Abstract
Background
Patients with inflammatory bowel disease (IBD) are often immunosuppressed and are at risk for reactivation of latent cytomegalovirus (CMV) infection. We examined the diagnostic yield from colon biopsies in IBD patients with suspected CMV infection.
Methods
Patients above 18 years of age who underwent testing for CMV on colon biopsies between January 1st, 2012, and December 31st, 2015, were identified from a pathology data base. A positive CMV result was included only if testing included both hematoxylin/eosin staining and immunohistochemistry from two or more biopsy samples.
Results
One hundred twenty-five patients met the inclusion criteria. Of these, 99 had a diagnosis of IBD: 30 with Crohn’s disease, 63 with ulcerative colitis, and 6 with indeterminate colitis. As regards treatment, 21.2% of the patients had biologic therapy alone, 13.1% received immunomodulators, and 11.1% were treated with combined biologic and immunomodulator therapy within 3 months of the colon biopsy. In addition, 32.3% of the patients were on steroids. Of the 99 IBD patients, only 1 had biopsy-proven CMV colitis.
Conclusion
The yield from colon biopsies with hematoxylin/eosin staining and immunohistochemistry to test for CMV in IBD flare is very low. Further multicenter studies with large numbers of patients are needed to compare all testing modalities in the same cohort of patients. This may help identify which subgroup of IBD patients are likely to benefit from specific modalities of CMV testing, with potential cost-saving implications.
Keywords: Cytomegalovirus, inflammatory bowel disease, biopsy, immunosuppression
Introduction
Despite the known association between inflammatory bowel disease (IBD) flare-up and cytomegalovirus (CMV) reactivation, the extent of the clinical impact of this association is still being debated [1]. Several studies and meta-analyses have suggested a deleterious role of CMV reactivation in IBD flare-up. On the other hand, other studies have described CMV as a simple marker of inflammation of the gastrointestinal tract, and considered it as an “innocent bystander” [2].
CMV is classified under the Herpes viridae family and has a tropism for inflammatory tissue. After initial infection, it enters a latent phase that lasts the lifetime of the host [3]. Patients with IBD are often immunosuppressed. This is the result of multiple factors, including malnutrition, immunosuppressive therapy and impairment of immune function. As a result, IBD patients are at risk for reactivation of latent CMV infection [4]. The first case of ulcerative colitis (UC)-associated CMV infection was reported in 1961 in a patient with viral cytoplasmic inclusions on colon biopsies [5].
Various methods are used to detect CMV reactivation. Quantitative whole-blood and plasma CMV polymerase chain reaction (PCR), as well as histological analysis of colon biopsies have shown a powerful correlation with reactivation [6,7]. They are often considered the gold standard [8]. The second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in IBD recommended the use of immunohistochemistry and tissue PCR to detect the virus in immunomodulatory refractory IBD patients [9]. Screening for CMV infection is not required before starting immunomodulator therapy. In patients with acute colitis unresponsive to steroids, CMV should be excluded, preferably by tissue PCR or IHC, before therapy is adjusted [9]. A viral load of >250 copies/mg has been shown to be a predictor of steroid-resistant disease [10].
In this study, we evaluated the yield of CMV testing on colon biopsies in a single tertiary care center with a large cohort of IBD patients.
Patients and methods
We conducted a retrospective review of pathology reports of colon biopsies tested for CMV between January 2012 and December 2015. A positive CMV test was included if identification was based on both hematoxylin and eosin (H&E) staining, and immunohistochemistry (IHC) from two or more levels of a biopsy sample. Patients were identified by a search of the anatomical pathology computer software Sunquest CoPathPlus v4.1.1, using the terms CMV, cytomegalovirus, colon, large intestine, large bowel, small intestine, small bowel, positive cytopathic effect, colitis, UC, and Crohn’s disease.
All patients (IBD and non-IBD) aged over 18 years who had CMV testing on colon biopsies during the study period were included. Prisoners and pregnant patients were excluded from the study. The medical records of patients who met the inclusion criteria were reviewed. Clinical and demographic data were collected, including age, sex, ethnicity and type of IBD. Additional data were collected on IBD-related surgical complications, as well as comorbidities including chronic kidney disease, liver cirrhosis and human immunodeficiency virus infection.
Previous and current biologic therapy (vedolizumab, anti-tumor necrosis factor-α agents), immunomodulators, and steroid use within three months of the biopsy was also documented.
The study was approved by the Institutional Review Board of Allegheny Health Network.
Statistical analysis
Statistical analysis was performed using STATA version 14 software (STATA, College Station, TX). Categorical variables are reported as number (percentage). Continuous variable are reported as mean ± standard deviation. Relationship between CMV positivity and categorical variables was analyzed using Fisher’s exact test. Two-sided P-value of 0.05 was used to determine statistical significance.
Results
A total of 125 patients who met the inclusion criteria were tested for CMV on the basis of colon biopsies between January 2012 and December 2015. Of these, 99 (79.2%) had IBD. Thirty patients had Crohn’s disease, 63 patients had UC, and 6 patients had indeterminate colitis. Table 1 summarizes the characteristics of the IBD patients. The mean age was 41.9±15.7 years and 57% of the patients were male. Of these 99 IBD patients, 21 (21.2%) were treated with biologic therapy alone, 13 (13.1%) were treated with immunomodulators alone, while 11 (11.1%) received combination therapy with biologics and immunomodulators within 3 months of the colon biopsy. Thirty-two patients (32.3%) were treated with steroids.
Table 1.
Characteristics of patients with IBD
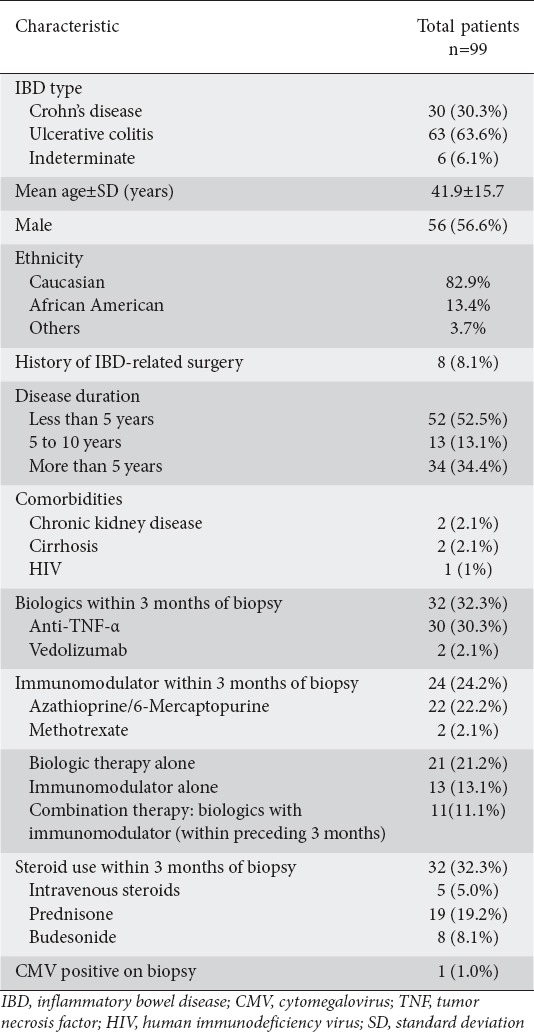
Only 1 of the 99 IBD patients (1.0%) had biopsy-proven CMV colitis. Six of the total cohort of 125 patients (4.8%) tested positive for CMV on colon biopsies. Of these 6 patients, 1 patient had UC as referenced above, 1 patient was post-renal transplant, 2 patients had hematological malignancies (chronic lymphocytic lymphoma on high-dose steroids and acute myeloid leukemia), 1 patient had graft versus host disease (GVHD), and 1 patient had ischemic colitis.
Discussion
Current clinical practice in IBD includes testing for CMV in acute flare and in IBD patients who are refractory to treatment [9,11]. Histological diagnosis is considered the “gold standard” for diagnosing end-organ disease in CMV infection (Fig. 1). IHC with monoclonal antibodies directed against CMV antigen increases the diagnostic yield of CMV compared to routine H&E staining. The sensitivity of IHC for detecting CMV infection is reported to be approximately 93%. PCR may enhance the diagnostic yield, but the specificity of this test in detecting active CMV infection is low [8].
Figure 1.
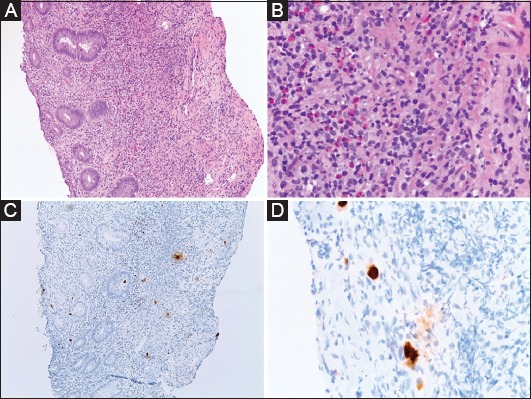
(A) Low magnification (10×), hematoxylin & eosin stain, showing fragments of severely ulcerated colon mucosa. The tissue consists of a mixture of fibrinopurulent exudate and an inflammatory infiltrate that includes lymphocytes, neutrophils, eosinophils and plasma cells. (B) High magnification (40×) multiple cells, showing enlarged nuclei with an intra-nuclear magenta inclusions. (C, D) Immunohistochemical staining (10×, 40×) for cytomegalovirus is positive on the intranuclear inclusions
There is significant discordance in yield among the various methods for CMV testing during active flare in IBD patients. In a previous study by Robiln et al, 42 patients hospitalized for moderate to severe UC on intravenous steroids were tested for CMV. IgG antibodies to CMV were present in 25 of 42 patients (59.5%). CMV DNA at a threshold of 10 copies/mg was detected in the inflamed tissue of 16 patients (38.0%). All the patients with CMV DNA in inflamed tissue were seropositive for anti-CMV IgG antibodies. None of these patients exhibited anti-CMV IgM antibodies and none of them had positive histological markers of CMV infection, tested by both H&E staining and IHC [10]. These results highlight the discordance in testing results and support the notion that CMV may be an innocent bystander in this setting. While it is tempting to infer that the addition of other testing modalities may be helpful, the specificity of PCR for detecting active infection is low as, highlighted by the negative CMV IgM antibodies in the above study.
Our study had a few limitations. It was a retrospective study from a single institution and our results may not be widely generalizable. Only one patient with IBD had positive CMV testing; thus, a subgroup analysis comparing type of IBD and immunosuppressive therapy was not performed. The low yield of CMV infection in our study population also precluded accurate comparison of CMV infection in patients with IBD versus those patients who did not have IBD. Another limitation was that, although CMV testing was performed by two methods from colon biopsy, it was not with CMV PCR and did not evaluate CMV IgG/IgM antibody status. However, as previously reported, the specificity of CMV PCR testing in active infection is low.
Matsuoka et al noted in their study that CMV reactivation in UC may resolve without antiviral treatment [2]. In their study, testing for CMV was performed in peripheral blood, which is not diagnostic of colon CMV infection [10]. We do not believe this phenomenon impacted the results noted in our study. Furthermore, 37.5% of patients with gastrointestinal CMV disease may not show inclusions on histology [12]. The addition of IHC with monoclonal antibodies directed against CMV immediate early antigen increases the diagnostic yield for CMV compared to routine H&E staining [13,14].
Previous reports have indicated an association between CMV infection and steroid use. In our study, 32 of the 99 IBD patients were treated with some form of steroids. Of these, only one patient tested positive for CMV.
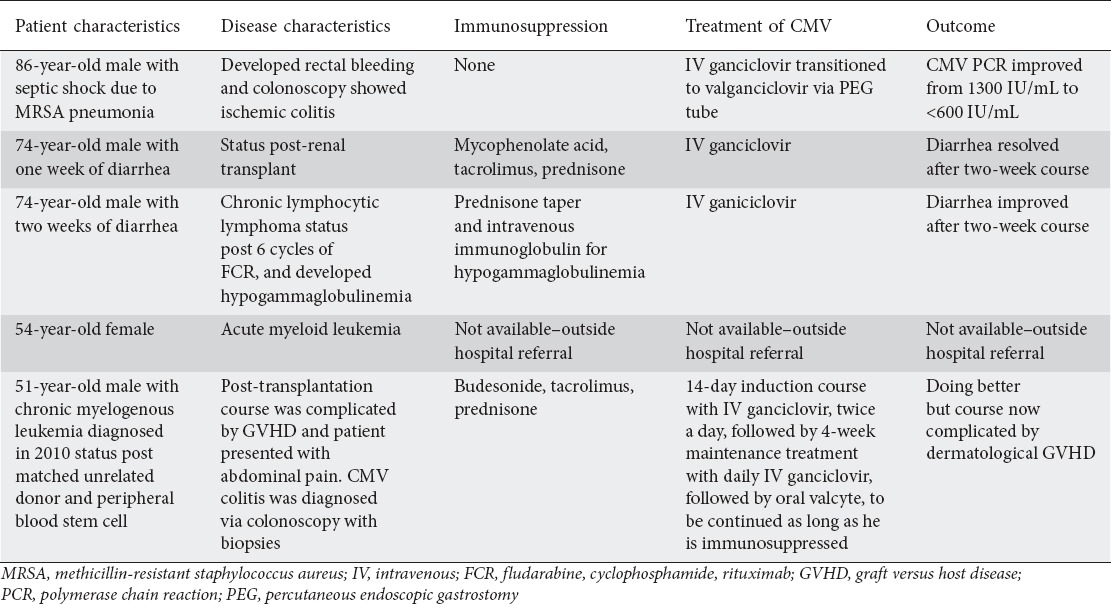
Another recent report with a larger cohort of patients (n=687), also showed that only 5.4% of patients were found to be CMV positive [15]. Although the patient population in this study was not limited to IBD patients, there was no specific comparison amongst patients with IBD versus those who did not have IBD. In our study, 5 of the 26 patients who did not have IBD tested positive for CMV infection. One patient was post-renal transplant, 2 patients had hematological malignancies (chronic lymphocytic lymphoma on high-dose steroids and acute myeloid leukemia), 1 patient had GVHD, and 1 patient had ischemic colitis. Details of the characteristics and clinical course of these patients are given in Table 2.
Table 2.
Clinical course of patients without inflammatory bowel disease found positive for cytomegalovirus (CMV)
Our study suggests that the yield from testing for CMV in patients with IBD disease flare-ups, based on colon biopsies with H&E and IHC, is very low. Further multicenter studies with large numbers of patients are needed to compare all testing modalities in the same cohort of patients. This may help identify which subgroup of IBD patients are likely to benefit from specific modalities of CMV testing, with potential cost-saving implications.
Summary Box.
What is already known:
Despite the known association between inflammatory bowel disease (IBD) flare-up and cytomegalovirus (CMV) reactivation, the extent of the clinical impact of this association is still being debated
Current guidelines recommend the use of immunohistochemistry and tissue CMV PCR to detect the virus in immunosuppressed-refractory IBD patients
Previously, some studies have suggested a deleterious role of CMV reactivation in IBD flare-up. On the other hand, other reports have described CMV as a simple marker of inflammation of the gastrointestinal tract, considering it an “innocent bystander”
What the new findings are:
Our study suggests that the yield from testing for CMV in IBD disease flare-up, based on colon biopsies with hematoxylin/eosin staining and immunohistochemistry, is low
The utility of CMV testing is only helpful when clinical suspicion is very high
Further multicenter studies with large numbers of patients are needed to compare CMV infection in patients with IBD on different treatment regimens. This may help identify which subgroup of IBD patients are likely to benefit from CMV testing, with potential cost-saving implications
Biography
Allegheny Health Network, Pittsburgh, PA; University of Texas Medical Branch, Galveston, TX, USA
Footnotes
Conflict of Interest: None
References
- 1.Pillet S, Pozzetto B, Jarlot C, Paul S, Roblin X. Management of cytomegalovirus infection in inflammatory bowel diseases. Dig Liver Dis. 2012;44:541–548. doi: 10.1016/j.dld.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Matsuoka K, Iwao Y, Mori T, et al. Cytomegalovirus is frequently reactivated and disappears without antiviral agents in ulcerative colitis patients. Am J Gastroenterol. 2007;102:331–337. doi: 10.1111/j.1572-0241.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 3.Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med. 1993;119:924–935. doi: 10.7326/0003-4819-119-9-199311010-00010. [DOI] [PubMed] [Google Scholar]
- 4.Papadakis KA, Tung JK, Binder SW, et al. Outcome of cytomegalovirus infections in patients with inflammatory bowel disease. Am J Gastroenterol. 2001;96:2137–2142. doi: 10.1111/j.1572-0241.2001.03949.x. [DOI] [PubMed] [Google Scholar]
- 5.Powell RD, Warner NE, Levine RS, Kirsner JB. Cytomegalic inclusion disease and ulcerative colitis;report of a case in a young adult. Am J Med. 1961;30:334–340. doi: 10.1016/0002-9343(61)90105-x. [DOI] [PubMed] [Google Scholar]
- 6.Yoshino T, Nakase H, Ueno S, et al. Usefulness of quantitative real-time PCR assay for early detection of cytomegalovirus infection in patients with ulcerative colitis refractory to immunosuppressive therapies. Inflamm Bowel Dis. 2007;13:1516–1521. doi: 10.1002/ibd.20253. [DOI] [PubMed] [Google Scholar]
- 7.Criscuoli V, Casa A, Orlando A, et al. Severe acute colitis associated with CMV: a prevalence study. Dig Liver Dis. 2004;36:818–820. doi: 10.1016/j.dld.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Kandiel A, Lashner B. Cytomegalovirus colitis complicating inflammatory bowel disease. Am J Gastroenterol. 2006;101:2857–2865. doi: 10.1111/j.1572-0241.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 9.Rahier JF, Magro F, Abreu C, et al. European Crohn’s and Colitis Organisation (ECCO) Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443–468. doi: 10.1016/j.crohns.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Roblin X, Pillet S, Oussalah A, et al. Cytomegalovirus load in inflamed intestinal tissue is predictive of resistance to immunosuppressive therapy in ulcerative colitis. Am J Gastroenterol. 2011;106:2001–2008. doi: 10.1038/ajg.2011.202. [DOI] [PubMed] [Google Scholar]
- 11.Kornbluth A, Sachar DB Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–523. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- 12.Cotte L, Drouet E, Bissuel F, Denoyel GA, Trepo C. Diagnostic value of amplification of human cytomegalovirus DNA from gastrointestinal biopsies from human immunodeficiency virus-infected patients. J Clin Microbiol. 1993;31:2066–2069. doi: 10.1128/jcm.31.8.2066-2069.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beaugerie L, Cywiner-Golenzer C, Monfort L, et al. Definition and diagnosis of cytomegalovirus colitis in patients infected by human immunodeficiency virus. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:423–429. doi: 10.1097/00042560-199704150-00005. [DOI] [PubMed] [Google Scholar]
- 14.Chemaly RF, Yen-Lieberman B, Castilla EA, et al. Correlation between viral loads of cytomegalovirus in blood and bronchoalveolar lavage specimens from lung transplant recipients determined by histology and immunohistochemistry. J Clin Microbiol. 2004;42:2168–2172. doi: 10.1128/JCM.42.5.2168-2172.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juric-Sekhar G, Upton MP, Swanson PE, Westerhoff M. Cytomegalovirus (CMV) in gastrointestinal mucosal biopsies: should a pathologist perform CMV immunohistochemistry if the clinician requests it? Hum Pathol. 2017;60:11–15. doi: 10.1016/j.humpath.2016.09.009. [DOI] [PubMed] [Google Scholar]


