Abstract
Subgroup analyses of a randomized global phase II study of axitinib showed objective response rate of 66% and median progression‐free survival of 27.6 months in treatment‐naïve Japanese patients with metastatic renal cell carcinoma (RCC). This analysis evaluated overall survival (OS) and safety in 44 Japanese patients and compared the results with 169 non‐Japanese patients. In addition, baseline characteristics for predictive factors that may influence OS in first‐line metastatic RCC were explored in all patients using a Cox proportional hazard model. With median follow‐up of 33 months, fewer than half (16 of 44) of the Japanese patients had died and median OS was not reached (95% confidence interval [CI], 38.8 months–not estimable), whereas 107 of 169 (63%) non‐Japanese patients had died and median OS was 33.9 months (95% CI, 28.9–42.7). Estimated 1‐year, 2‐year and 3‐year survival probability (95% CI) was 86.4% (76.2–96.5), 75.0% (62.2–87.8) and 68.2% (54.4–81.9), respectively, in Japanese patients, and was higher than that in non‐Japanese patients (75.1% [68.4–81.8], 62.1% [54.5–69.7] and 47.2% [39.3–55.1], respectively). The updated safety analysis did not reveal any new adverse events of concern among Japanese or non‐Japanese patients. The multivariate analysis identified that lower baseline Eastern Cooperative Oncology Group performance status, lower baseline tumor burden, and longer time from histopathological diagnosis to treatment were significant positive predictors of OS. The current analysis confirmed the clinical activity of axitinib in treatment‐naïve Japanese patients with metastatic RCC, with an acceptable toxicity profile.
Keywords: Antiangiogenic agent, axitinib, clinical trial phase II, Japanese, renal cell carcinoma
Over the past decade, the landscape of treatment options for advanced and metastatic renal cell carcinoma (RCC) has evolved significantly with the approval of several targeted agents, including vascular endothelial growth factor (VEGF) receptor tyrosine kinase inhibitors (TKI), anti‐VEGF monoclonal antibody, mammalian target of rapamycin inhibitors, and anti‐programmed death 1 monoclonal antibody. Axitinib, a potent and selective inhibitor of VEGF receptors 1–3, was approved in 2012 for the treatment of advanced RCC after failure of one prior therapy, based on a significantly longer progression‐free survival (PFS) compared with sorafenib in a head‐to‐head randomized phase III Axitinib Second‐line (AXIS) trial.1 Although an improved PFS with axitinib treatment remained in the follow‐up analysis, it did not translate to a longer survival benefit.2
In treatment‐naïve patients with metastatic RCC in a randomized open‐label phase III trial, the difference in median PFS between axitinib and sorafenib did not reach significance3 and no survival advantage was observed with axitinib over sorafenib.4 However, axitinib showed antitumor activity with an acceptable safety profile. The National Comprehensive Cancer Network now includes axitinib among the first‐line treatment options for metastatic unresectable RCC, in addition to its well‐established position in the second‐line setting.5
Antitumor activity and the safety of axitinib for metastatic RCC in the first‐line setting was also investigated in a multinational, randomized phase II trial, in which the effect of axitinib dose titration on efficacy and safety was evaluated prospectively.6 The study showed that a statistically higher proportion of patients in the axitinib dose‐titration group achieved an objective response compared with the placebo dose‐titration group, providing evidence for the clinical benefit of individualized dose titration in some patients. Furthermore, median overall survival (OS) was found to be numerically longer in patients who received axitinib dose titration compared with those who received placebo dose titration.7
Although the efficacy and safety of axitinib have previously been shown in Japanese patients with metastatic RCC in the second‐line setting on the basis of a Japanese phase II study8, 9 and a subgroup analysis of the AXIS trial,10 there has been no such report for the first‐line setting. To investigate whether axitinib is efficacious and safe in Japanese patients with metastatic RCC in the first‐line setting and, if so, whether Japanese patients achieve better efficacy outcomes than non‐Japanese patients, we conducted a subgroup analysis from this multinational, randomized phase II trial. The analysis indicated that axitinib is effective, with median PFS exceeding 2 years, and is well tolerated in treatment‐naïve Japanese patients with metastatic RCC.11 The objective response rate (95% CI) in Japanese patients was 66% (50–80) compared with 44% (36–52) in non‐Japanese patients, providing further evidence for more favorable clinical outcomes in axitinib‐treated Japanese patients. The aim of the current analysis was to evaluate the OS and the safety of treatment with first‐line axitinib in Japanese patients with metastatic RCC in this phase II study. In addition, we investigated potential predictive values of baseline characteristics for OS using the data from all patients enrolled in this study.
Patients and Methods
Study design, patients and treatment
The study design and patient eligibility criteria have been described in detail elsewhere.6, 11 In brief, patients aged ≥18 years with histologically confirmed metastatic RCC with a component of clear cell histology were enrolled from six countries, including Japan, in this multicenter, double‐blind, randomized phase II study.
All patients received axitinib 5 mg b.i.d. during a 4‐week lead‐in period. Patients who met the randomization criteria at the end of the lead‐in period were stratified by Eastern Cooperative Oncology Group performance status (ECOG PS) and randomly assigned (1:1) to axitinib with or without titration. The randomization criteria were: blood pressure ≤150/90 mm Hg, absence of drug‐related grade 3 or 4 adverse events (AE) according to the NCI Common Terminology Criteria for Adverse Events (NCI‐CTCAE) v3.0, no axitinib dose reductions, and use of no more than two concurrent antihypertensive medications for 2 consecutive weeks. Patients who did not meet the randomization criteria continued on axitinib in a non‐randomized arm.
Following the lead‐in period, patients in the axitinib‐titration or placebo‐titration arm had their daily dose titrated to 7 mg b.i.d. (i.e. 5 mg axitinib plus either 2 mg axitinib or placebo). If patients tolerated the 7 mg b.i.d. dose by meeting the dose titration (i.e. randomization) criteria for 2 consecutive weeks, the dose could then be increased to a maximum of 10 mg b.i.d. (i.e. 5 mg axitinib plus either 5 mg axitinib or placebo). The axitinib dose could also be reduced from 5 mg b.i.d. to 3 mg b.i.d., and then to 2 mg b.i.d., if necessary, to manage axitinib‐related grade ≥3 toxicities or hypertension while on maximal antihypertensive medications. Both patients and investigators were blinded to the drug (axitinib or placebo) used in dose titration. Study treatments were administered to patients in 4‐week cycles.
The primary endpoint of the study, the comparison of the objective response rate between the two randomized arms, has been reported previously.6 Secondary endpoints included PFS, OS and safety.
The study protocol was approved at each study center by an institutional review board or independent ethics committee, and the study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization guidelines on Good Clinical Practice, and applicable local regulatory requirements. Written, informed consent was obtained from each patient.
Assessments
Radiological tumor assessments were conducted by investigators according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.0 at screening; after 8, 16 and 24 weeks of treatment; and every 12 weeks thereafter. Safety was monitored throughout the study and AE were graded per NCI‐CTCAE v3.0. Blood pressure was monitored at each clinic visit and at home by patients twice daily before study drug administration. Survival status was collected every 3 months after the follow‐up study visit, which was 28 days after the last dose.
Statistical analyses
The calculation of the sample size required for the primary endpoint and statistical analyses have been described previously.6 Median OS, survival rate, and their 95% CI were estimated using the Kaplan–Meier method, and comparisons between Japanese versus non‐Japanese patients and by ECOG PS (0 vs ≥1) were done using unstratified and stratified log‐rank tests, respectively, and hazard ratio (HR) and 95% CI were provided. Ad hoc analyses to assess baseline predictive factors were performed using the Cox proportional hazard model. Each variable was tested in a univariate analysis with the Wald test, and the final model was constructed using a stepwise procedure with a 5% significance level.
Results
Patient disposition, baseline characteristics and treatment
A total of 44 patients from Japan and 169 non‐Japanese patients from five other countries were enrolled in the study (Fig. 1). One Japanese and nine non‐Japanese patients discontinued the study treatment during the lead‐in period because of disease progression, withdrawal of consent, or other reasons. After the lead‐in period, 11 Japanese and 101 non‐Japanese patients were assigned to either the axitinib or the placebo titration arm. In total, 32 Japanese and 59 non‐Japanese continued axitinib in the non‐randomized arm. At the time of follow‐up analysis (data cutoff date: 4 November 2014), 36 of 44 (82%) Japanese and 157 of 169 (93%) non‐Japanese patients had discontinued study treatment, mostly because of disease progression.
Figure 1.
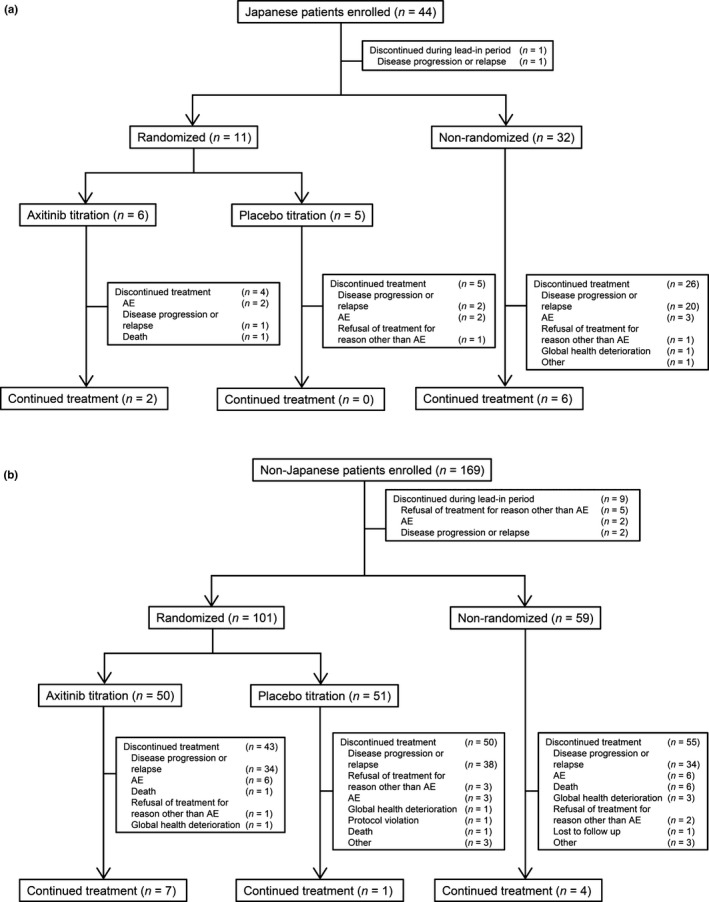
Trial profile: (a) Japanese and (b) non‐Japanese patients. AE, adverse event.
The demographics and baseline characteristics of overall Japanese versus non‐Japanese patients are summarized in Table 1.11 The median age of Japanese patients was 5 years older than non‐Japanese patients, but Japanese patients had more favorable baseline prognosis, with ECOG PS 0, fewer metastases and smaller tumor size. There was no significant difference in the percentage of Japanese versus non‐Japanese patients who had prior nephrectomy.
Table 1.
Demographics and baseline characteristics in the overall Japanese versus non‐Japanese patients†
| Japanese | Non‐Japanese | P‐value | |
|---|---|---|---|
| n = 44 | n = 169 | ||
| Age, median (range), years | 66 (42–81) | 61 (28–87) | 0.0231‡ |
| Sex, n (%) | |||
| Male | 30 (68) | 113 (67) | 1.0000§ |
| Female | 14 (32) | 56 (33) | |
| Race, n (%) | |||
| White | 0 | 162 (96) | <0.0001§ |
| Asian | 44 (100) | 2 (1) | |
| Black | 0 | 2 (1) | |
| Other | 0 | 3 (2) | |
| Height, mean (SD), cm | 162 (9) | 172 (10) | <0.0001‡ |
| Weight, mean (SD), kg | 61 (12) | 83 (18) | <0.0001‡ |
| ECOG PS, n (%)¶ | |||
| 0 | 37 (84) | 99 (59) | 0.0015§ |
| ≥1 | 7 (16) | 70 (41) | |
| Prior nephrectomy, n (%) | |||
| Yes | 37 (84) | 146 (86) | 0.8077§ |
| No | 7 (16) | 23 (14) | |
| Number of metastatic sites, n (%) | |||
| 1 | 17 (39) | 24 (14) | 0.0003†† |
| 2 | 13 (30) | 44 (26) | |
| 3 | 6 (14) | 46 (27) | |
| ≥4 | 8 (18) | 55 (33) | |
| Metastatic sites (lung versus lung + others), n (%) | |||
| Lung only | 9 (20) | 16 (9) | 0.0627§ |
| Lung + others | 35 (80) | 153 (91) | |
| Metastatic sites (individual), n (%) | |||
| Lung | 30 (68) | 119 (70) | 0.8538§ |
| Lymph node | 13 (30) | 86 (51) | 0.0169§ |
| Kidney | 13 (30) | 37 (22) | 0.3194§ |
| Liver | 6 (14) | 47 (28) | 0.0766§ |
| Adrenal | 3 (7) | 46 (27) | 0.0042§ |
| Bone | 7 (16) | 30 (18) | 1.0000§ |
| Pancreas | 1 (2) | 4 (2) | 1.0000§ |
| Time from histopathological diagnosis to treatment, median (range), weeks | |||
| 56 (0.1–952) | 23 (0.1–1338) | 0.9223‡‡ | |
| Time from metastatic diagnosis to treatment, median (range), weeks | |||
| 7 (0.9–263) | 8 (0.7–456) | 0.4476‡‡ | |
| Sum of longest diameter for target lesion, median (range), mm | |||
| 75 (10–376) | 99 (10–466) | 0.0013‡‡ | |
| Presence of metastases (de novo) at initial diagnosis, n (%) | |||
| No | 25 (57) | 94 (56) | 1.0000§ |
| Yes | 19 (43) | 75 (44) | |
†Tomita Y et al. (2011).11 Available from http://jjco.oxfordjournals.org/content/46/11/1031.long. ‡Using Student's t‐test. §Using Fisher's exact test. ¶Per case report forms and the last measure taken prior to dosing. One non‐Japanese patient had ECOG PS 2. ††Using Cochran–Armitage trend exact test. ‡‡Using Wilcoxon test. ECOG PS, Eastern Cooperative Oncology Group performance status; SD, standard deviation.
Because a significantly higher percentage of Japanese than non‐Japanese patients (73 vs 35%) were not assigned to dose titration arms and remained on or below the starting 5 mg b.i.d. in the non‐randomized arm, patient baseline characteristics were also compared between Japanese and non‐Japanese patients in the non‐randomized arm (Table 2). As in the overall population, Japanese patients in the non‐randomized arm had more favorable prognosis at baseline than non‐Japanese patients, but there was no longer any significant difference between the two groups with regard to patient age.
Table 2.
Demographics and baseline characteristics in Japanese versus non‐Japanese patients in the non‐randomized arm
| Japanese | Non‐Japanese | P‐value | |
|---|---|---|---|
| n = 32 | n = 59 | ||
| Age, median (range), years | 63 (43–79) | 63 (47–87) | 0.7777† |
| Sex, n (%) | |||
| Male | 19 (59) | 36 (61) | 1.0000‡ |
| Female | 13 (41) | 23 (39) | |
| Race, n (%) | |||
| White | 0 | 55 (93) | <0.0001‡ |
| Asian | 32 (100) | 1 (2) | |
| Black | 0 | 2 (3) | |
| Other | 0 | 1 (2) | |
| Height, mean (SD), cm | 161 (9) | 171 (10) | <0.0001† |
| Weight, mean (SD), kg | 61 (13) | 87 (18) | <0.0001† |
| ECOG PS, n (%)¶ | |||
| 0 | 27 (84) | 36 (61) | 0.0313‡ |
| ≥1 | 5 (16) | 23 (39) | |
| Prior nephrectomy, n (%) | |||
| Yes | 28 (88) | 53 (90) | 0.7367‡ |
| No | 4 (13) | 6 (10) | |
| Number of metastatic sites, n (%) | |||
| 1 | 13 (41) | 9 (15) | 0.0045§ |
| 2 | 10 (31) | 14 (24) | |
| 3 | 3 (9) | 18 (31) | |
| ≥4 | 6 (19) | 18 (31) | |
| Metastatic sites (lung versus lung + others), n (%) | |||
| Lung only | 7 (22) | 8 (14) | 0.3781‡ |
| Lung + others | 25 (78) | 51 (86) | |
| Metastatic sites (individual), n (%) | |||
| Lung | 20 (63) | 47 (80) | 0.0869‡ |
| Lymph node | 7 (22) | 29 (49) | 0.0137‡ |
| Kidney | 9 (28) | 10 (17) | 0.2806‡ |
| Liver | 5 (16) | 14 (24) | 0.4281‡ |
| Adrenal | 2 (6) | 15 (25) | 0.0267‡ |
| Bone | 4 (13) | 9 (15) | 1.0000‡ |
| Pancreas | 1 (3) | 2 (3) | 1.0000‡ |
| Time from histopathological diagnosis to treatment, median (range), weeks | |||
| 56 (0.1–952) | 42 (0.1–1338) | 0.8127†† | |
| Time from metastatic diagnosis to treatment, median (range), weeks | |||
| 7 (1.3–263) | 9 (1.3–325) | 0.2854†† | |
| Sum of longest diameter for target lesion, median (range), mm | |||
| 65 (11–376) | 89 (10–379) | 0.0218†† | |
| Presence of metastases (de novo) at initial diagnosis, n (%) | |||
| No | 19 (59) | 34 (58) | 1.0000‡ |
| Yes | 13 (41) | 25 (42) | |
†Using Student's t‐test. ‡Using Fisher's exact test. ¶Per case report forms and the last measure taken prior to dosing. One non‐Japanese patient had ECOG PS 2. §Using Cochran–Armitage trend exact test. ††Using Wilcoxon test. ECOG PS, Eastern Cooperative Oncology Group performance status; SD, standard deviation.
Treatment duration was comparable between the axitinib titration, the placebo titration and the non‐randomized arms among non‐Japanese patients, as previously reported.11 However, among Japanese patients, treatment duration was substantially longer with the axitinib titration and non‐randomized arm than with the placebo titration arm. In general, Japanese patients received treatment longer than non‐Japanese patients, and had more frequent dose reductions. Relative dose intensity was also lower among Japanese than non‐Japanese patients.
Efficacy
At the data cutoff date for the follow‐up analysis, the median duration of follow‐up was 33 months (range, 1–60) in all patients and 44 months (range, 5–51) in Japanese patients. Fewer than half (n = 16 of 44) of Japanese patients had died, and, thus, median OS was not reached (95% CI, 38.8–not estimable). The HR for OS in Japanese versus non‐Japanese patients was 0.489 (95% CI, 0.281–0.850; stratified, two‐sided P = 0.0099; Fig. 2). Estimated survival probability (95% CI) in Japanese patients was 86.4% (76.2–96.5) at 1 year, 75.0% (62.2–87.8) at 2 years and 68.2% (54.4–81.9) at 3 years (Table 3).
Figure 2.
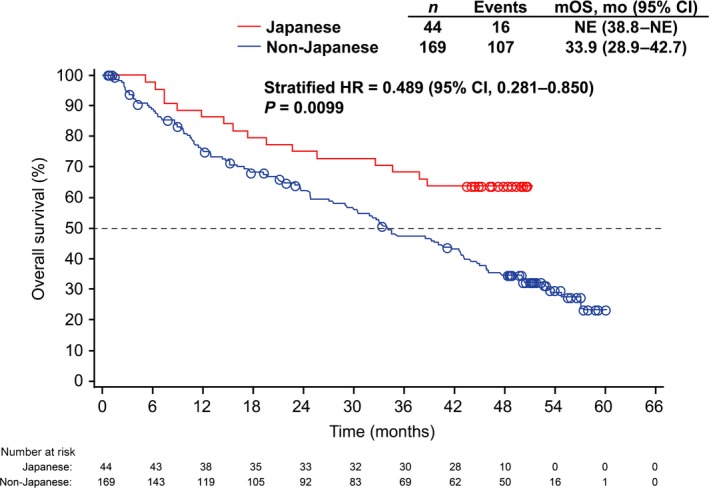
Kaplan–Meier estimates for overall survival in Japanese and non‐Japanese patients. CI, confidence interval; HR, hazard ratio; mo, month; mOS, median overall survival, NE, not estimable.
Table 3.
Survival probability at 1, 2 and 3 years in Japanese versus non‐Japanese patients
| Survival probability, % (95% CI) | Total | ECOG PS 0 | ECOG PS ≥1 | |||
|---|---|---|---|---|---|---|
| Japanese n = 44 | Non‐Japanese n = 169 | Japanese n = 37 | Non‐Japanese n = 99 | Japanese n = 7 | Non‐Japanese n = 70 | |
| 1‐year | 86.4 (76.2–96.5) | 75.1 (68.4–81.8) | 91.9 (83.1–100.0) | 84.3 (77.0–91.6) | 57.1 (20.5–93.8) | 61.4 (49.5–73.3) |
| 2‐year | 75.0 (62.2–87.8) | 62.1 (54.5–69.7) | 81.1 (68.5–93.7) | 71.0 (61.8–80.3) | 42.9 (6.2–79.5) | 48.8 (36.6–61.0) |
| 3‐year | 68.2 (54.4–81.9) | 47.2 (39.3–55.1) | 75.7 (61.9–89.5) | 58.2 (47.9–68.4) | 28.6 (0–62.0) | 31.5 (20.1–42.9) |
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status.
A total of 107 of 169 (63%) non‐Japanese patients had died at the cutoff date and 62 were censored. Median OS among non‐Japanese patients was 33.9 months (95% CI, 28.9–42.7), with generally lower estimated 1‐year, 2‐year and 3‐year survival probability than for Japanese patients (Table 3).
Because a higher percentage of Japanese patients had ECOG PS 0 compared with non‐Japanese patients, OS was compared between Japanese and non‐Japanese patients after stratifying for ECOG PS (Fig. 3). Median OS was not estimable in Japanese patients with ECOG PS 0 compared with 42.7 months (95% CI, 34.5–52.6) in non‐Japanese patients with ECOG PS 0. The HR (Japanese versus non‐Japanese patients) was 0.451 (95% CI, 0.235–0.866; unstratified, two‐sided P = 0.0140). However, among those with ECOG PS ≥1, there was no significant difference in OS between Japanese and non‐Japanese patients. Similarly, 1‐year, 2‐year and 3‐year survival probability was higher in Japanese than non‐Japanese patients with ECOG PS 0, whereas no differences were observed between Japanese and non‐Japanese patients when comparing those with ECOG PS ≥1 (Table 3).
Figure 3.
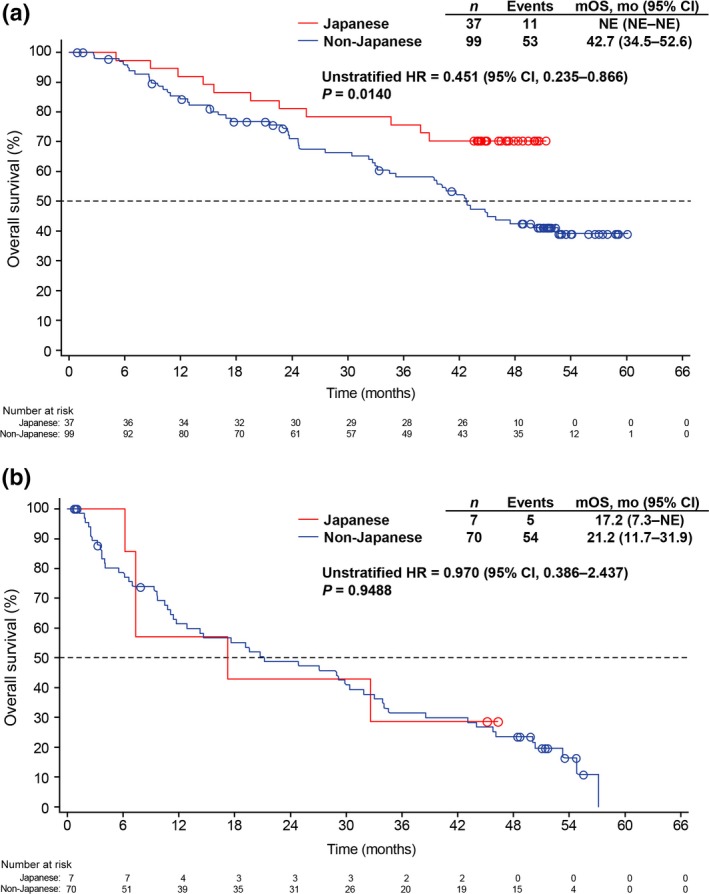
Kaplan–Meier estimates for overall survival stratified by ECOG PS: (a) ECOG PS 0 in Japanese and non‐Japanese patients and (b) ECOG PS ≥1 in Japanese and non‐Japanese patients. CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; mo, month; mOS, median overall survival; NE, not estimable.
The efficacy outcomes (PFS and OS) between Japanese and non‐Japanese patients were additionally evaluated in the non‐randomized arm because more Japanese patients were in the non‐randomized than randomized arm. Comparable to the results obtained with the overall Japanese versus non‐Japanese patients, PFS and OS (Fig. 4a,b, respectively) were significantly longer in Japanese than non‐Japanese patients in the non‐randomized arm. Furthermore, 1‐year, 2‐year and 3‐year survival probabilities were substantially higher among Japanese than non‐Japanese patients in the non‐randomized arm (data not shown).
Figure 4.
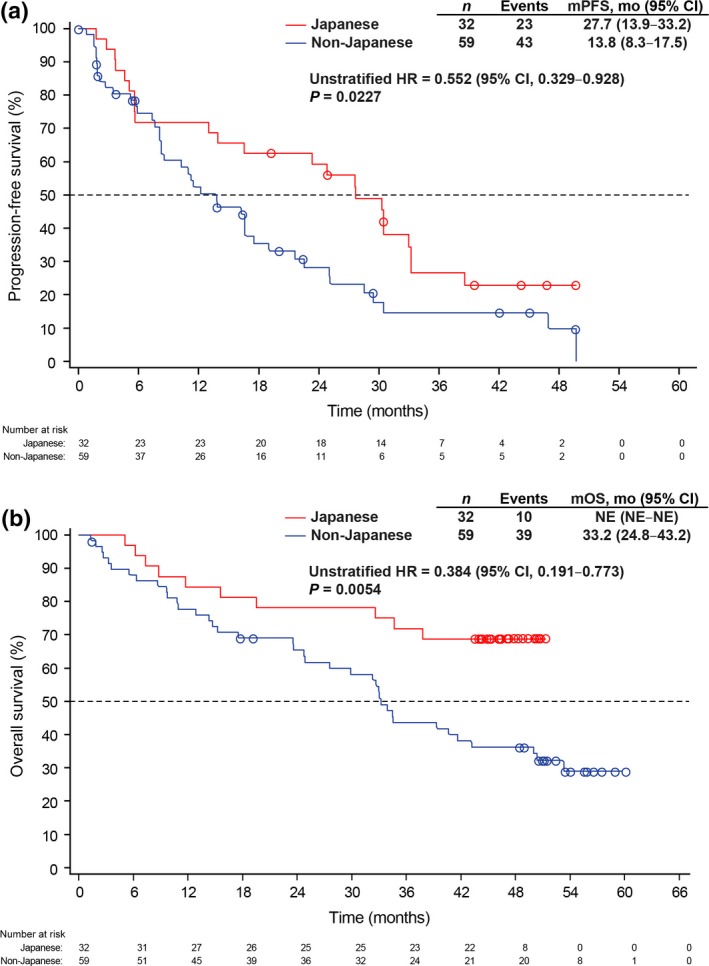
Kaplan–Meier estimates for (a) progression‐fee survival and (b) overall survival in Japanese and non‐Japanese patients in the non‐randomized arm. CI, confidence interval; HR, hazard ratio; mo, month; mOS, median overall survival; mPFS, median progression‐free survival; NE, not estimable.
Follow‐up systemic therapy
A higher percentage of Japanese than non‐Japanese patients received any follow‐up systemic therapy (75 vs 52%; Table 4). There was a tendency for Japanese patients to receive more frequent follow‐up systemic therapies than non‐Japanese patients: 31, 11 and 14% of Japanese patients received two, three or more than four follow‐up systemic therapies, respectively, compared with 11, 8 and 3% of non‐Japanese patients. Everolimus, sunitinib and sorafenib were preferred follow‐up systemic therapeutic agents in Japan, whereas everolimus and sunitinib were most frequently used in non‐Japanese patients from other regions.
Table 4.
Follow‐up systemic therapy in Japanese versus non‐Japanese patients
| Japanese | Non‐Japanese | |
|---|---|---|
| n = 36† | n = 157† | |
| Number of regimen, n (%) | ||
| Any | 27 (75) | 82 (52) |
| 1 | 7 (19) | 46 (29) |
| 2 | 11 (31) | 18 (11) |
| 3 | 4 (11) | 13 (8) |
| 4 | 4 (11) | 4 (3) |
| 5 | 0 | 1 (1) |
| 6 | 1 (3) | 0 |
| Type of medication, n (%) | ||
| Everolimus | 16 (44) | 35 (22) |
| Sunitinib | 9 (25) | 34 (22) |
| Sorafenib | 9 (25) | 9 (6) |
| Axitinib | 7 (19) | 5 (3) |
| Temsirolimus | 6 (17) | 8 (5) |
| Interferon‐α | 4 (11) | 4 (3) |
| Pazopanib | 3 (8) | 17 (11) |
†The number of patients who discontinued study treatment.
Safety
As reported previously,11 hypertension, diarrhea and fatigue were the most common treatment‐emergent, all‐causality, all‐grade AE in both Japanese (91, 75 and 50%, respectively) and non‐Japanese (59, 56 and 50%, respectively) patients treated with axitinib in this updated analysis. Hand–foot syndrome, hypothyroidism, dysphonia and proteinuria were also prevalent in both Japanese and non‐Japanese patients, but incidence rates were higher in Japanese (all‐grade: 73, 68, 68 and 64%, respectively) than non‐Japanese (all‐grade: 21, 26, 33 and 22%, respectively) patients. Compared with the previous safety analysis, the nature of AE remained the same and no new AE of concern were observed, but the incidence rates for several AE increased slightly among Japanese and non‐Japanese patients. The AE that increased by ≥5% were nasopharyngitis (from 32% to 39%) and nausea (from 25% to 30%) in Japanese patients, with none increasing by ≥5% in non‐Japanese patients.
Predictive factors for overall survival
The predictive potential of baseline characteristics for OS was evaluated in all 213 patients. The univariate analysis identified several baseline characteristics that were associated with longer OS: Asian race, ECOG PS 0, prior nephrectomy, fewer number of metastases, metastasis to lung only (compared with metastasis to lung plus other organs), time from histopathological diagnosis to treatment ≥1 year, sum of the longest diameter of target lesions (i.e. baseline tumor burden) ≤median (89 mm in all patients), absence of de novo metastasis at initial diagnosis, baseline lactate dehydrogenase ≤1.5× upper limit of normal, and baseline hemoglobin ≥lower limit of normal (Table 5). A proportionality in median OS and HR was observed with regard to the number of metastatic sites: median OS was 57.2, 54.8, 38.8 and 22.9 months in patients with 1, 2, 3 and ≥4 metastatic sites, respectively, with corresponding HR of 1, 1.366, 2.209 and 3.538. With regard to individual metastatic sites, involvement of lung, lymph node, liver, or bone was predictive of shorter survival (see Table S1). In the multivariate analysis, baseline ECOG PS 0, baseline tumor size ≤median, and time from histopathological diagnosis to treatment ≥1 year remained significant (P ≤ 0.0003) predictors for longer OS (Table 6).
Table 5.
Univariate analysis of predictive factors for overall survival in all patients (N = 213)
| n | mOS, month (95% CI) | Hazard ratio (95% CI)† | P‐value‡ | |
|---|---|---|---|---|
| Age, years | ||||
| <65 | 136 | 34.7 (24.7–44.0) | 1 | |
| ≥65 | 77 | 47.5 (33.9–NE) | 0.692 (0.472–1.014) | 0.0592 |
| Sex | ||||
| Male | 143 | 38.8 (32.3–50.1) | 1 | |
| Female | 70 | 39.6 (27.6–50.0) | 1.115 (0.770–1.616) | 0.5634 |
| Race | ||||
| White | 162 | 33.9 (27.6–42.8) | 1 | |
| Asian | 46 | NE (38.8–NE) | 0.489 (0.292–0.820) | 0.0066 |
| Black | 2 | 36.8 (33.0–40.6) | 1.327 (0.327–5.396) | 0.6922 |
| Other | 3 | 54.8 (4.1–54.8) | 0.818 (0.201–3.321) | 0.7782 |
| Body weight, kg | ||||
| ≤65 | 56 | 39.6 (25.5–52.6) | 1 | |
| >65 to ≤76 | 51 | 39.3 (22.9–NE) | 0.883 (0.531–1.470) | 0.6325 |
| >76 to ≤89 | 53 | 34.5 (24.7–54.8) | 0.868 (0.532–1.417) | 0.5720 |
| >89 | 53 | 41.6 (29.9–NE) | 0.866 (0.529–1.418) | 0.5675 |
| ECOG PS | ||||
| 0 | 136 | 50.0 (40.6–NE) | 1 | |
| ≥1 | 77 | 20.8 (11.7–31.9) | 2.484 (1.741–3.543) | <0.0001 |
| Prior nephrectomy | ||||
| Yes | 183 | 42.5 (34.5–50.3) | 1 | |
| No | 30 | 21.6 (10.5–34.1) | 2.139 (1.367–3.348) | 0.0009 |
| Number of metastatic sites | ||||
| 1 | 41 | 57.2 (50.0–NE) | 1 | |
| 2 | 57 | 54.8 (35.2–NE) | 1.366 (0.720–2.591) | 0.3401 |
| 3 | 52 | 38.8 (24.8–44.7) | 2.209 (1.203–4.058) | 0.0106 |
| ≥4 | 63 | 22.9 (11.7–32.7) | 3.538 (1.980–6.321) | <0.0001 |
| Metastatic sites (lung versus lung + others) | ||||
| Lung only | 25 | 57.2 (37.8–NE) | 1 | |
| Lung + others | 188 | 34.7 (28.9–43.2) | 2.057 (1.076–3.930) | 0.0291 |
| Time from histopathological diagnosis to treatment, years | ||||
| ≥1 | 88 | 57.2 (42.7–NE) | 1 | |
| <1 | 125 | 28.9 (17.6–39.3) | 2.270 (1.547–3.330) | <0.0001 |
| Time from metastatic diagnosis to treatment, years | ||||
| ≥1 | 20 | 40.1 (24.7–NE) | 1 | |
| <1 | 193 | 39.3 (32.6–46.1) | 1.050 (0.577–1.908) | 0.8736 |
| Sum of longest diameter for target lesion§ | ||||
| ≤Median¶ | 107 | 54.8 (44.0–NE) | 1 | |
| >Median¶ | 105 | 23.7 (14.5–33.9) | 2.624 (1.817–3.789) | <0.0001 |
| Presence of metastases (de novo) at initial diagnosis | ||||
| No | 119 | 50.3 (40.1–NE) | 1 | |
| Yes | 94 | 28.9 (19.5–37.8) | 2.019 (1.413–2.885) | 0.0001 |
| Baseline LDH†† | ||||
| ≤1.5 × ULN | 198 | 40.6 (33.9–47.5) | 1 | |
| >1.5 × ULN | 11 | 9.8 (3.7–30.4) | 3.149 (1.588–6.243) | 0.0010 |
| Baseline hemoglobin | ||||
| ≥LLN | 119 | 43.2 (38.6–57.2) | 1 | |
| <LLN | 94 | 30.4 (17.2–40.6) | 1.563 (1.097–2.227) | 0.0135 |
†1 equals reference. ‡Using Wald test. §One patient did not have measurable disease at baseline. ¶Median equals 89 mm in all patients. ††Values were missing for 4 patients. CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; LLN, lower limit of normal; mOS, median overall survival; NE, not estimable; ULN, upper limit of normal.
Table 6.
| Hazard ratio (95% CI) | P‐value§ | |
|---|---|---|
| ECOG PS | ||
| ≥1 vs 0 | 1.956 (1.356–2.822) | 0.0003 |
| Time from histopathological diagnosis to treatment, years | ||
| <1 vs ≥1 | 2.079 (1.410–3.065) | 0.0002 |
| Sum of longest diameter for target lesion | ||
| >Median¶ versus ≤median¶ | 2.197 (1.503–3.211) | <0.0001 |
†Final model constructed by a stepwise method with a 0.05 significance level. ‡Analysis based on 208 patients because 1 patient did not have measurable disease at baseline and 4 patients did not have LDH data. §Using Wald test. ¶Median equals 89 mm in all patients. CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status.
Discussion
The previous subgroup analysis of this phase II trial of axitinib suggests that Japanese patients achieved better efficacy than non‐Japanese patients and there was a similar safety profile in first‐line treatment of metastatic RCC.11 The current analysis was conducted to further evaluate survival benefit of axitinib in Japanese versus non‐Japanese patients previously untreated for metastatic RCC. In addition, we have investigated predictive values of baseline characteristics for OS using the data from all 213 patients enrolled in this trial. The study found several key findings: first, OS was significantly (stratified HR 0.489; P = 0.0099) longer in the overall Japanese than the overall non‐Japanese patients. Second, when comparing Japanese and non‐Japanese patients in the non‐randomized arm, PFS, OS and survival probability were significantly longer for Japanese than non‐Japanese patients, similar to the results seen in the overall Japanese versus non‐Japanese patients. The majority of Japanese patients were in the non‐randomized arm; thus, the longer PFS and OS in overall Japanese patients in the non‐randomized arm contributed to the longer PFS and OS in the overall Japanese patients. Third, no new safety concerns were observed in Japanese or non‐Japanese patients. Fourth, several baseline factors may be predictive for OS in patients treated with first‐line axitinib.
The reasons for longer OS and higher survival probability observed in axitinib‐treated Japanese than non‐Japanese patients, whether in the overall population or in the non‐randomized arm, are likely multifactorial and may include patient‐related factors, such as more favorable baseline characteristics (e.g. ECOG PS 0, small tumor size and fewer metastases), as shown in Tables 1 and 2. In addition, treatment‐related factors, such as longer treatment duration and more frequent use of follow‐up therapies, have undoubtedly contributed to the better clinical outcomes for Japanese patients. More favorable baseline characteristics of Japanese patients may be explained by early diagnosis of metastatic disease through rigorous and extensive medical examination of patients with RCC by Japanese physicians followed by immediate and frequent treatments. Despite longer duration of axitinib treatment, the updated safety assessments did not reveal any new AE of significance in Japanese patients. As previously described, the nature of AE was similar between Japanese and non‐Japanese patients, with minor differences in incidence rates for some AE.11 The pharmacokinetics seemingly did not contribute to the differences in axitinib efficacy between Japanese and non‐Japanese because the range for the maximum observed plasma concentration as well as drug exposure at steady‐state generally overlapped between Japanese and non‐Japanese patients.11
The current analysis confirmed that axitinib has antitumor activity in treatment‐naïve Japanese patients with metastatic RCC. The 3‐year survival probability of 68.2% with first‐line axitinib and a median OS not reached after a median follow‐up of 44 months in Japanese patients are higher than that previously observed with sunitinib or sorafenib. For example, in treatment‐naïve Japanese patients with metastatic RCC treated with sorafenib (n = 172) or sunitinib (n = 99) for ≥2 months in a routine clinical setting, the 3‐year OS rate was 48.8% and the median OS was 33.1 months.12 In an open‐label phase II study, the median OS was 33.1 months (95% CI, 14.8–not reached) in 25 treatment‐naïve Japanese patients with metastatic RCC treated with sunitinib 50 mg orally once daily on a 4‐week‐on/2‐week‐off schedule.13 Median OS in first‐line treatment with sorafenib or sunitinib in Japanese patients with metastatic RCC was less than 3 years in these reports, whereas, in the current study, median OS in first‐line treatment with axitinib exceeded 3 years (lower limit of 95% CI was 38.8 months). To date, there has been no report on median OS for Japanese patients treated with pazopanib since its approval in 2014 by the Ministry of Health, Labour and Welfare in Japan. Thus, cross‐study comparison of the limited data seems to suggest that axitinib may achieve a longer median OS compared with sorafenib or sunitinib in Japanese patients, but without a prospective head‐to‐head study no definitive conclusions can be drawn. Of note, in a prospective phase II study of combination therapy of sorafenib and interferon‐α conducted in Japanese patients with metastatic RCC, the 3‐year survival rate was 64.5% and median OS, which had not been reached after median follow‐up of 21.3 months, without increasing the incidence of AE.14 A combination therapy of TKI, including axitinib, with immunotherapy may offer additional improvement in OS.
This study has some limitations. First, this was a post hoc exploratory analysis. Second, although the study clearly demonstrated the benefit of axitinib in treating Japanese patients with metastatic RCC in the first‐line setting, the effect of axitinib titration compared with placebo titration on efficacy outcomes could not be confirmed due to the small sample sizes in the axitinib and placebo titration arms (6 and 5 patients, respectively).
To date, there have not been any validated biomarkers that may help identify patients with metastatic RCC who would achieve better clinical outcomes from targeted therapies, including axitinib, in either first‐line or second‐line settings. The univariate analysis in this study has indicated several baseline characteristics to be potentially predictive of OS in treatment‐naïve patients with metastatic RCC treated with axitinib. The factors were the same as those previously found to be strongly associated with PFS,11 except prior nephrectomy and baseline lactate dehydrogenase. In the multivariate analysis, baseline ECOG PS, time from histopathological diagnosis to treatment, and tumor burden remained significant. It is noteworthy that ECOG PS was found to be associated with improved OS, but not with PFS when other relevant factors were taken into account. Some of the factors that prolonged OS in Japanese patients treated with axitinib, such as prior nephrectomy, number of metastatic sites, or metastasis to liver, lymph node or bone, have also been reported to affect OS in Japanese patients treated with first‐line sunitinib or sorafenib in univariate analyses.12, 15 In these reports, the Memorial Sloan Kettering Cancer Center (MSKCC) classification,16 C‐reactive protein level and early tumor shrinkage, in addition to liver metastasis, were shown to be independently associated with OS in multivariate analyses. In the current study, blood samples for determination of serum calcium concentrations were not collected and, hence, the effect of the MSKCC or the International Metastatic Renal Cell Carcinoma Database Consortium17 classification could not be evaluated.
In conclusion, the current analysis confirmed that first‐line treatment of axitinib has clinical activity in Japanese patients with metastatic RCC, including a higher 3‐year survival rate and a significantly longer OS in Japanese than non‐Japanese patients. Such a long OS observed in Japanese patients with metastatic RCC has not been previously reported for this population. In addition, axitinib is safe and well tolerated in Japanese patients. Furthermore, the study identified several potential predictive factors for OS in patients treated with axitinib as first‐line therapy.
Disclosure Statement
Mototsugu Oya has received research funding and honoraria from Pfizer, Novartis and Ono, and honoraria from Bayer. Yoshihiko Tomita has received research funding from Pfizer, and honoraria from Pfizer, Novartis, Ono and Bristol‐Myers Squibb. Satoshi Fukasawa has no conflict of interest to declare. Nobuo Shinohara has received research funding from Pfizer and Astellas, and honoraria from Pfizer, Bayer, Novartis and GlaxoSmithKline. Tomonori Habuchi has received research funding from Pfizer, Novartis, Bayer, Daiichi Sankyo, Chugai, AstraZeneca, Kyowa Hakko Kirin, Kissei, Torii, Astellas and Takeda, and honoraria from Pfizer, Novartis, Janssen, Takeda, Astellas and AstraZeneca. Brian I. Rini has served as a consultant for and received research funding from Pfizer. Yosuke Fujii, Yoichi Kamei, Yoshiko Umeyama and Angel H. Bair are employed by Pfizer and Umeyama and Bair own stock in Pfizer. Hirotsugu Uemura has received research funding from Pfizer, and honoraria from Pfizer, Bayer, Novartis, Ono and Bristol‐Myers Squibb. The study was sponsored by Pfizer. Pfizer was involved in study design, and collection, analysis and interpretation of the data. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publicaiton.
Supporting information
Table S1. Univariate analysis of baseline individual metastatic sites for overall survival in all patients (N = 213).
Acknowledgments
We thank Hisanaga Ohashi of Pfizer for data collection and Hiroko Godai of Pfizer for data analysis. Medical writing support was provided by Mariko Nagashima of Engage Scientific Solutions (Southport, CT, USA) and was funded by Pfizer.
Cancer Sci 108 (2017) 1231–1239
This trial is registered at ClinicalTrials.gov, NCT00835978.
Funding Information
This study was sponsored by Pfizer.
References
- 1. Rini BI, Escudier B, Tomczak P et al Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011; 378: 1931–9. [DOI] [PubMed] [Google Scholar]
- 2. Motzer RJ, Escudier B, Tomczak P et al Axitinib versus sorafenib as second‐line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 2013; 14: 552–62. [DOI] [PubMed] [Google Scholar]
- 3. Hutson TE, Lesovoy V, Al‐Shukri S et al Axitinib versus sorafenib as first‐line therapy in patients with metastatic renal‐cell carcinoma: a randomised open‐label phase 3 trial. Lancet Oncol 2013; 14: 1287–94. [DOI] [PubMed] [Google Scholar]
- 4. Hutson TE, Al‐Shukri S, Stus VP et al Axitinib versus sorafenib in first‐line metastatic renal cell carcinoma: overall survival from a randomized phase III trial. Clin Genitourin Cancer 2017; 15: 72–6. [DOI] [PubMed] [Google Scholar]
- 5. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Kidney Cancer version 3.2016. 2016. [Cited 14 September 2016.] Available from URL: https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf.
- 6. Rini BI, Melichar B, Ueda T et al Axitinib with or without dose titration for first‐line metastatic renal‐cell carcinoma: a randomised double‐blind phase 2 trial. Lancet Oncol 2013; 14: 1233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rini BI, Tomita Y, Melichar B et al Overall survival analysis from a randomized phase II study of axitinib with or without dose titration in first‐line metastatic renal cell carcinoma. Clin Genitourin Cancer 2016; 14: 499–503. [DOI] [PubMed] [Google Scholar]
- 8. Tomita Y, Uemura H, Fujimoto H et al Key predictive factors of axitinib (AG‐013736)‐induced proteinuria and efficacy: a phase II study in Japanese patients with cytokine‐refractory metastatic renal cell Carcinoma. Eur J Cancer 2011; 47: 2592–602. [DOI] [PubMed] [Google Scholar]
- 9. Eto M, Uemura H, Tomita Y et al Overall survival and final efficacy and safety results from a Japanese phase II study of axitinib in cytokine‐refractory metastatic renal cell carcinoma. Cancer Sci 2014; 105: 1576–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ueda T, Uemura H, Tomita Y et al Efficacy and safety of axitinib versus sorafenib in metastatic renal cell carcinoma: subgroup analysis of Japanese patients from the global randomized Phase 3 AXIS trial. Jpn J Clin Oncol 2013; 43: 616–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tomita Y, Fukasawa S, Oya M et al Key predictive factors for efficacy of axitinib in first‐line metastatic renal cell carcinoma: subgroup analysis in Japanese patients from a randomized, double‐blind phase II study. Jpn J Clin Oncol 2016; 46: 1031–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miyazaki A, Miyake H, Harada KI, Inoue TA, Fujisawa M. Prognostic outcome in patients treated with tyrosine kinase inhibitors as first‐line molecular‐targeted therapy for metastatic renal cell carcinoma: experience in real‐world clinical practice in Japan. Mol Clin Oncol 2015; 3: 601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tomita Y, Shinohara N, Yuasa T et al Overall survival and updated results from a phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma. Jpn J Clin Oncol 2010; 40: 1166–72. [DOI] [PubMed] [Google Scholar]
- 14. Eto M, Kawano Y, Hirao Y et al Phase II clinical trial of sorafenib plus interferon‐alpha treatment for patients with metastatic renal cell carcinoma in Japan. BMC Cancer 2015; 15: 667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miyake H, Miyazaki A, Imai S, Harada K, Fujisawa M. Early tumor shrinkage under treatment with first‐line tyrosine kinase inhibitors as a predictor of overall survival in patients with metastatic renal cell carcinoma: a retrospective multi‐institutional study in Japan. Target Oncol 2016; 11: 175–82. [DOI] [PubMed] [Google Scholar]
- 16. Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon‐alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 2002; 20: 289–96. [DOI] [PubMed] [Google Scholar]
- 17. Heng DY, Xie W, Regan MM et al Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor‐targeted agents: results from a large, multicenter study. J Clin Oncol 2009; 27: 5794–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariate analysis of baseline individual metastatic sites for overall survival in all patients (N = 213).


