Abstract
Treating advanced or recurrent melanoma remains a challenge. Cancer cells can evade the immune system by blocking T‐cell activation through overexpression of the inhibitory receptor programmed death 1 (PD‐1) ligands. The PD‐1 inhibitor nivolumab blocks the inhibitory signal in T cells, thus overcoming the immune resistance of cancer cells. Nivolumab has shown promising anticancer activity in various cancers. We carried out a single‐arm, open‐label, multicenter, phase II study to investigate the efficacy and safety of nivolumab in previously untreated Japanese patients with advanced melanoma. Twenty‐four patients with stage III/IV or recurrent melanoma were enrolled and received i.v. nivolumab 3 mg/kg every 2 weeks until disease progression or unacceptable toxicity. The primary endpoint was overall response rate evaluated by an independent radiology review committee. The independent radiology review committee‐assessed overall response rate was 34.8% (90% confidence interval, 20.8–51.9), and the overall survival rate at 18 months was 56.5% (90% confidence interval, 38.0–71.4). Treatment‐related adverse events (AEs) of grade 3 or 4 only occurred in three patients (12.5%). Two patients discontinued nivolumab because of AEs, but all AEs were considered manageable by early diagnosis and appropriate treatment. Subgroup analyses showed that nivolumab was clinically beneficial and tolerable regardless of BRAF genotype, and that patients with treatment‐related select AEs and with vitiligo showed tendency for better survival. In conclusion, nivolumab showed favorable efficacy and safety profiles in Japanese patients with advanced or recurrent melanoma, with or without BRAF mutations. (Trial registration no. JapicCTI‐142533.)
Keywords: Immune checkpoint inhibitor, Japanese patients, melanoma, nivolumab, programmed death 1 (PD‐1) inhibitor
Advanced or recurrent melanoma is a challenging disease to treat. Dacarbazine was approved for use in the USA in 1975, since when it has been a standard therapy for advanced melanoma; however, the efficacy of dacarbazine monotherapy is unsatisfactory.1, 2 Melanoma is still associated with high mortality, despite recent advancements in systemic therapy that have improved the 10‐year survival rate of patients with distant metastatic melanoma from <10% in 20013 to approximately 30% in 2009.4 Effective, alternative therapy options are therefore needed.
Immune checkpoint inhibitors have become a recent focus of anticancer drug discovery. T‐cell activation is tightly regulated by the balance between positive and negative signals, allowing T cells to recognize and respond to pathogens while maintaining self‐tolerance.5 Checkpoint receptors are expressed in T cells and induce inhibitory signals following receptor binding. However, cancer cells overexpress immune checkpoint ligands that inhibit T‐cell activation, allowing the cells to escape immune system attack,6 whereas antagonists of such receptors can increase antigen‐specific T‐cell immune responses against tumor cells. Programmed death 1 and cytotoxic T‐lymphocyte‐associated antigen‐4 are two of the most intensively investigated target receptors in cancer immunotherapy research. Unlike other antibody‐based cancer therapies, immune checkpoint inhibitors do not target tumor cells directly, but rather modulate lymphocytes to enhance the body's own anticancer activities.
Nivolumab is a fully human mAb that inhibits the PD‐1 checkpoint receptor. Expression of PD‐1 on the T‐cell surface is upregulated following activation,7 and the PD‐1 pathway negatively regulates effector T‐cell activity following ligand binding. Tumor cells usually overexpress the PD‐1 ligands, PD‐L1 and PD‐L2,8, 9 on the cell surface, thus acquiring immune resistance. Indeed, the expression level of PD‐L1 was shown to correlate with tumor growth in primary melanomas.10 Blockade of PD‐1 by nivolumab thus represents a promising approach for enhancing the antimelanoma T cell immune response, and thereby improving clinical endpoints.11, 12, 13
The present study investigated the efficacy and safety of nivolumab in previously untreated Japanese patients with advanced melanoma.
Material and Methods
Patients
This was a single‐arm, open‐label, multicenter, phase II study to evaluate the efficacy and safety of nivolumab. Eligible patients were at least 20 years old, with histopathologically confirmed, previously untreated malignant melanoma that were unresectable stage III/IV or recurrent, an Eastern Cooperative Oncology Group performance status score of 0 or 1,14 a predicted survival of at least 3 months, and adequate organ function. At least one tumor had to be measurable by imaging, as defined using the RECIST guidelines (version 1.1)15 14 days before enrollment. Enrollment of patients with previous adjuvant therapies was allowed. Patients with the following conditions were not enrolled: (i) history of hypersensitivity to other antibody‐based medications; (ii) remaining influence of previous radiation or resection therapies; (iii) chronic or recurrent autoimmune diseases; (iv) genotyping for BRAF mutation not possible; (v) melanomas with primary tumors in the esophagus or rectum; (vi) presence of double cancer, except completely resected cancers (basal cell carcinoma, squamous cell carcinoma of stage 1, intraepithelial carcinoma, intramucosal carcinoma, or superficial bladder cancer) or other cancers without recurrence for 5 years; (vii) primary or metastatic lesions in brain or meninges; or (viii) interstitial lung disease or pulmonary fibrosis. All patients provided tumor biopsy specimens for gene analyses. The BRAF V600 mutation was detected using real‐time PCR (Cobas 4800 BRAF V600 Mutation Test; Roche Diagnostics, Branford, CT, USA).
The study protocol was approved by the institutional review board at each study site. The study was carried out in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guideline for Good Clinical Practice. Written informed consent was provided by all participants before the first treatment.
Interventions
The study consisted of three stages: screening, intervention, and post‐treatment follow‐up. After the screening stage, eligible patients were enrolled and received i.v. nivolumab 3 mg/kg every 2 weeks in each 6‐week cycle until progressive disease (PD) or unacceptable toxicity was observed. Treatment was discontinued immediately when any of following discontinuation criteria was met at any time during the intervention stage: (i) complete response (CR) based on RECIST guidelines, except patients with anticipated recurrence assessed by investigators; (ii) PD based on RECIST guidelines, and no further clinical benefit expected; (iii) clinical symptoms indicating cancer progression; (iv) interstitial lung disease of grade ≥2 regardless of the relationship to nivolumab; (v) AEs of grade ≥3 of which the relationship to nivolumab was not ruled out; or (vi) AEs (eye pain and visual acuity reduced) of grade ≥2 that were not ruled out for their relationship to nivolumab and not recovered after topical treatment. Tumors were evaluated at the end of the 6‐week regimen to determine if the treatment should be continued. The follow‐up stage began when the treatment was discontinued or no new cycle was started.
Assessment
Efficacy endpoints
Tumor images were obtained using computed tomography or magnetic resonance imaging at screening, and at the end of every 6‐week treatment cycle from the 1st to 9th cycles, and thereafter at the end of every other 6‐week cycle, and also at discontinuation of the treatment and on the 28th day of the follow‐up period. These images were used to classify the overall response into four categories, based on the RECIST guidelines (version 1.1). The primary endpoint was the ORR, defined as the proportion of patients with CR or PR, assessed by an IRC. Secondary endpoints were the ORR assessed by investigators at each study site, OS, PFS, duration of response, disease‐control rate, and change in tumor size.
Safety endpoints
Safety was assessed by recording AEs, evaluated by vital signs, and the results of 12‐lead electrocardiograms and clinical tests, collected at predefined time points. Adverse events were graded using the Common Terminology Criteria for Adverse Events version 4.0. The frequency of treatment‐related select AEs, defined as AEs with potential immunological causes, was also recorded.
Statistical analysis
Demographic characteristics were described as the summary statistics of the safety set, which comprised patients who had received nivolumab at least once. Efficacy endpoints were analyzed in the full‐analysis set, which comprised evaluable patients in the safety set who continued to fulfill the major eligibility criteria. The proportions of patients and two‐sided 90% CIs were calculated for the ORR and disease‐control rate. The OS and PFS were reported as medians and two‐sided 90% CIs, estimated using the Kaplan–Meier method. The proportions of patients with CR, PR, SD, PD, and not evaluable were calculated, and two‐sided 90% CIs were calculated for CR, PR, and SD. The proportion of patients who showed ORR for 12 months or longer was estimated as the durable response rate using Kaplan–Meier methods. Safety was analyzed in the safety set.
Patients were stratified into two groups based on BRAF genotypes, and subgroup analyses were carried out to determine the nivolumab efficacy and safety endpoints in patients with BRAF wild‐type and mutant, respectively. In addition, we undertook a posteriori subgroup analyses for several factors that could influence the efficacy of nivolumab. Median OS and PFS with two‐sided 90% CIs were estimated for subgroups using the Kaplan–Meier method and compared using unstratified log–rank tests and unstratified Cox proportional hazards models.
The planned sample size was 20 patients. An earlier phase II study in previously treated Japanese patients who received nivolumab once every 3 weeks showed a response rate of 22.9%,16 which were therefore set as the expected response rate for the present study. The threshold response rate was set as 6.0%, estimated based on the response rate to dacarbazine. Using these estimates, the sample size was determined to 20 patients, with a statistical power of 80% ensured to detect the response rate in a one‐sided test with a 5.0% significance level. The required sample size for patients with BRAF wild‐type was calculated as 14, using the same estimates with a statistical power of 70%, and the estimated sample size for patients with BRAF mutant was six, so that at least one patient would achieve a response with approximately 80% probability.
Results
Patients and treatment
A total of 24 patients from nine study centers participated from May to October 2014, with a data cut‐off date of February 29, 2016. The demographic and baseline characteristics are summarized in Table 1. Eighteen patients (75%) had BRAF wild‐type, and 6 (25%) had BRAF mutant. All 24 patients received nivolumab, however, one patient was excluded from the full analysis set because the patient met one of the exclusion criteria (double cancer) after enrollment (Fig. 1). Six patients were receiving treatment at the cut‐off point and continued with further treatment. Of the remaining 18 patients, 15 were in the follow‐up stage at cut‐off, because of PD (nine patients, 37.5%), clinical symptoms indicating cancer progression (two patients, 8.3%), or the physician's decision (four patients, 16.7%). The other three patients discontinued the study without entering the follow‐up stage because of disease progression. A median of 23 doses (range, 2–46) of nivolumab were administered, with a median treatment duration of 11.9 months (range 0.5–21.0). The median follow‐up was 18.8 months (range, 2.0–21.5 months).
Table 1.
Demographics and baseline characteristics of Japanese patients with previously untreated advanced melanoma given nivolumab (n = 24)
| Characteristic | Nivolumab (n = 24) |
|---|---|
| Sex | |
| Male | 14 (58.3) |
| Female | 10 (41.7) |
| Age, years | |
| <65 | 13 (54.2) |
| ≥65 | 11 (45.8) |
| Median (range), years | 63.0 (26–81) |
| Performance status (ECOG) | |
| 0 | 16 (66.7) |
| 1 | 8 (33.3) |
| Stage | |
| IV | 3 (12.5) |
| Recurrent | 21 (87.5) |
| Melanoma type | |
| Lentigo maligna | 0 (0.0) |
| Superficial spreading | 6 (25.0) |
| Nodular | 1 (4.2) |
| Acral lentiginous | 7 (29.2) |
| Other | 10 (41.7) |
| Previous resection | |
| Yes | 23 (95.8) |
| Previous radiation therapy | |
| Yes | 3 (12.5) |
| Number of previous adjuvant therapies | |
| 0 | 9 (37.5) |
| 1 | 7 (29.2) |
| ≥2 | 8 (33.3) |
| BRAF V600 status | |
| Mutation | 6 (25.0) |
| Wild‐type | 18 (75.0) |
Data given as n (%) unless otherwise stated. ECOG, Eastern Cooperative Oncology Group.
Figure 1.
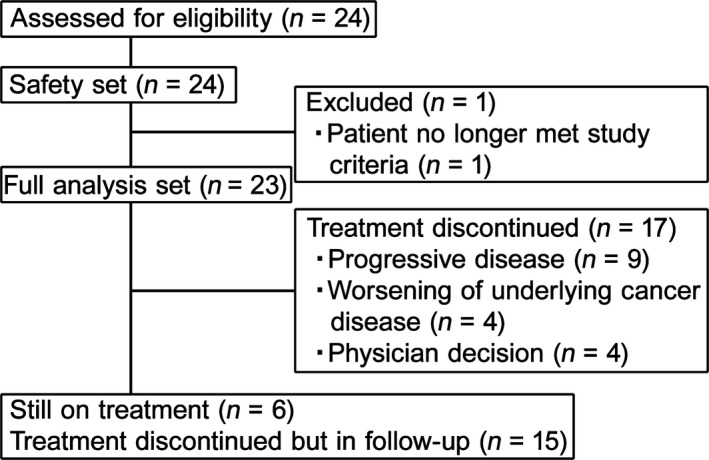
Patient disposition during the study (n = 23).
Efficacy
The IRC‐assessed and investigator‐assessed ORRs are summarized in Table 2. The IRC‐assessed ORR was 34.8% (90% CI, 20.8, 51.9) and the investigator‐assessed ORR was 43.5% (90% CI, 28.1, 60.3), indicating similar results for both assessment methods. The lower limits of the 90% CI in both IRC‐assessed and investigator‐assessed ORRs were higher than the threshold response rate of 6.0% estimated using dacarbazine data. The best overall responses assessed by IRC were CR in two patients (8.7%), PR in six patients (26.1%), and SD in seven patients (30.4%), giving a disease‐control rate (CR+PR+SD) of 65.2%.
Table 2.
Response and survival of Japanese patients with previously untreated advanced melanoma given nivolumab (n = 23)
| IRC assessed, n (%) | Investigator assessed, n (%) | |
|---|---|---|
| Total patients | 23 (100.0) | 23 (100.0) |
| Best overall response | ||
| CR | 2 (8.7) | 0 (0.0) |
| PR | 6 (26.1) | 10 (43.5) |
| SD | 7 (30.4) | 8 (34.8) |
| PD | 7 (30.4) | 5 (21.7) |
| No lesion founda | 1 (4.3) | 0 (0.0) |
| Overall response rate (CR+PR) | 8 (34.8) | 10 (43.5) |
| 90% CI, % | 20.8, 51.9 | 28.1, 60.3 |
| Disease control rate (CR+PR+SD) | 15 (65.2) | 18 (78.3) |
| 90% CI, % | 48.1, 79.2 | 61.6, 89.0 |
| Duration of response (IRC assessed) | ||
| Median, months | Not reached | – |
| Range, months | 1.4–17.1 | – |
| Progression‐free survival | ||
| Median, months | 5.9 | 9.8 |
| 90% CI, months | 2.8, 12.2 | 2.8, – |
| Rate at 12 months, % | 38.3 | 42.7 |
| 90% CI, % | 21.8, 54.6 | 25.5, 58.9 |
| Rate at 18 months, % | 28.7 | 37.9 |
| 90% CI, % | 14.3, 44.9 | 21.5, 54.3 |
| Overall survival | ||
| Median, months | Not reached | |
| 90% CI, months | 12.02, – | |
| Rate at 12 months, % | 16 (69.6) | |
| 90% CI, % | 50.8, 82.3 | |
| Rate at 18 months, % | 13 (56.5) | |
| 90% CI, % | 38.0, 71.4 | |
Measurable lesion was found by investigator on site but not by independent radiology review committee (IRC) throughout the study. –, Censored value; CI, confidence interval; CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
The median OS was not reached, and the proportion of patients surviving at 18 months was 56.5% (Table 2, Fig. 2). The median PFS evaluated by IRC was 5.9 months (90% CI, 2.8, 12.2) (Table 2, Fig. 3).
Figure 2.
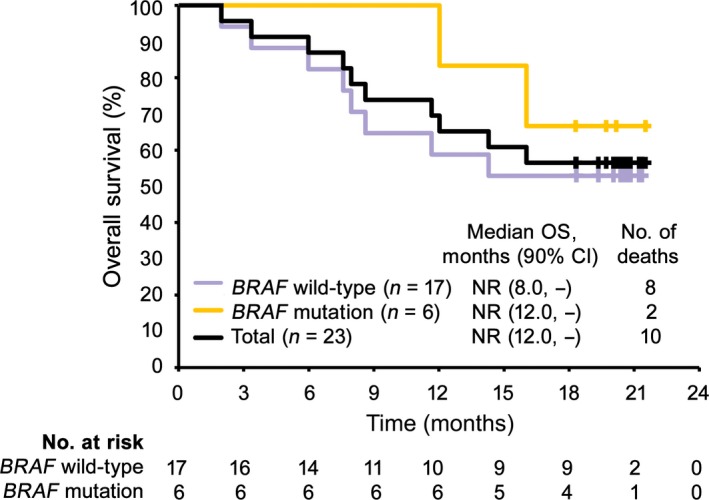
Kaplan–Meier analysis of overall survival (OS) in Japanese patients with previously untreated advanced melanoma given nivolumab (n = 23). Purple, yellow, and black lines represent patients with BRAF wild‐type, patients with BRAF mutation, and total patients, respectively. CI, confidence interval; NR, not reached.
Figure 3.
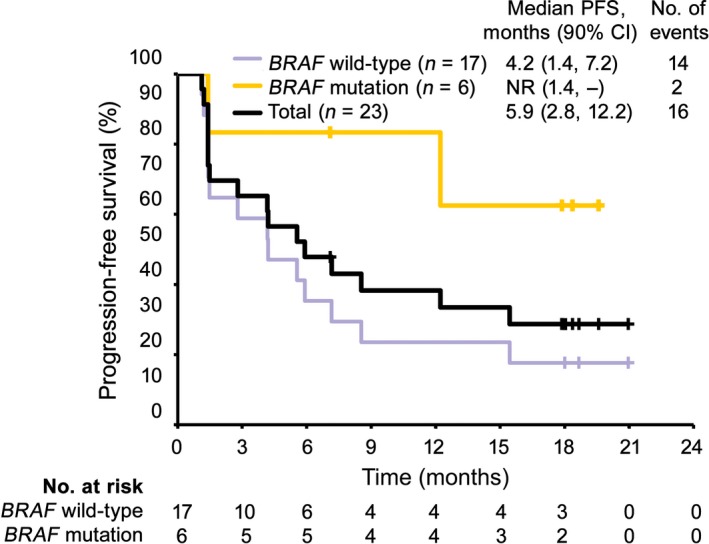
Kaplan–Meier analysis of progression‐free survival (PFS) in Japanese patients with previously untreated advanced melanoma given nivolumab (n = 23), estimated using data evaluated by independent radiology review committee. Purple, yellow, and black lines represent patients with BRAF wild‐type, patients with BRAF mutation, and total patients, respectively. CI, confidence interval; NR, not reached.
A decrease in target‐tumor diameter was observed in more than half the patients (Fig. 4), and patients who had decreased tumor diameter also had long antitumor effects (Fig. 5, 6). Response was observed in eight patients and was persistent in five of eight patients until the cut‐off date. The durable response rate for 12 months, estimated by Kaplan–Meier methods, was 71.4%.
Figure 4.
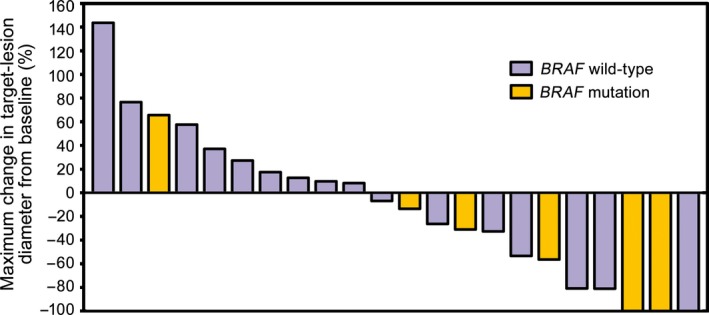
Maximum change in target‐lesion diameter in relation to BRAF genotype in Japanese patients with previously untreated advanced melanoma given nivolumab (n = 23). Maximum changes in target‐lesion diameter from baseline evaluated by independent radiology review committee. Purple and yellow bars represent patients with BRAF wild‐type and mutation, respectively.
Figure 5.
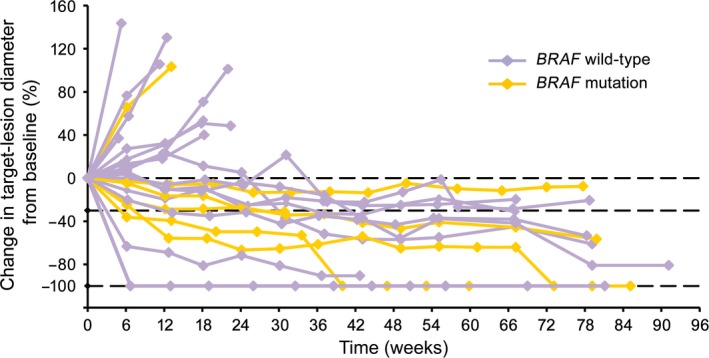
Change in target‐lesion diameter over time in relation to BRAF genotype in Japanese patients with previously untreated advanced melanoma given nivolumab (n = 23). Change in target‐lesion diameter evaluated by independent radiology review committee. Purple and yellow plots represent patients with BRAF wild‐type and mutation, respectively.
Figure 6.
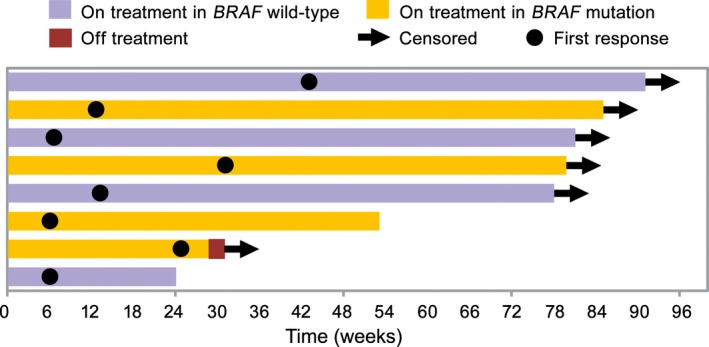
Time to and duration of response in Japanese patients with previously untreated advanced melanoma given nivolumab (n = 23), in relation to BRAF genotype. Time to and duration of response evaluated by independent radiology review committee. Purple and yellow bars represent patients with BRAF wild‐type and BRAF mutant, respectively, on treatment. Arrow, date of censor; brown bar, patients off treatment; closed circle, date of first response.
Safety
Adverse events were reported in 22 patients (91.7%), including AEs of grade ≥3 in five (20.8%) patients. Treatment‐related AEs were found in 20 patients (83.3%), including three (12.5%) with treatment‐related AEs grade ≥3 (Table 3). No death occurred during the study period. The most commonly observed treatment‐related AEs were vitiligo (nine patients, 37.5%), pruritus (six patients, 25.0%), hypothyroidism (six patients, 25.0%), and malaise (six patients, 25.0%). Serious treatment‐related AEs were reported in three patients (12.5%), including colitis, abnormal hepatic function, renal impairment, and pleural effusion. Treatment was temporarily interrupted in two of these patients (colitis and renal impairment). Two patients (8.3%) discontinued the study because of treatment‐related AEs (colitis and pleural effusion). Treatment‐related select AEs were found in seven patients (29.2%). The only treatment‐related select AE of grade ≥3 was colitis (4.2%), which was observed twice in the same patient, who recovered from the first episode after study‐drug withdrawal and treatment with corticosteroids, but had another episode of colitis 146 days later, after which study treatment was discontinued. The second episode of colitis improved 71 days after its initial appearance.
Table 3.
Treatment‐related adverse events (AEs) in Japanese patients with previously untreated advanced melanoma given nivolumab (n = 23)
| All grades, n (%) | Grade ≥3, n (%) | |
|---|---|---|
| Overall | 20 (83.3) | 3 (12.5) |
| Treatment‐related AEs observed in ≥10% of patients | ||
| Vitiligo | 9 (37.5) | 0 (0.0) |
| Pruritus | 6 (25.0) | 0 (0.0) |
| Hypothyroidism | 6 (25.0) | 0 (0.0) |
| Malaise | 6 (25.0) | 0 (0.0) |
| Weight decreased | 3 (12.5) | 0 (0.0) |
| Appetite decreased | 3 (12.5) | 0 (0.0) |
| Arthralgia | 3 (12.5) | 0 (0.0) |
| Rash maculo‐papular | 3 (12.5) | 0 (0.0) |
| Treatment‐related AEs leading to discontinuation of treatment | ||
| Colitis | 1 (4.2) | 1 (4.2) |
| Pleural effusion | 1 (4.2) | 0 (0.0) |
| Treatment‐related serious AEs | ||
| Colitis | 1 (4.2) | 1 (4.2) |
| Hepatic function abnormal | 1 (4.2) | 0 (0.0) |
| Renal impairment | 1 (4.2) | 1 (4.2) |
| Pleural effusion | 1 (4.2) | 0 (0.0) |
| Treatment‐related select AEs | ||
| Endocrine disorders | 7 (29.2) | 0 (0.0) |
| Infusion reactions | 0 (0.0) | 0 (0.0) |
| Gastrointestinal toxicity | 2 (8.3) | 1 (4.2) |
| Hepatotoxicity | 1 (4.2) | 0 (0.0) |
| Pulmonary toxicity | 1 (4.2) | 0 (0.0) |
| Nephrotoxicity | 0 (0.0) | 0 (0.0) |
| Skin toxicity | 11 (45.8) | 0 (0.0) |
BRAF subgroup analyses
Among 23 patients, 17 (73.9%) had BRAF wild‐type and six (26.1%) had BRAF mutation. The IRC‐evaluated ORRs were 23.5% in patients with BRAF wild‐type (90% CI, 11.0, 43.3), and 66.7% in patients with a BRAF mutation (90% CI, 34.7, 88.3) (Table 4). Regardless of BRAF genotype, the lower limit of the 90% CI was higher than the threshold response rate of 6.0% estimated using dacarbazine data. The OS rates at 18 months were 52.9% and 66.7% in patients with BRAF wild‐type and mutation, respectively (Table 4, Fig. 2). The median OS was not reached in either subgroup. The median PFS was 4.2 months in patients with BRAF wild‐type, but was not reached in patients with BRAF mutation (Table 4, Fig. 3).
Table 4.
Response and survival in Japanese patients with previously untreated advanced melanoma given nivolumab, grouped according to wild‐type and mutant BRAF, assessed by independent radiology review committee (IRC)
| BRAF wild‐type, n (%) | BRAF mutation, n (%) | |
|---|---|---|
| Total patients | 17 (73.9) | 6 (26.1) |
| Best overall response | ||
| CR | 1 (5.9) | 1 (16.7) |
| PR | 3 (17.6) | 3 (50.0) |
| SD | 6 (35.3) | 1 (16.7) |
| PD | 6 (35.3) | 1 (16.7) |
| No lesion founda | 1 (5.9) | 0 (0.0) |
| Overall response rate (CR+PR) | 4 (23.5) | 4 (66.7) |
| 90% CI, % | 11.0, 43.3 | 34.7, 88.3 |
| Disease control rate (CR+PR+SD) | 10 (58.8) | 5 (83.3) |
| 90% CI, % | 39.3, 75.9 | 49.8, 96.2 |
| Progression‐free survival (IRC‐assessed) | ||
| Median, months | 4.21 | Not reached |
| 90% CI, months | 1.41, 7.16 | 1.41, – |
| Rate at 12 months, % | 23.5 | 83.3 |
| 90% CI, % | 9.3, 41.5 | 38.8, 96.5 |
| Rate at 18 months, % | 17.6 | 62.5 |
| 90% CI, % | 5.8, 34.8 | 21.2, 86.7 |
| Overall survival | ||
| Median, months | Not reached | Not reached |
| 90% CI, months | 7.95, – | 12.02, – |
| Rate at 12 months, % | 58.8 | 100.0 |
| 90% CI, % | 37.0, 75.4 | 100.0, 100.0 |
| Rate at 18 months, % | 52.9 | 66.7 |
| 90% CI, % | 31.7, 70.3 | 27.0, 88.2 |
Measurable lesion was found by investigator on site but not by IRC throughout the study. –, Censored value; CI, confidence interval;CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Treatment‐related AEs were reported in 15 (83.3%) and five (83.3%) patients with BRAF wild‐type and mutant, respectively, and the safety profiles were similar in both genotype subgroups. However, these data were obtained from a small number of patients and therefore need to be carefully interpreted.
Other subgroup analyses
A posteriori subgroup analyses were carried out to identify possible factors associated with nivolumab efficacy (Table 5). Median OS was not reached (90% CI, 16.0, –) during the study period in patients with normal level (≤ULN) of LDH (determined in clinical tests) (n = 16). Median OS in patients with abnormal level (>ULN) of LDH (n = 7) was 11.7 months (90% CI, 3.4, –) (hazard ratio, 0.30; 90% CI, 0.10, 0.85) (Fig. 7a). Seven of eight responders experienced treatment‐related select AEs, and the median OS was higher in patients with treatment‐related select AEs (n = 13) (not reached; 90% CI, 0.00, –) compared with patients without treatment‐related select AEs (n = 10) (median, 8.3 months; 90% CI, 3.4, 14.3) (hazard ratio, 0.10; 90% CI, 0.03, 0.36) (Fig. 7b). Notably, 66.7% of patients who developed vitiligo (included vitiligo vulgaris) responded to nivolumab, compared with only 14.3% without vitiligo. The median OS was not reached in patients with vitiligo (n = 9), but was 11.8 months (90% CI, 7.6, 16.0) in patients without vitiligo (n = 14) (Fig. 7c). Generally, patients with normal LDH, treatment‐related select AEs, or vitiligo had longer PFS (Fig. 7d–f).
Table 5.
Subgroup analyses of overall response rate (ORR) in Japanese patients with previously untreated advanced melanoma given nivolumab (n = 23)
| Patients, n (%) | ORR (IRC‐assessed), % (90% CI) | |
|---|---|---|
| Serum lactate dehydrogenase | ||
| Normal (≤ULN) | 6/16 | 37.5 (20.8, 57.8) |
| Abnormal (>ULN) | 2/7 | 28.6 (10.0, 59.1) |
| Treatment‐related select adverse events | ||
| Present | 7/13 | 53.8 (32.5, 73.9) |
| Absent | 1/10 | 10.0 (2.3, 34.8) |
| Vitiligo as a treatment‐related adverse event (including vitiligo vulgaris) | ||
| Present | 6/9 | 66.7 (39.8, 85.8) |
| Absent | 2/14 | 14.3 (4.8, 35.3) |
Cl, confidence interval; IRC, independent radiology review committee; ULN, upper limit of the normal range.
Figure 7.
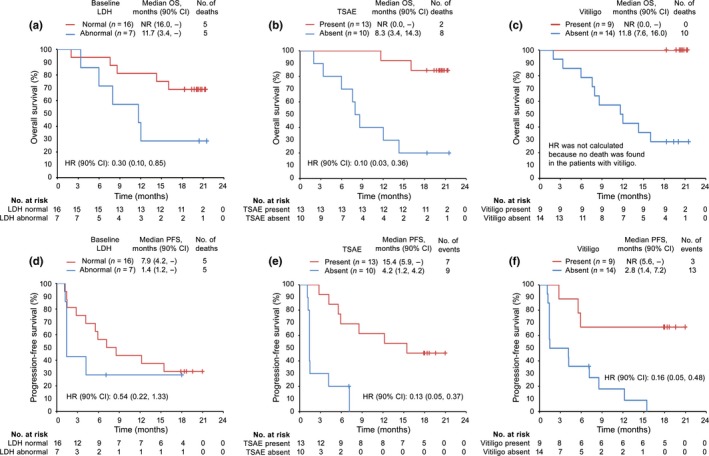
Overall survival (OS; upper panels) and progression‐free survival (PFS; lower panels) estimated by Kaplan–Meier analyses in subgroups of Japanese patients with previously untreated advanced melanoma given nivolumab (n = 23). OS (a) and PFS (d) in subgroups stratified by lactate dehydrogenase (LDH) levels at baseline (normal [≤ upper limit of the normal range [ULN], abnormal [>ULN]). Red and blue lines represent patients with low and high levels of LDH, respectively. OS (b) and PFS (e) in subgroups stratified by treatment‐related select adverse events (TSAEs). Red and blue lines represent patients with and without TSAEs, respectively. OS (c) and PFS (f) in subgroups stratified by vitiligo during treatment. Red and blue lines represent patients with and without vitiligo, respectively. CI, confidence interval; HR, hazard ratio; NR, not reached.
Discussion
Programmed death 1 inhibitors have been tested in clinical studies and have shown encouraging antitumor activities and tolerability in a wide range of advanced or refractory cancers, including renal cell carcinoma,17, 18 non‐small‐cell lung cancer,19, 20, 21 Hodgkin's or non‐Hodgkin's lymphoma,22, 23 ovarian cancer,24 and melanoma.11, 12, 13
In this study we investigated the efficacy and safety of the PD‐1‐blocking mAb nivolumab in Japanese patients with previously untreated advanced or recurrent melanoma. The efficacy in responding patients was sustainable, with the median OS not being reached during the course of this study. These results showed that nivolumab had good efficacy and was clinically more beneficial than standard dacarbazine therapy in Japanese patients with previously untreated advanced or recurrent melanoma.
The safety profile of nivolumab was similar to that observed in previous large, international, phase III studies.11, 12 All treatment‐related AEs in the present study were considered to be manageable by early diagnosis and appropriate treatment, such as with corticosteroids. These results indicated that nivolumab was tolerable in Japanese patients with previously untreated advanced melanoma.
We also compared the efficacy and safety profiles of nivolumab in patients with and without BRAF mutations. Nivolumab showed effective anti‐\tumor activity regardless of BRAF genotype, with apparently better OS and PFS in patients with BRAF mutant, although the sample sizes were insufficient to draw a statistically relevant conclusion. Our results were consistent with previous studies that reported clinical benefits of nivolumab, regardless of BRAF genotype.25, 26 Melanomas have been reported to be more aggressive and resistant to chemotherapy in patients with BRAF mutations,27, 28, 29 and the available therapeutic options have been limited in these patients. Nivolumab may thus represent a promising option for these patients.
High pretreatment serum LDH has been associated with shorter survival in patients with metastatic melanoma,30, 31 and the similar tendency was observed in our study. Nevertheless, some patients with high LDH levels showed a response to nivolumab, suggesting that it might offer an effective therapeutic option in melanoma patients with elevated LDH.
Patients experiencing treatment‐related select AEs and vitiligo showed a tendency for better survival in nivolumab‐treated patients in the current study. Immune‐related AEs have previously been shown to be characteristic of PD‐1 inhibitors,32, 33 and have a reported association with increased survival.32 However, it cannot be ruled out that non‐responder patients discontinued nivolumab so early that immune‐related AEs never appeared.34
The present study was limited by the small study group and the lack of a control group. However, several controlled clinical studies have previously been carried out in patients from the USA and Europe.11, 12 The efficacy and safety profiles of nivolumab in the current study were similar to those in previous trials, suggesting that the clinical data from those controlled clinical studies were likely to be applicable to the current Japanese study group.
The present study did not address the antitumor activity of nivolumab in relation to tumor PD‐L1 status. Furthermore, although nivolumab alone showed an ORR >30% in this study, combination therapy with another checkpoint inhibitor with a different mechanism of action, such as the cytotoxic T‐lymphocyte‐associated antigen‐4 inhibitor ipilimumab, may further enhance the therapeutic benefit,6, 13 and patients may respond differently.11, 35
In conclusion, nivolumab given at a dose of 3 mg/kg once every 2 weeks is tolerable and shows favorable anticancer activity in Japanese patients with previously untreated advanced or recurrent melanoma, irrespective of BRAF mutation status.
Disclosure Statement
Honoraria was received from Ono Pharmaceutical Co., Ltd. (N.Y., Y.K., H.U., T.T.), Takeda Pharmaceutical Co., Ltd. (N.Y.), Bristol‐Myers Squibb (N.Y., Y.K., H.U.), Chugai Pharmaceutical Co., Ltd. (N.Y., Y.K., H.U., T.T.), and Boehringer Ingelheim (N.Y.). Research funding was received from Ono Pharmaceutical Co., Ltd. (N.Y., Y.K., H.M.), Bristol‐Myers Squibb (N.Y., Y.K., H.M.), Chugai Pharmaceutical Co., Ltd. (Y.K., H.M.), MSD (Y.K.), Glaxo Smith Kline (Y.K.), and Novartis Pharma (N.Y., H.M.). The other authors have no conflict of interest. This study was funded by ONO Pharmaceutical, Japan, including the writing and editing service provided by ASCA Corporation, Japan. The study was planned, conducted, and analyzed independently by the authors.
Abbreviations
- AE
adverse event
- CI
confidence interval
- CR
complete response
- IRC
independent radiology review committee
- LDH
lactate dehydrogenase
- ORR
overall response rate
- OS
overall survival
- PD
progressive disease
- PD‐1
programmed death 1
- PD‐L
programmed death 1 ligand
- PFS
progression‐free survival
- PR
partial response
- RECIST
Response Evaluation Criteria in Solid Tumors
- SD
stable disease
- ULN
upper limit of the normal range
Acknowledgments
The authors are grateful to the patients and their families, and to the investigators, nurses, and staff members who participated in this study. We also acknowledge the statistical support of Eiichiro Morishima (Ono Pharmaceutical Co. Ltd., Osaka, Japan). The work was funded by Ono Pharmaceutical Co. Ltd.
Cancer Sci 108 (2017) 1223–1230
Funding Information
Ono Pharmaceutical Co. Ltd.
References
- 1. Avril MF, Aamdal S, Grob JJ et al Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. J Clin Oncol 2004; 22: 1118–25. [DOI] [PubMed] [Google Scholar]
- 2. Middleton MR, Grob JJ, Aaronson N et al Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol 2000; 18: 158–66. [DOI] [PubMed] [Google Scholar]
- 3. Balch CM, Buzaid AC, Soong SJ et al Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 2001; 19: 3635–48. [DOI] [PubMed] [Google Scholar]
- 4. Balch CM, Gershenwald JE, Soong SJ et al Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009; 27: 6199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Freeman GJ, Long AJ, Iwai Y et al Engagement of the PD‐1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000; 192: 1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992; 11: 3887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dong H, Strome SE, Salomao DR et al Tumor‐associated B7‐H1 promotes T‐cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8: 793–800. [DOI] [PubMed] [Google Scholar]
- 9. Latchman Y, Wood CR, Chernova T et al PD‐L2 is a second ligand for PD‐1 and inhibits T cell activation. Nat Immunol 2001; 2: 261–8. [DOI] [PubMed] [Google Scholar]
- 10. Hino R, Kabashima K, Kato Y et al Tumor cell expression of programmed cell death‐1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 2010; 116: 1757–66. [DOI] [PubMed] [Google Scholar]
- 11. Weber JS, D'Angelo SP, Minor D et al Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti‐CTLA‐4 treatment (CheckMate 037): a randomised, controlled, open‐label, phase 3 trial. Lancet Oncol 2015; 16: 375–84. [DOI] [PubMed] [Google Scholar]
- 12. Robert C, Long GV, Brady B et al Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372: 320–30. [DOI] [PubMed] [Google Scholar]
- 13. Larkin J, Chiarion‐Sileni V, Gonzalez R et al Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oken MM, Creech RH, Tormey DC et al Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5: 649–55. [PubMed] [Google Scholar]
- 15. Eisenhauer EA, Therasse P, Bogaerts J et al New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 16. Yamazaki N, Tahara H, Uhara H, Moroi Y, Kiyohara Y. Phase 2 study of nivolumab (Anti‐PD‐1; ONO‐4538/BMS‐936558) in patients with advanced melanoma [abstract no. 3738]. Eur J Cancer. 2013; 49 (Suppl 2): S868. [Google Scholar]
- 17. McDermott DF, Drake CG, Sznol M et al Survival, durable response, and long‐term safety in patients with previously treated advanced renal cell carcinoma receiving Nivolumab. J Clin Oncol 2015; 33: 2013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Motzer RJ, Escudier B, McDermott DF et al Nivolumab versus everolimus in advanced renal‐cell carcinoma. N Engl J Med 2015; 373: 1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brahmer J, Reckamp KL, Baas P et al Nivolumab versus Docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015; 373: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gettinger SN, Horn L, Gandhi L et al Overall survival and long‐term safety of nivolumab (anti‐programmed death 1 antibody, BMS‐936558, ONO‐4538) in patients with previously treated advanced non‐small‐cell lung cancer. J Clin Oncol 2015; 33: 2004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ansell SM, Lesokhin AM, Borrello I et al PD‐1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015; 372: 311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lesokhin AM, Ansell SM, Armand P et al Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol 2016; 34: 2698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamanishi J, Mandai M, Ikeda T et al Safety and antitumor activity of anti‐PD‐1 antibody, nivolumab, in patients with platinum‐resistant ovarian cancer. J Clin Oncol 2015; 33: 4015–22. [DOI] [PubMed] [Google Scholar]
- 25. Weber JS, Kudchadkar RR, Yu B et al Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab‐refractory or ‐naive melanoma. J Clin Oncol 2013; 31: 4311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Larkin J, Lao CD, Urba WJ et al Efficacy and safety of nivolumab in patients with BRAF V600 mutant and BRAF wild‐type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA Oncol 2015; 1: 433–40. [DOI] [PubMed] [Google Scholar]
- 27. Long GV, Menzies AM, Nagrial AM et al Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol 2011; 29: 1239–46. [DOI] [PubMed] [Google Scholar]
- 28. Flaherty KT, Puzanov I, Kim KB et al Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 2010; 363: 809–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arozarena I, Sanchez‐Laorden B, Packer L et al Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP‐specific phosphodiesterase PDE5A. Cancer Cell 2011; 19: 45–57. [DOI] [PubMed] [Google Scholar]
- 30. Gogas H, Eggermont AM, Hauschild A et al Biomarkers in melanoma. Ann Oncol 2009; 20 Suppl 6: vi8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakamura Y, Kitano S, Takahashi A et al Nivolumab for advanced melanoma: pretreatment prognostic factors and early outcome markers during therapy. Oncotarget 2016; 7: 77404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Freeman‐Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune‐related adverse events and association with outcomes. Clin Cancer Res 2016; 22: 886–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Belum VR, Benhuri B, Postow MA et al Characterisation and management of dermatologic adverse events to agents targeting the PD‐1 receptor. Eur J Cancer 2016; 60: 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weber JS, Hodi FS, Wolchok JD et al Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol 2016; 35: 785–92. [DOI] [PubMed] [Google Scholar]
- 35. Bowyer S, Prithviraj P, Lorigan P et al Efficacy and toxicity of treatment with the anti‐CTLA‐4 antibody ipilimumab in patients with metastatic melanoma after prior anti‐PD‐1 therapy. Br J Cancer 2016; 114: 1084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


