy M. EULAR; 2014. Can We Replace Temporal Artery Biopsy with Cranial Ultrasound for the Diagnosis of Giant Cell Arteritis? a Retrospective Cohort Study of the Diagnostic Utility of Ultrasound in Routine Clinical Practice. Abstract OP0056. [Google Scholar]