Abstract
The effect of socioeconomic factors on receipt of definitive treatment and survival outcomes in non‐metastatic head and neck squamous cell carcinoma (HNSCC) remains unclear. Eligible patients (n = 37 995) were identified from the United States Surveillance, Epidemiology and End Results (SEER) database between 2007 and 2012. Socioeconomic factors (i.e., median household income, education level, unemployment rate, insurance status, marital status and residence) were included in univariate/multivariate Cox regression analysis; validated factors were used to generate nomograms for cause‐specific survival (CSS) and overall survival (OS), and a prognostic score model for risk stratification. Low‐ and high‐risk groups were compared for all cancer subsites. Impact of race/ethnicity on survival was investigated in each risk group. Marital status, median household income and insurance status were included in the nomograms for CSS and OS, which had higher c‐indexes than the 6th edition TNM staging system (all P < 0.001). Based on three disadvantageous socioeconomic factors (i.e., unmarried status, uninsured status, median household income <US $65 394), the prognostic score model generated four risk subgroups with scores of 0, 1, 2 or 3, which had significantly separated CSS/OS curves (all P < 0.001). Low‐risk patients (score 0–1) were more likely to receive definitive treatment and obtain better CSS/OS than high‐risk patients (score 2–3). Chinese and non‐Hispanic black patients with high‐risk socioeconomic status had best and poorest CSS/OS, respectively. Therefore, marital status, median household income and insurance status have significance for predicting survival outcomes. Low‐risk socioeconomic status and Chinese race/ethnicity confer protective effects in HNSCC.
Keywords: Head and neck squamous cell carcinoma, nomogram, SEER, socioeconomic, survival
Head and neck squamous cell carcinoma (HNSCC), a malignancy arising in the mucosal lining of the oral cavity, pharynx and larynx, is the seventh most common cancer worldwide with an annual incidence of approximately 690 000 cases.1 An estimated 61 760 cases were diagnosed in the United States in 2016.2 Patient characteristics, tumor characteristics and molecular markers affect prognosis in non‐metastatic HNSCC.3 Although multidisciplinary treatment involving surgery, radiotherapy and chemotherapy is the mainstay of curative management for non‐metastatic HNSCC, treatment is guided by clinicopathologic information that mainly reflects tumor/molecular features. Moreover, varied survival outcomes are commonly observed among patients with different socioeconomic status receiving the same treatment.4, 5, 6, 7
Higher socioeconomic status (e.g., higher income/education level) has been reported to be associated with a lower incidence and better survival outcomes in HNSCC.4, 5, 6, 7, 8, 9, 10, 11 However, socioeconomic status had non‐significant effects in several retrospective studies after adjusting for covariates.12, 13 Moreover, the effects of socioeconomic status cannot be entirely explained by differences in the distributions of smoking and alcohol consumption,8, 9, 10, 11 which have long been recognized as the major risk factors for HNSCC.14, 15 Therefore, the associations between socioeconomic status and survival outcomes of HNSCC remain unclear and require a comprehensive large‐scale investigation of detailed socioeconomic factors.
Aggressive treatment for HNSCC can induce severe adverse outcomes, including mastication dysfunction, altered speech and facial disfigurement, which greatly affect physical and mental health and impede patients return to society. Social support, such as spousal support, has been proven to be a cost‐efficient method to improve survival in HNSCC.16 Race/ethnicity strongly reflects cultural background and has an influence on diet, customs and lifestyle. Racial/ethnic disparities have been noted to have significant17, 18 and non‐significant effects4, 5 on the incidence, management and survival of HNSCC. Therefore, marital status and race/ethnicity should be included in assessment of the impact of socioeconomic factors in HNSCC.
No proven screening methods, except visual inspection in high‐risk regions for oral cavity cancer, are known to exist for HNSCC.3 Thus, the ability to identify vulnerable patients who have disadvantaged socioeconomic status is important to develop individualized risk stratification and guide targeted interventions. In this study, we established nomograms and a prognostic score model based on socioeconomic factors to predict survival outcomes in non‐metastatic HNSCC.
Materials and Methods
Data source and patient selection
The Surveillance, Epidemiology and End Results (SEER) database released in April, 2015 was used to extract data on patients diagnosed with HNSCC between 2007 and 2012 for the present study. The year 2007 was selected as the first year, as several covariates were introduced to the database in 2007. Sponsored by the National Cancer Institute, the SEER program collects demographic, clinicopathologic and survival data from eighteen population‐based cancer registries (SEER‐18) in the United States. Since the SEER‐18 covers 27.8% of the population in the US with a typical distribution, it is thought to be representative of the US population as a whole.19 We used SEER*Stat software, version 8.2.1 (National Cancer Institute, Bethesda, MD, USA) to extract per‐patient data on 75 301 patients diagnosed with HNSCC between 2007 and 2012 from the SEER‐18. Ineligible cases were excluded according to the following criteria: (i) patients with metastatic HNSCC or prior malignancy; (ii) age of diagnosis <18 years‐old or unknown; (iii) patients not newly‐ or pathologically‐diagnosed; and (iv) patients with missing data on important variables, such as TN category, marital status and insurance record.
Study variables and outcomes
The socioeconomic factors assessed in this study were: median household income, education level, unemployment rate, residence, marital status and insurance status; the first four variables were determined at the county‐level. Data on median household income was obtained using the 2007 Poverty and Median Income Estimates from the US Census Bureau.20 The Economic Research Service of the US Department of Agriculture was used to obtain additional data, including 2006–2010 education levels, 2003 Rural‐Urban Continuum Codes and the 2010 unemployment rate.21 Education level represents the percentage of patients aged ≥25 years with at least a high school diploma. Residence was characterized as metro area, non‐metro urban area and non‐metro rural area according to the 2003 Rural‐Urban Continuum Codes. Marital status was classified as married, single (never married), separated/divorced and widowed; insurance status, as insured and uninsured.
Demographic and clinical variables included age at diagnosis, gender, race/ethnicity, clinical stage, TN category and definitive treatment. Race/ethnicity was classified as non‐Hispanic white, non‐Hispanic black, Hispanic and Chinese. Clinical stage and TN category were measured using the 6th edition of the American Joint Committee on Cancer staging system. Due to the lack of relevant information about chemotherapy or systemic therapy in the SEER database, treatment strategy was classified as a bivariate value, namely definitive treatment (i.e., surgery and/or radiotherapy) or no definitive treatment. According to the ICD‐10 site codes, the cancer subsite was classified as the nasopharynx (C11), oropharynx (C09‐C10), hypopharynx (C12‐C13), larynx (C32) and oral cavity (C00‐C06 and C14).
The primary outcomes of this study were cause‐specific survival (CSS) and overall survival (OS). CSS was defined as the time from the date of diagnosis until death due to HNSCC in the absence of other causes. OS was defined as the duration from the date of diagnosis to death, with no restrictions on the cause of death. The secondary outcome was whether the patients received definitive treatment.
Statistical analysis
All statistical analyses and figures were generated using SPSS, version 22.0 (SPSS Inc., Chicago, IL, USA) or the rms package in R version 3.3.2 (http://www.r-project.org/), unless otherwise specified. All P‐values were two‐sided with significance defined at <0.05. Follow‐up times were reported as median values and interquartile ranges (IQR). Descriptive statistics provided as continuous variables were converted into categorical variables according to IQR (i.e., age at diagnosis, median household income, unemployment rate and education level).
Multivariate logistic regression analysis was used to explore the effect of socioeconomic factors on receipt of definitive treatment after adjustment for age at diagnosis, gender, race/ethnicity, cancer subsite, T category and N category. Multivariate Cox regression analyses were performed to quantify the effect of socioeconomic factors on survival outcomes after adjustment for the aforementioned covariates plus definitive treatment. Variables with P < 0.05 in univariate Cox analysis were entered into multivariate Cox analysis to validate their significance using a backward stepwise algorithm.22 Cumulative 5‐year CSS and OS rates were calculated using the Kaplan–Meier method and compared using the log‐rank test.23
Nomograms for CSS and OS were generated based on multivariate Cox analysis. The final model selection was determined using a backward stepdown selection process based on the Akaike information criterion (AIC).24 Concordance index (c‐index) values were used to measure discriminative ability, and compared using the rcorrp.cens function in R. A higher c‐index indicates a better ability to separate patients with different survival outcomes. Calibration curves were assessed graphically by plotting the observed rates against the nomogram‐predicted probabilities via a bootstrap method with 1000 resamples. A prognostic score model was developed using the socioeconomic factors validated in multivariate Cox analysis. The score for each patient was equal to their total number of disadvantageous socioeconomic factors. The cut‐off score used to define high‐risk and low‐risk patients with respect to primary and secondary outcomes was identified using receiver‐operating characteristic (ROC) curve analysis; the optimal cut‐off score should have the greatest Youden's index value, which is equal to the sum of sensitivity and specificity minus 1. Forest plots were generated using Microsoft Excel (Microsoft Inc., Redmond, WA, USA) via Neyeloff's method25 to summarize the adjusted hazard ratios/odds ratios (AHRs/AORs) and the 95% confidence intervals (CIs) for the associations between socioeconomic status (high‐risk versus low‐risk) and receipt of definitive treatment, CSS and OS, as appropriate.
Results
Patient characteristics and effect of socioeconomic factors on CSS, OS and receipt of definitive treatment
The baseline characteristics of the 37 995 eligible patients with non‐metastatic HNSCC are shown in Table 1. Median follow‐up was 24 months (IQR = 10–44 months). Median age was 60 years (IQR = 52–69 years) with a male‐to‐female ratio of approximately 3:1. The distribution of the included patients throughout the United States is shown in Figure S1.
Table 1.
Univariate and multivariate Cox analysis of the effect of socioeconomic factors on CSS and OS in non‐metastatic HNSCC
| Variable | Patient no. (%) | CSS | OS | ||
|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||
| HR (95% CI) | HR (95% CI)† | HR (95% CI) | HR (95% CI)† | ||
| Age at diagnosis, year | |||||
| 18–52 | 8535 (22.5) | Reference | Reference | Reference | Reference |
| 53–60 | 9764 (25.7) | 1.28 (1.20–1.38)** | 1.20 (1.12–1.29)** | 1.39 (1.31–1.48)** | 1.30 (1.22–1.39)** |
| 61–69 | 9803 (25.8) | 1.40 (1.31–1.51)** | 1.48 (1.37–1.59)** | 1.63 (1.53–1.74)** | 1.68 (1.57–1.79)** |
| ≥70 | 9893 (26.0) | 2.06 (1.93–2.21)** | 2.45 (2.28–2.64)** | 2.70 (2.55–2.86)** | 3.08 (2.89–3.29)** |
| Gender | |||||
| Male | 27 837 (73.3) | Reference | – | Reference | Reference |
| Female | 10 158 (26.7) | 0.96 (0.91–1.01) | – | 0.95 (0.91–0.99)* | 0.92 (0.88–0.96)** |
| Race/ethnicity | |||||
| Non‐Hispanic white | 29 683 (78.1) | Reference | Reference | Reference | Reference |
| Non‐Hispanic black | 3832 (10.1) | 1.55 (1.45–1.66)** | 1.07 (1.00–1.15)* | 1.47 (1.39–1.55)** | 1.07 (1.01–1.14)* |
| Hispanic | 3719 (9.8) | 1.11 (1.03–1.20)* | 0.92 (0.85–0.99)* | 0.98 (0.92–1.05) | 0.83 (0.77–0.89)** |
| Chinese | 761 (2.0) | 0.75 (0.62–0.90)* | 0.76 (0.62–0.93)* | 0.61 (0.52–0.73)** | 0.62 (0.51–0.75)** |
| Marital status | |||||
| Married | 21 244 (55.9) | Reference | Reference | Reference | Reference |
| Single | 7719 (20.3) | 1.67 (1.58–1.77)** | 1.33 (1.25–1.41)** | 1.55 (1.48–1.63)** | 1.34 (1.27–1.41)** |
| Separated/divorced | 5456 (14.4) | 1.72 (1.61–1.84)** | 1.34 (1.25–1.43)** | 1.70 (1.61–1.79)** | 1.38 (1.31–1.46)** |
| Widowed | 3576 (9.4) | 2.23 (2.08–2.39)** | 1.50 (1.39–1.62)** | 2.38 (2.25–2.52)** | 1.56 (1.47–1.66)** |
| Insurance status | |||||
| Uninsured | 7083 (18.6) | Reference | Reference | Reference | Reference |
| Insured | 30 912 (81.4) | 0.53 (0.51–0.56)** | 0.69 (0.65–0.73)** | 0.57 (0.55–0.60)** | 0.66 (0.63–0.70)** |
| Median household income‡ | |||||
| <Quartile 1 (US $47 685) | 9416 (24.8) | Reference | Reference | Reference | Reference |
| <Quartile 2 (US $55 942) | 9430 (24.8) | 1.00 (0.93–1.06) | 0.97 (0.91–1.04) | 0.93 (0.88–0.98)* | 0.94 (0.89–1.00)* |
| <Quartile 3 (US $65 394) | 9428 (24.8) | 0.95 (0.89–1.01) | 1.00 (0.94–1.07) | 0.90 (0.86–0.95)** | 0.97 (0.91–1.02) |
| ≥Quartile 3 (US $65 394) | 9721 (25.6) | 0.79 (0.74–0.84)** | 0.85 (0.80–0.91)** | 0.77 (0.73–0.82)** | 0.85 (0.80–0.90)** |
| Unemployment rate‡ | |||||
| ≥Quartile 3 (12.5%) | 12 661 (33.3) | Reference | Reference | Reference | Reference |
| <Quartile 3 (12.5%) | 6653 (17.5) | 0.94 (0.88–1.01) | 1.02 (0.95–1.09) | 0.97 (0.92–1.02) | 1.01 (0.95–1.07) |
| <Quartile 2 (10.8%) | 10 012 (26.4) | 0.87 (0.82–0.92)** | 0.99 (0.92–1.05) | 0.89 (0.85–0.94)** | 0.98 (0.93–1.04) |
| <Quartile 1 (9.0%) | 8669 (22.8) | 0.79 (0.74–0.84)** | 0.94 (0.88–1.01) | 0.85 (0.80–0.89)** | 0.96 (0.91–1.02) |
| Residence‡ | |||||
| Metro area | 33 296 (87.6) | Reference | – | Reference | – |
| Non‐metro urban area | 4094 (10.8) | 0.96 (0.91–1.05) | – | 1.03 (0.97–1.10) | – |
| Non‐metro rural area | 605 (1.6) | 0.97 (0.81–1.17) | – | 1.03 (0.89–1.20) | – |
| Education level‡ | |||||
| <Quartile 1 (79.6%) | 9469 (24.9) | Reference | Reference | Reference | Reference |
| <Quartile 2 (86.4%) | 9540 (25.1) | 0.97 (0.91–1.03) | 0.98 (0.91–1.05) | 1.00 (0.95–1.05) | 0.98 (0.92–1.05) |
| <Quartile 3 (89.3%) | 9549 (25.1) | 0.91 (0.85–0.97)* | 0.98 (0.91–1.07) | 0.94 (0.89–0.99)* | 1.00 (0.94–1.08) |
| ≥Quartile 3 (89.3%) | 9437 (24.8) | 0.79 (0.74–0.84)** | 0.94 (0.86–1.04) | 0.83 (0.79–0.88)** | 0.98 (0.91–1.06) |
| Cancer subsite | |||||
| Nasopharynx | 1457 (3.8) | Reference | Reference | Reference | Reference |
| Oropharynx | 6278 (16.5) | 0.76 (0.67–0.87)** | 0.68 (0.59–0.78)** | 0.82 (0.73–0.92)** | 0.71 (0.63–0.80)** |
| Hypopharynx | 1475 (3.9) | 2.44 (2.12–2.81)** | 1.52 (1.31–1.76)** | 2.62 (2.31–2.97)** | 1.53 (1.35–1.75)** |
| Larynx | 10 477 (27.6) | 1.03 (0.91–1.17) | 1.12 (0.98–1.28) | 1.30 (1.16–1.45)** | 1.19 (1.06–1.33)* |
| Oral cavity | 18 308 (48.2) | 0.93 (0.83–1.05) | 1.04 (0.91–1.18) | 1.09 (0.98–1.21) | 1.06 (0.95–1.19) |
| T category§ | |||||
| T1 | 13 791 (36.3) | Reference | Reference | Reference | Reference |
| T2 | 11 739 (30.9) | 2.20 (2.05–2.36)** | 1.84 (1.71–1.98)** | 1.78 (1.68–1.88)** | 1.58 (1.49–1.67)** |
| T3 | 5889 (15.5) | 3.86 (3.58–4.15)** | 2.84 (2.63–3.07)** | 2.85 (2.69–3.02)** | 2.25 (2.12–2.40)** |
| T4 | 6576 (17.3) | 5.93 (5.53–6.35)** | 4.04 (3.75–4.34)** | 4.08 (3.86–4.31)** | 3.04 (2.87–3.23)** |
| N category§ | |||||
| N0 | 20 038 (52.7) | Reference | Reference | Reference | Reference |
| N1 | 5791 (15.2) | 2.11 (1.98–2.25)** | 1.86 (1.74–1.99)** | 1.66 (1.58–1.75)** | 1.58 (1.50–1.67)** |
| N2 | 11 121 (29.3) | 2.28 (2.16–2.40)** | 2.02 (1.91–2.14)** | 1.72 (1.64–1.79)** | 1.67 (1.60–1.76)** |
| N3 | 1045 (2.8) | 3.14 (2.81–3.51)** | 2.75 (2.45–3.09)** | 2.35 (2.13–2.59)** | 2.28 (2.06–2.53)** |
| Definitive treatment¶ | |||||
| No | 2587 (6.8) | Reference | Reference | Reference | Reference |
| Yes | 35 408 (93.2) | 0.21 (0.20–0.23)** | 0.29 (0.27–0.31)** | 0.24 (0.23–0.25)** | 0.32 (0.30–0.33)** |
*P < 0.050. **P‐value ≤ 0.001. †HRs for socioeconomic factors were adjusted for age at diagnosis, gender, race/ethnicity, cancer subsite, T category, N category and definitive treatment. ‡All data are county‐level; education level represents the percentage of patients aged ≥25 years with at least a high school diploma. §The classification was based on the 6th edition of the TNM staging system. ¶Definitive treatment consisted of surgery and/or radiotherapy. CI, confidence interval; CSS, cause‐specific survival; HNSCC, head and neck squamous cell carcinoma; HR, hazard ratio; N, node; OS, overall survival; T, tumor.
Univariate and multivariate Cox analyses of the effect of socioeconomic factors on CSS and OS are shown in Table 1. Only marital status, median household income and insurance status were validated to have significance for CSS and OS. Unmarried status, uninsured status and relatively lower median household income (<US $65 394) had negative effects on survival outcomes. Moreover, all demographics and clinical characteristics were significant, except for “gender” with respect to CSS. In addition, apart from the unemployment rate, all socioeconomic factors were significantly associated with definitive treatment (Table S1). Patients with insurance, higher median household income and higher education level were more likely to receive definitive treatment; unmarried patients and patients from non‐metro areas were less likely to receive definitive treatment.
Development and internal validation of nomograms for OS and DFS
The prognostic nomograms for CSS and OS at 3‐ and 5‐years in non‐metastatic HNSCC are presented in Figure 1(a,b). Calibration plots revealed excellent agreement between the nomogram‐predicted probabilities and actual observations of 3‐ and 5‐year CSS and OS (Fig. 1c–d). Individually, the nomograms for CSS and OS had significantly higher c‐indexes than the 6th edition TNM staging system (0.744 vs 0.706, P < 0.001; 0.725 vs 0.668, P < 0.001; Table 2). Nomograms for CSS and OS that individually excluded race/ethnicity, marital status, median household income, or insurance status yielded generally lower c‐indexes than the corresponding original nomograms, except for the nomogram for CSS that excluded race/ethnicity (0.744 vs 0.744, P = 1.000; Table 2).
Figure 1.
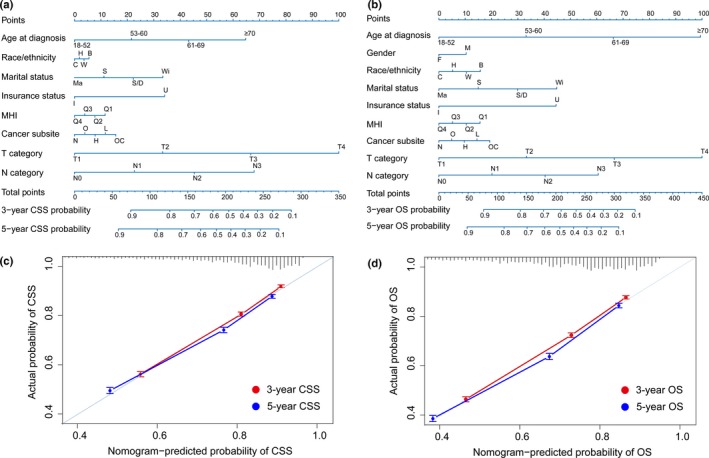
Prognostic nomograms (a, b) and calibration plots of survival probabilities at 3‐/5‐years (c, d) in patients with non‐metastatic head and neck squamous cell carcinoma (HNSCC). The left panel represents the nomogram and calibration plots for cause‐specific survival (CSS) (a, c); the right panel represents the nomogram and calibration plots for OS (b, d). Points for each variable were calculated by drawing a vertical straight line from a patient's variable value upward to the axis labeled “Points.” A vertical straight line is draw downward from the value located on the axis of “Total points” to estimate 3‐ and 5‐year survival. In calibration plots, nomogram‐predicted CSS/OS is plotted on the x‐axis; actual CSS/OS is plotted on the y‐axis. Dash lines falling along the 45‐degree line represent the ideal calibration models in which the predicted probabilities are identical to the observed probabilities. Vertical bars represent 95% confidence intervals. B, non‐Hispanic black; C, Chinese; F, female; H, Hispanic; H, hypopharynx; I, insured; L, larynx; M, male; Ma, married; MHI, median household income; N, nasopharynx; O, oropharynx; OC, oral cavity; OS, overall survival; Q, quartile; S, single; S/D, separated/divorced; U, uninsured; W, non‐Hispanic white; Wi, widowed.
Table 2.
C‐indexes for the nomograms and 6th edition TNM staging system in patients with non‐metastatic HNSCC
| Items | CSS | OS | ||
|---|---|---|---|---|
| C‐index (95% CI) | P‐value | C‐index (95% CI) | P‐value | |
| Nomogram | 0.744 (0.739–0.749) | Reference | 0.725 (0.721–0.729) | Reference |
| The 6th edition TNM staging system | 0.706 (0.701–0.711) | <0.001 | 0.668 (0.664–0.672) | <0.001 |
| Nomogram (excluding race/ethnicity) | 0.744 (0.739–0.749) | 1.000 | 0.722 (0.718–0.726) | 0.299 |
| Nomogram (excluding marital status) | 0.739 (0.734–0.744) | 0.166 | 0.719 (0.715–0.723) | 0.038 |
| Nomogram (excluding insurance status) | 0.737 (0.732–0.742) | 0.052 | 0.717 (0.713–0.721) | 0.006 |
| Nomogram (excluding median household income) | 0.742 (0.737–0.747) | 0.579 | 0.722 (0.718–0.726) | 0.299 |
CI, confidence interval; CSS, cause‐specific survival; HNSCC, head and neck squamous cell carcinoma; OS, overall survival; TNM, Tumor‐Node‐Metastasis.
Establishment and application of a prognostic score model
Three disadvantageous socioeconomic factors: unmarried status, uninsured status and median household income <US $65 394 were used to establish a prognostic score model. Therefore, the score for each patient could be 0, 1, 2 or 3, indicating a gradually increasing risk of death. For the subgroups with scores of 0 (n = 5407), 1 (n = 17 188), 2 (n = 11 280) and 3 (n = 4 120), the 5‐year cumulative CSS rates were 81.2%, 76.4%, 67.0% and 55.4%, and the 5‐year cumulative OS rates were 72.3%, 65.8%, 54.3% and 42.6%, respectively. The OS and CSS curves of all four risk subgroups were significantly separated (all P < 0.001; Fig. 2a,b).
Figure 2.
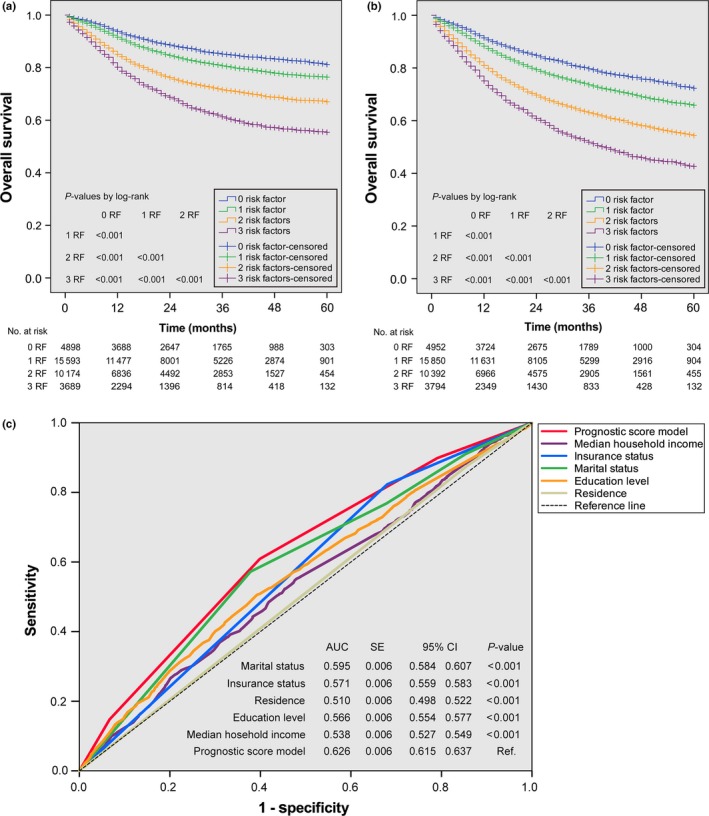
Kaplan–Meier survival curves for CSS (a) and OS (b) and receiver‐operating characteristic curves for receipt of definitive treatment (c) based on the prognostic score model in patients with non‐metastatic HNSCC. Two top curves are stratified by the number of risk factors. AUC, area under the curve; CI, confidence interval; CSS, cause‐specific survival; HNSCC, head and neck squamous cell carcinoma; No., number; OS, overall survival; RF, risk factors; SE, standard error.
The efficacy of the prognostic score model for predicting the receipt of definitive treatment as a secondary outcome was shown in Figure 2(c). The area under the curve (AUC) for the prognostic score model was 0.626, which was significantly higher than the AUC of any individual socioeconomic factor (all P < 0.001). A cut‐off score of 1.5 resulted in the highest Youden's index with a sensitivity of 0.61 and specificity of 0.60 with respect to receipt of definitive treatment. Thus, the four risk subgroups were condensed into two risk groups, i.e., low‐risk (score = 0–1; n = 22 595 patients) and high‐risk (score = 2–3; n = 15 400); these risk groups were applicable to both the primary and secondary outcomes of this study. As shown in Figure 3, after adjustment for demographic and clinical characteristics, low‐risk patients with non‐metastatic HNSCC were more likely to undergo definitive treatment than high‐risk patients (AOR = 2.02, 95% CI = 1.85–2.20, P < 0.001), an association that remained significant when each cancer subsite was evaluated individually (all P < 0.001). After adjustment for the same covariates plus definitive treatment, low‐risk patients had significantly better CSS and OS than high‐risk patients (AHR = 0.63, 95% CI = 0.60–0.66, P < 0.001; AHR = 0.63, 95% CI = 0.60–0.65, P < 0.001, respectively), this effect remained significant for all cancer subsites evaluated (all P ≤ 0.011).
Figure 3.
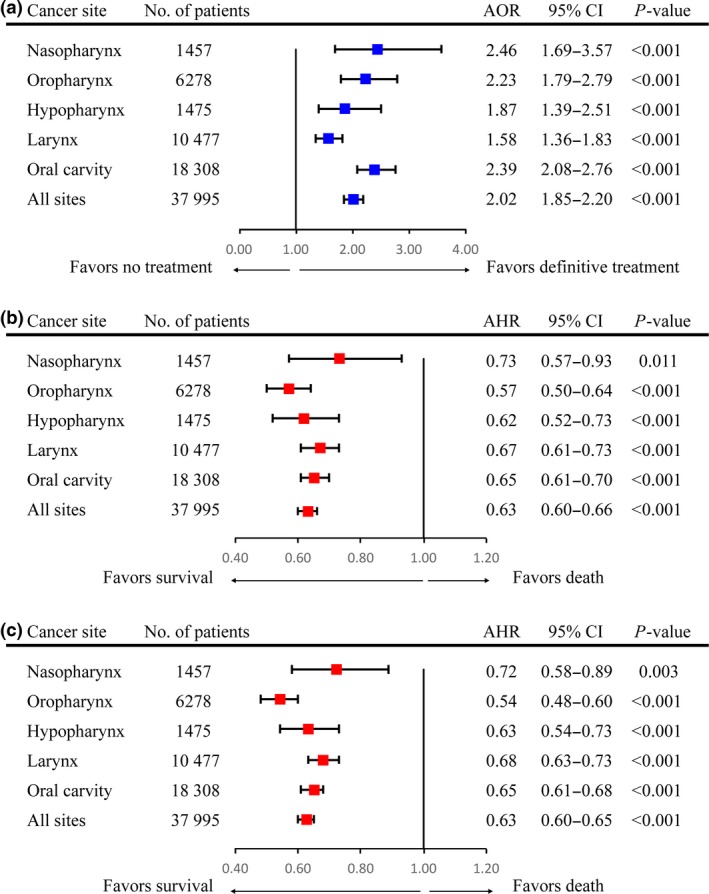
Forest plots depicting AHRs/AORs and 95% CIs of the association between socioeconomic status (high‐risk versus low‐risk) and receipt of definitive treatment (a), CSS (b) and OS (c). Squares represent AHRs/AORs with 95% CIs indicated by horizontal bars. AHR, adjusted hazard ratio; AOR, adjusted odds ratio; CI, confidence interval; CSS, cause‐specific survival; No., number; OS, overall survival.
Impact of race/ethnicity on CSS and OS in the low‐risk and high‐risk groups
In the low‐risk group, non‐Hispanic black patients had poorer CSS than other races/ethnicities (all P ≤ 0.001); a non‐significant difference in CSS was observed between the non‐Hispanic white, Hispanic American and Chinese American subgroups (all P > 0.050; Fig. 4a). In the high‐risk group, Chinese American patients and non‐Hispanic black patients had the best and poorest CSS, respectively, compared to other races/ethnicities (all P < 0.001). Non‐Hispanic white patients had equivalent CSS to Hispanic patients (P = 0.748; Fig. 4b). As shown in Figure 4(c–d), all of the OS curves for patients with different races/ethnicities were significantly separated (all P ≤ 0.004), except for those of non‐Hispanic white and Hispanic American patients in the low‐risk group (P = 0.036). Chinese Americans and non‐Hispanic black patients achieved the best and poorest OS, respectively.
Figure 4.
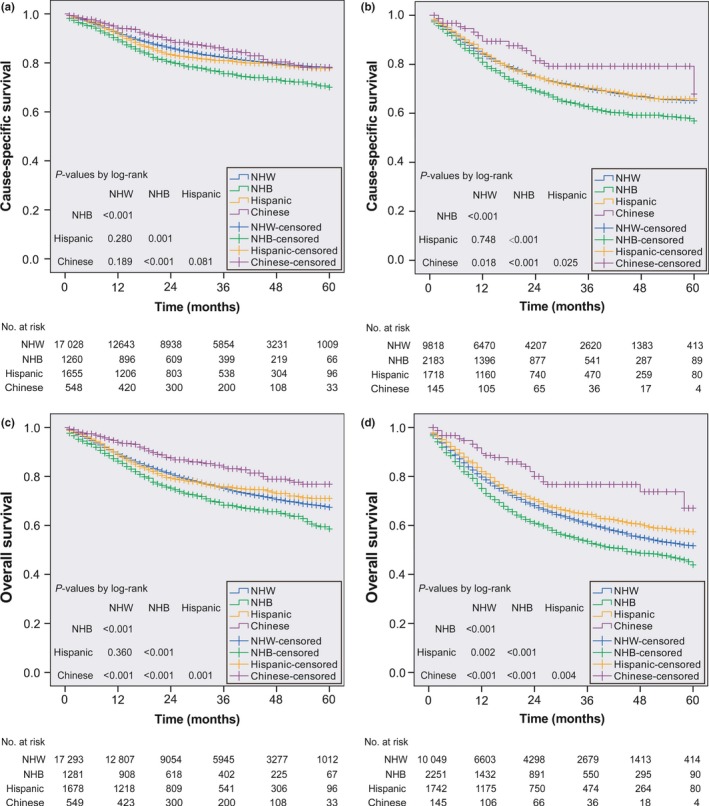
Kaplan–Meier survival curves for CSS (a, b) and OS (c, d) in patients with non‐metastatic HNSCC stratified by race/ethnicity. The left panel represents the survival curves in low‐risk patients (a, c); the right panel represents the survival curves in high‐risk patients (b, d). HNSCC, head and neck squamous cell carcinoma; CSS, cause‐specific survival; NHB, non‐Hispanic black; NHW, non‐Hispanic white; No., number; OS, overall survival.
Discussion
To the best of our knowledge, this is the first attempt to establish nomograms for CSS and OS and a prognostic score model based on socioeconomic factors for patients with non‐metastatic HNSCC. Importantly, we used the prognostic score model to generate risk stratifications, demonstrate the protective effect of low‐risk socioeconomic status, and elucidate the role of race/ethnicity in survival. The present study provides important information to assist development of health‐related policies and indicates the necessity of targeted social support‐based interventions for high‐risk patients, such as those with unmarried status, uninsured status and low income.
Disparities in socioeconomic status and race/ethnicity can confer different survival outcomes in many malignancies.4, 5, 6, 7, 8, 9, 10, 11, 17, 18 In general, patients with advantaged socioeconomic status are more likely to “die with cancer” compared to patients with disadvantaged socioeconomic status who are more likely to “die by cancer.” A previous large pooled analysis of 31 case‐control studies from 27 countries reported low levels of income and educational attainment were significantly associated with the risk of HNSCC.8 However, since this was a multinational study, there was a lack of standardized measurement for data processing between studies from different countries. Moreover, the large number of missing values reduced the reliability of the pooled analysis. As a nation of immigrants, the United States has a population of diverse races/ethnicities. The SEER program of the United States uses unified standardization to collect and organize per‐patient data. Therefore, the SEER‐18 is a suitable data source for the present study to assess the effect of socioeconomic factors and race/ethnicity in non‐metastatic HNSCC.
Higher household income and insured status can provide better financial support that enables patients to receive more timely treatment at superior, specialized hospitals. As a type of social support, married status has protective effects in many malignancies.26, 27, 28, 29 Aizer et al. individually analyzed 10 leading causes of cancer‐related deaths (including HNSCC) in the United States, and reported married status conferred survival benefits. Moreover, marriage conferred a greater survival advantage than the published survival advantage reported for chemotherapy in HNSCC.30 Several possible mechanisms may explain the relationship between married status and survivorship. Firstly, married status represents strong support from family members (e.g., spouse, children, close relatives), who can provide financial aid and meticulous heath care for patients with cancer. Therefore, married patients have better compliance to radical therapies and medical recommendations compared to unmarried patients.31 Secondly, according to the social readjustment rating scale created by Holmes and Rahe, the death of a spouse, divorce and marital separation rank as the first to third leading factors that confer psychological distress on individuals.32 Psychological disorders, such as despair, depression and anxiety, have been proven to induce health‐related problems that affect the longevity of patients with cancer.33 In the present study, it is noteworthy that education level, unemployment rate and residence had non‐significant effects on survival outcomes in non‐metastatic HNSCC, in contradiction to several previous studies that reported significant effects for these socioeconomic factors.4, 9 This discrepancy may be related to the different geographical origins of these studies. Countries with unbalanced developmental levels have different backgrounds in many respects, such as education level, residence type and other socioeconomic factors. Moreover, categorical variables (e.g., residence) in previous listed studies were classified according to specific standards depending on the data source, which may also lead to different outcomes.
Race/ethnicity seems to have a small effect in the nomogram for CSS; the c‐index value and 95% CI of the nomogram for CSS remained unchanged after eliminating “race/ethnicity” from the model. However, conclusions on the effect of race/ethnicity should be drawn with caution. A previous study focusing on patients in Florida indicated African American and non‐Hispanic patients had significantly poorer survival rates than White and Hispanic patients, respectively (P < 0.001 and 0.020, respectively).17 On the other hand, non‐significant differences in the survival outcomes of White, Black and Hispanic/other patients have been reported (P = 0.051).4 Thus, we further investigated the effect of race/ethnicity in each risk group to further elucidate this issue. Chinese American patients with high‐risk socioeconomic status obtained a greater survival benefit than other races/ethnicities, while non‐Hispanic black patients were less likely to enjoy longevity regardless of whether they had low‐risk or high‐risk socioeconomic status. This result may be due mainly to genetic factors, since several epidemiological studies have indicated Chinese patients with nasopharyngeal carcinoma have a survival advantage compared to non‐Hispanic white/black patients.34, 35, 36 In addition, even though oral cancer is prevalent among Asian populations, Chinese patients have a lower risk and later onset of oral cancer than Indian and Malaysian populations.37
This study has several limitations that must be taken into account. Firstly, not all data related to socioeconomic factors could be provided by the SEER database. Thus, we performed analyses by combining per‐patient data from the SEER database and county‐level data from other data sources. Moreover, the SEER database does not record detailed etiological or therapeutic information for HNSCC, including smoking, alcohol consumption, human papillomavirus type‐16 (HPV‐16) infection and chemotherapy regimens. Especially the information on the use of both tobacco and alcohol and the HPV infection, which have significant influence on survival outcomes in HNSCC.8, 9, 10, 11, 38 The absence of relevant data reduces the ability to assess the importance of these factors in HNSCC, as well as their potential interaction. Secondly, changes in socioeconomic factors (e.g., marital status) may occur after registering to the database or during treatment. The quality and stability of marital status also have significant influence on health.39 Last but not least, our results may not apply to patients with HNSCC in other countries, in that many socioeconomic factors vary significantly between countries. Moreover, although internal validation showed excellent agreement between the calibration plots, external validation could not be carried out due to a lack of data from other populations. However, the present study highlights the predictive effect of socioeconomic factors on the survival outcomes of patients with HNSCC. In the future, studies investigating the value of socioeconomic factors in HNSCC are needed in other populations. The three major risk factors for HNSCC, i.e., tobacco smoking, alcohol consumption and HPV infection, are suggested to be incorporated in the database by expanding the inclusion of relevant information (e.g., medical record, self‐report form and follow‐up data), or using additional data sources (e.g., local annual consumption of tobacco and/or wine).
Disclosure Statement
The authors have no conflict of interest.
Abbreviations
- AHR
adjusted hazard ratio
- AIC
Akaike information criterion
- AOR
adjusted odds ratio
- AUC
area under the curve
- CI
confidence interval
- c‐index
concordance index
- CSS
cause‐specific survival
- HNSCC
head and neck squamous cell carcinoma
- HPV
human papillomavirus
- IQR
interquartile range
- OS
overall survival
- ROC
receiver‐operating characteristic
- SEER
Surveillance, Epidemiology and End Results
Supporting information
Fig. S1. Distribution throughout the United States of the 37 995 patients from the SEER database included in this study.
Table S1. Multivariate logistic regression analysis of the effect of socioeconomic factors on receipt of definitive treatment in non‐metastatic HNSCC.
Acknowledgments
This work was supported by grants from the National Science & Technology Pillar Program during the Twelfth Five‐year Plan Period (No. 2014BAI09B10), the Science and Technology Project of Guangzhou City, China (No. 14570006), the Planned Science and Technology Project of Guangdong Province, China (No. 2013B020400004), the Health & Medical Collaborative Innovation Project of Guangzhou City, China (No. 201400000001), and the National Natural Science Foundation of China (No. 81230056).
Cancer Sci 108 (2017) 1253–1262
Funding Information
Health & Medical Collaborative Innovation Project of Guangzhou City, China (Grant/Award Number: No. 201400000001), Science and Technology Project of Guangzhou City, China (Grant/Award Number: No. 14570006), Planned Science and Technology Project of Guangdong Province, China (Grant/Award Number: No. 2013B020400004), National Science & Technology Pillar Program during the Twelfth Five‐year Plan Period (Grant/Award Number: No. 2014BAI09B10), National Natural Science Foundation of China (Grant/Award Number: No. 81230056).
References
- 1. Ferlay J, Soerjomataram I, Dikshit R et al Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–86. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30. [DOI] [PubMed] [Google Scholar]
- 3. Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet 2008; 371: 1695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choi SH, Terrell JE, Fowler KE et al Socioeconomic and other demographic disparities predicting survival among head and neck cancer patients. PLoS ONE 2016; 11: e0149886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Du XL, Liu CC. Racial/Ethnic disparities in socioeconomic status, diagnosis, treatment and survival among medicare‐insured men and women with head and neck cancer. J Health Care Poor Underserved 2010; 21: 913–30. [DOI] [PubMed] [Google Scholar]
- 6. Kuo TJ, Chu CH, Tang PL, Lai YC. Effects of geographic area and socioeconomic status in Taiwan on survival rates of head and neck cancer patients after radiotherapy. Oral Oncol 2016; 62: 136–8. [DOI] [PubMed] [Google Scholar]
- 7. de Graeff A, de Leeuw JR, Ros WJ, Hordijk GJ, Blijham GH, Winnubst JA. Sociodemographic factors and quality of life as prognostic indicators in head and neck cancer. Eur J Cancer 2001; 37: 332–9. [DOI] [PubMed] [Google Scholar]
- 8. Conway DI, Brenner DR, McMahon AD et al Estimating and explaining the effect of education and income on head and neck cancer risk: INHANCE consortium pooled analysis of 31 case‐control studies from 27 countries. Int J Cancer 2015; 136: 1125–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boing AF, Antunes JL, de Carvalho MB et al How much do smoking and alcohol consumption explain socioeconomic inequalities in head and neck cancer risk? J Epidemiol Community Health 2011; 65: 709–14. [DOI] [PubMed] [Google Scholar]
- 10. Conway DI, McKinney PA, McMahon AD et al Socioeconomic factors associated with risk of upper aerodigestive tract cancer in Europe. Eur J Cancer 2010; 46: 588–98. [DOI] [PubMed] [Google Scholar]
- 11. Johnson S, McDonald JT, Corsten MJ. Socioeconomic factors in head and neck cancer. J Otolaryngol Head Neck Surg 2008; 37: 597–601. [PubMed] [Google Scholar]
- 12. Chu KP, Habbous S, Kuang Q et al Socioeconomic status, human papillomavirus, and overall survival in head and neck squamous cell carcinomas in Toronto, Canada. Cancer Epidemiol 2016; 40: 102–12. [DOI] [PubMed] [Google Scholar]
- 13. Conway DI, McMahon AD, Smith K et al Components of socioeconomic risk associated with head and neck cancer: a population‐based case–control study in Scotland. Br J Oral Maxillofac Surg 2010; 48: 11–7. [DOI] [PubMed] [Google Scholar]
- 14. Blot WJ, McLaughlin JK, Winn DM et al Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res 1988; 48: 3282–7. [PubMed] [Google Scholar]
- 15. Szymanska K, Hung RJ, Wunsch‐Filho V et al Alcohol and tobacco, and the risk of cancers of the upper aerodigestive tract in Latin America: a case–control study. Cancer Causes Control 2011; 22: 1037–46. [DOI] [PubMed] [Google Scholar]
- 16. Inverso G, Mahal BA, Aizer AA, Donoff RB, Chau NG, Haddad RI. Marital status and head and neck cancer outcomes. Cancer 2015; 121: 1273–8. [DOI] [PubMed] [Google Scholar]
- 17. Molina MA, Cheung MC, Perez EA et al African American and poor patients have a dramatically worse prognosis for head and neck cancer: an examination of 20,915 patients. Cancer 2008; 113: 2797–806. [DOI] [PubMed] [Google Scholar]
- 18. Gourin CG, Podolsky RH. Racial disparities in patients with head and neck squamous cell carcinoma. Laryngoscope 2006; 116: 1093–106. [DOI] [PubMed] [Google Scholar]
- 19. Number of persons by race and Hispanic ethnicity for SEER participants (2010 census data). The Surveillance, Epidemiology and End Results (SEER) program. National Cancer Institute; [Cited 26 December 2016.] Available from URL: http://seer.cancer.gov/registries/data.html. [Google Scholar]
- 20. United States Census: Small Area Income and Poverty Estimates. [Cited 26 December 2016.] Available from URL: http://www.census.gov/did/www/saipe/data/statecounty/data/2004.html.
- 21. United States Department of Agriculture, Economic Research Service. [Cited 26 December 2016.] Available from URL: https://www.ers.usda.gov/data-products/county-level-data-sets.aspx.
- 22. Cox D. Regression models and life‐tables. J R Stat Soc Series B Stat Methodol 1972; 34: 187–220. [Google Scholar]
- 23. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–81. [Google Scholar]
- 24. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996; 15: 361–87. [DOI] [PubMed] [Google Scholar]
- 25. Neyeloff JL, Fuchs SC, Moreira LB. Meta‐analyses and Forest plots using a microsoft excel spreadsheet: step‐by‐step guide focusing on descriptive data analysis. BMC Res Notes 2012; 5: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Costa LJ, Brill IK, Brown EE. Impact of marital status, insurance status, income, and race/ethnicity on the survival of younger patients diagnosed with multiple myeloma in the United States. Cancer 2016; 122: 3183–90. [DOI] [PubMed] [Google Scholar]
- 27. He XK, Lin ZH, Qian Y, Xia D, Jin P, Sun LM. Marital status and survival in patients with primary liver cancer. Oncotarget 2016; https://doi.org/10.18632/oncotarget.11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shi RL, Qu N, Lu ZW, Liao T, Gao Y, Ji QH. The impact of marital status at diagnosis on cancer survival in patients with differentiated thyroid cancer. Cancer Med 2016; 5: 2145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qiu M, Yang D, Xu R. Impact of marital status on survival of gastric adenocarcinoma patients: results from the Surveillance Epidemiology and End Results (SEER) Database. Sci Rep 2016; 6: 21098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aizer AA, Chen MH, McCarthy EP et al Marital status and survival in patients with cancer. J Clin Oncol 2013; 31: 3869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta‐analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 2000; 160: 2101–7. [DOI] [PubMed] [Google Scholar]
- 32. Holmes TH, Rahe RH. The Social Readjustment Rating Scale. J Psychosom Res 1967; 11: 213–8. [DOI] [PubMed] [Google Scholar]
- 33. Phillips AC, Carroll D, Der G. Negative life events and symptoms of depression and anxiety: stress causation and/or stress generation. Anxiety Stress Coping 2015; 28: 357–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun LM, Li CI, Huang EY, Vaughan TL. Survival differences by race in nasopharyngeal carcinoma. Am J Epidemiol 2007; 165: 271–8. [DOI] [PubMed] [Google Scholar]
- 35. Wang Y, Zhang Y, Ma S. Racial differences in nasopharyngeal carcinoma in the United States. Cancer Epidemiol 2013; 37: 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bouchardy C, Parkin DM, Khlat M. Cancer mortality among Chinese and South‐East Asian migrants in France. Int J Cancer 1994; 58: 638–43. [DOI] [PubMed] [Google Scholar]
- 37. Zain RB, Ikeda N, Razak IA et al A national epidemiological survey of oral mucosal lesions in Malaysia. Community Dent Oral Epidemiol 1997; 25: 377–83. [DOI] [PubMed] [Google Scholar]
- 38. Gillison ML, D'Souza G, Westra W et al Distinct risk factor profiles for human papillomavirus type 16‐positive and human papillomavirus type 16‐negative head and neck cancers. J Natl Cancer Inst 2008; 100: 407–20. [DOI] [PubMed] [Google Scholar]
- 39. Jaremka LM, Glaser R, Malarkey WB, Kiecolt‐Glaser JK. Marital distress prospectively predicts poorer cellular immune function. Psychoneuroendocrinology 2013; 38: 2713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Distribution throughout the United States of the 37 995 patients from the SEER database included in this study.
Table S1. Multivariate logistic regression analysis of the effect of socioeconomic factors on receipt of definitive treatment in non‐metastatic HNSCC.


