Abstract
The circadian variation of sensory and motor symptoms with increasing severity in the evening and at night is a key diagnostic feature/symptom of the restless legs syndrome (RLS). Even though many neurological diseases have shown a strong nexus between motor and cognitive symptoms, it has remained unclear whether cognitive performance of RLS patients declines in the evening and which neurophysiological mechanisms are affected by the circadian variation. In the current study, we examined daytime effects (morning vs. evening) on cognitive performance in RLS patients (n = 33) compared to healthy controls (n = 29) by analyzing flanker interference effects in combination with EEG and source localization techniques. RLS patients showed larger flanker interference effects in the evening than in the morning (p = .023), while healthy controls did not display a comparable circadian variation. In line with this, the neurophysiological data showed smaller N1 amplitudes in RLS patients compared to controls in the interfering task condition in the evening (p = .042), but not in the morning. The results demonstrate diurnal cognitive changes in RLS patients with intensified impairments in the evening. It seems that not all dopamine-regulated cognitive processes are altered in RLS and thus show daytime-dependent impairments. Instead, the daytime-related cognitive impairment emerges from attentional selection processes within the extra-striate visual cortex, but not from later cognitive processes such as conflict monitoring and response selection.
Keywords: Restless legs syndrome (RLS), Cognition, Circadian variation, Flanker interference effect, Attentional selection, EEG
Highlights
-
•
RLS patients have larger flanker interference effect in the evening.
-
•
RLS patients have enhanced impairment of attentional selection in the evening.
-
•
Nocturnal attentional impairment relies on the extra-striate visual cortex.
-
•
Conflict monitoring and response selection are not affected by RLS.
1. Introduction
The restless legs syndrome (RLS) is a sensory-motor disorder causing a strong urge to move when staying at rest, especially in the evening and at night. In spite of the circadian variation of sensory and motor symptoms with increasing severity at night, potential associated diurnal changes of cognitive performance have not yet been investigated in RLS patients. RLS is strongly associated with a dopaminergic dysregulation and low doses of dopamine agents effectively reduce RLS symptoms (Allen et al., 2009, Cervenka et al., 2006, Clemens et al., 2006, Earley et al., 2013, Hening et al., 1999, Trenkwalder et al., 2005, Trenkwalder and Paulus, 2010). Taking into account that dopamine plays an important role in both motor (Albin et al., 1995) and cognitive functions (Nieoullon, 2002), RLS patients should presumably also suffer from cognitive dysfunction. By means of various neuropsychological tests, cognitive deficits of RLS patients in the domains of attention, verbal fluency, and executive function have been shown in previous studies (Fulda et al., 2010, Fulda et al., 2011, Pearson et al., 2006). Given that dopamine acts as a part of the circadian timing system (Domínguez-López et al., 2014, Garcia-Borreguero et al., 2004b, Kawano et al., 1990, Videnovic and Golombek, 2013, Wilkes et al., 1981), cognitive deficits of RLS patients may also vary with the time of the day. Based on the circadian pattern of motor symptoms, we assume that RLS patients have enhanced cognitive dysfunctions in the evening as compared to the morning. Cognitive processes such as selective attention, cognitive control, and response selection, are strongly modulated by dopamine (Falkenstein et al., 2006, Nieoullon, 2002, Russell et al., 1995, Sagvolden, 2000, Wylie et al., 2005, Wylie et al., 2009) and may therefore be vulnerable to the changed dopaminergic circadian rhythms in RLS patients (Earley et al., 2006, Garcia-Borreguero et al., 2004a) and consequentially show strong timing effects/fluctuations during the day. To test this hypothesis, merely applying standard neuropsychological tasks would be problematic as it would remain unclear which underlying neurophysiological mechanisms are impaired in RLS patients. EEG and event-related potentials (ERPs) provide an excellent approach to this problem. Using EEG, Jung et al. (Jung et al., 2011) found attentional dysfunction in RLS patients compared to healthy controls, but this study did not provide insights into the underlying functional neuroanatomical structures and no hints for possible circadian effects.
The aim of the current study was to determine which cognitive neurophysiological sub-processes within the cascade from early attentional stimulus processing to response selection mechanisms are modulated by the disease-relevant circadian rhythm of RLS patients and which functional neuroanatomical networks contribute to this daytime effect.
Therefore, we combined EEG with source localization techniques (i.e., sLORETA) and examined daytime effects (morning vs evening) on cognitive functions in RLS patients compared to healthy controls. Dopamine-regulated cognitive processes such as selective attention, conflict monitoring and response selection can be examined by means of a flanker task (Eriksen and Eriksen, 1974, Wylie et al., 2009). The flanker task has been proved to be well-suited to investigate dopamine-related cognitive functions (Beste et al., 2008a, Beste et al., 2008b, Beste et al., 2015, Beste et al., 2017). Here, selective attentional processing is necessary to select task-relevant information and to suppress distracting information (Beste et al., 2008a, Cagigas et al., 2007). As conflict arises in the condition with distracting information, effective conflict monitoring is required for correct response selection as the response tendencies caused by flankers need to be suppressed (Eimer et al., 1995, Ridderinkhof et al., 1995, Wylie et al., 2009). Previous studies have reported that Parkinson patients, who also suffer from dopaminergic dysfunctions, showed an increased flanker interference effect (Praamstra et al., 1998, Praamstra et al., 1999, Willemssen et al., 2011, Wylie et al., 2009). This suggests that RLS patients should also show increased interference effects and that this impairment should be stronger in the evening.
An increased impairment in attentional selection reflected by the N1 ERP (Beste et al., 2010, Herrmann and Knight, 2001, Hillyard and Anllo-Vento, 1998, Luck et al., 2000, Schneider et al., 2012) as well as later cognitive processes such as conflict monitoring reflected by the N2 (Folstein and Van Petten, 2008, Kopp et al., 1996, Tillman and Wiens, 2011) and stimulus-response mapping reflected by the P3 (Verleger et al., 2005) might be found among RLS patients in the evening. Furthermore, a reduced D2 receptor binding has been found in RLS patients (Michaud et al., 2002, Staedt et al., 1993, Staedt et al., 1995a, Staedt et al., 1995b, Turjanski et al., 1999). As D2 autoreceptors are especially sensitive in the evening (Domínguez-López et al., 2014) and blockade of D2 receptors reduces acetylcholine efflux (Moore et al., 1999), a reduced D2 receptor binding in RLS patients can exert a strong influence on acetylcholine distribution in the evening. Since cholinergic activity directly contributes to the modification of receptive field properties or the suppression of contextual information, a marked impact on early attentional processes reflected by P1 and N1 may be evident in RLS patients. Apart from this, the extension of the dopaminergic cortical innervation in the rostro-caudal direction is related to the cognitive capacities such as sensorimotor integration (Nieoullon, 2002) and this anterior-posterior communication is impaired in RLS patients (Choi et al., 2012). Based thereon, the early attentional processes which are associated with posterior areas are likely to be more severely concerned than cognitive processes, which are related to frontal areas.
2. Methods
2.1. Patients and controls
N = 33 adult patients with a stable medication treatment for at least 4 weeks were recruited from the Sleep disorders outpatient clinic of the Department of Neurology, Carl Gustav Carus University hospital in Dresden, Germany. The patients had a confirmed RLS diagnosis based on the International Restless Legs Syndrome Study Group (IRLSSG) diagnostic criteria (Allen et al., 2014) and showed no other neurological or psychiatric diseases. N = 33 individually age- (± 3 years) and gender-matched healthy control participants were recruited for comparison with the RLS patients. The control group was free of any neurological or psychiatric diseases. Patients and controls were tested in the morning (beginning between 8 and 9 am) and in the evening (beginning between 5 and 6 pm). The time of the first appointment (first testing in the evening or morning) was randomly assigned to the patient group an 1:1 matched controls were assigned to the same order as their RLS counterpart. The time between the two appointments was maximal one week to make sure that the individual performance variation between two appointments could only marginally be affected by disease progression. Four controls had to be excluded due to data quality problems. In total, the data of 33 patients (age: 65.21 ± 1.78, 25 female, 19 patients had their first appointment in the morning) and 29 controls (age: 64.41 ± 1.60, 22 female, 18 controls had their first appointment in the morning) were analyzed. Of note, we made efforts to balance the order of appointments so that daytime effects would not be confounded with learning effects. n = 25 or 75.8% of RLS subjects were under dopaminergic treatment with Levodopa or dopamine agonists. Dopaminergic medication was discontinued at least 24 h prior to each appointment to minimize effects of the medication. Given that all of the patients took their RLS-medication only in the evening, they typically took the last dosage about 36 h prior to the morning appointment and at about 24 h prior to their evening appointments. For prolonged release medication like L-Dopa/benserazide retarde or rotigotine patches, we asked the patients to abstain from using them for at least 24 h prior to each appointment. Due to compliance issues and patient safety, no RLS patient was asked to abstain from their RLS medication for > 3 days. The n = 8 or 24.2% of the patients who took opioids or antidepressants, were not to discontinue during the study due to safety reasons. Patients who took benzodiazepines were not included in this study to avoid potential confounding by manipulations of other neurotransmitter systems that are potentially involved in RLS (Winkelman et al., 2014). RLS patients that reported ever having experienced RLS symptoms before noon, or reported RLS symptoms during the morning appointment indicating treatment-related augmentation were excluded from the study. The study was approved by the institutional review board of the Medical faculty of the TU Dresden in Germany (EK 27012014) and conducted in accordance with the declaration of Helsinki. All participants received a reimbursement of 60 € for taking part in the study.
2.2. Questionnaires and neuropsychological assessment
The International RLS Study Group RLS Rating Scale (IRLS) was used to measure the severity of RLS symptoms (Walters et al., 2003) in patients. All participants were required to complete the Beck Depression Inventory (BDI) (Beck et al., 1961) and the fatigue scale for motor and cognitive functions (FSMC) (Penner et al., 2009). A self-assessment Morningness-Eveningness questionnaire (MEQ) was used to evaluate inter-individual differences in the circadian rhythms (Horne and Ostberg, 1976). In addition, sleep duration and sleep quality prior to each appointment were rated by participants. Sleep quality was rated by means of a three-level Likert item (“poor”, “moderate”, “good” with a distributed value of − 1, 0, and 1, respectively). This study's neuropsychological battery included the following tests: 1). Verbal learning and memory retention test (VLMT) involving immediate retention and long-term verbal memory (C. Helmstaedter et al., 2001) 2) Test d2-Revision (Brickenkamp et al., 2010) assessing selective and sustained attention. 3) The stroop color and word test determining the individual's cognitive flexibility (Jensen and Rohwer, 1966). 4) Benton visual retention test assessing visual perception and visual memory (Benton, 1945).
2.3. EEG task
To measure attentional and conflict monitoring processing, a flanker task (Kopp et al., 1996) was applied. In this task, three vertically arranged stimuli were presented. The target stimulus (arrowhead) was presented in the center pointing either to the left or right. It was flanked by two adjacent, vertically aligned arrowheads (one above and one below the target) that pointed either in the same (compatible) or in the opposite (incompatible) direction as the target (see Fig. 1). The subjects had to determine the direction of the target stimulus (the central arrowhead) by pressing the left and right Ctrl buttons on a regular computer keyboard using their left and right index fingers. Compatible (75%) and incompatible stimuli (25%) were presented randomly. To exert time pressure, a warning tone was presented if the subjects did not respond within 450 ms. The flankers preceded the target by 200 ms. The target was then presented for 300 ms and simultaneously switched off together with the flankers. A fixation cross was presented at the center of the screen during the response-stimulus interval, which was randomly varied between 900 and 1300 ms. The experiment consisted of 384 trials divided into 4 equally sized blocks. Participants were encouraged to respond as quickly and accurately as possible.
Fig. 1.
Experimental paradigm. Each trial began with the presentation of two flanker stimuli both pointing either to the left or right. After 200 ms, the target-stimulus was then presented in the center for 300 ms and simultaneously switched off together with the flankers. Flankers and target pointed either in the same (compatible) or in the opposite (incompatible) direction. The subjects had to determine the direction of the target-stimulus (the central arrowhead) by pressing the left and right Ctrl-buttons. Compatible (75%) and incompatible stimuli (25%) were presented randomly. The response-stimulus interval was randomly varied between 900 and 1300 ms.
2.4. EEG recording and analysis
The EEG was recorded from 60 Ag–AgCl electrodes at equidistant positions with a sampling rate of 500 Hz. The reference electrode was located at Fpz and the ground electrode was located at θ = 58, ф = 78. Electrode impedances were kept below 5 kΩ. The recorded data were down-sampled off-line to 256 Hz using spline interpolation and a band-pass filter from 0.5 to 20 Hz with a slope of 48 db/oct each was applied. A manual raw data inspection was implemented to remove technical artifacts and irregular facial movement artifacts, while periodically occurring artifacts such as pulse artifacts, horizontal and vertical eye movements were subsequently detected and corrected using an independent component analysis (ICA; infomax algorithm). Afterwards, flanker-locked segments of trials with correct responses were separately formed for all conditions. Segments started 200 ms prior to the locking point (flanker onset) and ended 1200 ms thereafter. Next, an automated artifact rejection procedure was applied to remove all the segments the amplitudes of which were below − 100 μV or above 100 μV, or which had value differences of > 200 μV in a 200 ms interval, or < 0.5 μV in a 100 ms interval. After that, a current source density (CSD) transformation was applied to eliminate the reference potential from the data (Perrin et al., 1989). Aside from eliminating the reference potential, the CSD transformation is known to serve as a spatial filter (Nunez and Pilgreen, 1991), which attenuates possible effects of volume conduction (Cohen, 2014, Vidal et al., 2015) and helps to identify electrodes best reflecting different ERPs (Nunez and Pilgreen, 1991, Tenke and Kayser, 2012). A baseline correction was then set to a time interval from − 200 ms to 0 ms before the segments were separately averaged for each condition. After that, electrodes P7, P8, P9, P10, Cz, PO1, and PO2 were selected on the basis of the scalp topography of the different ERP components. All ERP components were quantified by extracting the mean amplitude of brief time intervals centered around the respective peaks. The P1 and N1 ERPs were quantified at electrodes P7, P8, P9 and P10 following the flanker (P1: 90–100 ms; N1: 155–170 ms) and following the target stimulus (P1: 310–320 ms; N1: 400–430 ms). At electrode Cz, the N2 ERPs were quantified by extracting the mean amplitude of the time interval from 520 ms to 550 ms. At electrodes PO1 and PO2, the P3 ERPs were quantified by using the time interval from 510 ms to 540 ms. All ERP components were quantified relative to the baseline. The choice of electrodes was statistically validated using the method used by Mückschel et al. (Mückschel et al., 2014). This procedure revealed the same electrodes as identified by visual inspection.
To identify functional neuroanatomical structures that are (differentially) modulated by daytime effects and the experimental conditions in RLS patients, we used sLORETA (standardized low resolution brain electromagnetic tomography) (Pascual-Marqui, 2002). sLORETA reveals high convergence with fMRI data and neuronavigated EEG/TMS studies, which underlines the validity of the sources estimated using sLORETA (Dippel and Beste, 2015, Sekihara et al., 2005). sLORETA gives a single linear solution to the inverse problem based on extra-cranial measurements without a localization bias (Pascual-Marqui, 2002, Sekihara et al., 2005). For sLORETA, the intracerebral volume is partitioned into 6239 voxels at 5 mm spatial resolution. The standardized current density at each voxel is calculated in a realistic head model (Fuchs et al., 2002) using the MNI152 template (Mazziotta et al., 2001). In this study, the voxel-based sLORETA images were compared between patients and controls using the sLORETA-built-in voxel-wise randomization tests with 2000 permutations, based on statistical nonparametric mapping (SnPM). Voxels with significant differences (p < 0.01, corrected for multiple comparisons) between contrasted conditions were located in the MNI-brain.
2.5. Statistical analysis
Independent t-tests were used to compare psychometric scores of patients and controls (BDI, MEQ, FSMC, sleep duration and quality). Data assessed by the neuropsychological battery and behavioral as well as neurophysiological data of the EEG task were analyzed using separate mixed effects ANOVAs comprising the within-subject factors daytime (morning vs. evening), condition (compatible vs. incompatible – wherever applicable), and electrode (wherever applicable). Group (patients vs. controls) and first appointment (participants whose first appointment was in the morning vs. in the evening) were used as between-subjects factors. Separate ANOVAs were calculated for each behavioral and neurophysiological measure. Greenhouse–Geisser correction was applied whenever necessary. Values are provided as means ± SEMs. Post-hoc tests were Bonferroni-corrected whenever necessary.
3. Results
We compared the behavioral and neurophysiological data obtained from our flanker task between RLS patients and healthy controls to investigate which cognitive processes and their underlying neurophysiological mechanisms were impaired in RLS patients. Since our focus was on group differences, we report all effects, which did not include the group factor (i.e. group-unrelated main effects and interactions) in the supplement (see supporting information).
3.1. Questionnaires and neuropsychological assessment
Clinical characteristics of the patients including the neuropsychological data are shown in Table 1.
Table 1.
subject characteristics, neuropsychological scores for RLS patients and controls, and p-value for group comparision.
| RLS (n = 33) |
Control (n = 29) |
Group difference (P-value) |
|
|---|---|---|---|
| Age | 65.21 ± 1.78 | 64.41 ± 1.60 | |
| First appointment (In the morning %) |
57.6% | 62.1% | |
| Sex (Female %) | 75.8% | 75.9% | |
| RLS medication | Levodopa, Pramipexol, Ropinirol, Rotigotin | ||
| IRLS | 26.42 ± 1.24 (severe RLS symptoms) |
||
| Sleep duration (hour) | 5.61 ± 0.25 | 7.03 ± 0.15 | p < 0.001 |
| Sleep quality | 0.00 ± 0.10 | 0.67 ± 0.09 | p < 0.001 |
| BDI | 11.14 ± 1.43 | 4.13 ± 0.71 | p < 0.001 |
| FSMC (total) | 52.39 ± 3.02 | 31.93 ± 2.00 | p < 0.001 |
| FSMC (cognitive) | 26.31 ± 1.54 | 15.07 ± 0.96 | p < 0.001 |
| FSMC (motoric) | 26.09 ± 1.65 | 16.86 ± 1.10 | p < 0.001 |
| MEQ | 60.44 ± 1.38 | 58.68 ± 2.25 | p = 0.494 |
| Stroop word (msec) | 14.90 ± 0.46 | 14.86 ± 0.49 | p = 0.957 |
| Stroop color (msec) | 20.84 ± 0.56 | 21.03 ± 0.60 | p = 0.819 |
| Stroop conflict (msec) | 41.49 ± 2.24 | 37.10 ± 2.40 | p = 0.186 |
| d2-R | 123.00 ± 5.33 | 141.01 ± 5.65 | p = 0.024 |
| Benton | 12.45 ± 0.25 | 12.56 ± 0.31 | p = 0.797 |
| VLMT-reproduction (working memory) |
11.06 ± 0.35 | 12.01 ± 0.37 | p = 0.067 |
| VLMT-reproduction (long-term memory) |
3.21 ± 0.32 | 3.15 ± 0.35 | p = 0.903 |
| VLMT-reorganization (long-term memory) |
12.46 ± 0.32 | 12.97 ± 0.35 | p = 0.287 |
| VLMT-reproduction (vulnerability to interference) |
3.60 ± 0.35 | 3.23 ± 0.37 | p = 0.472 |
IRLS: International RLS Rating Scale; BDI: Beck Depression Inventory; FSMC: Fatigue Scale for Motor and Cognitive functions; MEQ: Morningness-Eveningness Questionaire. Lower scores represent greater eveningness and higher scores represent greater morningness; VLMT: Verbal Learning and Memory retention Test.
3.2. Behavioral data
For the accuracy/errors in percent, no group-related effects were found (all F < 3.02; all p > 0.088).
For the RTs, the mixed effects ANOVA revealed an interaction effect of “daytime x condition x group” (F(1,57) = 4.62; p = 0.036; η2 = 0.075) showing that the interaction of “daytime x condition” was only found in the patient group (F(1,32) = 5.69; p = 0.023; η2 = 0.151), but not in controls (F(1,27) = 0.29; p = 0.595; η2 = 0.011). Further analyses for the patients showed that there was a larger condition difference in the evening (incompatible-compatible: 81 ms ± 6) than in the morning (incompatible-compatible: 73 ms ± 6) (t(32) = 2.39; p = 0.023). No other significant group-related effects were found (all F < 3.62; all p > 0.062). To rule out that the group related effects were based on motor restrictions or sleep disturbances observed in the RLS patients, we calculated correlations between IRLS scores, fatigue, sleep duration, and sleep quality of the patients with their RTs. No significant correlations were found (all | r | < 0.30, all p > 0.084; Bonferroni-corrected significance threshold here is p = 0.006). Regarding the neuropsychological test, group differences were only found in the d2-R test (see Table 1). Interestingly, the RTs in the flanker task were significantly correlated with the d2-R performance scores. Participants who had higher scores in the d2-R test also responded faster in the flanker task (r = − 0.331, p = 0.010). Taken together, only RLS patients showed larger RT-based condition differences (compatible vs incompatible) in the evening than in the morning.
Given that the RLS patients were receiving different types of medical treatment, which might have potentially biased their behavioral performance, we furthermore conducted a Kruskal-Wallis tests to check whether there were any differences in accuracy or RTs between different treatment types. Contrasting L-Dopa (n = 7), dopamine agonists (n = 8), multiple pharmacological treatments for RLS (n = 10), and no pharmacological treatment (n = 7). Importantly, we found no significant differences/medication effects in any of the tested behavioral measures (all p ≥ 0.408).
3.3. Neurophysiological data of the flanker task
3.3.1. Early attentional processing
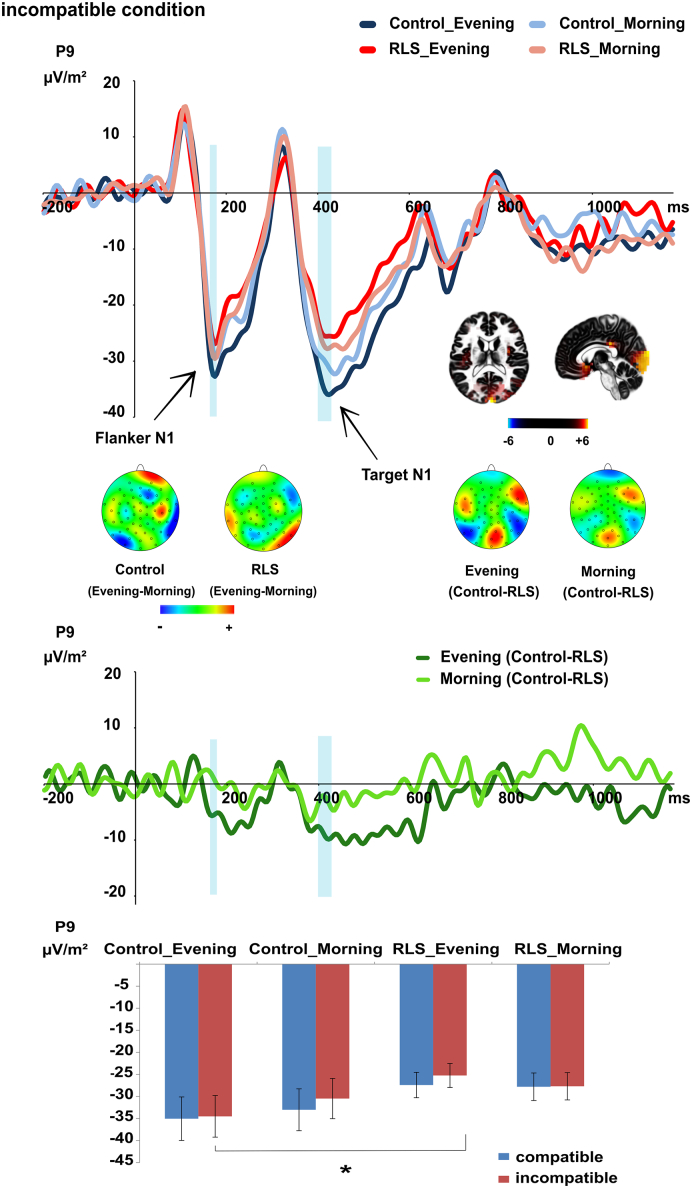
The P1 and N1 ERPs are shown in Fig. 2.
Fig. 2.
The N1 ERP evoked by the incompatible condition at electrode P9. Time point zero denotes the onset of the flanker stimuli; the target stimulus was presented 200 ms later. The flanker-elicited N1 showed a daytime effect (evening > morning) in the healthy controls but not in the RLS patients. The target-elicited N1 showed a significant group difference (controls > RLS patients) in the evening but not in the morning. This daytime-related group difference was rooted in extra-striate-visual cortex (BA 18). Group difference curves calculated for morning and evening appointments are depicted below in dark (evening) and light (morning) green separately. As shown in the middle of the figure, the group difference (controls-RLS) was larger in the evening (dark green) than in the morning (light green). The time intervals used for quantification of the flanker- and target-elicited N1 are denoted in semi-transparent blue color. The mean values and standard errors of the target N1 at electrode P9 for all conditions are plotted in a bar chat. Significant comparison is pointed out with *. For a comprehensive figure of P1 and N1 ERPs evoked by the flanker and target stimuli at all electrodes P7/P8/P9/P10 (mean value) and in all conditions (incompatible vs compatible) please refer to the supplemental material Fig. S1. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
For the flanker-elicited P1 at electrodes P7/P8/P9/P10, no group-related effects were significant (all F < 3.55; all p > 0.065). For the flanker-elicited N1 at electrodes P7/P8/P9/P10, an interaction of “daytime x group” (F(1,57) = 4.50; p = 0.038; η2 = 0.073) was found. While healthy controls had a larger flanker N1 amplitude in the evening (− 41.78 μV/m2 ± 4.20) than in the morning (− 38.19 μV/m2 ± 4.06) (t(28) = − 2.09; p = 0.046), patients did not show such daytime effects (t(31) = 1.29; p = 0.208; morning: − 37.19 μV/m2 ± 4.28; evening: − 34.62 μV/m2 ± 4.03). No other group-related effects were significant (all F < 2.17; all p > 0.094).
Analyzing the target-elicited P1 at electrodes P7/P8/P9/P10, no group-related effects were significant (all F < 2.63; all p > 0.052). For the target-evoked N1 at electrodes P7/P8/P9/P10, an interaction of “daytime x group x condition x electrode” (F(3171) = 2.70; p = 0.047; η2 = 0.045) was found showing an interaction effect of “daytime x group x condition” at electrode P9 (F(1,59) = 4.44; p = 0.039; η2 = 0.070), but no interactions at other electrodes (all F < 1.43; all p > 0.237). At electrode P9, an independent t-test revealed that controls (− 34.52 μV/m2 ± 4.72) had larger target N1 amplitudes than patients (− 25.25 μV/m2 ± 2.72) only in the incompatible condition in the evening (t(60) = − 1.75; p = 0.042) (all other comparisons: all | t | ≤ 1.38; p ≥ 0.085). The sLORETA analysis revealed that this difference between the patient group and the control group in the incompatible condition in the evening was due to activity differences in the extra-striate visual cortex (BA18), where controls had a larger activation than RLS patients. No other group-related effects were significant (all F < 3.42; all p > 0.069).
3.3.2. Conflict processing
The N2 ERPs are shown in Fig. 3.
Fig. 3.
The N2 ERP at electrode Cz (a). The N2 showed a significant daytime effect (morning > evening). Condition difference curves (incompatible-compatible) separately calculated for controls and RLS patients in the morning as well as in the evening appointments (b). The condition differences did not vary between groups or between daytimes. The observed condition differences in RLS patients were comparable to controls in the morning as well as in the evening. The time interval used for quantification of the target-related N2 is denoted in semi-transparent blue color. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
For the N2 at electrode Cz, an interaction effect of “condition x group x daytime x first appointment” (F(1,57) = 4.41; p = 0.040; η2 = 0.072) was found showing an interaction of “condition x daytime x group” among participants who had their evening appointment first (F(1,23) = 5.65; p = 0.026; η2 = 0.197), but not in participants who had their first appointment in the morning (F(1,34) = 0.41; p = 0.529; η2 = 0.012). Further analyses for the participants who had their first appointment in the evening revealed that only healthy controls had a larger target N2 in the incompatible condition (− 23.68 μV/m2 ± 3.92) than the compatible condition (− 14.33 μV/m2 ± 4.05) in the morning (at their second appointment) (t(10) = 2.99; p = 0.014) but not in the evening (at their first appointment) (t(10) = 1.06; p = 0.313). In contrast, patients did not show any condition differences in the morning (at their second appointment) (t(10) = 1.09; p = 0.297) or in the evening (at their first appointment) (t(10) = 0.621; p = 0.546) In short, only healthy controls who had their first appointment in the evening showed a condition difference (incompatible > compatible) at their second appointment. No other significant group-related effects were revealed (all F < 3.99; all p > 0.051). For the N2, Bayesian analysis was performed to further confirm the lack of the key interaction of “daytime x group x condition”. Other than regular ANOVAs, Bayesian analyses reveal the probability of the null hypothesis being true, given the observed data (Masson, 2011, Wagenmakers, 2007). Given the data D obtained in this study, this possibility was p(H0 | D) = 78%, which provides positive evidence for the null hypothesis holding true according to the criteria provided by Raftery (Raftery, 1995).
3.3.3. Stimulus evaluation, response selection, and context updating
The P3 ERPs are shown in Fig. 4.
Fig. 4.
The P3 ERP at electrodes PO1/PO2 (mean value) (a). The P3 revealed a significant condition effect (incompatible < compatible). Condition difference curves (incompatible-compatible) separately calculated for controls and RLS patients in the morning as well as in the evening appointments (b). The condition differences did not vary between groups or between daytimes. The observed condition differences in RLS patients were comparable to controls both in the morning and in the evening. The time interval used for quantification of the target-associated P3 is denoted in semi-transparent blue color. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Analyzing the P3 at electrodes PO1/PO2, no group-related effects were found (all F < 2.86; all p > 0.096). For the P3, the Bayesian analysis revealed that the probability of the null hypothesis being true, given the obtained data, was p(H0 | D) = 88%, which also supports the null hypothesis that the interaction of “daytime x group x condition” was not present (Raftery, 1995).
4. Discussion
In the current study, we examined whether and how circadian variations affect cognitive processes in RLS patients, putting a focus on the modulated neurophysiological mechanisms. Given that an essential diagnostic feature of RLS is the presence of circadian symptom variations, we hypothesized that like the motor symptoms, cognitive deficits of RLS patients might be intensified in the evening. Our behavioral data showed that the RLS patients suffered from a cognitive decline in the evening, which could not be explained by RLS severity, fatigue, or impaired sleep quality. Also, the medication of the participants did not cause any significant performance differences within the patient group. RLS patients showed larger RT differences between the incompatible and the compatible condition (i.e. a larger flanker interference effect) in the evening than in the morning while healthy controls did not display a comparable circadian variation. Based thereon, the allocation of selective attention to relevant information, conflict monitoring or response control, which were all required in the interfering task condition, could be strongly impaired among RLS patients in the evening. Besides, RTs in the flanker task were significantly correlated to performance scores of the d2-R test, in which participants were required to select relevant stimuli and to ignore distractors. As the task requirement of d2-R test is similar to that of the flanker task, in which participants were asked to focus on the target and to suppress the attention to flanker stimuli surrounding it the correlation between two task performances may provide important hint that the decreased performance of RLS patients in the evening strongly relies on attentional selection. As RLS patients did not show a general slowing of RTs in the evening, the poor performance of the patients in the evening could not result from motor restrictions or dysfunctions associated with RLS (as further underpinned by the lack of significant correlations between RLS symptom severity and behavioral measures). This interpretation is supported by our neurophysiological data, which showed that controls had a larger target N1 than the RLS patients in the incompatible condition when the task was conducted in the evening. The N1 has been reported to reflect attentional selection processes such as focusing on task-relevant stimuli (Herrmann and Knight, 2001, Hillyard and Anllo-Vento, 1998, Luck et al., 1990). The smaller N1 indicates that the attentional selection processes of RLS patients were impaired in the evening. This is consistent with previous findings that attentional selection is more demanding in conflict situations where adjustments in attentional selection processes help to resolve conflicts (Botvinick et al., 2001, Chmielewski et al., 2014). The source localization analysis revealed that the group differences between RLS patients and controls observed in the evening resulted from smaller activation of the extra-striate visual cortex (BA18), which contributes to selecting the important and filtering out the irrelevant information (Desimone, 1998, Herrmann and Knight, 2001, Kastner et al., 1998), in RLS patients compared to controls. Furthermore, attentional impairments shown by patients in the evening were supported by the daytime-related group differences reflected by the flanker N1. A general condition-independent circadian variation was only found in the healthy controls. The controls had a larger N1 in the evening than in the morning indicating that attentional involvement was intensified to compensate reduced alertness in the evening (Blatter and Cajochen, 2007, Dijk et al., 1992), so that the performance in the evening could still equal that of the morning. In contrast, RLS patients have seemingly failed to enhance attentional processes to achieve such compensation in the evening, ultimately resulting in worse performance in the evening than in the morning. Since there were no group differences in terms of MEQ scores (see Table 1), we rejected the alternative explanation that the performance impairments of RLS patients in the evening were due to chronotype differences. It is possible that the poor cognitive performance of the RLS patients in the evening could be due to dopaminergic dysfunction (Kähkönen et al., 2002, Nieoullon, 2002, Shine et al., 2011). Matching this, studies show that the early attentional processing in the extra-striate cortex can be top-down regulated by frontal cortex (Barceló et al., 2000, Herrmann and Knight, 2001). As striatal dopamine modulate the fontal activity through distinct cortico-basal ganglia circuits (Chudasama and Robbins, 2006, Haber, 2016), dopamine may indirectly affect the early attentional modulation. Aside from this, striatal dopamine may directly affect visual processing through possible connections with the visual cortex (Beste et al., 2008a, Silkis, 2007). Dopamine is involved in regulating circadian rhythms (Domínguez-López et al., 2014, Garcia-Borreguero et al., 2004b, Kawano et al., 1990, Videnovic and Golombek, 2013, Wilkes et al., 1981) and the diurnal variation of dopamine is characterized by a peak in the morning and nadir in the evening (Barrière et al., 2005, Kawano et al., 1990, Wilkes et al., 1981). Given that RLS patients show greater circadian changes in CSF dopaminergic measures (Barrière et al., 2005, Earley et al., 2006, Garcia-Borreguero et al., 2004a), it is well possible that this abnormality in dopamine-related circadian rhythm has an influence on attentional selection processes. A reduced D2 receptor binding has been reported among RLS patients (Michaud et al., 2002, Staedt et al., 1993, Staedt et al., 1995a, Staedt et al., 1995b, Turjanski et al., 1999) and blockade of D2 receptors attenuates acetylcholine efflux (Moore et al., 1999), which also plays an important role in the attention system (Sarter et al., 2006). Inasmuch as the sensitivity of D2 autoreceptors is higher in darkness (evening phases) (Domínguez-López et al., 2014), a reduced D2 receptor binding of patients may impact the cholinergic system more intensively in the evening, resulting in the observed attentional deficits. These may emerge because of close interactions of the cholinergic and dopaminergic system. Aside from this, Choi et al. (Choi et al., 2012) reported a weaker anterior-posterior interregional interaction in the RLS patients, which may be caused by an alteration in gray matter (Unrath et al., 2007) and dopaminergic dysfunction (Allen and Earley, 2001). This disturbance of interregional interactions might explain the observed activation differences in the extra-striate visual cortex and the resulting deficits in attentional selection processes.
Strikingly, unlike in other dopamine-related diseases such as Huntington disease (HD) (Beste et al., 2008a), no disease-related modulation of the N2 and P3 components was found. On this account, it may be stated that conflict monitoring (Folstein and Van Petten, 2008), context-updating (Polich, 2007) and stimulus-response mapping (Twomey et al., 2015, Verleger et al., 2005) are less affected by RLS. This lack of effects was further substantiated by the bayesian analysis. Conflict monitoring is assumed to be a function of anterior cingulate cortex (ACC) (Botvinick et al., 2001) and decreased N2 amplitudes observed in HD have been attributed to ACC dysfunction (Beste et al., 2007). As known, striatum and frontal cortex are connected via different functional circuits. While the dorsal striatum is more strongly connected to the prefrontal cortex, the ventral striatum has stronger connections to the limbic cortex including ACC (Chudasama and Robbins, 2006, Haber, 2016). But while HD patients suffer from a degeneration of the neostriatum, RLS patients show a different pattern where decreases in D2 receptors are mainly found in dorsal striatum rather than ventral striatum (Earley et al., 2013, Michaud et al., 2002, Turjanski et al., 1999). This may explain why conflict monitoring is less affected by RLS. Taken together, it seems that not all dopamine-regulated cognitive processes, such as later cognitive processes reflected by the N2 and P3 (Nieoullon, 2002, Polich, 2007, Schultz, 1998), are altered by the disorder and show daytime-dependent impairments. Instead, daytime-related cognitive impairments were restricted to attentional selection processes. Consistent with previous studies (Beste et al., 2008a, Beste et al., 2010, Willemssen et al., 2009, Willemssen et al., 2011), participants performed worse in the incompatible condition as compared to the compatible condition, which was underlined by our neurophysiological data. High salience, low attentional readiness and demand of conflict monitoring in the incompatible condition may account for the larger P1, the smaller N1, and the larger N2 in the incompatible condition (Folstein and Van Petten, 2008, Hillyard and Anllo-Vento, 1998, Knight, 1997). Inasmuch as an elevated P3 was normally observed in conditions with low frequency and with high demand on cognitive processing (Kok, 2001), the larger P3 in the compatible condition appeared counterintuitive. An explanation of this could be the overlapping of N2 and P3 time intervals. Although different activations of N2 and P3 were observed at distinct electrodes in the topography, the results of the N2 and P3 should therefore be interpreted with caution.
With respect to the medication, a few limitations should however be discussed. While the dopaminergic RLS medication was discontinued early enough to ensure that no patient was under direct effects of dopaminergic medication, patients who used opioids and antidepressants were encouraged to continue their medication for their own safety. Asking the patients to abstain from their medication for longer periods of time prior to their appointments would sure have been beneficial in case of retarded medication and dopamine agonists, but it would have drastically reduced the RLS patients' compliance and would furthermore have caused sleep deficits which might also have affected behavioral performance. While non-parametric testing proved that there were no behavioral differences between the medication groups, the heterogeneity of the sample may still have contributed to the rather large observed variance. In this context, it also needs to be noted that general conclusions on medication-induced differences in RLS patients cannot be drawn from our data, because the subgroups were way too small/underpowered to reliably generalize the lack of differences found in our study to the entire population of RLS patients. Also, there are studies which have shown that dopaminergic medication may delay simple reaction times in patients with Parkinson's disease (Müller et al., 2000, Müller et al., 2001, Müller et al., 2002). We however deem it very unlikely to have had similar effects in our sample as there was no general slowing of responses in the patient group (i.e. no main effect of group in the RT analyses). Yet, further studies in larger patient cohorts and especially in non-medicated RLS patients should be conducted to further elucidate the impact of the disease itself and the impact of the RLS medication on cognitive function in these patients. Another limitation of this study is that we measured sleep disturbance of RLS patients based on self-reports. Applying objective measures can provide more objective and detailed information about sleep duration and stability as well as quantification of arousals, which is increased in RLS (Allen et al., 2013, Winkelman et al., 2009) as well as periodic limb movements (PLMS), which occur in most RLS patients (Allen et al., 2005, Garcia-Borreguero, 2006). Moreover, it would be interesting the future studies to compare the cognitive performance between RLS patients with different phenotypes, pain symptoms or different comorbidities like obstructive sleep apnea (OSA).
5. Conclusion
To the best of our knowledge, this is the first study showing circadian cognitive impairments among RLS patients. The amplification of impairments in the evening seems to be restricted to attentional selection processes within the extra-striate visual cortex. In contrast, other dopamine-regulated cognitive processes such as conflict monitoring and response selection did not show any circadian changes. This suggests that in RLS patients, daytime-related attentional deficits rely on the changed circadian dopaminergic rhythm and its close interaction to the cholinergic system as well as disturbed interregional communication.
Disclosure Statement
W.S. has received an unrestricted research grant from TEVA and travel grants from UCB Pharma and TEVA not related to this manuscript. None of the other authors has declared any conflict of interest.
Acknowledgments
This study is supported by Else Kröner-Fresenius-Stiftung (2014_A197).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2017.06.018.
Appendix A. Supplementary data
The group-unrelated effects
References
- Albin R.L., Young A.B., Penney J.B. The functional anatomy of disorders of the basal ganglia. Trends Neurosci. 1995;18:63–64. [PubMed] [Google Scholar]
- Allen R.P., Earley C.J. Restless legs syndrome: a review of clinical and pathophysiologic features. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 2001;18:128–147. doi: 10.1097/00004691-200103000-00004. [DOI] [PubMed] [Google Scholar]
- Allen R.P., Walters A.S., Montplaisir J., Hening W., Myers A., Bell T.J., Ferini-Strambi L. Restless legs syndrome prevalence and impact: REST general population study. Arch. Intern. Med. 2005;165:1286–1292. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- Allen R.P., Connor J.R., Hyland K., Earley C.J. Abnormally increased CSF 3-Ortho-methyldopa (3-OMD) in untreated restless legs syndrome (RLS) patients indicates more severe disease and possibly abnormally increased dopamine synthesis. Sleep Med. 2009;10:123–128. doi: 10.1016/j.sleep.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R.P., Barker P.B., Horská A., Earley C.J. Thalamic glutamate/glutamine in restless legs syndrome: increased and related to disturbed sleep. Neurology. 2013;80:2028–2034. doi: 10.1212/WNL.0b013e318294b3f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R.P., Picchietti D.L., Garcia-Borreguero D., Ondo W.G., Walters A.S., Winkelman J.W., Zucconi M., Ferri R., Trenkwalder C., Lee H.B., International Restless Legs Syndrome Study Group Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Med. 2014;15:860–873. doi: 10.1016/j.sleep.2014.03.025. [DOI] [PubMed] [Google Scholar]
- Barceló F., Suwazono S., Knight R.T. Prefrontal modulation of visual processing in humans. Nat. Neurosci. 2000;3:399–403. doi: 10.1038/73975. [DOI] [PubMed] [Google Scholar]
- Barrière G., Cazalets J.R., Bioulac B., Tison F., Ghorayeb I. The restless legs syndrome. Prog. Neurobiol. 2005;77:139–165. doi: 10.1016/j.pneurobio.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Benton A.L. A visual retention test for clinical use. Arch. Neurol. Psychiatr. 1945;54:212–216. doi: 10.1001/archneurpsyc.1945.02300090051008. [DOI] [PubMed] [Google Scholar]
- Beste C., Saft C., Yordanova J., Andrich J., Gold R., Falkenstein M., Kolev V. Functional compensation or pathology in cortico-subcortical interactions in preclinical Huntington's disease? Neuropsychologia. 2007;45:2922–2930. doi: 10.1016/j.neuropsychologia.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Beste C., Saft C., Andrich J., Gold R., Falkenstein M. Stimulus-response compatibility in Huntington's disease: a cognitive-neurophysiological analysis. J. Neurophysiol. 2008;99:1213–1223. doi: 10.1152/jn.01152.2007. [DOI] [PubMed] [Google Scholar]
- Beste C., Saft C., Konrad C., Andrich J., Habbel A., Schepers I., Jansen A., Pfleiderer B., Falkenstein M. Levels of error processing in Huntington's disease: a combined study using event-related potentials and voxel-based morphometry. Hum. Brain Mapp. 2008;29:121–130. doi: 10.1002/hbm.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beste C., Baune B.T., Falkenstein M., Konrad C. Variations in the TNF-α Gene (TNF-α-308G → a) affect attention and action selection mechanisms in a dissociated fashion. J. Neurophysiol. 2010;104:2523–2531. doi: 10.1152/jn.00561.2010. [DOI] [PubMed] [Google Scholar]
- Beste C., Mückschel M., Elben S., Hartmann C.J., McIntyre C.C., Saft C., Vesper J., Schnitzler A., Wojtecki L. Behavioral and neurophysiological evidence for the enhancement of cognitive control under dorsal pallidal deep brain stimulation in Huntington's disease. Brain Struct. Funct. 2015;220:2441–2448. doi: 10.1007/s00429-014-0805-x. [DOI] [PubMed] [Google Scholar]
- Beste C., Mückschel M., Rosales R., Domingo A., Lee L., Ng A., Klein C., Münchau A. Striosomal dysfunction affects behavioral adaptation but not impulsivity-evidence from X-linked dystonia-parkinsonism. Mov. Disord. Off. J. Mov. Disord. Soc. 2017;32:576–584. doi: 10.1002/mds.26895. [DOI] [PubMed] [Google Scholar]
- Blatter K., Cajochen C. Circadian rhythms in cognitive performance: methodological constraints, protocols, theoretical underpinnings. Physiol. Behav. 2007;90:196–208. doi: 10.1016/j.physbeh.2006.09.009. http://dx.doi.org/10.1016/j.physbeh.2006.09.009 Includes a Special Section on Chronobiology Aspects of the Sleep--Wake Cycle and Thermoreregulation. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Braver T.S., Barch D.M., Carter C.S., Cohen J.D. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brickenkamp R., Schmidt-Atzert L., Liepmann D. Hogrefe; Göttingen: 2010. Test d2-Revision. [Google Scholar]
- Cagigas X.E., Vincent Filoteo J., Stricker J.L., Rilling L.M., Friedrich F.J. Flanker compatibility effects in patients with Parkinson's disease: impact of target onset delay and trial-by-trial stimulus variation. Brain Cogn. 2007;63:247–259. doi: 10.1016/j.bandc.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenka S., Pålhagen S.E., Comley R.A., Panagiotidis G., Cselényi Z., Matthews J.C., Lai R.Y., Halldin C., Farde L. Support for dopaminergic hypoactivity in restless legs syndrome: a PET study on D2-receptor binding. Brain J. Neurol. 2006;129:2017–2028. doi: 10.1093/brain/awl163. [DOI] [PubMed] [Google Scholar]
- Chmielewski W.X., Mückschel M., Roessner V., Beste C. Expectancy effects during response selection modulate attentional selection and inhibitory control networks. Behav. Brain Res. 2014;274:53–61. doi: 10.1016/j.bbr.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Choi J.W., Ko D., Lee G.-T., Jung K.-Y., Kim K.H. Reduced neural synchrony in patients with restless legs syndrome during a visual oddball task. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y., Robbins T.W. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol. Psychol. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Clemens S., Rye D., Hochman S. Restless legs syndrome: revisiting the dopamine hypothesis from the spinal cord perspective. Neurology. 2006;67:125–130. doi: 10.1212/01.wnl.0000223316.53428.c9. [DOI] [PubMed] [Google Scholar]
- Cohen M.X. MIT Press; 2014. Analyzing Neural Time Series Data: Theory and Practice. [Google Scholar]
- Desimone R. Visual attention mediated by biased competition in extrastriate visual cortex. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1998;353:1245–1255. doi: 10.1098/rstb.1998.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk D.-J., Duffy J.F., Czeisler C.A. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J. Sleep Res. 1992;1:112–117. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Dippel G., Beste C. A causal role of the right inferior frontal cortex in implementing strategies for multi-component behaviour. Nat. Commun. 2015;6:6587. doi: 10.1038/ncomms7587. [DOI] [PubMed] [Google Scholar]
- Domínguez-López S., Howell R.D., López-Canúl M.G., Leyton M., Gobbi G. Electrophysiological characterization of dopamine neuronal activity in the ventral tegmental area across the light-dark cycle. Synap. N. Y. 2014;68:454–467. doi: 10.1002/syn.21757. [DOI] [PubMed] [Google Scholar]
- Earley C.J., Hyland K., Allen R.P. Circadian changes in CSF dopaminergic measures in restless legs syndrome. Sleep Med. 2006;7:263–268. doi: 10.1016/j.sleep.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Earley C.J., Kuwabara H., Wong D.F., Gamaldo C., Salas R.E., Brašić J.R., Ravert H.T., Dannals R.F., Allen R.P. Increased synaptic dopamine in the putamen in restless legs syndrome. Sleep. 2013;36:51–57. doi: 10.5665/sleep.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M., Hommel B., Prinz W. S-R compatibility and response selection. Acta Psychol. (Amst.) 1995;90:301–313. http://dx.doi.org/10.1016/0001-6918(95)00022-M Discrete and Continuous Information Processing. [Google Scholar]
- Eriksen B.A., Eriksen C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974;16:143–149. [Google Scholar]
- Falkenstein M., Willemssen R., Hohnsbein J., Hielscher H. Effects of stimulus-response compatibility in Parkinson's disease: a psychophysiological analysis. J. Neural Transm. 2006;113:1449–1462. doi: 10.1007/s00702-005-0430-1. [DOI] [PubMed] [Google Scholar]
- Folstein J.R., Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs M., Kastner J., Wagner M., Hawes S., Ebersole J.S. A standardized boundary element method volume conductor model. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2002;113:702–712. doi: 10.1016/s1388-2457(02)00030-5. [DOI] [PubMed] [Google Scholar]
- Fulda S., Beitinger M.E., Reppermund S., Winkelmann J., Wetter T.C. Short-term attention and verbal fluency is decreased in restless legs syndrome patients. Mov. Disord. Off. J. Mov. Disord. Soc. 2010;25:2641–2648. doi: 10.1002/mds.23353. [DOI] [PubMed] [Google Scholar]
- Fulda S., Szesny N., Ising M., Heck A., Grübl A., Lieb R., Reppermund S. Further evidence for executive dysfunction in subjects with RLS from a non-clinical sample. Sleep Med. 2011;12:1003–1007. doi: 10.1016/j.sleep.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Garcia-Borreguero D. Time to REST: epidemiology and burden. Eur. J. Neurol. 2006;13(Suppl. 3):15–20. doi: 10.1111/j.1468-1331.2006.01587.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Borreguero D., Larrosa O., Granizo J.J., de la Llave Y., Hening W.A. Circadian variation in neuroendocrine response to L-dopa in patients with restless legs syndrome. Sleep. 2004;27:669–673. [PubMed] [Google Scholar]
- Garcia-Borreguero D., Serrano C., Larrosa O., Granizo J.J. Circadian effects of dopaminergic treatment in restless legs syndrome. Sleep Med. 2004;5:413–420. doi: 10.1016/j.sleep.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Haber S.N. Corticostriatal circuitry. Dialogues Clin. Neurosci. 2016;18:7–21. doi: 10.31887/DCNS.2016.18.1/shaber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter C., Lendt M., Lux S. Hogrefe Verl. Für Psychol; Gött: 2001. Verbaler Lern- und Merkfähigkeitstest. [Google Scholar]
- Hening W., Allen R., Earley C., Kushida C., Picchietti D., Silber M. The treatment of restless legs syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine Review. Sleep. 1999;22:970–999. [PubMed] [Google Scholar]
- Herrmann C.S., Knight R.T. Mechanisms of human attention: event-related potentials and oscillations. Neurosci. Biobehav. Rev. 2001;25:465–476. doi: 10.1016/s0149-7634(01)00027-6. [DOI] [PubMed] [Google Scholar]
- Hillyard S.A., Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proc. Natl. Acad. Sci. U. S. A. 1998;95:781–787. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne J.A., Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Jensen A.R., Rohwer W.D., Jr. The stroop color-word test: a review. Acta Psychol. 1966;25:36–93. doi: 10.1016/0001-6918(66)90004-7. [DOI] [PubMed] [Google Scholar]
- Jung K.-Y., Koo Y.-S., Kim B.-J., Ko D., Lee G.-T., Kim K.H., Im C.H. Electrophysiologic disturbances during daytime in patients with restless legs syndrome: further evidence of cognitive dysfunction? Sleep Med. 2011;12:416–421. doi: 10.1016/j.sleep.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Kähkönen S., Ahveninen J., Pekkonen E., Kaakkola S., Huttunen J., Ilmoniemi R.J., Jääskeläinen I.P. Dopamine modulates involuntary attention shifting and reorienting: an electromagnetic study. Clin. Neurophysiol. 2002;113:1894–1902. doi: 10.1016/s1388-2457(02)00305-x. [DOI] [PubMed] [Google Scholar]
- Kastner S., Weerd P.D., Desimone R., Ungerleider L.G. Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science. 1998;282:108–111. doi: 10.1126/science.282.5386.108. [DOI] [PubMed] [Google Scholar]
- Kawano Y., Kawasaki T., Kawazoe N., Abe I., Uezono K., Ueno M., Fukiyama K., Omae T. Circadian variations of urinary dopamine, norepinephrine, epinephrine and sodium in normotensive and hypertensive subjects. Nephron. 1990;55:277–282. doi: 10.1159/000185975. [DOI] [PubMed] [Google Scholar]
- Knight R.T. Distributed cortical network for visual attention. J. Cogn. Neurosci. 1997;9:75–91. doi: 10.1162/jocn.1997.9.1.75. [DOI] [PubMed] [Google Scholar]
- Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557–577. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- Kopp B., Rist F., Mattler U. N200 in the flanker task as a neurobehavioral tool for investigating executive control. Psychophysiology. 1996;33:282–294. doi: 10.1111/j.1469-8986.1996.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Luck S.J., Heinze H.J., Mangun G.R., Hillyard S.A. Visual event-related potentials index focused attention within bilateral stimulus arrays. II. Functional dissociation of P1 and N1 components. Electroencephalogr. Clin. Neurophysiol. 1990;75:528–542. doi: 10.1016/0013-4694(90)90139-b. [DOI] [PubMed] [Google Scholar]
- Luck S.J., Woodman G.F., Vogel E.K. Event-related potential studies of attention. Trends Cogn. Sci. 2000;4(11):432–440. doi: 10.1016/s1364-6613(00)01545-x. [DOI] [PubMed] [Google Scholar]
- Masson M.E.J. A tutorial on a practical Bayesian alternative to null-hypothesis significance testing. Behav. Res. Methods. 2011;43:679–690. doi: 10.3758/s13428-010-0049-5. [DOI] [PubMed] [Google Scholar]
- Mazziotta J., Toga A., Evans A., Fox P., Lancaster J., Zilles K., Woods R., Paus T., Simpson G., Pike B., Holmes C., Collins L., Thompson P., MacDonald D., Iacoboni M., Schormann T., Amunts K., Palomero-Gallagher N., Geyer S., Parsons L., Narr K., Kabani N., Le Goualher G., Boomsma D., Cannon T., Kawashima R., Mazoyer B. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philos. Trans. R. Soc. Lond. Ser. B. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud M., Soucy J.-P., Chabli A., Lavigne G., Montplaisir J. SPECT imaging of striatal pre- and postsynaptic dopaminergic status in restless legs syndrome with periodic leg movements in sleep. J. Neurol. 2002;249:164–170. doi: 10.1007/pl00007859. [DOI] [PubMed] [Google Scholar]
- Moore H., Fadel J., Sarter M., Bruno J.P. Role of accumbens and cortical dopamine receptors in the regulation of cortical acetylcholine release. Neuroscience. 1999;88:811–822. doi: 10.1016/s0306-4522(98)00261-9. [DOI] [PubMed] [Google Scholar]
- Mückschel M., Stock A.-K., Beste C. Psychophysiological mechanisms of interindividual differences in goal activation modes during action cascading. Cereb. Cortex N. Y. 2014;1991(24):2120–2129. doi: 10.1093/cercor/bht066. [DOI] [PubMed] [Google Scholar]
- Müller T., Benz S., Przuntek H. Choice reaction time after levodopa challenge in parkinsonian patients. J. Neurol. Sci. 2000;181:98–103. doi: 10.1016/s0022-510x(00)00436-6. [DOI] [PubMed] [Google Scholar]
- Müller T., Benz S., Börnke C. Delay of simple reaction time after levodopa intake. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2001;112:2133–2137. doi: 10.1016/s1388-2457(01)00653-8. [DOI] [PubMed] [Google Scholar]
- Müller T., Benz S., Przuntek H. Apomorphine delays simple reaction time in Parkinsonian patients. Parkinsonism Relat. Disord. 2002;8:357–360. doi: 10.1016/s1353-8020(01)00046-3. [DOI] [PubMed] [Google Scholar]
- Nieoullon A. Dopamine and the regulation of cognition and attention. Prog. Neurobiol. 2002;67:53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Nunez P.L., Pilgreen K.L. The spline-Laplacian in clinical neurophysiology: a method to improve EEG spatial resolution. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 1991;8:397–413. [PubMed] [Google Scholar]
- Pascual-Marqui R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find. Exp. Clin. Pharmacol. 2002;24(Suppl D):5–12. [PubMed] [Google Scholar]
- Pearson V.E., Allen R.P., Dean T., Gamaldo C.E., Lesage S.R., Earley C.J. Cognitive deficits associated with restless legs syndrome (RLS) Sleep Med. 2006;7:25–30. doi: 10.1016/j.sleep.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Penner I.K., Raselli C., Stöcklin M., Opwis K., Kappos L., Calabrese P. The Fatigue Scale for Motor and Cognitive Functions (FSMC): validation of a new instrument to assess multiple sclerosis-related fatigue. Mult. Scler. Houndmills Basingstoke Engl. 2009;15:1509–1517. doi: 10.1177/1352458509348519. [DOI] [PubMed] [Google Scholar]
- Perrin F., Pernier J., Bertrand O., Echallier J.F. Spherical splines for scalp potential and current density mapping. Electroencephalogr. Clin. Neurophysiol. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praamstra P., Stegeman D.F., Cools A.R., Horstink M.W.I.M. Reliance on external cues for movement initiation in Parkinson's disease. Evidence from movement-related potentials. Brain. 1998;121:167–177. doi: 10.1093/brain/121.1.167. [DOI] [PubMed] [Google Scholar]
- Praamstra P., Plat E.M., Meyer A.S., Horstink M.W.I.M. Motor cortex activation in Parkinson's disease: dissociation of electrocortical and peripheral measures of response generation. Mov. Disord. 1999;14:790–799. doi: 10.1002/1531-8257(199909)14:5<790::aid-mds1011>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Raftery A.E. Bayesian model selection in social research. Sociol. Methodol. 1995;25:111–163. [Google Scholar]
- Ridderinkhof K.R., van der Molen M.W., Bashore T.R. Limits on the application of additive factors logic: violations of stage robustness suggest a dual-process architecture to explain flanker effects on target processing. Acta Psychol. (Amst.) 1995;90:29–48. http://dx.doi.org/10.1016/0001-6918(95)00031-O Discrete and Continuous Information Processing. [Google Scholar]
- Russell V., de Villiers A., Sagvolden T., Lamm M., Taljaard J. Altered dopaminergic function in the prefrontal cortex, nucleus accumbens and caudate-putamen of an animal model of attention-deficit hyperactivity disorder--the spontaneously hypertensive rat. Brain Res. 1995;676:343–351. doi: 10.1016/0006-8993(95)00135-d. [DOI] [PubMed] [Google Scholar]
- Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD) Neurosci. Biobehav. Rev. 2000;24:31–39. doi: 10.1016/s0149-7634(99)00058-5. [DOI] [PubMed] [Google Scholar]
- Sarter M., Gehring W.J., Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res. Rev. 2006;51:145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Schneider D., Beste C., Wascher E. Attentional capture by irrelevant transients leads to perceptual errors in a competitive change detection task. Front. Psychol. 2012;3:164. doi: 10.3389/fpsyg.2012.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Sekihara K., Sahani M., Nagarajan S.S. Localization bias and spatial resolution of adaptive and non-adaptive spatial filters for MEG source reconstruction. NeuroImage. 2005;25:1056–1067. doi: 10.1016/j.neuroimage.2004.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J.M., Halliday G.M., Naismith S.L., Lewis S.J.G. Visual misperceptions and hallucinations in Parkinson's disease: dysfunction of attentional control networks? Mov. Disord. 2011;26:2154–2159. doi: 10.1002/mds.23896. [DOI] [PubMed] [Google Scholar]
- Silkis I. A hypothetical role of cortico-basal ganglia-thalamocortical loops in visual processing. Biosystems. 2007;89:227–235. doi: 10.1016/j.biosystems.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Staedt J., Stoppe G., Kögler A., Munz D., Riemann H., Emrich D., Rüther E. Dopamine D2 receptor alteration in patients with periodic movements in sleep (nocturnal myoclonus) J. Neural Transm. Gen. Sect. 1993;93:71–74. doi: 10.1007/BF01244940. [DOI] [PubMed] [Google Scholar]
- Staedt J., Stoppe G., Kögler A., Riemann H., Hajak G., Munz D.L., Emrich D., Rüther E. Nocturnal myoclonus syndrome (periodic movements in sleep) related to central dopamine D2-receptor alteration. Eur. Arch. Psychiatry Clin. Neurosci. 1995;245:8–10. doi: 10.1007/BF02191538. [DOI] [PubMed] [Google Scholar]
- Staedt J., Stoppe G., Kögler A., Riemann H., Hajak G., Munz D.L., Emrich D., Rüther E. Single photon emission tomography (SPET) imaging of dopamine D2 receptors in the course of dopamine replacement therapy in patients with nocturnal myoclonus syndrome (NMS) J. Neural Transm. Gen. Sect. 1995;99:187–193. doi: 10.1007/BF01271478. [DOI] [PubMed] [Google Scholar]
- Tenke C.E., Kayser J. Generator localization by current source density (CSD): implications of volume conduction and field closure at intracranial and scalp resolutions. Clin. Neurophysiol. 2012;123:2328–2345. doi: 10.1016/j.clinph.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillman C.M., Wiens S. Behavioral and ERP indices of response conflict in Stroop and flanker tasks. Psychophysiology. 2011;48:1405–1411. doi: 10.1111/j.1469-8986.2011.01203.x. [DOI] [PubMed] [Google Scholar]
- Trenkwalder C., Paulus W. Restless legs syndrome: pathophysiology, clinical presentation and management. Nat. Rev. Neurol. 2010;6:337–346. doi: 10.1038/nrneurol.2010.55. [DOI] [PubMed] [Google Scholar]
- Trenkwalder C., Paulus W., Walters A.S. 2005. The Restless Legs Syndrome 4, 465–475. [DOI] [PubMed] [Google Scholar]
- Turjanski N., Lees A.J., Brooks D.J. Striatal dopaminergic function in restless legs syndrome: 18F-dopa and 11C-raclopride PET studies. Neurology. 1999;52:932–937. doi: 10.1212/wnl.52.5.932. [DOI] [PubMed] [Google Scholar]
- Twomey D.M., Murphy P.R., Kelly S.P., O'Connell R.G. The classic P300 encodes a build-to-threshold decision variable. Eur. J. Neurosci. 2015;42:1636–1643. doi: 10.1111/ejn.12936. [DOI] [PubMed] [Google Scholar]
- Unrath A., Juengling F.D., Schork M., Kassubek J. Cortical grey matter alterations in idiopathic restless legs syndrome: an optimized voxel-based morphometry study. Mov. Disord. Off. J. Mov. Disord. Soc. 2007;22:1751–1756. doi: 10.1002/mds.21608. [DOI] [PubMed] [Google Scholar]
- Verleger R., Jaskowski P., Wascher E. 2005. Evidence for an Integrative Role of P3b in Linking Reaction to Perception. [Google Scholar]
- Vidal F., Burle B., Spieser L., Carbonnell L., Meckler C., Casini L., Hasbroucq T. Linking EEG signals, brain functions and mental operations: advantages of the Laplacian transformation. Int. J. Psychophysiol. 2015;97:221–232. doi: 10.1016/j.ijpsycho.2015.04.022. http://dx.doi.org/10.1016/j.ijpsycho.2015.04.022 On the benefits of using surface Laplacian (current source density) methodology in electrophysiology. [DOI] [PubMed] [Google Scholar]
- Videnovic A., Golombek D. Circadian and sleep disorders in Parkinson's disease. Exp. Neurol. 2013;243:45–56. doi: 10.1016/j.expneurol.2012.08.018. http://dx.doi.org/10.1016/j.expneurol.2012.08.018 Circadian rhythms and sleep disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenmakers E.-J. A practical solution to the pervasive problems ofp values. Psychon. Bull. Rev. 2007;14:779–804. doi: 10.3758/bf03194105. [DOI] [PubMed] [Google Scholar]
- Walters A.S., LeBrocq C., Dhar A., Hening W., Rosen R., Allen R.P., Trenkwalder C., International Restless Legs Syndrome Study Group Validation of the international restless legs syndrome study group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–132. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- Wilkes M.M., Babaknia A., Hoff J.D., Quigley M.E., Fraus P.F., Yen S.S.C. Circadian rhythm in circulating concentration of dihydroxyphenylacetic acid in normal women. J. Clin. Endocrinol. Metab. 1981;52:608–611. doi: 10.1210/jcem-52-4-608. [DOI] [PubMed] [Google Scholar]
- Willemssen R., Müller T., Schwarz M., Falkenstein M., Beste C. Response monitoring in de novo patients with Parkinson's disease. PLoS One. 2009;4 doi: 10.1371/journal.pone.0004898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemssen R., Falkenstein M., Schwarz M., Müller T., Beste C. Effects of aging, Parkinson's disease, and dopaminergic medication on response selection and control. Neurobiol. Aging. 2011;32:327–335. doi: 10.1016/j.neurobiolaging.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Winkelman J.W., Redline S., Baldwin C.M., Resnick H.E., Newman A.B., Gottlieb D.J. Polysomnographic and health-related quality of life correlates of restless legs syndrome in the sleep heart health study. Sleep. 2009;32:772–778. doi: 10.1093/sleep/32.6.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman J.W., Schoerning L., Platt S., Jensen J.E. Restless legs syndrome and central nervous system gamma-aminobutyric acid: preliminary associations with periodic limb movements in sleep and restless leg syndrome symptom severity. Sleep Med. 2014;15:1225–1230. doi: 10.1016/j.sleep.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Wylie S.A., Stout J.C., Bashore T.R. Activation of conflicting responses in Parkinson's disease: evidence for degrading and facilitating effects on response time. Neuropsychologia. 2005;43:1033–1043. doi: 10.1016/j.neuropsychologia.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Wylie S.A., van den Wildenberg W.P.M., Ridderinkhof K.R., Bashore T.R., Powell V.D., Manning C.A., Wooten G.F. The effect of Parkinson's disease on interference control during action selection. Neuropsychologia. 2009;47:145–157. doi: 10.1016/j.neuropsychologia.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The group-unrelated effects