Abstract
Hox gene functions are intimately linked to correct developmental expression of the genes. The identification of cis-acting regulatory sequences and their associated trans-acting factors constitutes a key step in deciphering the mechanisms underlying the correct positioning of the functional domain of Hox genes along the anterior-posterior axis. We have identified DNA elements driving Hoxa5 regionalized expression in mice, using the 2.1-kb mesodermal enhancer (MES) localized in Hoxa5 3′ flanking sequences as a starting point. The MES sequence comprises regulatory elements targeting Hoxa5 expression in the limbs, the urogenital and gastrointestinal tracts, and the cervical-upper thoracic region of the prevertebral column. A 164-bp DNA fragment within the MES caudally restricts Hoxa5 expression at the level of prevertebra 10, corresponding to the posterior limit of its functional domain. Cdx proteins directly bind to this element in vitro via two conserved sites. Preventing Cdx binding by mutating the sites causes caudal expansion of the transgene expression domain. Of all three murine Cdx proteins that bind this element in vitro, Cdx4 has emerged as a potential regional posterior repressor of Hoxa5 expression. The restrictive control provided by Cdx interactions with Hoxa5 regulatory sequences may be one of the critical events in cervicothoracic axial specification.
The establishment of the morphological diversity of the body plan requires that genetic information be processed to ultimately dictate specific shapes in subsets of domains along the body axes. In this cascade of molecular events, Hox genes are key components that define how and where structures will be elaborated. This gene family encodes transcription factors that specify regional identities along the embryonic axes of diverse organisms. The murine genome contains 39 Hox genes organized into four clusters, HoxA to HoxD (35). The organization of Hox complexes is fundamental for the precise spatiotemporal regulation and function of each gene. A colinear relationship exists between the relative order of Hox genes on the chromosome, their expression domains along the anterior-posterior axis, and their temporal onset: genes located at the 3′ ends of the clusters are expressed earlier and more anteriorly in the embryo than those in more 5′ positions (16, 31). As a result, Hox gene expression domains are both spatially restricted and overlapping, extending from specific anterior boundaries to the caudal end of the embryo. Hox developmental functions were first extrapolated from these expression patterns. However, mutational analyses of murine Hox genes have shown that their domains of action are mainly restrained to the most anterior regions of their expression domains (4, 56). Hox gene functions are also intimately linked to correct developmental regulation of the genes, as illustrated by the homeotic transformations observed when Hox cis-acting regulatory elements are mutated (17, 22, 65).
Even though substantial information has been gained regarding Hox gene regulation, a better comprehension of the controls governing Hox gene expression is needed to decipher the mechanistic basis responsible for Hox gene function in axial patterning. Hox gene regulation is thought to be achieved by a combination of strategies involving (i) chromatin remodeling (12), (ii) global enhancer sequences located outside the cluster and acting in a relatively non-promoter-specific manner (30, 33, 55), and (iii) an integrated regulation of neighboring genes through the sharing, competition, and/or selective use of defined local cis-acting sequences (23, 53; reviewed in reference 31). Extensive studies have been devoted to the identification of control elements, demonstrating that Hox gene expression can be recapitulated partly or entirely upon random integration in the genome of transgenes containing DNA regulatory elements (7, 10, 36, 45, 46, 48, 53, 61, 64). The common emerging picture is that the dynamic profile of Hox gene expression relies on the interpretation of positional information transduced by transcriptional factors interacting with both positive and repressive DNA sequences to restrict the enhancer activities to defined regional domains within specific expression boundaries (22, 44, 57). Among the upstream factors identified so far, caudal-related family members have emerged as key regulators (13, 19, 49, 62). The main evidence for Cdx protein involvement in Hox regulation comes from analyses of targeted mutations of these homeodomain-containing proteins in mice, as they result in skeletal defects akin to those previously observed in Hox mutant mice (14, 58, 62). In Cdx1−/−, Cdx2+/−, and Cdx1−/− Cdx2+/− compound mutants, the posterior shift in Hox gene boundaries correlates with the transformations observed along the anterior-posterior axis. Moreover, potential Cdx binding sites are found within the cis-regulatory regions of several Hox genes (13, 47, 54, 58). Finally, recent data substantiate the view that Cdx proteins may act as integrators of multiple signaling pathways elicited by either retinoic acid, Wnt, or fibroblast growth factors (8, 9, 25, 27, 28, 37, 49, 50). Consequently, Cdx proteins likely convey positional information from these signals to Hox genes (38).
To fully understand the regulatory events governing Hox gene expression, we are studying the Hoxa5 gene. This gene provides a good model system for deciphering the regional control of Hox regulation, as its loss-of-function mutation affects a well-defined subset of its axial expression domain (2, 29, 36). In the developing embryo, Hoxa5 expression extends from the caudal end to the posterior myelencephalon in the neural tube and to prevertebra (pv) 3 in the axial skeleton. Hoxa5 transcripts are also detected in the mesenchymal components of several organs, including the trachea, lungs, stomach, intestine, and kidneys (1, 2, 3, 5, 15, 21). Aside from morphological defects in foregut derivatives (1, 5, 40), targeted disruption of the Hoxa5 gene perturbs axial skeleton identity in a region confined between pv3 and pv10, the most anterior domain of Hoxa5 expression in the prevertebral column (2, 29). Several transcripts, of 1.8, 5.0, 9.5, and 11 kb in length, encompassing Hoxa5 coding sequences originate from the use of different promoters and from alternative splicing (29; Y. Coulombe and L. Jeannotte, unpublished data). As a result, the global Hoxa5 expression profile derives from a combination of the expression domains of all Hoxa5 transcriptional units initiating from both the proximal and the distal promoters. The major 1.8-kb transcript, which encodes the Hoxa5 270-amino-acid protein, is specifically expressed at high levels in the axial domain, where the Hoxa5 mutation exerts its effect (36).
The presence of complex and overlapping transcriptional units at the Hoxa5 locus implies the presence of dispersed and shared regulatory regions in the cluster. This prompted us to characterize the regulatory elements directing Hoxa5 developmental expression by using a transgenic approach. cis-acting sequences lying within an 11.1-kb genomic fragment can recapitulate the temporal expression and substantially reproduce the spatial pattern of the Hoxa5 1.8-kb transcript (36). Deletion analyses have revealed several DNA control elements, including a 604-bp brachial spinal cord enhancer (BSC) in 5′ flanking sequences (45, 61) and a 2.1-kb mesodermal enhancer (MES) downstream of Hoxa5 coding sequences, with the latter being essential for paraxial and lateral plate mesoderm expression in the cervical and upper thoracic regions (36). Additional regulatory sequences involved in Hoxa5 lung and gut expression have also been identified near the Hoxa4 gene (42). As a result of pursuing the characterization of the MES sequence by deletion and mutational analyses, we present evidence that the MES contains DNA elements that limit the Hoxa5 regional specific expression domain along the anterior-posterior axis and that Cdx proteins can directly interact with MES sequences to correctly position the Hoxa5 expression domain.
MATERIALS AND METHODS
Construction of Hoxa5/lacZ transgenes.
Construct 1 of this study, also named pLJ133, has been described previously (Fig. 1B) (construct 9 in reference 36). It contains the bacterial lacZ gene inserted into the SacI site of the first exon of the Hoxa5 gene, allowing translation of the lacZ open reading frame from the Hoxa5 AUG. Construct 1 was digested with HindIII to remove a 2.08-kb fragment from kb +2.85 to +4.93 and was self-ligated to produce an intermediate construct (pLJ143 [not shown]) containing a continuous XhoI-HindIII Hoxa5 genomic fragment extending from bp −235 to kb +2.85 that does not direct β-galactosidase expression, as previously shown (64). The insertion of a HindIII-PstI fragment (from kb +2.85 to +4.60) at the HindIII site (kb +2.85) of pLJ143 resulted in construct 2. Constructs 3 to 5 were generated by a series of deletions at the 3′ end of construct 1. Construct 1 was partially digested with Eco47III to produce construct 3 and with AvrII and XhoI to generate constructs 4 and 5, respectively. An 892-bp XhoI/HindIII filled-in fragment extending from kb +4.04 to +4.93 was inserted either at the HindIII site (kb +2.85) or at the XhoI site (bp −235) of pLJ143 to generate constructs 6 and 7, respectively. Construct 8 was derived from construct 1 by deleting the AvrII-Eco47III sequence, which was thereafter referred to as the AvEc 164-bp fragment located between kb +4.28 and +4.44. Construct 9 was obtained by cloning the AvEc 164-bp fragment into the HindIII (kb +2.85) site of pLJ143. For constructs 10, 11, and 12, nucleotide substitutions in the identified consensus Cdx binding sites were produced by an overlapping PCR strategy using synthetic oligonucleotide primers containing appropriate base changes (63). The modifications introduced are shown in Fig. 4A. The 2.08-kb HindIII fragment extending from kb +2.85 to +4.93 was subcloned, and the resulting construct was used as a template for synthetic primers. All introduced mutations were subsequently validated by sequencing. The plasmids containing the desired mutations were digested with HindIII, and the fragments were subcloned into the HindIII site (kb +2.85) of pLJ143 to generate constructs 10 (mut1), 11 (mut2), and 12 (mut1,2). All of the constructs described in the present study were made in pBluescript II SK(+) (Stratagene) and were purified by cesium chloride centrifugation.
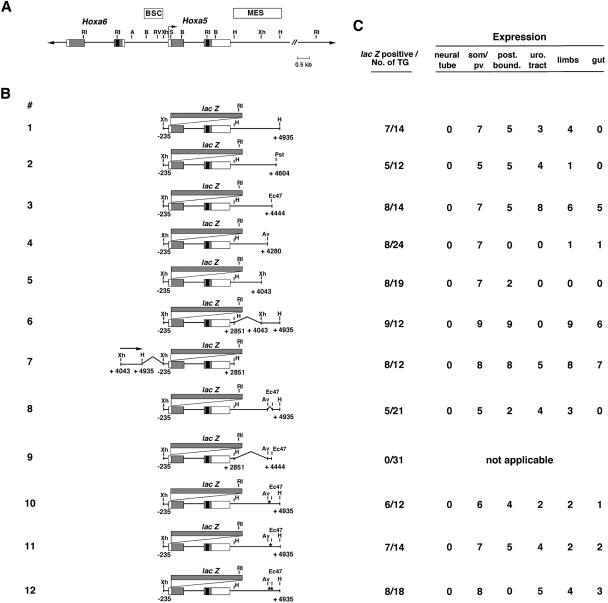
FIG. 1.
Characterization of regulatory regions of the Hoxa5 gene in transgenic mouse embryos. Schematics of reporter gene constructs and sites of expression are shown. (A) Partial restriction map of the Hoxa5 locus. Black boxes indicate homeoboxes of the Hoxa5 and Hoxa6 genes, gray boxes correspond to translated regions, and white boxes indicate transcribed regions. The boxes labeled BSC and MES denote the enhancer domains defined in references 36 and 61, respectively. (B) Diagram of the constructs used to generate transgenic embryos. The asterisks in constructs 10, 11, and 12 correspond to mutations of Cdx binding sites 1 and 2 (see Fig. 4 for the nucleotide sequences of these mutations). (C) Summary of transgenic expression analyses. The first column represents the number of lacZ-expressing embryos out of the total number of F0 transgenic embryos generated. The number of positively stained embryos for each structure listed is indicated. post. bound., defined upper thoracic posterior boundary along the prevertebral column; pv, prevertebrae; som, somites; uro. tract, urogenital tract. A, AccI; Av, AvrII; B, BglII; Ec47, Eco47III; H, HindIII; Pst, PstI; RI, EcoRI; RV, EcoRV; S, SacI; Xh, XhoI.
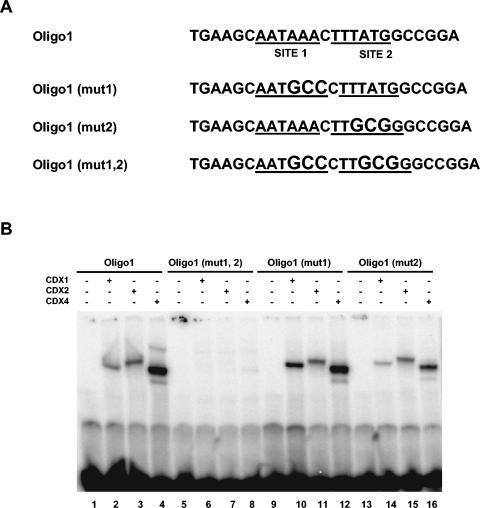
FIG. 4.
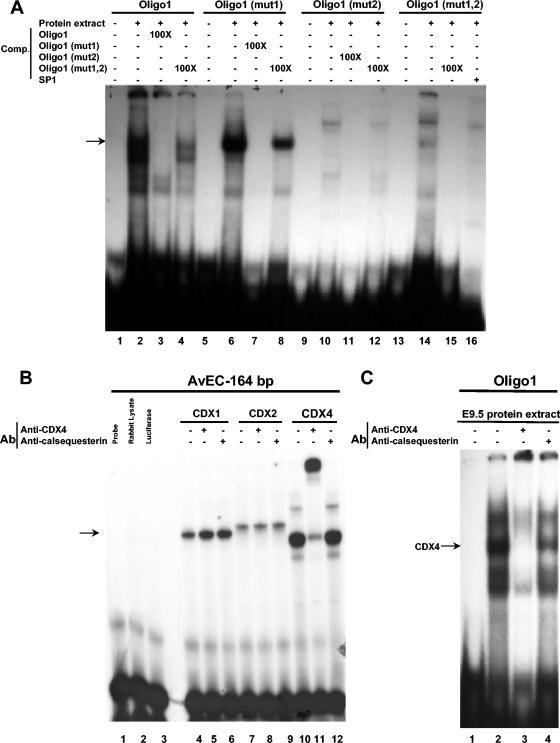
Both Cdx sites are involved in the binding of Cdx proteins to Oligo1. (A) Nucleotide sequences of wild-type and mutant versions of Oligo1. The two potential Cdx binding sites, sites 1 and 2, are underlined, and the introduced mutations are shown with larger letters. (B) EMSA with Cdx proteins produced in vitro and with different versions of Oligo1. The three Cdx proteins were able to bind Oligo1 (lanes 2, 3, and 4), Oligo1 (mut1) (lanes 10, 11, and 12), and Oligo1 (mut2) (lanes 14, 15, and 16), but no binding was observed with Oligo1 (mut1, 2) (lanes 6, 7, and 8).
Production and genotyping of transgenic mice.
All experiments were performed according to the guidelines of the Canadian Council on Animal Care and were approved by the institutional animal care committee. The Hoxa5/lacZ sequences from all constructs were isolated by a KpnI and NotI digestion to remove vector sequences and were purified on agarose gels. They were then injected into the pronuclei of fertilized eggs derived from C57BL/6 × C3H F1 hybrid intercrosses according to standard procedures (24). Transgenic founder embryos were recovered from foster mothers, genotyped by Southern analysis of yolk sac DNAs with a lacZ-specific probe to verify the integrity of the microinjected constructs, and analyzed for lacZ expression by β-galactosidase staining as previously described (36). Permanent mouse lines for construct 1 were also obtained and genotyped by the use of tail DNA.
The effect of Cdx proteins on Hoxa5/lacZ transgene expression was assessed by the use of either a Cdx1 mutant mouse line originally produced in the laboratory of P. Gruss (58) and provided by D. Lohnes or a Cdx2 mutant mouse line generated in the laboratory of F. Beck and provided by K. Chawengsaksophak (14). The Cdx1 and Cdx2 mutations were maintained in the 129/SvEv and ICR genetic backgrounds, respectively. In addition to the pLJ133-carrying established transgenic line (corresponding to construct 1 in the present study) (Fig. 1B), a permanent mouse line obtained previously for the pLJ57 Hoxa5/lacZ construct was tested (construct 2 in reference 36). The pLJ57 transgene contains a Hoxa5 genomic fragment extending from kb −2.13 to +7.19, with the lacZ reporter gene inserted into the first exon of the gene. To generate Cdx1+/− pLJ133+/− and Cdx1+/− pLJ57+/− animals, we set up matings between Cdx1−/− and Hoxa5/lacZ mice. Cdx1+/− pLJ133+/− and Cdx1+/− pLJ57+/− males were then bred with Cdx1+/− females to produce animals for all six possible genotypes. In parallel, pLJ133+/− or pLJ57+/− males were bred with Cdx2+/− females to generate animals for all four possible genotypes. The early embryonic lethality of Cdx2−/− mutants prevented the analysis of Cdx2−/− embryos carrying a Hoxa5/lacZ transgene (14). We also produced Cdx1+/− pLJ57+/− males that were bred with Cdx1+/− Cdx2+/− females to evaluate the impact of three Cdx mutated alleles on Hoxa5/lacZ transgene expression (Cdx1−/− Cdx2+/− pLJ57+/− specimens). The morning of vaginal plug detection was considered embryonic day 0.5 (E0.5). Embryos were obtained at various embryonic ages and stained for β-galactosidase activity. Yolk sac DNAs were used for genotyping of the lacZ and Cdx1 alleles by Southern blot analysis (58). The Cdx2 genotype was determined by PCR as described previously (62).
EMSA.
The expression vectors pSG5-Cdx1, pSG5-Cdx2, and pSG5-Cdx4 were provided by J. Deschamps (13). The TnT7 Quick coupled transcription-translation system (Promega Corporation) was used to produce each of the Cdx proteins in vitro, which were then analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and directly assayed by an electrophoretic mobility shift assay (EMSA). The AvEc 164-bp fragment was purified, end labeled with T4 polynucleotide kinase, and tested for Cdx1, Cdx2, and Cdx4 binding by EMSA. A combination of double-stranded end-labeled oligonucleotides covering the following regions were also tested: (i) the 5′ half of the AvEc 164-bp fragment, from nucleotides 1 to 86 (see Fig. 3B); (ii) the 3′ half of the AvEc 164-bp fragment, from nucleotides 67 to 164; and (iii) sequences encompassing Cdx binding sites, including 5′CCGGATTTAACTTTAGTCTTTGAAT3′, 5′TTGAAAGGGGTTAAAGCGCT3′, and Oligo1. We focused on two consensus Cdx binding sites, sites 1 and 2 present in Oligo1, that showed Cdx binding activity. EMSAs were performed with double-stranded end-labeled oligonucleotides harboring either the wild-type sequence 5′TGAAGCAATAAACTTTATGGCCGGA3′ (Oligo1) or sequences with mutated binding sites, i.e., 5′TGAAGCAATGCCCTTTATGGCCGGA3′ [Oligo1 (mut1)], 5′TGAAGCAATAAACTTGCGGGCCGGA3′ [Oligo1 (mut2)], and 5′TGAAGCAATGCCCTTGCGGGCCGGA3′ [Oligo1 (mut1,2)] (nucleotide substitutions are underlined). Binding reactions containing 0.5 to 4 ng of probe (100,000 cpm), 1 μl of Cdx protein, 1 μg of poly(dI-dC), and 100 ng of yeast tRNA prepared in a buffer comprising 15 mM HEPES (pH 7.9), 50 mM NaCl, 80 μM ZnCl2, 800 μM dithiothreitol, 0.5% NP-40, 2 mM MgCl2, and 3% Ficoll were equilibrated for 10 min at 25°C and separated by electrophoresis through a 6% polyacrylamide (29:1) gel containing 0.25× Tris-borate-EDTA. The specificity of Cdx binding was assessed by the addition of a 100-fold excess of unlabeled probe prior to the addition of the radiolabeled probe. A 23-bp doubled-stranded DNA fragment of the sucrase-isomaltase promoter, SIF1 (5′GTGCAATAAAACTTTATGAGTAA3′), which contains two sequences interacting with the Cdx2 protein, was used as a positive control and as a competitor for some binding assays (59, 60). Two unrelated double-stranded DNA fragments (TB2 and SP1) were also used as competitors. The sequence of the 97-bp double-stranded oligonucleotide TB2 used for this study was 5′TGGCAAACCGACCCCAACCTCTACACAAAGCCCAGAGGGGATACAAAGCCGGGGACCCGAAGTTGTTGTACCCGATTCTAAGTCACCACCTCCCCCG3′, and the sequence of the 22-bp double-stranded oligonucleotide SP1 was 5′ATTCGATCGGGGCGGGGCGAGC3′.
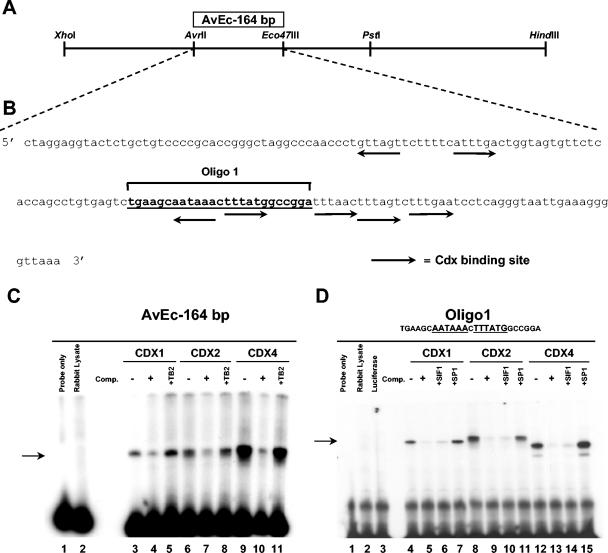
FIG. 3.
Characterization of the AvrII-Eco47III 164-bp fragment. (A) Restriction map of the 892-bp XhoI/HindIII fragment, extending from kb +4.04 to +4.93 in the 3′ half of the MES regulatory region. The box denotes the location of the AvEc 164-bp regulatory region defined by transgenic studies. (B) Complete sequence of the AvEc 164-bp fragment. Arrows indicate the positions and orientations of potential Cdx binding sites, while the bold underlined sequence corresponds to Oligo1 used in EMSA experiments. Oligo1 contains two potential Cdx binding sites. (C) The capacity of the AvEc 164-bp fragment to bind Cdx proteins produced in vitro was tested by EMSA (lanes 3, 6, and 9). Specific binding complexes were competed by a 100-fold excess of unlabeled probe (lanes 4, 7, and 10). The specificity of Cdx protein binding was also assessed by the addition of a 100-fold excess of an unrelated primer, TB2 (lanes 5, 8, and 11). (D) EMSA with Cdx proteins produced in vitro and with Oligo1 (lanes 4, 8, and 12). The specificity of Cdx protein binding to Oligo1 was assessed by the addition of a 100-fold excess of unlabeled Oligo1 probe (lanes 5, 9, and 13), a SIF1 positive control (lanes 6, 10, and 14), or an unrelated SP1 primer (lanes 7, 11, and 15) to the binding reactions. Comp., competitor; SIF1, sucrase-isomaltase factor 1.
Supershift assays.
E9.5 whole-cell extracts were prepared as described previously (13). Binding reactions containing 0.5 to 4 ng of probe (100,000 cpm), 20 mM HEPES (pH 8), 60 mM KCl, 1 mM MgCl2, 0.5 mM dithiothreitol, 0.1 mM EDTA, 10% glycerol, and 1 μg of poly(dI-dC) were incubated for 15 min at 25°C. The Cdx4 antibody (provided by C. Wright) was then added, and the incubation was continued for an additional 20 min at 25°C. The reaction products were separated by electrophoresis through a 6% polyacrylamide (29:1) gel containing 0.25× Tris-borate-EDTA and 2.5% glycerol. The specificity of antibody binding was assessed by the use of a control antibody raised against skeletal muscle calsequesterin obtained from E. Lonergan. Supershift assays were also done with Cdx1 and Cdx2 antibodies obtained from D. Lohnes.
RESULTS
Deletion analysis of Hoxa5 MES.
We previously identified a 5.17-kb DNA region encompassing the Hoxa5 locus (construct 1) (Fig. 1B) that can specifically direct expression in mesodermal derivatives of the cervical-upper thoracic domain (36). This sequence includes a 2.1-kb HindIII fragment (MES) possessing enhancer properties and localized downstream of Hoxa5 coding sequences between kb +2.85 and +4.93. In E12.5 F0 transgenic embryos carrying construct 1, β-galactosidase expression was detected in the prevertebral column between pv3 and pv10/11, with a stronger signal in the pv3-6 region (Fig. 2A) (36). The urogenital tract and the forelimb buds also expressed the transgene, in addition to the hindlimbs in a few specimens (not shown). No transgene expression was detected in the neural tube since the BSC enhancer element was not included in construct 1 (Fig. 1) (61).
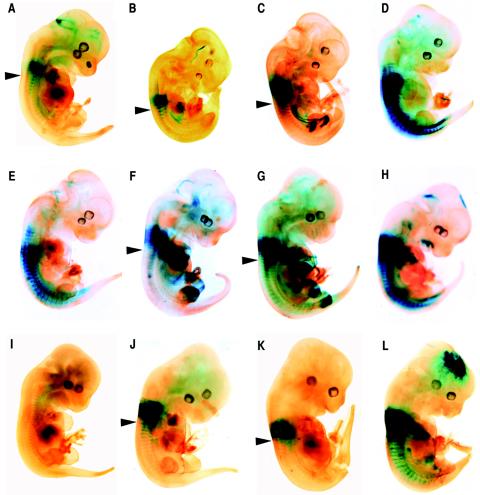
FIG. 2.
Representative transgenic E12.5 embryos stained for β-galactosidase activity showing the effects of different deletion constructs on the expression pattern of the Hoxa5/lacZ transgene. (A) Construct 1. (B) Construct 2. (C) Construct 3. (D) Construct 4. (E) Construct 5. (F) Construct 6. (G) Construct 7. (H) Construct 8. (I) Construct 9. (J) Construct 10. (K) Construct 11. (L) Construct 12. Arrowheads indicate the posterior boundary of strong transgene expression in the prevertebral column. Similar patterns of staining in the prevertebrae were observed for constructs 1, 2, 3, 6, 7, 10, and 11, with the presence of a posterior limit of expression at the upper thoracic level. This posterior restriction of expression along the anterior-posterior axis was lost with constructs 4, 5, 8, and 12. No lacZ expression was detected in F0 transgenic embryos with construct 9.
Deletions of the MES fragment were made to delineate the DNA elements involved in tissue-specific expression and were tested in E12.5 F0 transgenic embryos. The removal of 331 bp from the 3′ end of the MES resulted in a pattern similar to that observed with construct 1, except that expression in the mesenchymal condensations of the developing limb buds was detected in only one transgenic embryo (construct 2) (Fig. 1C and 2B; not shown). The deletion of an additional 160 bp (construct 3) resulted in an X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactoypyranoside) staining pattern comparable to that seen for constructs 1 and 2 (Fig. 2C). Furthermore, staining in the limb buds was recovered in six of eight F0 β-galactosidase-expressing transgenic embryos (Fig. 1C). In concordance with the delay in the formation of embryonic posterior structures versus more anterior ones, the forelimb buds appeared fully stained, whereas only the distal extremities of hindlimb buds, corresponding to the interdigital region, were positive. Construct 3 was also expressed in the guts of five F0 β-galactosidase-expressing transgenic embryos and in the urogenital tracts of all β-galactosidase-expressing embryos (Fig. 1C and 2C).
When an extra 164-bp DNA fragment was removed from the 3′ terminus of the MES (construct 4), all of the F0 β-galactosidase-expressing embryos obtained showed strong X-Gal staining in the prevertebral column, from the anterior limit at pv3 to the caudal end of the embryo, indicating that the posterior restriction at pv10 was lost (Fig. 1 and 2D). This 3′ deletion also led to the absence of reporter gene expression in the developing limb buds, the urogenital system, and the gut for the majority of F0 β-galactosidase-expressing embryos, suggesting the presence of tissue-specific regulatory elements in the AvrII-HindIII 655-bp sequence. We also performed an additional 3′ deletion up to the XhoI site located at kb +4.04 that generated construct 5 (Fig. 1B). As observed for construct 4, most of the F0 β-galactosidase-expressing embryos expressed the transgene from pv3 down to the tail of the embryo (Fig. 2E). Furthermore, no transgene expression was detected in the urogenital tract, the limb buds, or the gut (Fig. 1C). Taken together, these results indicated that the 3′ half of the MES encompasses several cis-acting regulatory elements that are involved in specific regional control activities. The loss of posterior restriction along the prevertebral column also suggested that sequences between the AvrII (kb +4.28) and Eco47III (kb +4.44) restriction sites are involved in the positioning of the posterior boundary of expression at the upper thoracic level.
To further define the contribution of the 892-bp XhoI-HindIII DNA fragment to MES activity, we designed constructs carrying this DNA sequence either at the 3′ end (construct 6) (Fig. 1B) or at the 5′ end (construct 7) (Fig. 1B) of a Hoxa5/lacZ minimal construct. This Hoxa5/lacZ minimal vector included 3.09 kb of Hoxa5 genomic sequence, from bp −235 to kb +2.85, that on its own cannot direct transgene expression (64). Constructs 6 and 7 presented similar patterns of expression in the interdigital region and the mesenchymal condensations along the proximodistal axis of the limb buds and in the gut (Fig. 1C and 2F and G). Only F0 β-galactosidase-expressing embryos for construct 7 showed X-Gal staining in the urogenital tract (Fig. 1C). Like constructs 1, 2, and 3, constructs 6 and 7 drove β-galactosidase expression in the cervical region of the prevertebral column, with a well-established posterior limit in the upper thoracic region (Fig. 2F and G). The results described above suggested that the XhoI-HindIII sequence was able to direct region-specific expression independent of its position relative to the Hoxa5 promoter. However, the XhoI-HindIII fragment was unable to drive transgene expression when tested in front of a heterologous promoter (hsp68/lacZ) (not shown), suggesting that the Hox genomic context is essential for the regulatory action of the 3′ fragment of the MES region. These results also indicated that the genuine enhancer activity of the MES relies on its integrity.
Distinct MES regulatory sequences mediate Hoxa5/lacZ regional expression.
The 3′ sequential deletion of the MES suggested that the AvEc 164-bp fragment contains DNA regulatory elements that restrict Hoxa5/lacZ transgene expression to the cervical-upper thoracic region along the axis. We directly tested this hypothesis by removing the 164-bp sequence from construct 1 to generate construct 8 (Fig. 1B). Deletion of the AvEc 164-bp fragment resulted in a loss of posterior restriction along the prevertebral column for three of five F0 β-galactosidase-expressing transgenic embryos (Fig. 1C). Transgene expression was stronger in the cervical region, but the extent of the loss of posterior restriction varied from embryo to embryo (Fig. 2H). The loss of posterior restriction was the only difference in expression between constructs 1 and 8, as both constructs drove lacZ gene expression in the urogenital system and the limb buds but not in the gut (Fig. 1C and 2H). When linked to the Hoxa5/lacZ minimal vector (construct 9) (Fig. 1B), the AvEc 164-bp fragment did not direct any reporter gene expression, indicating that it has no enhancer properties (Fig. 1C and 2I). Altogether, these data show that the AvEc 164-bp fragment contains DNA elements that participate in the positioning of the posterior boundary of Hoxa5 expression at the upper thoracic level along the axial skeleton.
Cdx gene products bind in vitro to the AvrII-Eco47III 164-bp fragment.
To gain insight into critical sequences involved in axial regional specification, we investigated whether the AvEc 164-bp fragment contains binding sites for known trans-acting factors that may be involved in transducing positional information to Hox genes. By sequence comparisons with the TFsearch database (http://molsun1.cbrc.aist.go.jp/research/db/TFSEARCH.html), we found, among several binding sites of putative Hox transcriptional regulators, seven potential sites with high homology to the consensus (TTTATA/G) for the Cdx gene products (Fig. 3B) (39, 52, 59). Sequence comparisons of the surrounding Hoxa5 genomic environment revealed that Cdx consensus binding sites were predominantly localized in the MES region and that the majority of these Cdx binding sites were confined to the 3′ half of the AvEc 164-bp fragment (Fig. 3B). To establish whether Cdx proteins can bind the AvEc 164-bp fragment, we performed EMSAs with the three murine Cdx proteins, Cdx1, Cdx2, and Cdx4, which we produced in vitro (Fig. 3C). All three Cdx proteins formed binding complexes with the AvEc 164-bp fragment (Fig. 3C, lanes 3, 6, and 9). The specificity of binding was confirmed by competition studies with a 100-fold excess of unlabeled probe, which resulted in a substantial reduction of Cdx protein binding (Fig. 3C, lanes 4, 7, and 10), and by the addition of a 100-fold excess of an unrelated primer, TB2 (lanes 5, 8, and 11). Further characterization of the specific interactions of Cdx proteins with each half of the AvEc 164-bp fragment revealed that the Cdx1, Cdx2, and Cdx4 proteins bound specifically to the 3′ half of the fragment. No complex with the 5′ half of the AvEc 164-bp fragment was detected, despite the presence of two potential Cdx binding sites (not shown). To identify which sites from the 3′ half of the region were involved in binding activity, we tested synthetic oligonucleotides containing the potential Cdx binding sequences by an EMSA with the three proteins. We identified a 25-bp double-stranded oligonucleotide named Oligo1, which spans two Cdx consensus sites, as the only target for Cdx protein binding (Fig. 3D, lanes 4, 8, and 12). Indeed, the other three consensus sites for caudal-related proteins located in the 3′ half of the AvEc 164-bp fragment did not form any complexes with Cdx proteins (not shown). Cdx protein binding to Oligo1 was efficiently competed by the addition of a 100-fold excess of unlabeled probe (Fig. 3D, lanes 5, 9, and 13). The specificity of the interaction was further demonstrated by use of the competitor SIF1, a 23-bp double-stranded DNA fragment of the sucrase-isomaltase promoter which was previously shown to interact with Cdx2 (Fig. 3D, lanes 6, 10, and 14) (59). Finally, additional support for the specificity of binding was provided by the addition of a 100-fold excess of an unrelated SP1 primer (Fig. 3D, lanes 7, 11, and 15).
To compare the importance of each site for the formation of Cdx-Oligo1 complexes, we introduced three nucleotide substitutions into Cdx binding sites 1 and 2, either individually or in combination (Fig. 4A). While nucleotide substitutions in site 1 (mut1) or site 2 (mut2) did not alter the binding of the three Cdx proteins to Oligo1 (Fig. 4B, lanes 2 to 4, 10 to 12, and 14 to 16), their combined mutations (mut1,2) in Oligo1 abolished the formation of specific binding complexes (Fig. 4B, lanes 6 to 8).
To further establish the functional significance of the two Cdx binding sites, we produced E12.5 F0 Hoxa5/lacZ transgenic embryos to assess the effect on transgene expression of nucleotide substitutions at sites 1 and 2, either individually or in combination. These mutations were introduced into construct 1 to generate constructs 10, 11, and 12, respectively (Fig. 1B). Transgenic embryos for constructs 10 or 11 carrying individual mutations displayed the same regionally restricted expression pattern of lacZ staining as their cognate wild-type construct (Fig. 1C and 2A, J, and K). Expression in the urogenital system, the developing limb buds, and the gut was detected in some F0 β-galactosidase-expressing specimens (Fig. 1C). In contrast, all F0 β-galactosidase-expressing embryos carrying construct 12, with nucleotide substitutions in both binding sites, presented a lack of posterior restriction along the prevertebral column (Fig. 1C and 2L). X-Gal staining was stronger in the cervical region and extended caudally. Altogether, these results indicated that the Cdx gene products can interact with the AvEc 164-bp fragment via two Cdx consensus binding sites confined within a 25-bp sequence and that this interaction may participate in vivo in the positioning of a posterior boundary of expression in the upper thoracic region.
Hoxa5/lacZ transgene expression in Cdx mutant backgrounds.
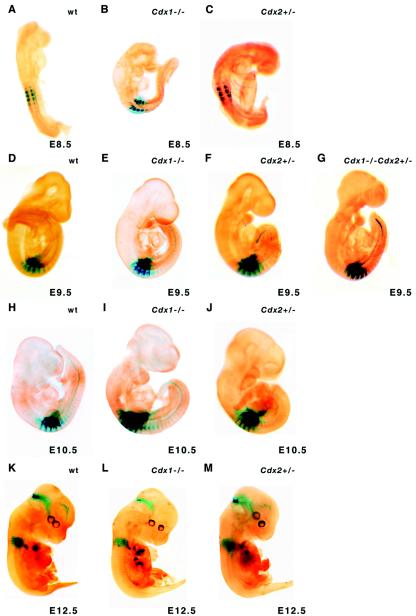
Experimental data have shown that the overexpression or loss of function of Cdx genes can affect Hox/lacZ transgene activity and directly regulates endogenous Hox gene expression upon interaction with Cdx binding sites in the flanking regions of Hox genes (13, 58, 62). To test the in vivo requirement of Cdx1 or Cdx2 gene function in the correct positioning of the Hoxa5 expression domain, we verified whether the expression of construct 1 could be altered in Cdx1 or Cdx2 mutant embryos. To do so, we introduced the pLJ133 Hox/lacZ transgene (corresponding to construct 1) into the Cdx1 or Cdx2 mutant background by successive matings. E12.5 transgenic embryos carrying the Hoxa5/lacZ transgene and either the Cdx1 or Cdx2 mutant allele were generated and stained for β-galactosidase activity. In Cdx1+/+ pLJ133+/− transgenic embryos, X-Gal staining was detected in the forelimb buds, the urogenital tract, and the cervical region of the prevertebral column, with a posterior limit at the upper thoracic level, similar to the staining obtained for construct 1 (Fig. 2A and 5K). The loss of Cdx1 function did not affect the expression pattern of the transgene, and no posterior shift of the caudal boundary along the prevertebral column could be observed for the majority of the specimens analyzed in the cohort (Cdx1−/− pLJ133+/−) (Fig. 5L). Although early embryonic lethality precluded the analysis of Cdx2−/− mutants, the loss of one Cdx2 allele causes alterations in vertebral identity at the anterior thoracic and posterior cervical levels (14). Cdx2+/− pLJ133+/− embryos displayed a lacZ staining profile similar to that of control and Cdx1−/− pLJ133+/− specimens (Fig. 5M), suggesting that the lack of both Cdx1 alleles or one Cdx2 allele does not affect the regional expression of construct 1.
FIG. 5.
Effect of Cdx1 and Cdx2 mutations on Hoxa5/lacZ transgene expression patterns. (A to C) pLJ57 transgene expression at E8.5. (D to F) pLJ57 transgene expression at E9.5. (H to J) pLJ57 transgene expression at E10.5. (K to M) pLJ133 (construct 1) expression at E12.5. The pLJ57 transgene reconstitutes Hoxa5 endogenous temporal expression. No major change in expression profile was observed between wild-type (wt), Cdx1−/−, and Cdx2+/− embryos at E8.5, E9.5, and E10.5. At E12.5, the pLJ133 expression profile for wild-type embryos (K) was identical to that observed for Cdx1−/− (L) and Cdx2+/− (M) embryos. pLJ57 transgene expression was also analyzed in a Cdx1−/− Cdx2+/− compound background. No change in the expression profile was detected at E9.5 (G).
Construct 1 can direct β-galactosidase activity in a narrow window of time, from E11 to E13 (J. Lapointe and L. Jeannotte, unpublished data). To assess the effect of the Cdx1 and Cdx2 mutations on the expression pattern of the Hoxa5/lacZ transgene at earlier stages of embryogenesis, we introduced the pLJ57 transgene into both Cdx mutant backgrounds. The pLJ57 construct allows lacZ expression from E8.0 to E8.25 and onward, reproducing the endogenous temporal expression of the Hoxa5 gene (36). E8.5, E9.5, and E10.5 transgenic embryos carrying both the pLJ57 transgene and the Cdx1 or Cdx2 mutant allele were analyzed. As reported for Cdx mutant embryos carrying the pLJ133 transgene, we did not detect noticeable variations in pLJ57 regional expression in the cohorts of Cdx1−/− pLJ57+/− and Cdx2+/− pLJ57+/− specimens at all embryonic ages analyzed (Fig. 5A to F and H to J). Since functional redundancy between the Cdx1 and Cdx2 genes can occur in Hox gene regulation (62), we also introduced the pLJ57 transgene into the Cdx1−/− Cdx2+/− mutant background. As shown for E9.5, no alteration of the transgene expression profile was noticed in Cdx1−/− Cdx2+/− pLJ57+/− embryos (Fig. 5G). An examination of the Hoxa5 endogenous expression pattern also failed to reveal any changes in Cdx1−/− embryos, thus validating the transgene expression studies (not shown). Taken together, these results indicate that a loss of Cdx1 function, a Cdx2 haploinsufficiency, or a combination of both does not perturb the regional expression of the Hoxa5 gene along the prevertebral column.
Cdx4-containing embryonic protein extracts form complexes with Oligo1.
The lack of genetic evidence supporting the involvement of the Cdx1 and Cdx2 genes in the control of Hoxa5 regional expression led us to evaluate whether the third member, Cdx4, could be implicated in Hoxa5 gene regulation. Since no Cdx4 mutant mouse line has been reported, we used a biochemical approach. First, we tested whether the Cdx gene products present in embryos possessed the ability to bind the Oligo1 sequence. We performed EMSAs with E9.5 embryo protein extracts and with Oligo1, Oligo1 (mut1), Oligo1 (mut2), and Oligo1 (mut1,2), used as probes (Fig. 6A). Proteins present in the extract were able to bind Oligo1, Oligo1 (mut1), and Oligo1 (mut2) (Fig. 6A, lanes 2, 6, and 10). In the last case, the different shift pattern suggested that other proteins may bind this mutated form. The specificity of binding was confirmed by competition studies with a 100-fold excess of unlabeled probe that resulted in the abolition of protein binding (Fig. 6A, lanes 3, 7, and 11). Moreover, the addition of a 100-fold excess of unlabeled Oligo1 (mut1,2) was unable to efficiently compete for protein binding (Fig. 6A, lanes 4, 8, and 12), suggesting that proteins from the embryo extract bind Oligo1 specifically via the two Cdx binding sites identified. An EMSA with Oligo1 (mut1,2) produced a specific pattern of bands revealing that different proteins can bind Oligo1 (mut1,2) (Fig. 6A, lane 14). This specificity of binding was confirmed by competition with a 100-fold excess of an unlabeled Oligo1 (mut1,2) probe, demonstrating that these proteins were indeed different from those binding Oligo1 (Fig. 6A, lane 15). The specificity of binding was also assessed by the addition of a 100-fold excess of the SP1 binding site (Fig. 6A, lane 16).
FIG. 6.
In vivo detection of Cdx protein binding to Oligo1 sequence. (A) EMSA with E9.5 embryonic protein extract and different versions of Oligo1 (lanes 2, 6, 10, and 14). The specificity of binding to Oligo1 was assessed by the addition of a 100-fold excess of unlabeled Oligo1 probe (lane 3). Similar results were observed for binding to Oligo1 (mut1) and Oligo1 (mut2) when we used unlabeled Oligo1 (mut1) (lane 7) and Oligo1 (mut2) (lane 11), respectively, as competitors. The addition of a 100-fold excess of unlabeled Oligo1 (mut1,2) was unable to efficiently compete for protein binding (lanes 4, 8, and 12). An EMSA with Oligo1 (mut1,2) produced a specific pattern of bands that was competed by an unlabeled Oligo1 (mut1,2) probe (lanes 14 and 15), revealing that the binding proteins differed from those binding Oligo1. The Oligo1 (mut1,2) binding specificity was also assessed by the addition of a 100-fold excess of the SP1 binding site (lane 16). (B) EMSA with in vitro-translated Cdx proteins and the AvEc 164-bp fragment (lanes 4, 7, and 10). In the presence of an anti-Cdx4 antibody, a supershift was only observed with the Cdx4 protein (lanes 5, 8, and 11). No supershift was observed when a control antibody (anti-calsequestrin) was used (lanes 6, 9, and 12). Anti-Cdx1 and anti-Cdx2 antibodies also produced specific supershifts with their respective proteins (not shown). (C) EMSA with an Oligo1 probe and an E9.5 embryonic protein extract (lane 2). A near-complete loss of the most abundant Oligo1-protein complex was observed in the presence of the Cdx4 antibody (lane 3), but not when a control antibody was added (lane 4). Ab, antibody; Comp., competitor.
To test if the E9.5 embryo protein complexes that bound the Oligo1 sequence contained Cdx proteins, we performed supershift EMSAs with E9.5 embryo protein extracts, Oligo1, and Cdx-specific antibodies. The specificity of each anti-Cdx antibody was assessed by EMSAs with the different Cdx proteins produced in vitro, as shown for the anti-Cdx4 antibody (Fig. 6B, lanes 5, 8, and 11). The addition of an anti-Cdx4 antibody to the embryo protein extract-Oligo1 reaction led to a near-complete loss of the most abundant Oligo1-protein complex (Fig. 6C, lanes 2 and 3). This may have been caused either by the inhibition of stable DNA-protein complex formation following antibody binding or by the strong alteration of the electrophoretic mobility of the DNA-protein-antibody complex. Such a result was not observed when an anti-Cdx1, anti-Cdx2, or control antibody (anticalsequesterin) was used (Fig. 6C, lane 4; not shown). Taken together, these results demonstrate that Cdx4 present in E9.5 embryo protein extracts can specifically bind the Oligo1 sequence. Thus, Cdx4 appears as a potential regulator of the correct positioning of the posterior boundary of Hoxa5 expression in the upper thoracic region along the axial skeleton.
DISCUSSION
MES sequence directs Hoxa5 expression in several structures.
The correct development of structures from the cervical and upper thoracic axial level necessitates the proper control of Hoxa5 gene expression. To define the mechanisms involved, we have refined our analysis of the MES region. In addition to directing expression in the skeleton at this axial level, the MES sequence encloses DNA elements in its 3′ half that are able to target transgene expression in the limb buds and in the urogenital and intestinal tracts (Fig. 1). Although the present work did not focus on the delimitation of these tissue-specific elements, insights can be gained from our deletion analysis. For instance, gut expression seems to result from the equilibrium between positive and negative sequences, located between the AvrII (kb +4.28) and Eco47III (kb +4.44) restriction sites and the Eco47III (kb +4.44) and HindIII (kb +4.93) restriction sites, respectively. Additional cis-acting sequences located in the proximity of the Hoxa4 gene are also required for gut expression, suggesting the coordinated action of several regulatory elements (42). As for the urogenital tract, we can infer that a combination of DNA elements, including the AvEc 164-bp fragment, is required for expression in this organ system. The control of limb bud expression also implicates interspersed elements in the XhoI (kb +4.04) to HindIII (kb +4.93) region. Our analysis clearly demonstrates that coordination between several cis-acting regulatory elements is needed to fully reproduce the correct spatial and temporal Hoxa5 gene profile. Further work will be needed to finely map these additional regulatory sequences.
Positioning of Hoxa5 posterior boundary depends on MES sequences.
The definition of adequate boundaries is indispensable for proper Hox gene action. The functional limits of Hoxa5, as delineated by the axial skeletal phenotype of Hoxa5 mutant mice, are restricted to the pv3-10 region (2, 29). Specific expression in this domain can be driven by an 892-bp XhoI-HindIII DNA fragment (constructs 6 and 7) (Fig. 1 and 2). Posterior restriction at the pv10 level is further delimitated by the AvEc 164-bp fragment enclosed within the XhoI-HindIII fragment, which acts as a repressor. We have shown that the pv3-10 region corresponds to the specific expression domain of the 1.8-kb transcript (36). This transcript is also the sole Hoxa5 transcript expressed in the mesenchyme of several structures that are affected by the mutation at this axial level, such as the respiratory tract, the thyroid gland, and the pectoral girdle (1, 2, 6, 40). Since this transcript is likely to carry the functional form of the Hoxa5 gene, its expression domain must be tightly regulated. The transgenes used for the present study reflect the regulation of the proximal promoter from which the 1.8-kb transcript is produced, as the distal promoter, which resides in the vicinity of the Hoxa7 gene and is responsible for transcription of the larger forms, was not included in the constructs (Y. Coulombe and L. Jeannotte, unpublished data). Consequently, this approach provides us with a useful means to elucidate the regulatory mechanisms governing Hoxa5 regional expression in its functional domain.
As proposed by Kmita and Duboule (31), axial patterning relies upon a given partitioning of Hox functional domains along the developing anterior-posterior axis. The concept of posterior prevalence states that posteriorly expressed Hox genes functionally prevail over more anterior genes. Hence, the functional readout of the Hox overlapping transcription domains may reflect the prominent function of the most caudal Hox protein produced in a particular region (16). Several strategies may be envisaged to explain the suppressive mechanism by which a posterior Hox gene imposes its functional dominance (31). In the present case, a transcription-based partitioning of Hoxa5 gene function may account for the restriction of the functional domain to the pv3-10 area. Thus, the control provided by the AvEc 164-bp fragment may be an important regulatory event in cervicothoracic axial specification through the constraint of the Hoxa5 expression domain. The Hoxb5 gene also displays a pattern of expression that represents the sum of multiple Hoxb5 transcripts (34). The Hoxb5 mutation exerts its effect in the most anterior expression domain of the gene, a region where the Hoxb5 protein is specifically found (51, 53). One may speculate that Hoxb5 gene expression is transcriptionally regulated similarly to its paralog Hoxa5 and that the Hoxb5 protein expression domain reflects the distribution of the Hoxb5 major transcript. On the other hand, posttranscriptional events whereby a Hox transcript is selectively destabilized in its posterior domain may be used to confine Hox gene expression, as demonstrated for the Hoxb4 gene (11). These strategies may coexist, but we do not have evidence that posttranscriptional control occurs in Hoxa5 spatial regulation (S. Tabariès and L. Jeannotte, unpublished data). While much emphasis has been given to the regulation of the establishment and maintenance of the anterior boundary of expression (e.g., see references 17, 22, 32, and 46), our present work underscores the importance of defining posterior boundaries as well.
Cdx gene products participate in restriction of Hox gene expression.
caudal-related genes are involved in anterior-posterior patterning by regulating members of the Hox gene family in various species (13, 18, 26, 28, 41, 58). Our demonstration that Cdx proteins physically interact with Hoxa5 regulatory sequences and that they likely participate in the definition of the Hoxa5 functional domain further confirms the importance of Cdx genes in axial patterning via Hox gene regulation. While Cdx proteins are mainly recognized for their role as positive regulators of Hox gene expression (13, 58, 62), our data show that murine Cdx proteins may also act to restrict Hox gene expression. This was also shown to be the case for Drosophila, in which caudal acts as a repressor of the Abd-B homeotic gene during analia structure formation (43). Moreover, the fact that Xcad overexpression in Xenopus embryos leads to the repression of anterior Hox genes while posterior Hox genes exhibit more anterior expression substantiates the notion of a repressive function for Cdx proteins (19, 28). Taken together, these data suggest that caudal-related gene products possess dual functions of posterior-promoting and anterior-suppressing activities toward Hox gene expression, as proposed by Isaacs and colleagues (28). This dual role would allow Cdx proteins to differentially modulate the expression of subsets of Hox genes, resulting in the definition of specific Hox functional domains that in turn would set up the correct posterior development of the organism. For Hoxa5, the binding of Cdx proteins to the AvEc 164-bp fragment would preclude its action posterior to the pv10 limit. However, Hoxa5 posterior restriction also depends on other regulators since the mutation of both Cdx binding sites did not result in a gain of posterior expression of the transgene that was as strong and as distal as that obtained for some of the deletions tested (construct 12 versus construct 4, 5, or 8) (Fig. 2D, E, H, and L). Recent data also support the notion that additional sequences located in the 3′ extremity of the MES region may cooperate with the AvEc 164-bp fragment for Hoxa5 regional expression (Tabariès and Jeannotte, unpublished data). Finally, since Cdx proteins may act as intermediates of retinoic acid, Wnt, and fibroblast growth factors (38), it remains to be defined if these signaling molecules impinge on the correct setting of Hoxa5 functional boundaries via Cdx transduction.
Despite the fact that the three murine Cdx proteins possess the ability to bind in vitro the Hoxa5 AvEc 164-bp fragment, Cdx4 emerged as the potential in vivo trans-acting factor for Hoxa5 regulation. The Cdx4 protein was also shown to regulate Hoxb8 expression (13). The Cdx1 and Cdx2 proteins can likely be excluded from Hoxa5 regulation based on biochemical and genetic evidence. The absence of a modification of the Hoxa5 expression pattern along the prevertebral column of single or compound Cdx1 and Cdx2 mutants was unanticipated considering that the homeotic transformations observed in Cdx mutants encompassed the functional domain of Hoxa5. However, although similarities exist between some of the skeletal transformations seen in Hoxa5 and Cdx mutants, most of the vertebrae affected do not display the same type of transformation. For instance, the predominant homeotic defect observed in Hoxa5 mutant mice is the posteriorization of the 7th cervical vertebra (C7) into a thoracic one. In contrast, in Cdx mutants, C7 adopts a C6 identity (2, 14, 29, 58, 62). Moreover, for Cdx2 heterozygous specimens, we could not exclude the possibility that enough Cdx2 protein remained to ensure proper Hoxa5 regulation. Unfortunately, to our knowledge, no Cdx4 mutant mouse line is available to genetically address the importance of Cdx4 in Hoxa5 gene regulation.
The three Cdx proteins are expressed in the embryo according to a gradient with a posterior maximum (38). Of these, Cdx4 is the most posteriorly expressed, and its expression ceases at E10.5 (20). Construct 1 and its derivatives did not reproduce the temporal expression pattern of the Hoxa5 endogenous gene, as they directed expression in a narrow time frame, from E11 to E13 (64; Lapointe and Jeannotte, unpublished data). Therefore, the X-Gal staining pattern observed in E12.5 F0 transgenic embryos was likely due to an earlier regulatory event directed by Cdx4. Indeed, in vivo detection of Cdx4 protein binding occurred with E9.5 embryo extracts but not with specimens from older embryos. Altogether, these data suggest that Cdx4 may be involved in the establishment of the correct Hoxa5 posterior boundary at the upper thoracic level.
In summary, our work gives insights into the mechanisms by which Cdx proteins, most likely Cdx4, directly participate in the posterior delimitation of the Hoxa5 functional domain. The data favor the view that a transcription-based partitioning of Hox gene function is a strategy for the establishment of posterior prevalence in the specification of the cervical-upper thoracic axial level.
Acknowledgments
We thank J. Aubin, J. Charron, and C. Séguin for helpful comments on the manuscript and M. Lemieux for technical assistance. We are also grateful to J. Deschamps for providing Cdx plasmids, P. Gruss and D. Lohnes for the Cdx1 mutant mouse line, F. Beck and K. Chawengsaksophak for the Cdx2 mutant mouse line, and E. Lonergan, C. Wright, and D. Lohnes for the calsequestrin, Cdx4, and Cdx1 and Cdx2 antibodies, respectively.
This work was supported by a grant from the National Institutes of Health (RO1-HD38463 to L.J. and C.K.T.). L.J. holds a Chercheur National Award from the Fonds de la Recherche en Santé du Québec.
REFERENCES
- 1.Aubin, J., M. Lemieux, M. Tremblay, J. Bérard, and L. Jeannotte. 1997. Early postnatal lethality in Hoxa-5 mutant mice is attributable to respiratory tract defects. Dev. Biol. 192:432-445. [DOI] [PubMed] [Google Scholar]
- 2.Aubin, J., M. Lemieux, M. Tremblay, R. R. Behringer, and L. Jeannotte. 1998. Transcriptional interferences at the Hoxa4/Hoxa5 locus: importance of correct Hoxa5 expression for the proper specification of the axial skeleton. Dev. Dyn. 212:141-156. [DOI] [PubMed] [Google Scholar]
- 3.Aubin, J., P. Chailler, D. Ménard, and L. Jeannotte. 1999. Loss of Hoxa5 gene function in mice perturbs intestinal maturation. Am. J. Physiol. 277:C965-C973. [DOI] [PubMed] [Google Scholar]
- 4.Aubin, J., and L. Jeannotte. 2001. Implication des gènes Hox dans les processus d'organogenèse chez les mammifères. Médecine/Science 17:54-62. [Google Scholar]
- 5.Aubin, J., U. Déry, M. Lemieux, P. Chailler, and L. Jeannotte. 2002. Stomach regional specification requires Hoxa5-driven mesenchymal-epithelial signaling. Development 129:4075-4087. [DOI] [PubMed] [Google Scholar]
- 6.Aubin, J., M. Lemieux, J. Moreau, J. Lapointe, and L. Jeannotte. 2002. Cooperation of Hoxa5 and Pax1 genes during formation of the pectoral girdle. Dev. Biol. 244:96-113. [DOI] [PubMed] [Google Scholar]
- 7.Behringer, R. R., D. A. Crotty, V. M. Tennyson, R. L. Brinster, R. D. Palmiter, and D. J. Wolgemuth. 1993. Sequences 5′ of the homeobox of the Hox-1.4 gene direct tissue-specific expression of lacZ during mouse development. Development 117:823-833. [DOI] [PubMed] [Google Scholar]
- 8.Béland, M., N. Pilon, M. Houle, K. Oh, J.-R. Sylvestre, P. Prinos, and D. Lohnes. 2004. Cdx1 autoregulation is governed by a novel Cdx1-LEF1 transcription complex. Mol. Cell. Biol. 24:5028-5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bel-Vialar, S., N. Itasaki, and R. Krumlauf. 2002. Initiating Hox gene expression: in the early chick neural tube differential sensitivity to FGF and RA signaling subdivides the HoxB genes in two distinct groups. Development 129:5103-5115. [DOI] [PubMed] [Google Scholar]
- 10.Bradshaw, M. S., C. S. Shashikant, H.-G. Belting, J. A. Bollekens, and F. H. Ruddle. 1996. A long-range regulatory element of Hoxc8 identified by using pClaster vector. Proc. Natl. Acad. Sci. USA. 93:2426-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brend, T., J. Gilthorpe, D. Summerbell, and P. W. Rigby. 2003. Multiple levels of transcriptional and post-transcriptional regulation are required to define the domain of Hoxb4 expression. Development 130:2717-2728. [DOI] [PubMed] [Google Scholar]
- 12.Chambeyron, S., and W. A. Bickmore. 2004. Chromatin decondensation and nuclear reorganization of the HoxB locus upon induction of transcription. Genes Dev. 18:1119-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charité, J., W. de Graaff, D. Consten, M. J. Reijnen, J. Korving, and J. Deschamps. 1998. Transducing positional information to the Hox genes: critical interaction of cdx gene products with position-sensitive regulatory elements. Development 125:4349-4358. [DOI] [PubMed] [Google Scholar]
- 14.Chawengsaksophak, K., R. James, V. E. Hammond, F. Kontgen, and F. Beck. 1997. Homeosis and intestinal tumours in Cdx2 mutant mice. Nature 386:84-87. [DOI] [PubMed] [Google Scholar]
- 15.Dony, C., and P. Gruss. 1987. Specific expression of the Hox1.3 homeobox gene in murine embryonic structures originating from or induced by the mesoderm. EMBO J. 6:2965-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duboule, D., and G. Morata. 1994. Colinearity and functional hierarchy among genes of the homeotic complexes. Trends Genet. 10:358-364. [DOI] [PubMed] [Google Scholar]
- 17.Dupé, V., M. Davenne, J. Brocard, P. Dollé, M. Mark, A. Dierich, P. Chambon, and F. M. Rijli. 1997. In vivo functional analysis of the Hoxa-1 3′ retinoic acid response element (3′RARE). Development 124:399-410. [DOI] [PubMed] [Google Scholar]
- 18.Ehran, L. A., and K. E. Yutzey. 2001. Anterior expression of the caudal homologue cCdx-B activates a posterior genetic program in avian embryos. Dev. Dyn. 221:412-421. [DOI] [PubMed] [Google Scholar]
- 19.Epstein, M., G. Pillemer, R. Yelin, J. K. Yisraeli, and A. Fainsod. 1997. Patterning of the embryo along the anterior-posterior axis: the role of the caudal genes. Development 124:3805-3814. [DOI] [PubMed] [Google Scholar]
- 20.Gamer, L. W., and C. V. Wright. 1993. Murine Cdx-4 bears striking similarities to the Drosophila caudal gene in its homeodomain sequence and early expression pattern. Mech. Dev. 43:71-81. [DOI] [PubMed] [Google Scholar]
- 21.Gaunt, S. J., P. L. Coletta, D. Pravtcheva, and P. T. Sharpe. 1990. Mouse Hox-3.4: homeobox sequence and embryonic expression patterns compared with other members of the Hox gene network. Development 109:329-339. [DOI] [PubMed] [Google Scholar]
- 22.Gérard, M., J. Y. Chen, H. Gronemeyer, P. Chambon, D. Duboule, and J. Zakany. 1996. In vivo targeted mutagenesis of a regulatory element required for positioning the Hoxd-11 and Hoxd-10 expression boundaries. Genes Dev. 10:2326-2334. [DOI] [PubMed] [Google Scholar]
- 23.Gould, A., A. Morrison, G. Sproat, R. A. White, and R. Krumlauf. 1997. Positive cross-regulation and enhancer sharing: two mechanisms for specifying overlapping Hox expression patterns. Genes Dev. 11:900-913. [DOI] [PubMed] [Google Scholar]
- 24.Hogan, B., R. S. Beddington, F. Constantini, and E. Lacy. 1994. Manipulating the mouse embryo: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 25.Houle, M., J. R. Sylvestre, and D. Lohnes. 2003. Retinoic acid regulates a subset of Cdx1 function in vivo. Development 130:6555-6567. [DOI] [PubMed] [Google Scholar]
- 26.Hunter, C. P., J. M. Harris, J. N. Maloof, and C. Kenyon. 1999. Hox gene expression in a single Caenorhabditis elegans cell is regulated by a caudal homolog and intercellular signals that inhibit wnt signaling. Development 126:805-814. [DOI] [PubMed] [Google Scholar]
- 27.Ikeya, M., and S. Takada. 2001. Wnt-3a is required for somite specification along the anteroposterior axis of the mouse embryo and for regulation of cdx-1 expression. Mech. Dev. 193:27-33. [DOI] [PubMed] [Google Scholar]
- 28.Isaacs, H. V., M. E. Pownall, and J. M. Slack. 1998. Regulation of Hox gene expression and posterior development by the Xenopus caudal homologue Xcad3. EMBO J. 17:3413-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeannotte, L., M. Lemieux, J. Charron, F. Poirier, and E. J. Robertson. 1993. Specification of axial identity in the mouse: role of the Hoxa-5 (Hox1.3) gene. Genes Dev. 7:2085-2096. [DOI] [PubMed] [Google Scholar]
- 30.Kmita, M., N. Fraudeau, Y. Hérault, and D. Duboule. 2002. Serial deletions and duplications suggest a mechanism for the collinearity of Hoxd genes in limbs. Nature 420:145-150. [DOI] [PubMed] [Google Scholar]
- 31.Kmita, M., and D. Duboule. 2003. Organizing axes in time and space; 25 years of colinear tinkering. Science 301:331-333. [DOI] [PubMed] [Google Scholar]
- 32.Knittel, T., M. Kessel, M. H. Kim, and P. Gruss. 1995. A conserved enhancer of the human and murine Hoxa-7 genes specifies the anterior boundary of expression during embryonal development. Development 121:1077-1088. [DOI] [PubMed] [Google Scholar]
- 33.Kondo, T., and D. Duboule. 1999. Breaking colinearity in the mouse HoxD complex. Cell 97:407-417. [DOI] [PubMed] [Google Scholar]
- 34.Krumlauf, R., P. W. H. Holland, J. H. McVey, and B. L. M. Hogan. 1987. Developmental and spatial patterns of expression of the mouse homeobox gene Hox2.1. Development 99:603-617. [DOI] [PubMed] [Google Scholar]
- 35.Krumlauf, R. 1994. Hox genes in vertebrate development. Cell 78:191-201. [DOI] [PubMed] [Google Scholar]
- 36.Larochelle, C., M. Tremblay, D. Bernier, J. Aubin, and L. Jeannotte. 1999. Multiple cis-acting regulatory regions are required for restricted spatio-temporal Hoxa5 gene expression. Dev. Dyn. 214:127-140. [DOI] [PubMed] [Google Scholar]
- 37.Lickert, H., C. Domon, G. Huls, C. Wehrle, I. Duluc, H. Clevers, B. I. Meyer, J. N. Freund, and R. Kemler. 2000. Wnt/(beta)-catenin signaling regulates the expression of the homeobox gene Cdx1 in embryonic intestine. Development 127:3805-3813. [DOI] [PubMed] [Google Scholar]
- 38.Lohnes, D. 2003. The Cdx1 homeodomain protein: an integrator of posterior signaling in the mouse. Bioessays 25:971-980. [DOI] [PubMed] [Google Scholar]
- 39.Margalit, Y., S. Yarus, E. Shapira, Y. Gruenbaum, and A. Fainsod. 1993. Isolation and characterization of target sequences of the chicken CdxA homeobox gene. Nucleic Acids Res. 21:4915-4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meunier, D., J. Aubin, and L. Jeannotte. 2003. Perturbed thyroid morphology and transient hypothyroidism symptoms in Hoxa5 mutant mice. Dev. Dyn. 227:367-378. [DOI] [PubMed] [Google Scholar]
- 41.Mlodzik, M., G. Gibson, and W. J. Gehring. 1990. Effects of ectopic expression of caudal during Drosophila development. Development 109:271-277. [DOI] [PubMed] [Google Scholar]
- 42.Moreau, J., and L. Jeannotte. 2002. Sequence analysis of a Hoxa4-Hoxa5 intergenic region including shared regulatory elements. DNA Seq. 13:203-209. [DOI] [PubMed] [Google Scholar]
- 43.Moreno, E., and G. Morata. 1999. Caudal is the Hox gene that specifies the most posterior Drosophila segment. Nature 400:873-877. [DOI] [PubMed] [Google Scholar]
- 44.Morrison, A., L. Ariza-McNaughton, A. Gould, M. Featherstone, and R. Krumlauf. 1997. HOXD4 and regulation of the group 4 paralog genes. Development 124:3135-3146. [DOI] [PubMed] [Google Scholar]
- 45.Nowling, T., W. Zhou, K. E. Krieger, C. Larochelle, M. C. Nguyen-Huu, L. Jeannotte, and C. K. Tuggle. 1999. Hoxa5 gene regulation: a gradient of binding activity to a brachial spinal cord element. Dev. Biol. 208:134-146. [DOI] [PubMed] [Google Scholar]
- 46.Oosterveen, T., K. Niederreither, P. Dollé, P. Chambon, F. Meijlink, and J. Deschamps. 2003. Retinoids regulate the anterior expression boundaries of 5′ Hoxb genes in posterior hindbrain. EMBO J. 22:262-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papenbrock, T., R. L. Peterson, R. S. Lee, T. Hsu, A. Kuroiwa, and A. Awgulewitsch. 1998. Murine Hoxc-9 gene contains a structurally and functionally conserved enhancer. Dev. Dyn. 212:540-547. [DOI] [PubMed] [Google Scholar]
- 48.Pöpperl, H., M. Bienz, M. Studer, S. K. Chan, S. Aparicio, S. Brenner, R. S. Mann, and R. Krumlauf. 1995. Segmental expression of Hoxb-1 is controlled by a highly conserved autoregulatory loop dependent upon exd/pbx. Cell 81:1031-1042. [DOI] [PubMed] [Google Scholar]
- 49.Pownall, M. E., A. S. Tucker, J. M. Slack, and H. V. Isaacs. 1996. eFGF, Xcad3 and Hox genes form a molecular pathway that establishes the anteroposterior axis in Xenopus. Development 122:3881-3892. [DOI] [PubMed] [Google Scholar]
- 50.Prinos, P., S. Joseph, K. Oh, B. I. Meyer, P. Gruss, and D. Lohnes. 2001. Multiple pathways governing Cdx1 expression during murine development. Dev. Biol. 239:257-269. [DOI] [PubMed] [Google Scholar]
- 51.Rancourt, D. E., T. Tsuzuki, and M. R. Capecchi. 1995. Genetic interaction between hoxb-5 and hoxb-6 is revealed by nonallelic noncomplementation. Genes Dev. 9:108-122. [DOI] [PubMed] [Google Scholar]
- 52.Rivera-Pomar, R., X. Lu, N. Perrimon, H. Taubert, and H. Jackle. 1995. Activation of posterior gap gene expression in the Drosophila blastoderm. Nature 376:253-256. [DOI] [PubMed] [Google Scholar]
- 53.Sharpe, J., S. Nonchev, A. Gould, J. Whiting, and R. Krumlauf. 1998. Selectivity, sharing and competitive interactions in the regulation of Hoxb genes. EMBO J. 17:1788-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shashikant, C. S., C. J. Bieberich, H. G. Belting, J. C. Wang, M. A. Borbely, and F. H. Ruddle. 1995. Regulation of Hoxc-8 during mouse embryonic development: identification and characterization of critical elements involved in early neural tube expression. Development 121:4339-4347. [DOI] [PubMed] [Google Scholar]
- 55.Spitz, F., F. Gonzalez, and D. Duboule. 2003. A global control region defines a chromosomal regulatory landscape containing the HoxD cluster. Cell 113:405-417. [DOI] [PubMed] [Google Scholar]
- 56.Stein, S., R. Fritsch, L. Lemaire, and M. Kessel. 1996. Checklist: vertebrate homeobox genes. Mech. Dev. 55:91-108. [DOI] [PubMed] [Google Scholar]
- 57.Studer, M., H. Pöpperl, H. Marshall, A. Kuroiwa, and R. Krumlauf. 1994. Role of a conserved retinoic acid response element in rhombomere restriction of Hoxb-1. Science 265:1728-1732. [DOI] [PubMed] [Google Scholar]
- 58.Subramanian, V., B. I. Meyer, and P. Gruss. 1995. Disruption of the murine homeobox gene Cdx1 affects axial skeletal identities by altering the mesodermal expression domains of Hox genes. Cell 83:641-653. [DOI] [PubMed] [Google Scholar]
- 59.Suh, E., L. Chen, J. Taylor, and P. G. Traber. 1994. A homeodomain protein related to caudal regulates intestine-specific gene transcription. Mol. Cell. Biol. 14:7340-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taylor, J. K., T. Levy, E. R. Suh, and P. G. Traber. 1997. Activation of enhancer elements by the homeobox gene Cdx2 is cell line specific. Nucleic Acids Res. 25:2293-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tuggle, C. K., J. Zakany, L. Cianetti, C. Peschle, and M. C. Nguyen-Huu. 1990. Region-specific enhancers near two mammalian homeobox genes define adjacent rostrocaudal domains in the central nervous system. Genes Dev. 4:180-189. [DOI] [PubMed] [Google Scholar]
- 62.van den Akker, A. E., S. Forlani, K. Chawengsaksophak, W. de Graaff, F. Beck, B. I. Meyer, and J. Deschamps. 2002. Cdx1 and Cdx2 have overlapping functions in anteroposterior patterning and posterior axis elongation. Development 129:2181-2193. [DOI] [PubMed] [Google Scholar]
- 63.Wang, W., and B. A. Malcolm. 1999. Two-stage PCR protocol allowing introductions of multiple mutation deletions and insertions using quick-change site-directed mutagenesis. BioTechniques 26:680-682. [DOI] [PubMed] [Google Scholar]
- 64.Zakany, J., C. K. Tuggle, M. D. Patel, and M. C. Nguyen-Huu. 1988. Spatial regulation of homeobox gene fusions in the embryonic central nervous system of transgenic mice. Neuron 1:679-691. [DOI] [PubMed] [Google Scholar]
- 65.Zakany, J., M. Gérard, B. Favier, and D. Duboule. 1997. Deletion of a HoxD enhancer induces transcriptional heterochrony leading to transposition of the sacrum. EMBO J. 16:4393-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]