Abstract
Nucleotide binding protein‐like, NUBPL, is an assembly factor for human mitochondrial complex I, which is the biggest member of the mitochondrial respiratory chain. However, the relationship between NUBPL and carcinoma progression remains unknown. In this study, NUBPL was characterized for its role in colorectal cancer (CRC) and the underlying molecular mechanisms. Data (n = 197) from the Oncomine database revealed that mRNA levels of NUBPL were remarkably overexpressed in CRC tissues compared with normal tissues. In addition, immunohistochemical analysis of 75 pairs of CRC and non‐tumor tissues showed that the expression level of NUBPL was significantly higher in CRC tissues, and its expression level was positively associated with lymph node metastasis (P = 0.028) and advanced staging (P = 0.030). Expression of NUBPL in metastatic lymph nodes of CRC patients was also detected by immunohistochemical staining and high expression levels of NUBPL were observed. Overexpression of NUBPL significantly promoted the migration and invasion ability of CRC cell lines SW480 and SW620, whereas knockdown of NUBPL lead to an opposite effect. Our further study found that NUBPL could induce epithelial–mesenchymal transition (EMT), characterized by downregulation of epithelial markers (E‐cadherin) and upregulation of mesenchymal markers (N‐cadherin and vimentin). Moreover, NUBPL was able to activate ERK, which is believed to promote EMT and tumor metastasis. Inhibition of ERK suppressed the NUBPL‐induced changes in EMT and cell motility. These data showed that NUBPL plays a vital role in CRC migration and invasion by inducing EMT and activating ERK. It might be a novel therapeutic target for CRC.
Keywords: Cadherins, colorectal neoplasms, neoplasm invasiveness, neoplasm metastasis, vimentin
Colorectal cancer (CRC) is the second leading cause of cancer death worldwide1 and over 90% of these deaths result from metastasis.2 However, effective treatments for patients with metastatic CRC are still unavailable. Hence, an improved understanding of the molecular mechanisms underlying metastasis may enhance therapeutic strategies or prevent early metastasis.
Double minute chromosomes (DMs) are considered to be associated with tumor metastasis or malignant phenotype. They are extrachromosomal circular DNA ranging from hundreds of kilobases to megabases in size.3, 4 Nucleotide binding protein‐like (NUBPL) was found amplified in human CRC cell line NCI‐H508, which is known to contain many DMs. Therefore, NUBPL might play a role in the development of CRC. NUBPL, also known as IND1 or huInd1, is an assembly factor for human mitochondrial complex I, which is a large ~1000‐kDa mitochondrial inner membrane enzyme composed of 45 protein subunits.5 The expression of NUBPL is closely correlated with complex I protein and activity levels. However, the relationship between NUBPL and tumorigenesis has not been described.
One of the major mechanisms of cancer cell metastasis includes epithelial–mesenchymal transition (EMT), which allows cells to leave the site of the primary tumor, invade surrounding tissues, disseminate through the lymphatic or hematogenous systems, and migrate to distant organs.6, 7 During the process of EMT, epithelial cells undergo a loss of polarity and cell–cell contact, and convert into mesenchymal cells with increased motility. Biochemically, the expression of epithelial markers such as E‐cadherin and α‐catenin are repressed and the expression of mesenchymal markers such as N‐cadherin and vimentin are upregulated.8 Reduction of E‐cadherin is a well‐established hallmark of EMT, and is also correlated with poor clinical prognosis in many kinds of cancers.9, 10, 11
In the present study, the expression of NUBPL was compared between CRC tissues and paired adjacent non‐tumor colorectal tissues. Additionally, the role of NUBPL in human CRC cell lines was investigated using various assays. Furthermore, the mechanism of NUBPL in tumor metastasis was also addressed.
Materials and Methods
Cell lines and cell culture
Colorectal cancer cell lines SW620 and HT29 were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Colorectal cancer cell lines SW480, NCI‐H716, T84, COLO320HSR, SK‐CO‐1, and NCI‐H508 were purchased from ATCC (Manassas, VA, USA). COLO320HSR, NCI‐H508, and NCI‐H716 cells were maintained in RPMI‐1640 medium, SW480 and SW620 cells in Leibovitz's L‐15 medium, SK‐CO‐1 cells in minimal essential medium, T84 cells in a 1:1 mixture of Ham's F12 medium and DMEM, and HT‐29 cells in DMEM containing high glucose. All cells were supplemented with 10% FBS.
Tissue microarray and immunohistochemistry
The tissue microarray used in this study was obtained from Outdo Biotech (Shanghai, China). This CRC tissue microarray contains 75 pairs of human CRC and their matched adjacent normal tissues. The lymph node metastatic sites of CRC cases were obtained from the Second Affiliated Hospital (Harbin Medical University, Harbin, China). The immunohistochemical staining was carried out using PowerVision Two‐Step Histostaining Reagent (Zhongshan Golden Bridge, Beijing, China) following the manufacturer's recommended protocol. Anti‐NUBPL antibody produced in rabbit was used at a dilution of 1:100 (HPA029203; Sigma‐Aldrich, St. Louis, MO, USA). Immunoreactivity was assessed independently by three researchers.
Western blot analysis
Colorectal cancer cells were dissolved in RIPA (Thermo Fisher Scientific, Waltham, MA, USA). Approximately 40 μg total protein was separated by 10% SDS‐PAGE and then transferred onto PVDF membranes (Millipore, Billerica, MA, USA). Membranes were blocked with 10% blocking solution (Roche, Basel‐Stadt, Switzerland) and incubated with primary antibodies overnight at 4°C, followed by incubation with secondary antibody for 1 h at room temperature. The bands were detected using the Odyssey Infrared Imaging System (LI‐COR Biosciences, Lincoln, NE, USA). The following primary antibodies were used: NUBPL (SAB1408017, Sigma‐Aldrich), E‐cadherin, α‐catenin, N‐cadherin, vimentin, fibronectin, α‐smooth muscle actin (α‐SMA) (all from Proteintech, Rosemont, IL, USA), ERK, phospho‐ERK (both from Cell Signaling Technology, Danvers, MA, USA), and GAPDH (Kangchen Bio‐tech, Shanghai, China).
Quantitative RT‐PCR
Total RNA was extracted from CRC cells using the High Pure RNA Isolation Kit (Roche) according to the manufacturer's instructions. The synthesis of cDNA from total RNA was carried out with the PrimeScript RT Reagent Kit Perfect Real Time (Takara, Dalian, China). Reverse transcription–PCR for quantification was undertaken with LightCycler 480 SYBR Green I Master (Roche). The specific primers for human NUBPL, E‐cadherin, α‐catenin, vimentin, ribronectin, and α‐SMA were as follows: NUBPL forward 5′‐GTTGTAGACATGCCACCAGGAA‐3′, reverse 5′‐CACGTGGACTCTGCGAAACA‐3′; E‐cadherin forward 5′‐TGGATAACCAGAATAAAGACCAAGTG‐3′, reverse 5′‐TCCTCCGAAGAAACAGCAAGA‐3′; α‐catenin forward 5′‐AATTGCTGAGGCAGGATCCA‐3′, reverse 5′‐GCCTTGACCTTGCTGCAGAT‐3′; vimentin forward 5′‐GCAGGAGGCAGAAGAATGGTA‐3′, reverse 5′‐GGGACTCATTGGTTCCTTTAAGG‐3′; fibronectin forward 5′‐GGGACCGTCAGGGAGAAAA‐3′, reverse 5′‐CGAGATATTCCTTCTGCCACTGTT‐3′; and α‐SMA forward 5′‐GGGACATCAAGGAGAAACTGTGT‐3′, reverse 5′‐TCTCTGGGCAGCGGAAAC‐3′. The expression levels were normalized using GAPDH: forward 5′‐ATCACTGCCACCCAGAAGAC‐3′, reverse 5′‐TTTCTAGACGGCAGGTCAGG‐3′.
Overexpression and RNA interference of NUBPL
The coding sequence region of human NUBPL was amplified and cloned into pEGFP‐C1 expression vector, which was kindly provided by Dr. ZH Zhong (Department of Microbiology, Harbin Medical University, Harbin, China). Three siRNAs targeting NUBPL and a negative control siRNA were purchased from RiboBio (Guangzhou, China). The transfection was carried out with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. The SW480 cells stably expressing NUBPL, namely the SW480‐NUBPL cells, were isolated by G418 selection after the cells were transfected with expression or control plasmids.
Wound‐healing assay
Cells were planted on 6‐well dishes and cultured as confluent monolayers. The cells were carefully scraped using a 10‐μL pipette tip and debris was removed by washing with PBS. Cell migration was evaluated at 48 h.
Cell migration and invasion assays
Cell suspension prepared with serum‐free media was loaded onto the upper chamber of a Transwell precoated with or without Matrigel (Corning, Bedford, MA, USA). Medium with 20% FBS was added to the lower chamber as a chemoattractant. The chambers were incubated for 24 h or 48 h, then the non‐migrated or non‐invaded cells in the upper chamber were removed by a cotton tip. The cells that had migrated or invaded to the bottom of the membrane were fixed, stained and counted.
Immunofluorescence staining
Cells were fixed with cold ethanol, treated with 0.2% Triton X‐100 and blocked with 5% BSA. The primary antibodies were added and incubated overnight at 4°C. Then cells were treated with fluorescence‐labeled secondary antibody and stained with DAPI.
Statistical analyses
The results are shown as mean ± SD and compared using Student's t‐test. The χ2‐test was used to determine the correlation between NUBPL expression and clinicopathologic features. Wilcoxon's signed‐rank test was used to analyze the expression differences between CRC and adjacent normal tissues. The statistical analysis was carried out using spss for Windows, version 19.0 (IBM, Armonk, NY, USA) and P < 0.05 was considered significant.
Results
Overexpression of NUBPL is identified in CRC tissues
To explore the relationship between NUBPL and CRC, we investigated the expression levels of NUBPL in CRC tissues and normal tissues. The mRNA expression of NUBPL was found elevated in CRC compared with normal colorectal tissues, as analyzed in three databases (n = 197) from Oncomine (GSE20916, GSE5206, and GSE9348) (Fig. 1a). Additionally, the expression pattern of NUBPL was studied by immunohistochemistry in 75 pairs of CRC tissues and adjacent non‐tumor tissues. The protein expression level of NUBPL was significantly higher in CRC tissues compared with their non‐tumor counterparts (P < 0.05; Fig. 1b), suggesting that NUBPL was frequently overexpressed in CRC.
Figure 1.
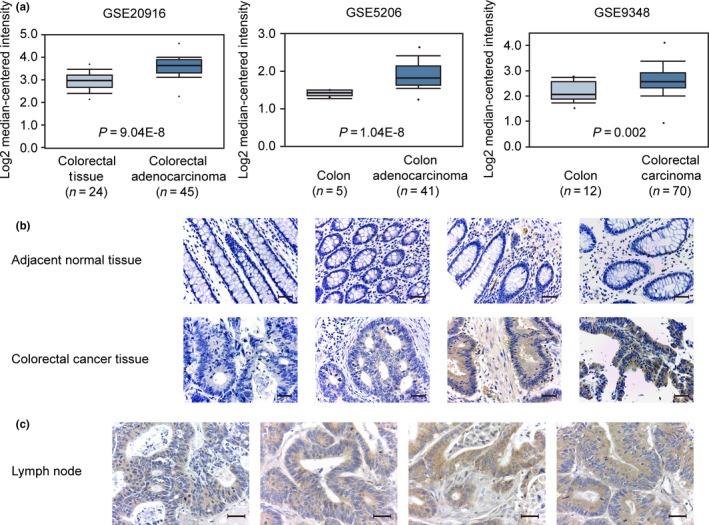
Expression status of nucleotide binding protein‐like (NUBPL) in colorectal carcinoma (CRC) tissues. (a) Gene expression analysis of NUBPL in CRC tissues and normal colorectal tissues. Three different datasets (GSE20916, GSE5206, and GSE9348) were obtained from the Oncomine database. (b) Representative immunohistochemical staining of NUBPL expression in CRC tissue and paired non‐tumor tissue (magnification, ×400). Scale bar = 400 μm. (c) Representative immunohistochemical staining of NUBPL expression in metastatic lymph node of CRC patients (magnification, ×400). Scale bar = 400 μm.
Moreover, correlation analysis using immunohistochemistry data revealed that the overexpression of NUBPL was positively associated with CRC lymph node metastasis (P = 0.028) and a more advanced clinical stage (P = 0.030; Table 1). Then NUBPL expression was detected in metastatic lymph node of CRC patients by immunohistochemical staining. High expression level of NUBPL was observed in metastatic lymph nodes (Fig. 1c). These results indicate that NUBPL may play a vital role in CRC metastasis.
Table 1.
Correlation between nucleotide binding protein‐like (NUBPL) expression and clinicopathologic features of colorectal carcinoma
| Variable | All cases | NUBPL expression | P‐value | |
|---|---|---|---|---|
| High | Low | |||
| Gender | ||||
| Female | 32 | 8 | 24 | 0.129† |
| Male | 43 | 18 | 25 | |
| Age, years | ||||
| <60 | 23 | 9 | 14 | 0.589† |
| ≥60 | 52 | 17 | 35 | |
| Lymph node metastasis | ||||
| − | 39 | 9 | 30 | 0.028† , * |
| + | 36 | 17 | 19 | |
| Distant metastasis | ||||
| − | 66 | 21 | 45 | 0.303‡ |
| + | 9 | 5 | 4 | |
| Differentiation | ||||
| 1–2 | 62 | 22 | 40 | 0.997‡ |
| 3 | 13 | 4 | 9 | |
| Staging | ||||
| I–II | 36 | 8 | 28 | 0.030† , * |
| III–IV | 39 | 18 | 21 | |
†Pearson's χ2‐test.‡Yates correction χ2‐test. *P < 0.05.
Expression of NUBPL in CRC cell lines
The expression of NUBPL in eight colorectal adenocarcinoma cell lines (SW620, SW480, NCI‐H716, NCIH508, T84, HT29, COLO320HSR, and SK‐CO‐1) was examined by quantitative RT‐PCR (qRT‐PCR). Results showed that SW620 and SW480 exhibited the lowest NUBPL expression among these cell lines (Fig. 2a). Therefore, they were used for the subsequent overexpression experiments with control plasmids and pEGFP‐C1‐NUBPL. The expression of NUBPL in SW620 and SW480 cells was upregulated after transfection as detected by Western blot analysis (Fig. 2b). Then, the NUBPL gene was further silenced by three targeted siRNAs (siNUBPL‐1, siNUBPL‐2, and siNUBPL‐3) in SW480‐NUBPL cells. Quantitative RT‐PCR (Fig. 2c) and Western blot analysis (Fig. 2d) results suggested that the expression levels of NUBPL in both mRNA and protein could be effectively silenced by siRNAs (siNUBPL‐1, siNUBPL‐2, and siNUBPL‐3).
Figure 2.
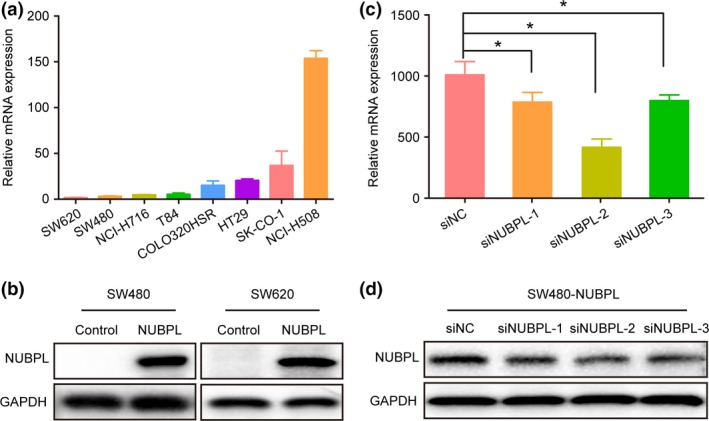
Nucleotide binding protein‐like (NUBPL) expression in colorectal carcinoma (CRC) cell lines. (a) NUBPL expression level in CRC cell lines. (b) Ectopic NUBPL expression in CRC cell lines SW480 and SW620. (c, d) Knockdown efficiency of NUBPL in SW480‐NUBPL cells by treatment with siNUBPL‐1, siNUBPL‐2, or siNUPBL‐3 as examined by quantitative RT‐PCR (c) and Western blot analysis (d). *P < 0.05.
Migration and invasion of CRC cells enhanced by NUBPL
As our clinical data revealed that NUBPL overexpression was correlated with lymph node metastasis, we hypothesized that NUBPL increases cell motility. In order to determine whether ectopic expression of NUBPL could alter CRC cell migration and invasion, we undertook a series of biological experiments using SW480 and SW620 cells. The wound‐healing assay showed that NUBPL‐transfected cells obtained a remarkably faster healing rate of the scratched “wound” compared with control cells (P < 0.05; Fig. 3a). Cell migration assay also showed that NUBPL could significantly increase cell motility compared with control cells (P < 0.05; Fig. 3b). In addition, Transwell invasion assay further revealed that NUBPL overexpression was able to promote cell invasion significantly (P < 0.05; Fig. 3c).
Figure 3.
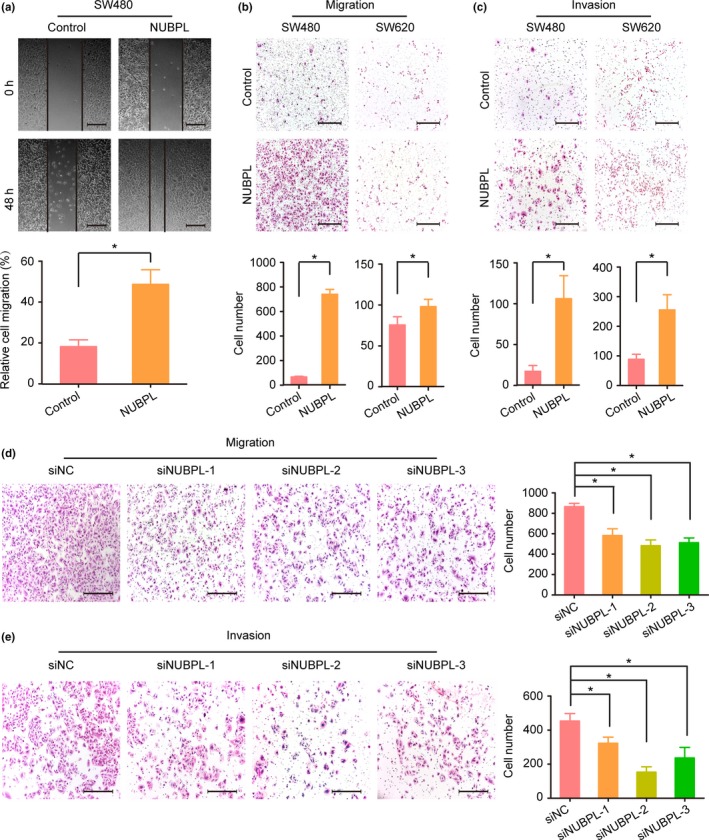
Effect of nucleotide binding protein‐like (NUBPL) overexpression or knockdown on colorectal carcinoma cell migration and invasion. (a) Wound‐healing assay detecting the motility of SW480‐NUBPL cells. Columns: mean ± SD of triplicate experiments. *P < 0.05, independent Student's t‐test. (b, c) Cell migration (b) and invasion (c) assays investigating the influence of NUBPL overexpression on SW480 and SW620 cells. Representative images of migrated or invaded cells are illustrated in the upper panels (magnification, ×100). Scale bar = 200 μm. Columns: mean ± SD of triplicate experiments. *P < 0.05, independent Student's t‐test. (d, e) Effect of NUBPL knockdown on SW480‐NUBPL cell migration (d) and invasion (e) in Transwell assays. Examples of cells migrated through the PET‐membrane or Matrigel‐coated Transwell are shown in the left panel (magnification, ×100). Scale bar = 200 μm. Columns: mean ±SD of triplicate experiments. siNC, negative control siRNA. *P < 0.05, independent Student's t‐test.
To determine whether the above functional changes were actually caused by NUBPL, we then knocked down the NUBPL expression in SW480‐NUBPL cells. As expected, both cell migratory (Fig. 3d) and invasive (Fig. 3e) abilities were significantly suppressed in NUBPL knockdown cells compared with the controls (P < 0.05). Collectively, these results suggest that NUBPL overexpression can lead to the aggressive phenotype of CRC cells.
Epithelial–mesenchymal transition induced by NUBPL in CRC
As NUBPL could remarkably enhance CRC cell motility, and EMT is one of the key events in cancer cell migration and invasion, the impact of NUBPL on EMT was investigated by detecting the expression levels of EMT markers. Results from Western blot analyses revealed that expression of E‐cadherin was clearly decreased when NUBPL was overexpressed; in contrast, the expression of two mesenchymal markers (N‐cadherin and vimentin) were elevated in SW480‐NUBPL cells compared with the control cells (Fig. 4a). Additionally, the Western blot results were confirmed by qRT‐PCR (Fig. 4b) and immunofluorescence staining assays (Fig. 4c). These data indicate that NUBPL overexpression could induce EMT.
Figure 4.
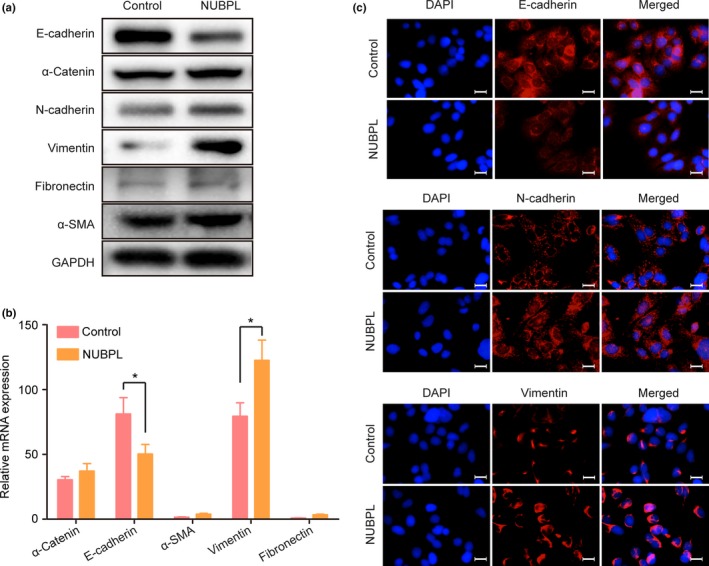
Promotion of epithelial–mesenchymal transition (EMT) by nucleotide binding protein‐like (NUBPL) in colorectal carcinoma cells. (a) Western blot analysis of protein expression of EMT markers in SW480‐Control and SW480‐NUBPL cells. (b) mRNA expression levels of EMT markers in SW480‐Control and SW480‐NUBPL cells as examined by quantitative RT‐PCR. Columns: mean ± SD of triplicate experiments. *P < 0.05, independent Student's t‐test. (c) Immunofluorescence staining detecting the expression of E‐cadherin, vimentin, and N‐cadherin in SW480 cells transfected with NUBPL.
Epithelial–mesenchymal transition promoted by NUBPL through activating ERK
It is reported that EMT and cell migration are regulated by MAPKs,12 so we investigated the mechanism underlying the promotion of EMT by NUBPL. The results showed that expression of phosphorylated ERK was increased (Fig. 5a). Further studies showed that ERK inhibitor could decrease N‐cadherin and vimentin expression and increase E‐cadherin expression in NUBPL‐transfected SW480 cells (Fig. 5b). Inhibition of ERK was also found to suppress migratory and invasive abilities of SW480‐NUBPL cells (Fig. 5c).
Figure 5.
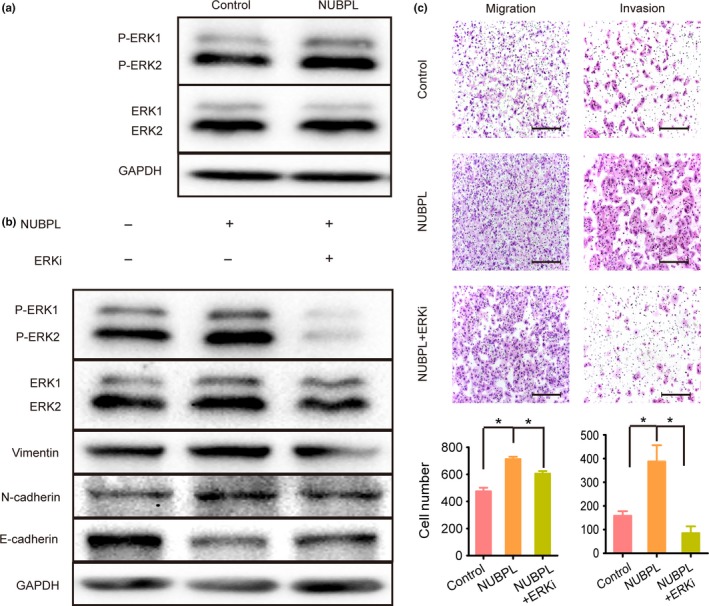
Effect of ERK inhibitor on nucleotide binding protein‐like (NUBPL)‐induced changes in epithelial–mesenchymal transition markers and colorectal carcinoma cell motility. (a) Phosphorylation of ERK in SW480‐NUBPL cells as investigated by Western blot. (b) ERK phosphorylation and E‐cadherin/N‐cadherin/vimentin expression as detected by Western blot analysis. SW480‐NUBPL cells were treated with the ERK inhibitor, SCH772984, for 24 h. (c) Cell migration and invasion assays investigating the influence of ERK inhibition. Representative images of migrated and invaded cells are shown in the upper panels (magnification, ×100). Scale bar = 200 μm. Columns: mean ± SD of triplicate experiments. *P < 0.05, independent Student's t‐test.
Discussion
Double minute chromosomes are extrachromosomal cytogenetic structures and commonly found to carry oncogenes in various cancers, including epidermal growth factor receptor13 in gliomas, C‐MYC14 in CRC, and EIF5A215 or RPL22L116 in ovarian cancer. In the report presented here, we identified a novel CRC metastasis‐related gene, namely NUBPL, that was also amplified on DMs. It is reported that NUBPL participates in the assembly of mitochondrial complex I, 5, 17 but its relationship with cancer has not been investigated. So far, the functions of the 45 subunits of complex I are not well understood. Recent studies reported that GRIM‐19, one of the subunits of complex I, is not only associated with mitochondrial metabolism, but is also involved in numerous cancers, such as hepatocellular cancer, renal cell carcinoma, and prostate cancer.18, 19, 20 This suggests that other subunits may also play a role in cancers.
In our study, the protein expression of NUBPL was first detected in a collection of CRC and adjacent non‐tumor tissues. Results from immunohistochemical staining revealed that the level of NUBPL expression was remarkably higher in CRC tissues compared with non‐tumor counterparts (P < 0.05). Moreover, analysis of three databases (GSE20916, GSE5206, and GSE9348) from Oncomine indicated that the mRNA expression of NUBPL is considerably higher in CRC compared with normal colorectal tissues (P < 0.05). Importantly, overexpression of NUBPL was significantly correlated with lymph node metastasis (P = 0.028) and advanced clinical stage (P = 0.030). High expression levels of NUBPL were also observed in lymph node metastatic cases. Taken together, these data strongly suggest that NUBPL is frequently overexpressed in CRC tissues and the high expression level of NUBPL may facilitate the metastatic phenotype.
To further understand the biological role of NUBPL in CRC, we studied the function of NUBPL in CRC cell lines, SW480 and SW620, using pEGFP‐NUBPL transfection. The results showed that ectopic expression of NUBPL strongly enhanced cell migration and invasion, and knockdown of NUBPL by siRNA further supported it. These data are consistent with the clinical analysis, suggesting that NUBPL is associated with CRC metastasis. Therefore, NUBPL is not only essential for assembly of complex I, but also vital in tumor progression.
Tumor metastasis is usually induced by EMT. Our data showed that expression of mesenchymal markers (N‐cadherin and vimentin) was increased and expression of epithelial marker (E‐cadherin) was decreased in CRC cells overexpressing NUBPL. Vimentin is an intermediate filament that is constitutively expressed in mesenchymal cells. Increased vimentin expression can facilitate cell migration and is commonly used as a canonical EMT marker in cancer. E‐cadherin is an important mediator of cell–cell adhesions in epithelial tissues. The E‐cadherin to N‐cadherin switch, which occurs during cancer progression, is used to monitor EMT.21 The loss of E‐cadherin and gain of N‐cadherin is considered a key step in the EMT process and is able to enhance metastatic behavior in various epithelial cancers.22 Thus, NUBPL probably undergoes EMT to achieve higher motility and invasiveness, promoting CRC metastasis.
Accumulating evidence suggests that EMT is one of the pathways mediated by MAPKs, and MAPK–ERK has been considered to be a determinant of EMT.23, 24 In the present study, we found that NUBPL could enhance the activation of ERK. Furthermore, inhibition of ERK was found to suppress EMT and cell motility in NUBPL‐transfected SW480 cells. These results indicate that NUBPL promotes EMT through the activation of ERK.
Collectively, NUBPL has been identified to be a novel metastasis‐related gene that plays an essential role in the progression of CRC by promoting EMT through the activation of ERK. These discoveries suggest NUBPL as a novel biomarker for CRC diagnosis, and also provide insights for the development of new anticancer therapies for better clinical outcomes for patients with CRC.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This study was supported by National Natural Science Foundation of China (Grant No. 81371617 to YJ), Outstanding Youth foundation of Heilongjiang Province of China (Grant No. JC201215 to YJ), Program for Changjiang Scholars and Innovative Research Team in University (IRT1230), and National Key Research and Development Program of China (2016YFC1000504).
Cancer Sci 108 (2017) 1169–1176
Funding Information
National Natural Science Foundation of China; Outstanding Youth Foundation of Heilongjiang Province of China; University of China; National Key Research and Development Program of China.
Contributor Information
Songbin Fu, Email: fusb@ems.hrbmu.edu.cn.
Yan Jin, Email: jinyan@ems.hrbmu.edu.cn.
References
- 1. Li Q, Liang X, Wang Y et al miR‐139‐5p inhibits the epithelial‐mesenchymal transition and enhances the chemotherapeutic sensitivity of colorectal cancer cells by downregulating BCL2. Sci Rep 2016; 6: 27157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu S, Zhang J, Xu F et al IGFBP‐rP1 suppresses epithelial‐mesenchymal transition and metastasis in colorectal cancer. Cell Death Dis 2015; 6: e1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barker PE. Double minutes in human tumor cells. Cancer Genet Cytogenet 1982; 5: 81–94. [DOI] [PubMed] [Google Scholar]
- 4. Bahr G, Gilbert F, Balaban G, Engler W. Homogeneously staining regions and double minutes in a human cell line: chromatin organization and DNA content. J Natl Cancer Inst 1983; 71: 657–61. [PubMed] [Google Scholar]
- 5. Sheftel AD, Stehling O, Pierik AJ et al Human ind1, an iron‐sulfur cluster assembly factor for respiratory complex I. Mol Cell Biol 2009; 29: 6059–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer 2004; 4: 448–56. [DOI] [PubMed] [Google Scholar]
- 7. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial‐mesenchymal transitions in development and disease. Cell 2009; 139: 871–90. [DOI] [PubMed] [Google Scholar]
- 8. Hur K, Toiyama Y, Takahashi M et al MicroRNA‐200c modulates epithelial‐to‐mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut 2013; 62: 1315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Byles V, Zhu L, Lovaas JD et al SIRT1 induces EMT by cooperating with EMT transcription factors and enhances prostate cancer cell migration and metastasis. Oncogene 2012; 31: 4619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Richmond PJ, Karayiannakis AJ, Nagafuchi A, Kaisary AV, Pignatelli M. Aberrant E‐cadherin and alpha‐catenin expression in prostate cancer: correlation with patient survival. Can Res 1997; 57: 3189–93. [PubMed] [Google Scholar]
- 11. Ross JS, Figge HL, Bui HX et al E‐cadherin expression in prostatic carcinoma biopsies: correlation with tumor grade, DNA content, pathologic stage, and clinical outcome. Mod Pathol 1994;7:835–41. [PubMed] [Google Scholar]
- 12. Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci 2004; 117: 4619–28. [DOI] [PubMed] [Google Scholar]
- 13. Canute GW, Longo SL, Longo JA et al The hydroxyurea‐induced loss of double‐minute chromosomes containing amplified epidermal growth factor receptor genes reduces the tumorigenicity and growth of human glioblastoma multiforme. Neurosurgery 1998; 42: 609–16. [DOI] [PubMed] [Google Scholar]
- 14. Von Hoff DD, McGill JR, Forseth BJ et al Elimination of extrachromosomally amplified MYC genes from human tumor cells reduces their tumorigenicity. Proc Natl Acad Sci USA 1992; 89: 8165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guan XY, Sham JS, Tang TC, Fang Y, Huo KK, Yang JM. Isolation of a novel candidate oncogene within a frequently amplified region at 3q26 in ovarian cancer. Can Res 2001; 61: 3806–9. [PubMed] [Google Scholar]
- 16. Wu N, Wei J, Wang Y et al Ribosomal L22‐like1 (RPL22L1) promotes ovarian cancer metastasis by inducing epithelial‐to‐mesenchymal transition. PLoS ONE 2015; 10: e0143659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bych K, Kerscher S, Netz DJ et al The iron‐sulphur protein Ind1 is required for effective complex I assembly. EMBO J 2008; 27: 1736–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kong D, Zhao L, Du Y et al Overexpression of GRIM‐19, a mitochondrial respiratory chain complex I protein, suppresses hepatocellular carcinoma growth. Int J Clin Exp Pathol 2014; 7: 7497–507. [PMC free article] [PubMed] [Google Scholar]
- 19. Alchanati I, Nallar SC, Sun P et al A proteomic analysis reveals the loss of expression of the cell death regulatory gene GRIM‐19 in human renal cell carcinomas. Oncogene 2006; 25: 7138–47. [DOI] [PubMed] [Google Scholar]
- 20. Zhang L, Gao L, Li Y et al Effects of plasmid‐based Stat3‐specific short hairpin RNA and GRIM‐19 on PC‐3M tumor cell growth. Clin Cancer Res 2008; 14: 559–68. [DOI] [PubMed] [Google Scholar]
- 21. Scanlon CS, Van Tubergen EA, Inglehart RC, D'Silva NJ. Biomarkers of epithelial‐mesenchymal transition in squamous cell carcinoma. J Dent Res 2013; 92: 114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Canel M, Serrels A, Frame MC, Brunton VG. E‐cadherin‐integrin crosstalk in cancer invasion and metastasis. J Cell Sci 2013; 126: 393–401. [DOI] [PubMed] [Google Scholar]
- 23. Elsum IA, Martin C, Humbert PO. Scribble regulates an EMT polarity pathway through modulation of MAPK‐ERK signaling to mediate junction formation. J Cell Sci 2013; 126: 3990–9. [DOI] [PubMed] [Google Scholar]
- 24. Buonato JM, Lazzara MJ. ERK1/2 blockade prevents epithelial‐mesenchymal transition in lung cancer cells and promotes their sensitivity to EGFR inhibition. Can Res 2014; 74: 309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]


