Abstract
Background
Increasing evidence has suggested that Helicobacter pylori (H. pylori) eradication might prevent the development of gastric cancer (GC). This systematic review and meta-analysis aimed to better explore the role of H. pylori eradication in preventing GC, with particular reference to patients with precancerous lesions at baseline histology.
Methods
Searches for human studies were performed through October 2016 and risk ratios (RRs), were obtained. Heterogeneity between studies was estimated using the Cochran Q test and I2 values, whereas the possibility of publication bias was estimated with funnel plots. Additionally, we performed subgroup and sensitivity analyses.
Results
In 26 studies suitable for meta-analysis (10 randomized controlled trials and 16 cohort studies) 52,363 subjects were included. The risk of GC among patients in whom H. pylori was successfully eradicated was significantly lower than that among controls: pooled RRs [95% CI] 0.56 [0.48-0.66], Z= -7.27, P=0.00001. This finding applied separately for randomized controlled trials (0.65 [0.51-0.84], Z= -3.33, P=0.0009) and for cohort studies (0.51 [0.42-0.62], Z= -6.63, P=0.00001). Concerning H. pylori eradication in patients with precancerous lesions, subgroup analyses showed that patients with non-atrophic or atrophic gastritis benefited from H. pylori eradication for the risk of GC development, whereas those with intestinal metaplasia or dysplasia did not.
Conclusion
H. pylori eradication is associated with a significantly lower risk of GC; this finding has significant implications for the prevention of this cancer. The benefit is maximized when H. pylori eradication is applied at early stages of the infection.
Keywords: H. pylori, eradication, gastric cancer, prevention, meta-analysis
Introduction
Despite a decline of gastric cancer (GC) in many countries in the developed world, GC remains among the most common causes of cancer death globally [1]. In fact, there is an increase in global mortality from the disease due to population growth and increasing life expectancy in the developing world [2]. Two major types are evident: The intestinal and the diffuse type. The intestinal type, the more common variant, shows a strong association with Helicobacter pylori (H. pylori) infection, as well as other factors such as cigarette smoking and diet (salted foods). The diffuse type is less common and is attributed to host-factor influences, such as mutations in the E-cadherin gene.
H. pylori infection affects approximately 50% of the global population [3] and it is the principal trigger of gastric carcinogenesis. It is believed to predispose to GC by inducing precancerous changes, i.e., atrophic gastritis (AG) and intestinal metaplasia (IM) [4,5]. There is evidence that subjects infected by H. pylori were between three and six times more prone to develop GC than uninfected controls [6-8]. These data were taken into account by the International Agency for Research on Cancer, which declared H. pylori as a class I human carcinogen [9]. Eradication of H. pylori can result in amelioration of gastric inflammation, halt the progression of gastric mucosal damage, prevent further H. pylori-induced DNA damage, improve gastric acid secretion, and restore the microbiome toward normal [10]. Because we can treat H. pylori with a course of antibiotic treatment, identifying and treating H. pylori infection could represent a strategy for reducing the disease burden of GC [11]. Thus, an earlier meta-analysis [12] that pooled randomized controlled trials (RCTs) conducted in asymptomatic infected individuals reported that H. pylori treatment could reduce the risk of GC in Asians.
As more data concerning the relationship of H. pylori treatment and GC prevention have become available from recent studies, we undertook the present meta-analysis to update and better define this relationship. We gave particular attention to studies that examined the role of H. pylori eradication in reducing the cancer risk in patients with precancerous lesions at baseline histology, i.e., AG, IM, and dysplasia (DYS).
Materials and methods
Study identification and extraction of data
The three authors performed extensive PubMed, MEDLINE and Embase database medical literature searches for human studies through October 2016, using suitable key words (“stomach neoplasms”[MeSH Terms] OR (“stomach”[All Fields] AND “neoplasms”[All Fields]) OR “stomach neoplasms”[All Fields] OR (“gastric”[All Fields] AND “cancer”[All Fields]) OR “gastric cancer”[All Fields]) AND (“helicobacter pylori” [MeSH Terms] OR (“helicobacter” [All Fields] AND “pylori” [All Fields]) OR “helicobacter pylori” [All Fields] OR “h pylori” [All Fields]) AND ((“therapy” [Subheading] OR “therapy” [All Fields] OR “treatment” [All Fields] OR “therapeutics” [MeSH Terms] OR “therapeutics” [All Fields]) OR eradication [All Fields]) AND (“prevention and control”[Subheading] OR (“prevention” [All Fields] AND “control” [All Fields]) OR “prevention and control” [All Fields] OR “prevention” [All Fields]). A full manual search of all review articles, recently published editorials and retrieved original studies was also performed. Two of the authors (TR and PP) independently extracted data from suitable studies using a standard form. Any disagreement was settled by further discussion. The meta-analysis was performed in agreement with PRISMA [13].
Selection criteria
Inclusion and exclusion criteria were defined prior to the literature search. Hence, the following criteria had to be met for studies to be included in this meta-analysis: 1) published as full articles or abstracts; 2) written in English; and 3) randomized controlled trials (RCTs) or observational studies with data on the association of H. pylori eradication and GC prevention. Exclusion criteria were the following: 1) studies not meeting the inclusion criteria; 2) studies without data for retrieval; 3) duplicate studies; and 4) the less informative publication of two on the same study.
Statistical analysis
Authors’ agreement on study selection was measured by the κ coefficient. The pooled risk ratio (RR) and the 95% confidence interval (CI) were calculated and results of individual studies were compared by employing the Mantel-Haenszel method (fixed-effects model) [14] or the DerSimonian and Laird method (random-effects model) [15]. Forest plots were constructed for the visual display of RRs of individual studies. Heterogeneity between studies was measured with the Cochran Q test [16] and was judged significant when the Q test had a P-value of less than 0.10 [17,18]. In addition, the I2 statistic was employed to estimate the rate of inconsistency in individual studies that could not be interpreted by chance [19]. When statistically significant heterogeneity was not present, pooled RRs (95% CI) were calculated using the fixed-effects model. If significant heterogeneity was present, pooled RRs (95% CI) were calculated using the random-effects model [17-19]. We also used cumulative meta-analysis to explore whether the association between H. pylori eradication and GC remained steady [18]. The Comprehensive Meta-Analysis software, version 2 (BIOSTAT INC., Englewood, NJ, USA), was used in all calculations throughout this meta-analysis.
Sensitivity analyses and publication bias
In case of significant heterogeneity, except for the random-effects model, sensitivity analyses were employed to judge the consistency of the results. Thus, to explore any significant influence of single studies on the pooled RR results, we performed the exclusion method by repeating the meta-analysis after excluding single studies one at a time [18]. Furthermore, as different populations and study designs may incorporate various biases, we employed subgroup analyses by stratifying all factors that could influence the overall results. In addition, we further explored possible heterogeneity using meta-regression analyses (methods of moments) [20]. The possibility of publication bias was examined by constructing funnel plots. These were constructed by plotting the log RRs vs. Standard Error (SE) of individual studies [20,21]. Their symmetry was measured by Egger’s regression test and the Begg and Mazumdar adjusted rank correlation test [22,23]. The adjustment for publication bias, i.e., calculation of the number of studies missing from the meta-analysis, was achieved by using the nonparametric ‘‘trim and fill’’ rank-based method of Duval and Tweedie [24].
Results
Descriptive assessment and characteristics of the studies
Agreement between the authors regarding the selection of suitable articles and data extraction was high: κ=0.93, 95% CI 0.83-1. A flow chart explaining the study selection is shown in Fig. 1. Of the 1122 titles initially found by the literature searches, 26 studies were found suitable for meta-analysis, 8 RCTs and 18 observational (cohort) studies [25-50]. There were 24 studies from Far East countries (13 from Japan, 5 from China, 5 from Korea, 1 from Taiwan), 1 from Colombia and 1 from Finland. Table 1 shows the characteristics of the 26 eligible studies for meta-analysis.
Figure 1.
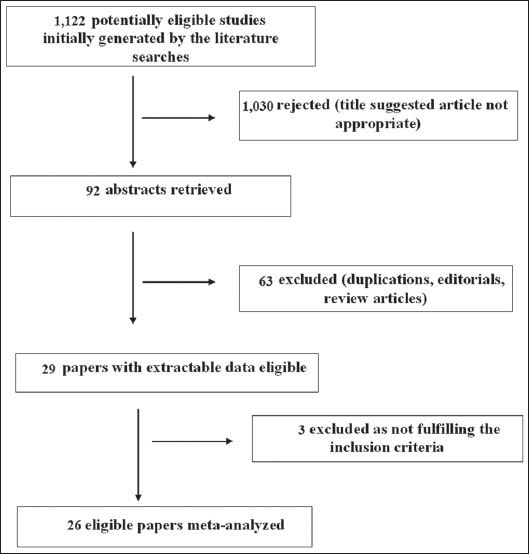
Flow diagram of the studies identified in this meta-analysis
Table 1.
The main characteristics of the studies included in the meta-analysis
GC risk in H. pylori treated patients and non-treated (controls)
A total of 22,619 patients and 29,744 controls were included in the 26 meta-analysis studies. There was no significant heterogeneity in the included studies: Q=23.09, df(Q)=25, I2=0%, P=0.57. Therefore, we applied the fixed-effects model, which yielded significant results: i.e., pooled RR result 0.56, 95% CI 0.48-0.66, test for overall effect Z= -7.27, P=0.00001 (Fig. 2). No publication bias was found, as seen in the funnel plot and its symmetry calculation: P=0.11 by adjusted rank correlation test and 0.14 by Egger’s regression test (Fig. 3).
Figure 2.
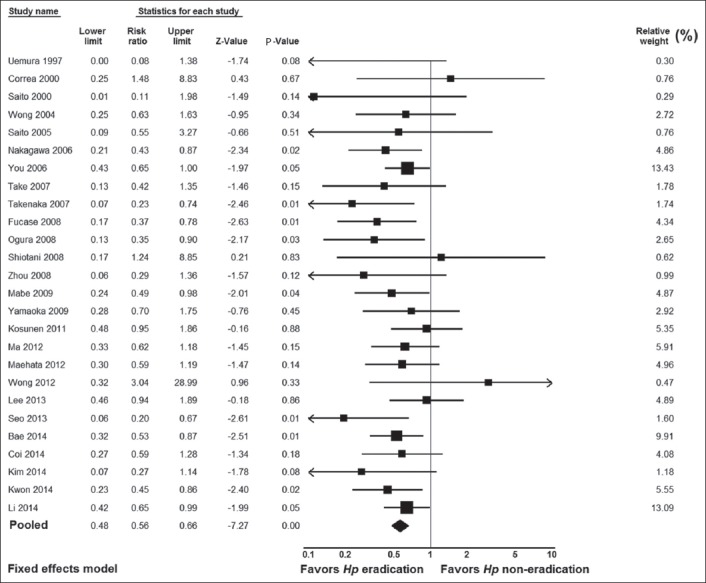
Forest plot showing individual and pooled risk ratios and 95% confidence intervals in studies comparing gastric cancer risk in Helicobacter pylori (Hp)-eradicated patients and in controls
Figure 3.
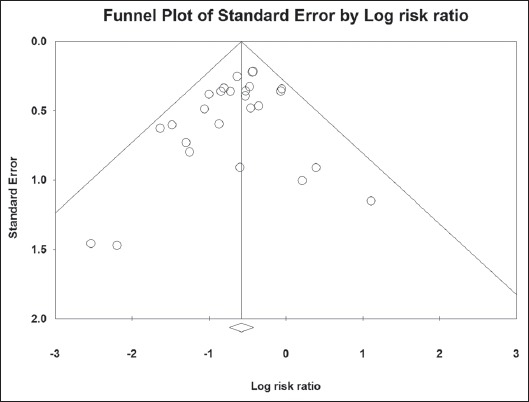
Funnel plot of all studies included in the meta-analysis. There is no evidence of publication bias (P=0.11 by Begg and Mazumdar adjusted rank correlation test and 0.14 by Egger’s regression test)
Subgroup and sensitivity analyses
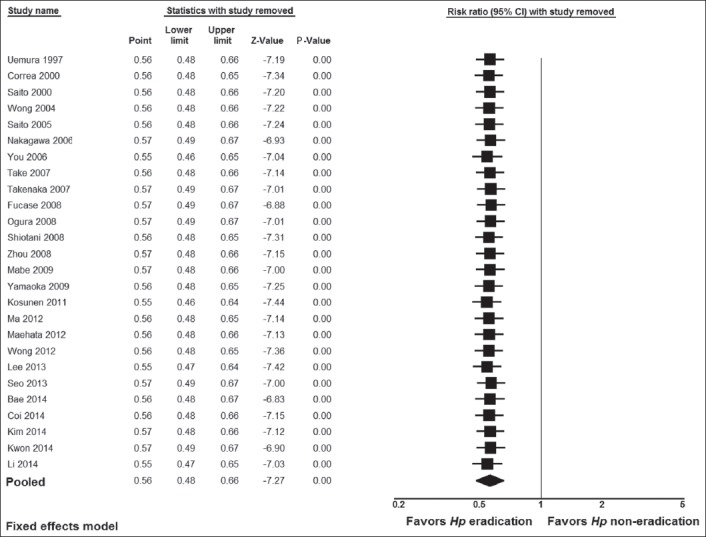
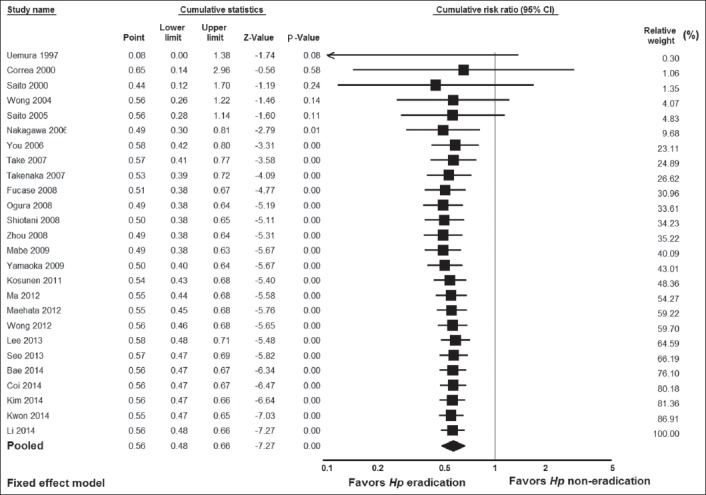
We performed subgroup analyses to examine whether the significant result found for all 26 studies would apply separately when the data were stratified according to study design (i.e., RCTs vs. cohort studies), geographical region, and kind of prevention, i.e., primary vs. secondary, according to whether or not early GC endoscopic resection was performed before H. pylori treatment was given to prevent the development of metachronous cancer. The results of these subgroup analyses are shown in Table 2. There were significant results for both groups of study design: i.e., 0.65 (0.51-0.84), Z= -3.33, P=0.0009 for RCTs, and 0.51 (0.42-0.62), Z= -6.63, P=0.00001 for cohort studies. Concerning geographical region, there were significant results for the Far East studies (0.54 [0.46-0.63], Z=-7.51, P=0.000001), whereas the results were not significant for the American study from Colombia (1.48 [0.25-8.83], Z=-0.43, P=0.67) or the European study from Finland (0. 95 [0.48-1.86], Z= -0.16, P=0.88). There were significant results for both primary (0.63 [0.52-0.77], Z= -4.62, P=0.000004) and secondary prevention (0.46 [0.36-0.60], Z= -5.91, P=0.000001). Additionally, more sensitivity analyses were utilized to detect the presence of any biases related to included populations and different study designs. The exclusion of any study did not alter the summary results substantially (Fig. 4). Furthermore, the results of the cumulative meta-analysis did not change over the years (Fig. 5). In addition, the meta-regression analyses showed no significant results for publication year (Z=0.67, P=0.49), but significant results for mean duration follow up (Z=1.96.57, P=0.049) (Fig. 6). The latter was expected as more cancers were noted in subjects who had longer duration of follow up.
Table 2.
Sub-group analyses by stratifying the data according to study design, geographical region and kind of prevention (primary vs. secondary)
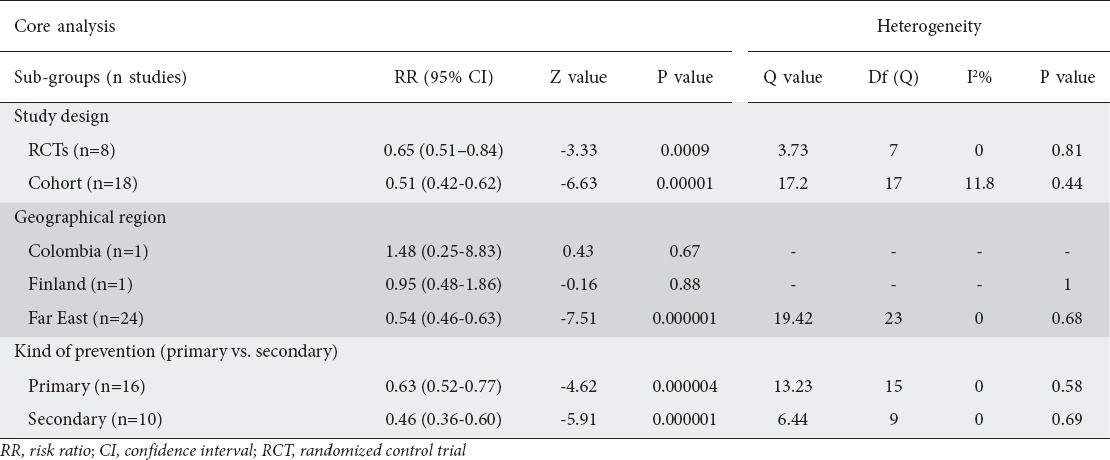
Figure 4.
Exclusion sensitivity plot showing the effect of exclusion of any study on the magnitude of the summary estimate
Figure 5.
Cumulative meta-analysis of studies ordered by year of publication
Figure 6.
(A) Meta-regression analysis showing regression of publication year on log risk ratio. (B) Meta-regression analysis showing regression of mean follow-up duration on log risk ratio. Circles represent each study in the meta-analysis
GC risk according to baseline histology
Five studies [26,28,31,43,46] examined the GC risk according to baseline histology and the results are shown in Fig. 7. These studies stratified baseline histology into two groups: i.e., a group of subjects with chronic non-AG (NAG) or AG, and a group of subjects with IM or DYS. The RR [95% CI] was significant in the NAG/AG group (0.28 [0.08-0.96], Z= -2.03, P=0.04), but not in the IM/DYS group (0.84 [0.55-1.28], Z= -0.83, P=0.41).
Figure 7.
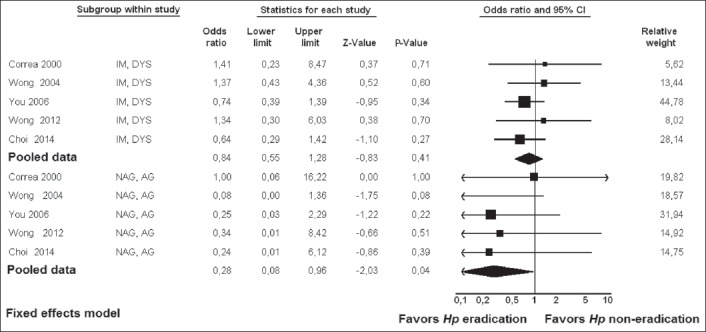
Forest plot showing individual and pooled risk ratios with 95% confidence intervals in studies comparing gastric cancer risk in Helicobacter pylori (Hp)-eradicated patients and in controls, according to baseline histology
NAG, non atrophic gastritis; AG, atrophic gastritis; IM, intestinal metaplasia; DYS, dysplasia
Discussion
Despite the decline in its incidence in recent decades, GC remains a major health concern in many parts of the world. The development of GC constitutes a complex cascade of events, characterized mainly by interactions between H. pylori infection, host factors and environmental influences, such as diet [51]. Indeed, H. pylori plays a crucial role in this process, as most non-cardia GCs develop from H. pylori-infected mucosa [52]. In the gastric mucosa, H. pylori initiates a cascade of events, which may lead to chronic AG and then to IM, DYS, and finally carcinoma [53]. Such a proposal is supported by a substantial number of studies performed in countries with a high incidence of GC. Indeed, in these countries H. pylori eradication has been proposed as a potentially important primary preventive strategy to reduce GC incidence. The results of this meta-analysis showed that H. pylori eradication significantly reduces the risk of GC development, in comparison to non-eradication. This evidence was robust, as evidenced by the various sensitivity and other analyses. In addition, these results confirm earlier meta-analyses [12,54]. These significant results applied for primary prevention and also for secondary prevention, i.e., when evaluating the effect of H. pylori treatment on the risk of metachronous GC development after resection of early GC. These results support the notion that mass H. pylori screening and eradication is a viable preventive strategy and should be implemented in high-risk populations, especially in those with an incidence rate higher than 150/100,000 person-years [55]. The number needed to treat varies depending on the GC risk in various populations being studied. Thus, it was calculated that it could be as low as 15 infected men from areas with a high risk of GC, such as China or Japan, in comparison to 250 in infected women from a low-risk area, such as the USA [12].
Some studies [26,28,31,43,46], examined the effect of H. pylori eradication on GC risk according to baseline histology. In these studies, baseline histology was stratified into two groups, i.e., a group of subjects with early histological events, namely chronic NAG or AG and a group of subjects with advanced histological events, namely IM or DYS. We meta-analyzed these data, and the results showed that the RR [95% CI] was significant in the NAG/AG group (0.28 [0.08-0.96], P=0.04), whereas in the IM/DYS group it was not (0.84 [0.55-1.28], P=0.41). These results suggest that H. pylori eradication represents a primary preventive strategy in a subset of subjects with NAG or AG. In contrast, H. pylori eradication in patients who have already developed advanced pre-neoplastic lesions, i.e., IM or DYS, does not prevent GC development. All this supports the notion that IM represents the “point of no return”, as was suggested previously [56,57] and was advocated by the recent Maastricht guidelines [58]. If we take into account that IM starts approximately in the fourth decade of life [59], all this could potentially mean that the implementation of H. pylori infection screening and treatment could be more effective well before the risk for GC starts increasing [60]. Thus, in Korea it was shown that H. pylori eradication might be most effective in the second decade of life [61].
The stability of the results over time, as shown in the cumulative meta-analysis chart and the lack of heterogeneity and publication bias, strengthens the results of this meta-analysis. However, there is a limitation in that 24 of the 26 meta-analyzed studies (92%) were conducted in far-eastern countries (13 in Japan, 5 in Korea, 5 in China, 1 in Taiwan) and only two were from other parts of the world (1 in Colombia, 1 in Finland). Therefore, more studies from non–far-eastern countries are needed to better define the role of H. pylori eradication in preventing GC. Additional limitations could be the fact that only studies written in English were included in this meta-analysis and the fact that baseline histology was available only in five studies. The latter stresses the need for more studies that take the baseline histology into account.
In conclusion, the results of this meta-analysis support the notion that H. pylori is a significant risk factor for GC. In addition, the results confirmed that H. pylori eradication is associated with a reduction in the incidence of GC and may therefore be the most viable strategy for GC prevention. However, as these results come mainly from East Asian countries, they must be interpreted with caution for European and North American countries; more studies from these parts of the world are needed to better define the role of H. pylori eradication in preventing GC.
Summary Box.
What is already known:
The International Agency for Research on Cancer declared Helicobacter pylori (H. pylori) as a class I human carcinogen and individuals who tested positive for H. pylori are at increased risk of developing gastric cancer (GC) compared with uninfected controls
A growing body of evidence has suggested that H. pylori eradication might prevent GC development
An earlier meta-analysis reported that H. pylori treatment could reduce the risk of GC in Asians
What the new findings are:
This meta-analysis evaluated updated data concerning the effect of H. pylori eradication on GC risk and in particular examined the effect of H. pylori eradication on GC risk according to baseline histology
The results support the notion that H. pylori is a significant risk factor for GC and that H. pylori eradication is associated with a reduction in the incidence of GC
H. pylori eradication represents a primary preventive strategy in a subset of subjects with non-atrophic or atrophic gastritis. However, H. pylori eradication in patients who have already developed advanced pre-neoplastic lesions, i.e., intestinal metaplasia or dysplasia, does not prevent GC development
Biography
Henry Dunant Hospital, Athens, Greece; Aldo Moro University, Bari Medical School, Bari, Italy
Footnotes
Conflict of Interest: None
References
- 1.Ferro A, Peleteiro B, Malvezzi M, et al. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50:1330–1344. doi: 10.1016/j.ejca.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008-2030): A population-based study. Lancet Oncol. 2012;13:790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 3.Correa P, Piazuelo MB. Helicobacter pylori infection and gastric adenocarcinoma. US Gastroenterol Hepatol Rev. 2011;7:59–64. [PMC free article] [PubMed] [Google Scholar]
- 4.Correa P. The gastric precancerous process. Cancer Surv. 1983;2:437–450. [Google Scholar]
- 5.Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2:58–60. doi: 10.1016/s0140-6736(75)90498-5. [DOI] [PubMed] [Google Scholar]
- 6.Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 7.Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 8.Forman D, Newell DG, Fullerton F, et al. Association between infection with Helicobacter pylori and risk of gastric cancer: Evidence from a prospective investigation. BMJ. 1991;302:1302–1305. doi: 10.1136/bmj.302.6788.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Agency for Research on Cancer. Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;61:177–241. [PMC free article] [PubMed] [Google Scholar]
- 10.Machado AM, Figueiredo C, Touati E, et al. Helicobacter pylori infection induces genetic instability of nuclear and mitochondrial DNA in gastric cells. Clin Cancer Res. 2009;15:2995–3002. doi: 10.1158/1078-0432.CCR-08-2686. [DOI] [PubMed] [Google Scholar]
- 11.Malfertheiner P, Megraud F, O’Morain CA, et al. European Helicobacter Study Group. Management of Helicobacter pylori infection—the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 12.Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: Systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174. doi: 10.1136/bmj.g3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Shamseer L, Clarke M, et al. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;8:101–129. [Google Scholar]
- 17.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Sutton AJ, Abrams KR, Jones DR, et al. Methods for meta-analysis in medical research. New York: John Wiley & Sons, Ltd; 2000. [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to meta-analysis. New York: John Wiley & Sons, Ltd; 2009. [Google Scholar]
- 21.Copas JB, Shi JQ. A sensitivity analysis for publication bias in systematic reviews. Stat Methods Med Res. 2001;10:251–265. doi: 10.1177/096228020101000402. [DOI] [PubMed] [Google Scholar]
- 22.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 25.Uemura N, Mukai T, Okamoto S, et al. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:639–642. [PubMed] [Google Scholar]
- 26.Correa P, Fontham ET, Bravo JC, et al. Chemoprevention of gastric dysplasia: Randomized trial of antioxidant supplements and anti-Helicobacter pylori therapy. J Natl Cancer Inst. 2000;92:1881–1888. doi: 10.1093/jnci/92.23.1881. [DOI] [PubMed] [Google Scholar]
- 27.Saito K, Arai K, Mori M, Kobayashi R, Ohki I. Effect of Helicobacter pylori eradication on malignant transformation of gastric adenoma. Gastrointest Endosc. 2000;52:27–32. doi: 10.1067/mge.2000.106112. [DOI] [PubMed] [Google Scholar]
- 28.Wong BC, Lam SK, Wong WM, et al. China Gastric Cancer Study Group. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: A randomized controlled trial. JAMA. 2004;291:187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 29.Saito D, Boku N, Fujioka T, et al. Impact of H. pylori eradication on gastric cancer prevention: Endoscopic results of the Japanese Intervention Trial. Gastroenterology. 2005;128:A4. [Google Scholar]
- 30.Nakagawa S, Asaka M, Kato M, et al. Helicobacter pylori eradication and metachronous gastric cancer after endoscopic mucosal resection of early gastric cancer. Aliment Pharmacol Ther. 2006;24(Suppl 4):214–218. [Google Scholar]
- 31.You WC, Brown LM, Zhang L, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Inst. 2006;98:974–983. doi: 10.1093/jnci/djj264. [DOI] [PubMed] [Google Scholar]
- 32.Take S, Mizuno M, Ishiki K, et al. Baseline gastric mucosal atrophy is a risk factor associated with the development of gastric cancer after Helicobacter pylori eradication therapy in patients with peptic ulcer diseases. J Gastroenterol. 2007;42(Suppl 17):21–27. doi: 10.1007/s00535-006-1924-9. [DOI] [PubMed] [Google Scholar]
- 33.Takenaka R, Okada H, Kato J, et al. Helicobacter pylori eradication reduced the incidence of gastric cancer, especially of the intestinal type. Aliment Pharmacol Ther. 2007;25:805–812. doi: 10.1111/j.1365-2036.2007.03268.x. [DOI] [PubMed] [Google Scholar]
- 34.Fukase K, Kato M, Kikuchi S, et al. Japan Gast Study Group. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: An open-label, randomised controlled trial. Lancet. 2008;372:392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 35.Ogura K, Hirata Y, Yanai A, et al. The effect of Helicobacter pylori eradication on reducing the incidence of gastric cancer. J Clin Gastroenterol. 2008;42:279–283. doi: 10.1097/01.mcg.0000248006.80699.7f. [DOI] [PubMed] [Google Scholar]
- 36.Shiotani A, Uedo N, Iishi H, et al. Predictive factors for metachronous gastric cancer in high-risk patients after successful Helicobacter pylori eradication. Digestion. 2008;78:113–119. doi: 10.1159/000173719. [DOI] [PubMed] [Google Scholar]
- 37.Zhou L. Ten-year follow-up study on the incidence of gastric cancer and the pathological changes of gastric mucosa after H. pylori eradication in China. Gastroenterology. 2008;134:A233. [Google Scholar]
- 38.Mabe K, Takahashi M, Oizumi H, et al. Does Helicobacter pylori eradication therapy for peptic ulcer prevent gastric cancer? World J Gastroenterol. 2009;15:4290–4297. doi: 10.3748/wjg.15.4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yanaoka K, Oka M, Ohata H, et al. Eradication of Helicobacter pylori prevents cancer development in subjects with mild gastric atrophy identified by serum pepsinogen levels. Int J Cancer. 2009;125:2697–2703. doi: 10.1002/ijc.24591. [DOI] [PubMed] [Google Scholar]
- 40.Kosunen TU, Pukkala E, Sarna S, et al. Gastric cancers in Finnish patients after cure of Helicobacter pylori infection: A cohort study. Int J Cancer. 2011;128:433–439. doi: 10.1002/ijc.25337. [DOI] [PubMed] [Google Scholar]
- 41.Ma JL, Zhang L, Brown LM, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst. 2012;104:488–492. doi: 10.1093/jnci/djs003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maehata Y, Nakamura S, Fujisawa K, et al. Long-term effect of Helicobacter pylori eradication on the development of metachronous gastric cancer after endoscopic resection of early gastric cancer. Gastrointest Endosc. 2012;75:39–46. doi: 10.1016/j.gie.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 43.Wong BC, Zhang L, Ma JL, et al. Effects of selective COX-2 inhibitor and Helicobacter pylori eradication on precancerous gastric lesions. Gut. 2012;61:812–818. doi: 10.1136/gutjnl-2011-300154. [DOI] [PubMed] [Google Scholar]
- 44.Seo JY, Lee DH, Cho Y, et al. Eradication of Helicobacter pylori reduces metachronous gastric cancer after endoscopic resection of early gastric cancer. Hepatogastroenterology. 2013;60:776–780. doi: 10.5754/hge12929. [DOI] [PubMed] [Google Scholar]
- 45.Bae SE, Jung HY, Kang J, et al. Effect of Helicobacter pylori eradication on metachronous recurrence after endoscopic resection of gastric neoplasm. Am J Gastroenterol. 2014;109:60–67. doi: 10.1038/ajg.2013.404. [DOI] [PubMed] [Google Scholar]
- 46.Choi J, Kim SG, Yoon H, et al. Eradication of Helicobacter pylori after endoscopic resection of gastric tumors does not reduce incidence of metachronous gastric carcinoma. Clin Gastroenterol Hepatol. 2014;12:793–800. doi: 10.1016/j.cgh.2013.09.057. [DOI] [PubMed] [Google Scholar]
- 47.Kim YI, Choi IJ, Kook MC, et al. The association between Helicobacter pylori status and incidence of metachronous gastric cancer after endoscopic resection of early gastric cancer. Helicobacter. 2014;19:194–201. doi: 10.1111/hel.12116. [DOI] [PubMed] [Google Scholar]
- 48.Kwon YH, Heo J, Lee HS, Cho CM, Jeon SW. Failure of Helicobacter pylori eradication and age are independent risk factors for recurrent neoplasia after endoscopic resection of early gastric cancer in 283 patients. Aliment Pharmacol Ther. 2014;39:609–618. doi: 10.1111/apt.12633. [DOI] [PubMed] [Google Scholar]
- 49.Lee YC, Chen TH, Chiu HM, et al. The benefit of mass eradication of Helicobacter pylori infection: A community-based study of gastric cancer prevention. Gut. 2013;62:676–682. doi: 10.1136/gutjnl-2012-302240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li WQ, Ma JL, Zhang L, et al. Effects of Helicobacter pylori treatment on gastric cancer incidence and mortality in subgroups. J Natl Cancer Inst. 2014;106:dju0116. doi: 10.1093/jnci/dju116. doi:10.1093/jnci/dju116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Houghton J, Wang TC. Helicobacter pylori and gastric cancer: A new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005;128:1567–1578. doi: 10.1053/j.gastro.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 52.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 53.Correa P. The epidemiology of gastric cancer. World J Surg. 1991;15:228–234. doi: 10.1007/BF01659057. [DOI] [PubMed] [Google Scholar]
- 54.Fuccio L, Zagari RM, Minardi ME, Bazzoli F. Systematic review: Helicobacter pylori eradication for the prevention of gastric cancer. Aliment Pharmacol Ther. 2007;25:133–141. doi: 10.1111/j.1365-2036.2006.03183.x. [DOI] [PubMed] [Google Scholar]
- 55.Lee YC, Chiang TH, Chou CK, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: A systematic review and meta-analysis. Gastroenterology. 2016;150:1113–1124. doi: 10.1053/j.gastro.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 56.Rokkas T, Pistiolas D, Sechopoulos P, Robotis I, Margantinis G. The long-term impact of Helicobacter pylori eradication on gastric histology: A systematic review and meta-analysis. Helicobacter. 2007;12(Suppl 2):32–38. doi: 10.1111/j.1523-5378.2007.00563.x. [DOI] [PubMed] [Google Scholar]
- 57.Wang J, Xu L, Shi R, et al. Gastric atrophy and intestinal metaplasia before and after Helicobacter pylori eradication: A meta-analysis. Digestion. 2011;83:253–260. doi: 10.1159/000280318. [DOI] [PubMed] [Google Scholar]
- 58.Malfertheiner P, Megraud F, O’Morain CA, et al. European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 59.Kim N, Park YS, Cho SI, et al. Prevalence and risk factors of atrophic gastritis and intestinal metaplasia in a Korean population without significant gastroduodenal disease. Helicobacter. 2008;13:245–255. doi: 10.1111/j.1523-5378.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 60.Liu CY, Wu CY, Lin JT, Lee YC, Yen AM, Chen TH. Multistate and multifactorial progression of gastric cancer: Results from community-based mass screening for gastric cancer. J Med Screen. 2006;13(Suppl 1):S2–S5. [PubMed] [Google Scholar]
- 61.Shin CM, Kim N, Yang HJ, et al. Stomach cancer risk in gastric cancer relatives: Interaction between Helicobacter pylori infection and family history of gastric cancer for the risk of stomach cancer. J Clin Gastroenterol. 2010;44:e34–e39. doi: 10.1097/MCG.0b013e3181a159c4. [DOI] [PubMed] [Google Scholar]


