Abstract
This is a presentation of a case of disseminated fungal infection in a renal transplant patient with Nannizziopsis obscura, a species not previously reported as having caused disseminated disease in humans and not previously reported in the UK. The fungus was isolated from a intramuscular collection and from a lymph node. The patient responded well to a course of posaconazole.
Keywords: Nannizziopsis, Renal transplant
1. Introduction
This is a presentation of a case of disseminated fungal infection in a renal transplant patient with Nannizziopsis obscura, a species not previously reported as having caused disseminated disease in humans and not previously reported in the UK.
A 34 year old African man on immunosuppressive medication for a renal transplant presented with back pain, a skin rash, fever and night sweats and imaging showed a soft tissue thoracic collection and subcarinal lymphadenopathy. The patient was initially suspected to have tuberculosis but subsequent culture of pus from the collection and tissue from the lymph node grew Nannizziopsis obscura.
There are only five previously reported cases of Nannizziopsis infection in humans, although infection with other Nannizziopsis species in reptiles is well described.
2. Case
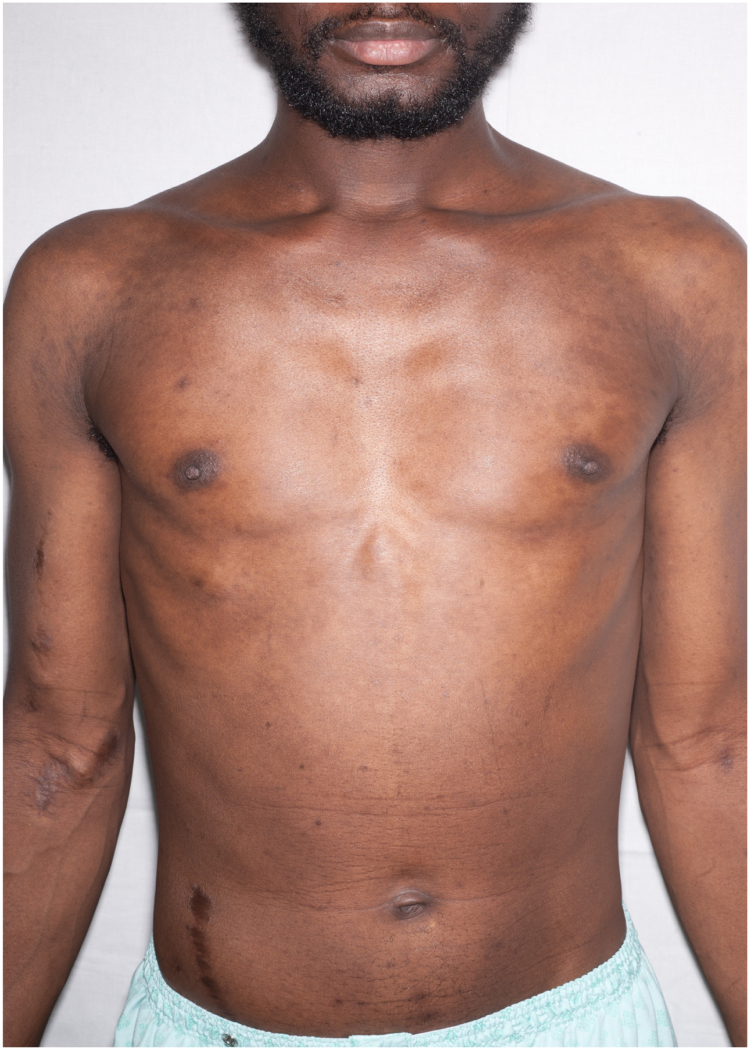
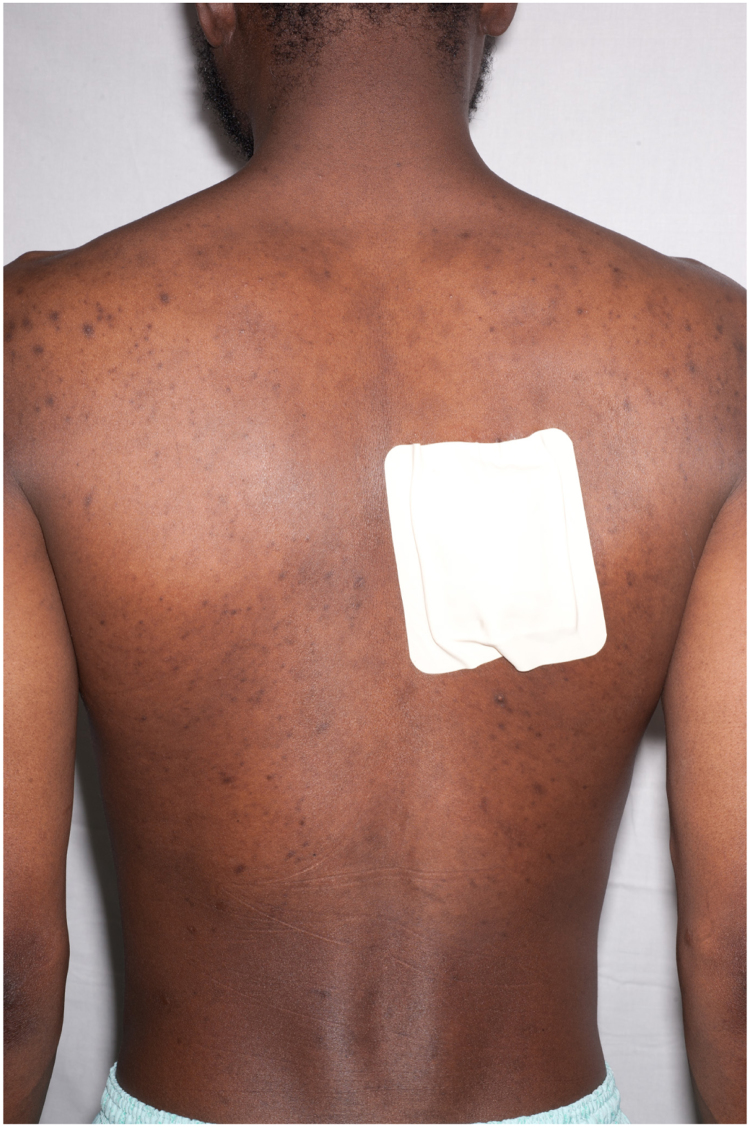
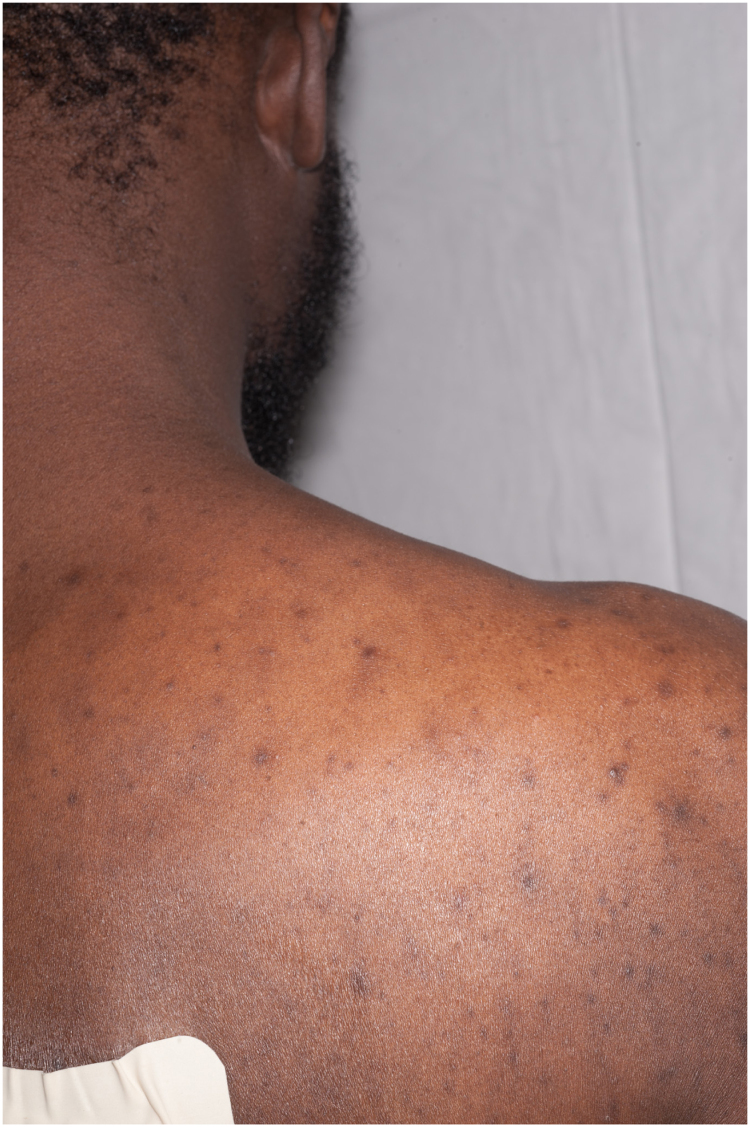
A 34 year old African man from the Gambia presented to hospital in June 2015 with several weeks of fever, night sweats, cough and wheeze, together with increasingly severe back pain from what appeared to be a paraspinal abscess. He had a rash across his trunk and several pigmented skin lesions on his feet and legs, which had developed over six months (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5).
Fig. 1.
Anterior view of truncal rash.
Fig. 2.
Posterior view of truncal rash; dressing at site of thoracic collection drainage.
Fig. 3.
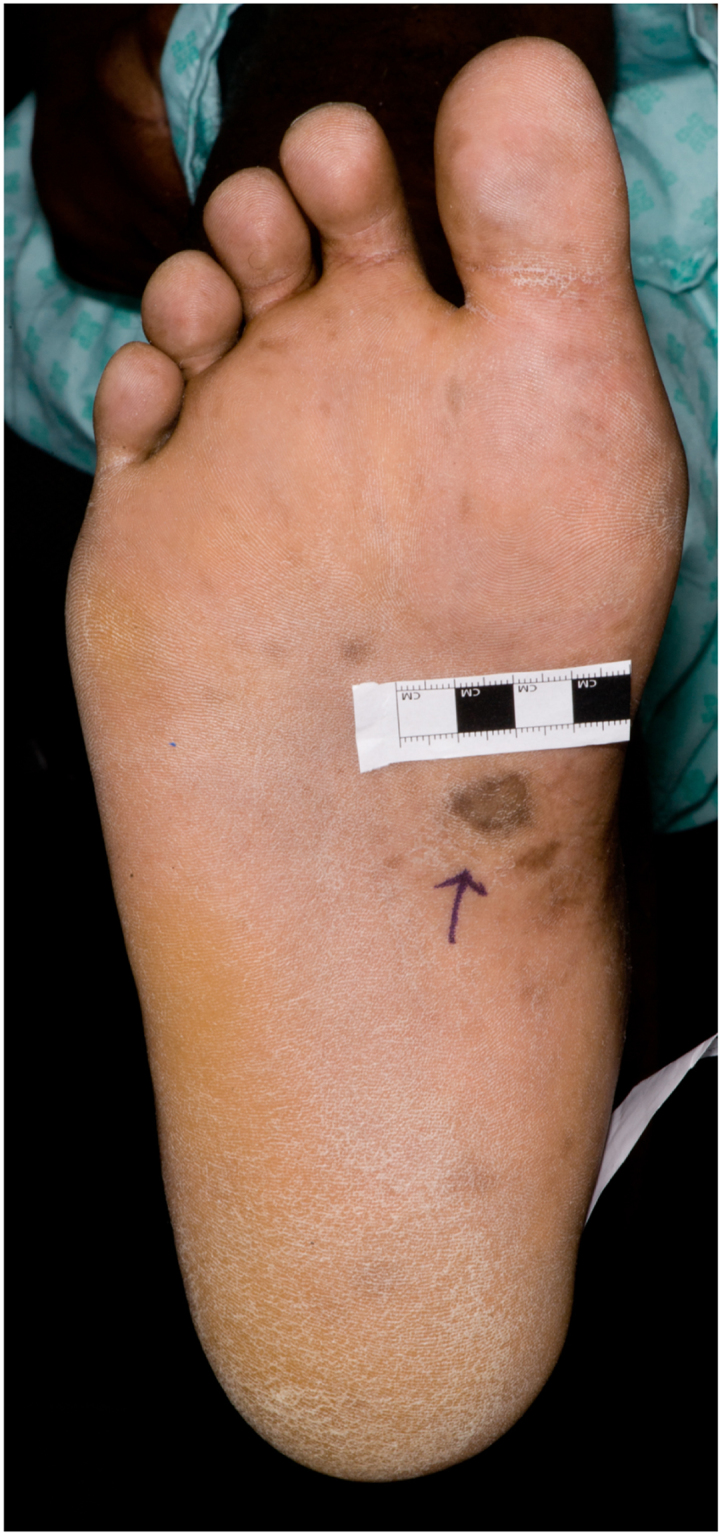
Plantar lesions, right foot.
Fig. 4.
Truncal rash, detail.
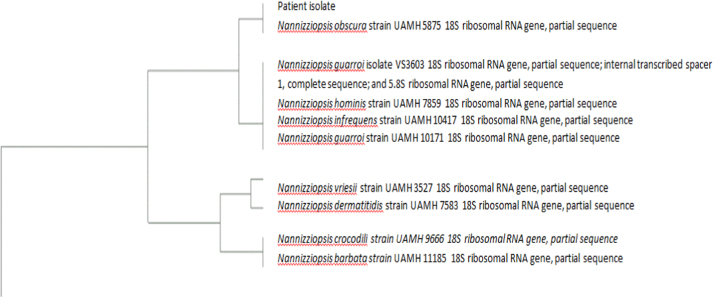
Fig. 5.
Phylogenetic tree containing patient isolate sequence (generated from the CBS database) [21].
He had end stage renal failure of unknown cause with renal transplantation in 2008. Following an episode of antibody-mediated rejection in March 2014, his immunosuppressive regimen had been intensified such that at the time of presentation he was on tacrolimus 12 mg once daily, prednisolone 7.5 mg once daily and mycophenylate 1 g twice daily. He was HIV negative.
He had been resident in the UK for several years but regularly made visits to the Gambia, most recently in March 2015.
On the day of admission, Day 0, he had a raised CRP of 124 mg/L and a significantly elevated alkaline phosphatase of 1182 iu/L, of predominantly bone origin. A CT thorax on Day 0 demonstrated subcarinal lymphadenopathy causing compression of the right main bronchus and a few pulmonary nodules. MRI spine on Day 2 showed a loculated soft tissue collection at T6-T8 level with no spinal involvement.
The clinical suspicion was of tuberculosis, although pus from the paraspinal collection obtained on Day 8 was smear negative for acid-alcohol-fast bacilli. Pus grew a Salmonella species but this result went unnoticed at the time. No other microorganisms were isolated at this point, although the sample was not sent for mycological culture on this occasion. Scrapings from the skin rash showed fungal elements but the rash was thought to be pityriasis versicolor and so this was not investigated further at this point. The patient was commenced on standard anti-tuberculous therapy on Day 10. He was discharged from hospital on Day 24 and followed up in the TB clinic on Day 38.
By August, the back pain and paraspinal swelling had worsened. The patient was readmitted on Day 53 and a repeat CT thorax revealed a 13 cm by 6 cm collection in the thoracic musculature extending from T5 to T10. The subcarinal node and pulmonary nodules seen in June were still present, as was the rash on his trunk.
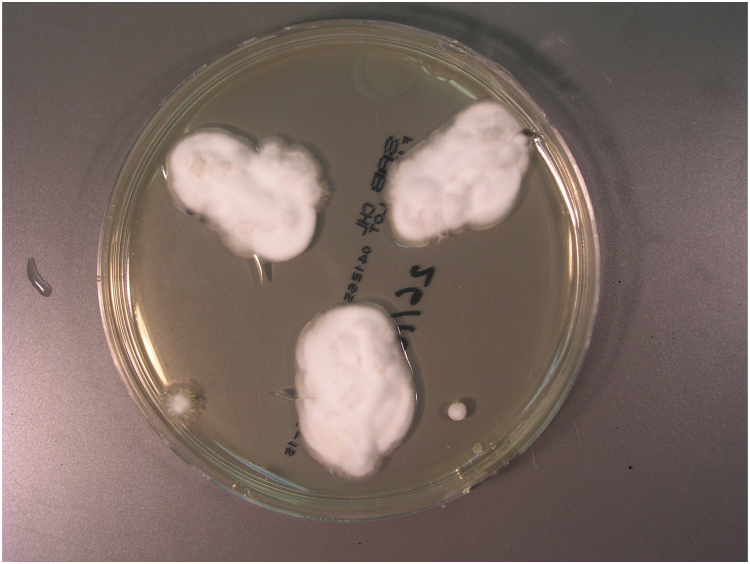
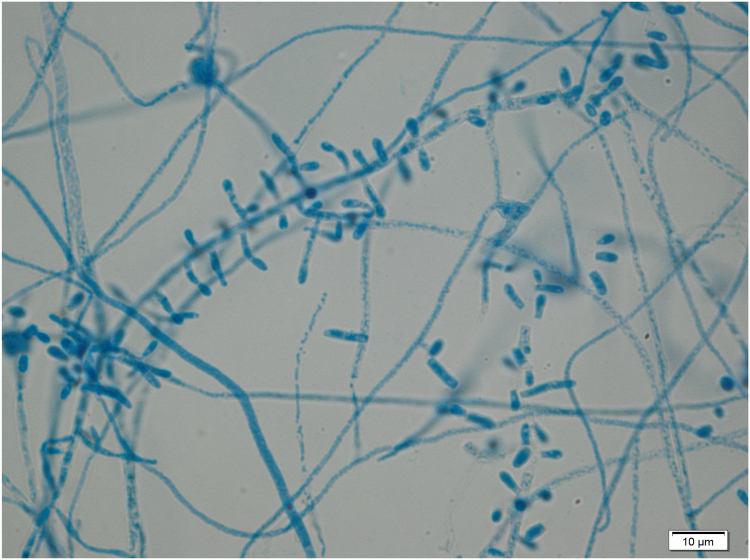
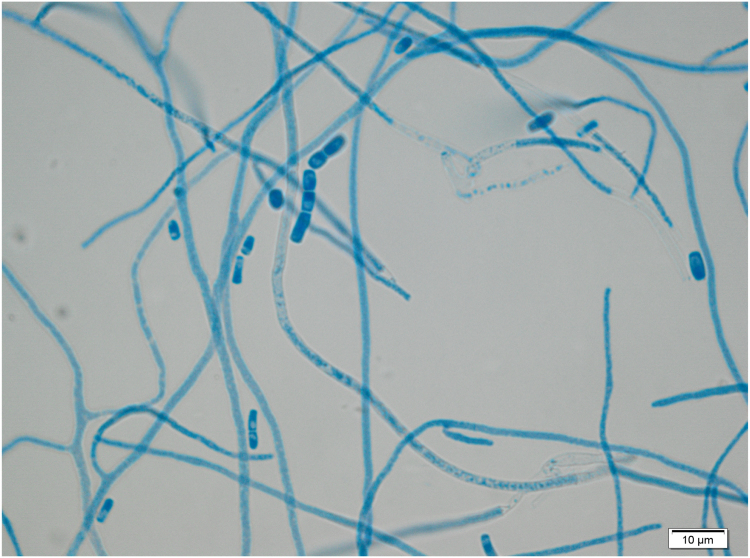
He underwent surgical drainage of the thoracic musculature collection on Day 55 and pus was examined microscopically and a few pseudohyphae or round fungal cells were seen. The sample was cultured on Sabouraud agar and incubated at 35 °C. White mould colonies grew following five days incubation (Fig. 6), and microscopy revealed club-shaped conidia borne on the sides of hyphae (Fig. 7) and cylindrical arthroconidia in chains (Fig. 8). DNA from the isolate was extracted, amplified and sequenced using universal fungal primers ITS5-F (5′- CCTTGTTACGACTTTTACTTCC-3′) and ITS4-R (5′-GCATATCAATAAGCGGAGGA-3′) [1]. A 787 nucleotide sequence was obtained with subsequent BLAST analysis identified closest alignment with Nannizziopsis obscura (100% identity, 100% query cover, 787/787) (accession KF466865.1). The fluid from this collection also grew Salmonella enteritidis.
Fig. 6.
Colonies of Nannizziopsis sp. on Sabouraud agar.
Fig. 7.
Lactophenol cotton blue staining of Nannizziopsis culture.
Fig. 8.
Microscopy of Nannizziopsis culture showing arthroconidia in chains.
The subcarinal node was biopsied under endobronchial ultrasound guidance on Day 64 and calcofluor staining revealed a mass of mycelium (Fig. 9). The sample was cultured on SAB agar at 35 °C and fine growth was observed after three days’ incubation. Examination of colonies revealed similar colony morphology and microscopic appearance to the Nannizziopsis spp. isolated from the thoracic collection. Calcofluor staining of the lymph node tissue showed fungal hyphae.
Fig. 9.
X100 Fluorescence microscopy of calcofluor stained tissue.
Scrapings were taken from the truncal rash and fungal elements were seen on microscopy although fungal cultures were negative.
The isolates from the muscle collection and the lymph node were identical and also identified as N. obscura. An isolate was also sent for sensitivity testing to the Public Health England Mycology Reference Laboratory in Bristol and the results are shown in Table 1.
Table 1.
Sensitivity testing results for Nannizziopsis spp.
| Antifungal | MIC mg/L | Interpretation |
|---|---|---|
| Amphotericin B | 0.125 | S |
| Posaconazole | 0.125 | S |
| Terbinafine | 0.125 | S |
| Itraconazole | 0.25 | S |
| Voriconazole | 0.25 | S |
| Anidulafungin | 4 | R |
It was considered that he had disseminated Nannizziopsis infection with secondary infection of the thoracic musculature collection with Salmonella enteritidis. The tuberculosis treatment was stopped on Day 62 and he was treated with ciprofloxacin 500 mg twice daily for six weeks and posaconazole 300 mg once daily. He responded well with resolution of his main symptoms within a few weeks. The thoracic collection, skin rash and skin lesions improved and CRP and alkaline phosphatase levels had normalised by the beginning of September 2015, when he was reviewed in clinic on Day 86. The patient completed a total of ten months of posaconazole therapy and at the time of writing, seven months after the last dose, he has remained well with no evidence of recurrence thus far.
3. Discussion
Nannizziopsis species belong to the Onygenaceae family which includes the dermatophytes. They are keratinophilic microfungi, the ecology of which is not well known.
Fungi previously referred to as the Chrysosporium anamorph of Nannizziopsis vriesii are known to cause dermatological lesions of reptiles, both in captivity and in the wild. (About 65 Chrysosporium species are currently accepted and are now better referred to by their teleomorphs and are found in a variety of genera such as Aphanoascus, Arthroderma or Nannizziopsis) [2]. Multiple cases of infection caused by these fungi have been reported with aggressive, pyogranulomatous lesions that can affect the skin, integument and musculoskeletal systems of snakes, chameleons, geckos, lizards and crocodiles [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]. These reports are mostly from the USA and Canada, but also Australia [5], Belgium [9], Spain [14], Scotland [8], and Russia [15].
The infections often prove fatal in reptiles and appear to be highly transmissible between animals kept in confinement together, but do not infect humans [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]. The clinical course tends to be of a rapidly progressing, deep, necrotic or granulomatous dermatomycosis that eventually disseminates and for which the outcome is usually fatal [16]. There are however reports of reptile infections being successfully treated with itraconazole or voriconazole [9].
Case reports of Nannizziopsis species infections in humans are rare and, as far as the authors are aware, there are no reports of disseminated infection with N. obscura, as was seen in this patient, although there have been isolated reports from America of disseminated infection in both immunocompetent and immunocompromised patients with N. hominis [16].
Sigler et al. at the University of Alberta microfungus laboratory studied six Nannizziopsis isolates from human samples (together with over forty isolates from reptiles) and from these isolates confidently identified three distinct species that have been found in humans; N. hominis, N. infrequens and N. obscura [16]. Five of these isolates are from cases which were reported and so for which clinical information is available. One of the isolates is from a case for which the clinical information is missing. The isolates and their related cases are described below.
N. infrequens was found in the bronchial lavage sample of an HIV positive 40 year old male patient being treated for pneumonia in Atlanta in 2004 with a CD4 count of 13. However, it was not thought to be pathogenic and the patient recovered without being treated with antifungals [17].
N. hominis was isolated in 1994 from a deep muscle mass on the right thigh, right groin, buttock and lung of an HIV positive male in California who died eight months after the initial isolation. The patient received itraconazole for the fungal infection. The isolate from the lungs had been tentatively identified initially as a Trichophyton species [16].
In 2000, a white mould was isolated from the swollen lymph nodes of an immunocompetent Nigerian man from Boston who presented with disseminated adenopathy following a trip to Nigeria. One of the isolates from this patient tested positive in the AccuProbe Blastomyces culture identification test and was referred on to a laboratory in Texas for further evaluation. Because of its Chrysosporium-like morphology, the isolate was then forwarded to specialists at the University of Alberta microfungus laboratory for further review. The patient was readmitted to the hospital six months later and found to have a disseminated fungal infection involving the heart (endocarditis), lungs, spleen, and kidneys. The fungus was not regrown, but the patient had been on itraconazole since the initial diagnosis and remained on the drug for two years. The original isolate in this case was thought to be N. hominis [16].
Sigler et al. also found that an isolate from disseminated disease in a Nigerian-American that had originally been identified as N. guarroi by Stchigel et al. appeared to represent another N. hominis isolate. The ITS sequence differed in only three positions and the isolate grew at 40 °C, whereas N. guarroi isolates did not grow at this temperature [16]. Stchigel et al. do not refer to the details of this case or make reference to any published reports of this case, so the details of where this isolate came from and the clinical context in which it occurred are not clear.
There has been one confirmed reported case of N. obscura infection in humans before and Sigler et al. identified one further possible case of N. obscura infection in their analysis too [16].
In 1984 a black African 24 year old male patient presented to orthopaedic surgeons in New York with swelling of his right ankle. X-rays showed a large lucency in the distal tibia and tissue samples were positive for what was identified at the time as a Chrysosporium species. The patient was not known to be immunocompromised but had travelled to Africa in 1975, at which time he had been involved in renovating a house, being exposed to lots of dust. He denied any pulmonary symptoms and apparently recovered following a four month course of amphotericin B [19]. Sigler et al. identified this isolate as N. obscura in their analysis in 2013 [16].
An isolate causing a brain infection in an HIV positive Nigerian male in Germany was identified as Chrysosporium anamorph of Nannizziopsis vriesii, but no details on the methods used to identify the fungus were provided [20]. This was a 38 year old man who presented to neurosurgeons in Hamburg in 2005 with an eight month history of parietal headaches, poor memory, left arm paraesthesiae and left sided focal seizures. His viral load was undetectable on antiretroviral treatment and he had a CD4 count of 102. MRI brain showed two large abscesses in the right hemisphere with associated oedema. The pus drained from these abscesses grew what was initially identified as N. vriesii and he apparently responded to treatment with voriconazole, steroids and anticonvulsant medication [20].
Stchigel et al. identified the isolate as N. vriesii [18], but its ability to grow at 40 °C, low ITS similarity with other N. vriesii isolates (93%) and the low support for the grouping with N. vriesii in both phylogenetic analyses by Sigler et al. make this identification questionable. Their sequence groups with N. obscura, which is recognised as a human pathogen, but differs at 14 positions along the ITS region [16]. As such, this may have been a case of N. obscura causing a brain abscess in an immunocompromised patient, but the identification of the pathogen remains not fully resolved (Table 2).
Table 2.
Summary of current and previously documented human cases of Nannizziopsis infection.
| Species | Disease, dissemination | Immunocompromise? | Reference |
|---|---|---|---|
| N. infrequens | Localised isolate. Bronchial wash specimen, M, 40 yr old, USA, IA, 2004. | HIV positive. | Sigler et al., 2013 (elucidated further from Brandt et al., 2005 who named isolate as Nannizziopsis vriesii) |
| N. hominis | Disseminated disease. Right thigh mass with lung lesion, M, USA, CA, 1994. | HIV positive. | Sigler et al., 2013 |
| N. hominis | Disseminated Inguinal node, Nigerian, M, 32 yr old, with disseminated adenopathy, USA, MA, 2000 | Immunocompetent. | Sigler et al., 2013 |
| N. obscura | Localised disease. Abscess right ankle, African, M, 24 yr old (isolated twice), USA, NY, 1984 |
Immunocompetent. | Sigler et al., 2013 (elucidated further from Stillwell et al. 1984 who named isolate as Chrysosporium spp) |
| N. vriesii | Disseminated disease. Lung infiltration and a brain abscess in a Nigerian, M, 38 yr old, Germany. 2005. | HIV positive. | Steininger et al., 2005 |
| N. obscura | Disseminated disease. Thoracic collection, lymphadenopathy and skin rash in Gambian, M, 34 yr old, UK. 2015. | Immunosuppressed for renal transplant. | This report, 2015. |
In conclusion, it appears that this patient is the first reported case of disseminated N. obscura infection and the first reported case of Nannizziopsis infection in humans in the UK.
It seems likely from the small pool of evidence in existence that the species of Nannizziopsis which cause disease in reptiles, such as N. vriesii and N. guarroi, are separate from the species which are pathogenic in humans, chiefly N. hominis and N. obscura. Risk factors for acquiring Nannizziopsis infection appear to be immunocompromise and travel to Africa. Although this patient had both risk factors for acquiring infection, the cases above demonstrate that infection can occur in the presence of only one risk factor.
The duration of antifungal therapy was difficult to determine. The patient's antifungal medication has now been discontinued, although due to his ongoing immunosuppression, there is potential risk for relapse.
His case demonstrates the importance of investigating for fungal infection in immunocompromised patients and of repeating investigations when initial tests are inconclusive.
Conflict of interest
There are none.
Ethical form
The authors have obtained written and signed consent from the patient to publish the details of his case. Please see attached ethical form.
Acknowledgements
Thanks to Tony English for the sequencing work and Dr Deborah Gascoyne-Binzi for discussions about the sequence analysis.
Many thanks to the patient for allowing us to publish the details of his case.
Thanks to the PHE Mycology Reference Centre at Bristol for the sensitivity testing.
References
- 1.White T.J., Bruns T., Lee S.J.W.T., Taylor J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc.: a Guide Methods Appl. 1990;18(1):315–322. [Google Scholar]
- 2.Seifert K., Morgan-Jones G., Gams W., Kendrick B. Centraalbureau voor Schimmelcultures; Utrecht, The Netherlands: 2011. The Genera of Hyphomycetes. [Google Scholar]
- 3.Pare J.A., Sigler L., Hunter D.B., Summerbell R.C., Smith D.A., Machin K.L. Cutaneous mycoses in chameleons caused by the Chrysosporium anamorph of Nannizziopsis vriesii (Apinis) Currah. J. Zoo Wildl. Med.: Off. Publ. Am. Assoc. Zoo. Vet. 1997;28(4):443–453. [PubMed] [Google Scholar]
- 4.Nichols D.K., Weyant R.S., Lamirande E.W., Sigler L., Mason R.T. Fatal mycotic dermatitis in captive brown tree snakes (Boiga irregularis) J. Zoo Wildl. Med.: Off. Publ. Am. Assoc. Zoo. Vet. 1999;30(1):111–118. [PubMed] [Google Scholar]
- 5.Thomas A.D., Sigler L., Peucker S., Norton J.H., Nielan A. Chrysosporium anamorph of Nannizziopsis vriesii associated with fatal cutaneous mycoses in the saltwater crocodile (Crocodylus porosus) Med. Mycol. 2002;40(2):143–151. doi: 10.1080/mmy.40.2.143.151. [DOI] [PubMed] [Google Scholar]
- 6.Bowman M.R., Pare J.A., Sigler L., Naeser J.P., Sladky K.K., Hanley C.S. Deep fungal dermatitis in three inland bearded dragons (Pogona vitticeps) caused by the Chrysosporium anamorph of Nannizziopsis vriesii. Med. Mycol. 2007;45(4):371–376. doi: 10.1080/13693780601188610. [DOI] [PubMed] [Google Scholar]
- 7.Abarca M.L., Martorell J., Castella G., Ramis A., Cabanes F.J. Cutaneous hyalohyphomycosis caused by a Chrysosporium species related to Nannizziopsis vriesii in two green iguanas (Iguana iguana) Med. Mycol. 2008;46(4):349–354. doi: 10.1080/13693780701851711. [DOI] [PubMed] [Google Scholar]
- 8.Eatwell K. Suspected fatal Chrysosporium anamorph of Nannizziopsis vriesii (CANV) dermatitis in an albino Boa constrictor (Constrictor constrictor) J. Small Anim. Pract. 2010;51(5):290. doi: 10.1111/j.1748-5827.2010.00942.x. [DOI] [PubMed] [Google Scholar]
- 9.Hellebuyck T., Baert K., Pasmans F., Van Waeyenberghe L., Beernaert L., Chiers K. Cutaneous hyalohyphomycosis in a girdled lizard (Cordylus giganteus) caused by the Chrysosporium anamorph of Nannizziopsis vriesii and successful treatment with voriconazole. Vet. Dermatol. 2010;21(4):429–433. doi: 10.1111/j.1365-3164.2010.00880.x. [DOI] [PubMed] [Google Scholar]
- 10.Toplon D.E., Terrell S.P., Sigler L., Jacobson E.R. Dermatitis and cellulitis in leopard geckos (Eublepharis macularius) caused by the Chrysosporium anamorph of Nannizziopsis vriesii. Vet. Pathol. 2013;50(4):585–589. doi: 10.1177/0300985812465324. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell M.A., Walden M.R. Chrysosporium anamorph nannizziopsis vriesii. An emerging fungal pathogen of captive and wild reptiles. Vet. Clin. North Am. - Exot. Anim. Pract. 2013;16(3):659–668. doi: 10.1016/j.cvex.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 12.E. Bicknese, Itraconazole treated CANV (Chrysosporium anamorph of Nannizziopsis vriesii) in green anacondas (Eunectes murinus murinus), Proceedings of the Association of Reptilian and Amphibian Veterinarians, Milwaukee, WI, 2009, pp. 157–158.
- 13.Volker Schmidt. Fungal infections in reptiles – An emerging problem. J. Exotic Pet Med. 2015;24(3):267–275. [Google Scholar]
- 14.Abarca M.L., Martorell J., Castella G., Ramis A., Cabanes F.J. Dermatomycosis in a pet inland bearded dragon (Pogona vitticeps) caused by a Chrysosporium species related to Nannizziopsis vriesii. Vet. Dermatol. 2009;20(4):295–299. doi: 10.1111/j.1365-3164.2009.00736.x. [DOI] [PubMed] [Google Scholar]
- 15.R.S. Ovchinnikov, M.G. Manoyan, A.G. Gaynullina, A.N. Panin, New and emerging fungal pathogens in companion animals in Russia. Mycoses. Conference: 6th Trends in Medical Mycology, TIMM Copenhagen Denmark. Conference Publication: (var.pagings). 56, 2013, pp. 87–88.
- 16.Sigler L., Hambleton S., Pare J.A. Molecular characterization of reptile Pathogens currently known as members of the chrysosporium anamorph of Nannizziopsis vriesii complex and relationship with some human-associated isolates. J. Clin. Microbiol. 2013;51(10):3338–3357. doi: 10.1128/JCM.01465-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandt M.E., Gaunt D., Iqbal N., McClinton S., Hambleton S., Sigler L. False-positive Histoplasma capsulatum Gen-Probe chemiluminescent test result caused by a Chrysosporium species. J. Clin. Microbiol. 2005;43:1456–1458. doi: 10.1128/JCM.43.3.1456-1458.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stchigel A.M., Sutton D.A., Cano-Lira J.F., Cabañes F.J., Abarca L., Tintelnot K. Phylogeny of chrysosporia infecting reptiles: proposal of the new family Nannizziopsiaceae and five new species. Persoonia. 2013;31:86–100. doi: 10.3767/003158513X669698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stillwell W.T., Rubin B.D., Axelrod J.L. Chrysosporium. a new causative agent in osteomyelitis. Clin. Orthop. 1984;184:190–192. [PubMed] [Google Scholar]
- 20.Steininger C., Lunzen J., van, Tintelnot K., Sobottka I., Rohde H., Horstkotte M.A. Mycotic brain abscess caused by opportunistic reptile pathogen. Emerg. Infect. Dis. 2005;11:349–350. doi: 10.3201/eid1102.040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centraalbureau voor Schimmelcultures (CBS) Fungal Biodiversity Center website 〈http://www.westerdijkinstitute.nl/Collections/BioloMICSSequences.aspx?File=all〉 (Accessed 17 May 2017).