Abstract
Patients with rheumatoid arthritis often develop methotrexate‐associated lymphoproliferative disorders (MTX‐LPD) during MTX treatment. MTX‐LPD occasionally regresses spontaneously after simply discontinuing MTX treatment. In patients without spontaneous regression, additional chemotherapy is required to avoid disease progression. However, the differences between spontaneous and non‐spontaneous regression have yet to be elucidated. To clarify the factors important for spontaneous regression, we analyzed the clinicopathological features of 51 patients with rheumatoid arthritis who developed MTX‐LPD (diffuse large B‐cell lymphoma [DLBCL]‐type [n = 34] and classical Hodgkin lymphoma [CHL]‐type [n = 17]). We examined the interval from MTX discontinuation to the administration of additional chemotherapy. The majority of DLBCL‐type MTX‐LPD patients (81%) exhibited remission with MTX discontinuation alone. In contrast, the majority of CHL‐type MTX‐LPD patients (76%) required additional chemotherapy. This difference was statistically significant (P = 0.001). However, overall survival was not significantly different between DLBCL‐type and CHL‐type (91% vs 94%, respectively; P > 0.05). Thus, the morphological differences in the pathological findings of MTX‐LPD may be a factor for spontaneous or non‐spontaneous regression after discontinuation of MTX.
Keywords: Epstein‐Barr virus, histological findings, methotrexate‐associated lymphoproliferative disorders, rheumatoid arthritis, spontaneous remission
Methotrexate (MTX) is an anti‐cancer agent that is classified as an anti‐folate and is used as an anti‐rheumatic drug. MTX‐associated lymphoproliferative disorders (MTX‐LPD) is a lymphoproliferative disease or lymphoma in patients treated with MTX for autoimmune diseases, such as rheumatoid arthritis (RA).1 MTX‐LPD was first reported in 1991 and was subsequently established as a disease concept owing to the reported increase in incidence with the increasing use of MTX for the treatment of RA.2 MTX‐LPD is classified as “other iatrogenic immunodeficiency‐associated LPDs” in the 4th edition of the World Health Organization classification.2
MTX‐LPD have various histopathological features. Among patients treated with MTX, the most commonly reported cases are diffuse large B‐cell lymphoma (DLBCL; 35–60%) and classical Hodgkin lymphoma (CHL; 12–25%)‐types.2 There are no histological differences compared with the lymphomas of RA patients who develop non‐MTX‐LPD.2, 3
Although the developmental mechanism of MTX‐LPD is poorly understood, it is assumed that immune disorders in patients with RA and immunosuppression associated with MTX might contribute to the development of MTX‐LPD. In the majority of patients with MTX‐LPD, activation of the Epstein‐Barr virus (EBV) has been detected,3, 4 EBV‐positive patients, and some other patients with MTX‐LPD, experience spontaneous regression of their lesions after simply discontinuing MTX treatment.5, 6, 7 In patients with MTX‐LPD that do not spontaneously regress, additional chemotherapy is required to avoid disease progression.7 However, the differences between spontaneous and non‐spontaneous regression have yet to be elucidated.
To clarify the factors important for spontaneous regression, we examined the histopathological features, IGH gene rearrangements, EBV‐encoded small RNA (EBER)‐positive rates, and the clinical course of RA patients who developed DLBCL‐type or CHL‐type MTX‐LPD.
Patients and Methods
Patients
We analyzed the clinicopathological features of 51 patients who had been diagnosed with MTX‐LPD (DLBCL‐type [n = 34] and CHL‐type [n = 17]) and whose records were selected from pathology files in the Department of Pathology at Okayama University (Okayama, Japan). We excluded patients with polymorphous‐type MTX‐LPD.
All patients received low‐dose MTX, and 17 patients (33%) received MTX with prednisolone. Five DLBCL‐type and 4 CHL‐type MTX‐LPD patients were treated with MTX in combination with other disease‐modifying anti‐rheumatic drugs (e.g., infliximab, etanercept, adalimumab, and bucillamine). In this study, 19 DLBCL‐type (56%) and 12 CHL‐type MTX‐LPD patients (71%) were included in the analysis of MTX administration. The median treatment duration was 119 (range, 8–192) months.
This study was approved by the Institutional Review Board of Okayama University (Okayama, Japan).
Clinical data
Data regarding the Eastern Cooperative Oncology Group performance status and clinical stage (CS) were collected from medical records.
Histological examination and in situ hybridization
Specimens were fixed in 10% formaldehyde and embedded in paraffin. Three‐micrometer‐thick sections were cut from the paraffin‐embedded tissue blocks and stained with hematoxylin and eosin.
Paraffin sections of each tissue sample were used for immunohistochemical staining with antibodies to CD3 (clone: LN10, 1:200; Novocastra Laboratories, Ltd., Newcastle upon Tyne, UK), CD5 (clone: 4C7, 1:100; Novocastra Laboratories, Ltd.), CD10 (clone: 56C6, 1:100; Novocastra Laboratories, Ltd.), CD15 (clone: Carb‐3, 1:50; DAKO, Glostrup, Denmark), CD20 (clone: L26, 1:100; DAKO), CD30 (clone: Ber‐H2, 1:40; DAKO), EBV nuclear antigen 2 (EBNA2) (clone: PE2, 1:100; Abcam, Cambridge, MA, USA), EBV latent membrane protein 1 (LMP‐1) (clone: CS1‐4, 1:50; Novocastra Laboratories, Ltd.), and Ki‐67 (clone: MIB‐1, 1:2500; DAKO). Staining was performed using the automated Bond Max Stainer (Leica Biosystems, Wetzlar, Germany).
The EBV was detected by in situ hybridization for EBER using the automated Bond Max Stainer (Leica Biosystems).
IGH gene rearrangement analysis
We scraped sections from the tumor area and placed them in 1× AmpliTaq Gold Buffer (Applied Biosystems, Inc., Foster City, CA, USA). DNA was extracted by incubating at 94°C for 45 min, using the automated Thermocycler GeneAmp PCR System 9700 (Applied Biosystems, Inc.). DNA was quantified using the NanoDrop ND‐1000 spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA). IGH gene rearrangement analysis was performed as previously described.8 The following primers were used in this study:
VH1‐FR2 (5′‐CTGGGTGCGACAGGCCCCTGGACAA‐3′),
VH2‐FR2 (5′‐TGGATCCGTCAGCCCCCAGGGAAGG‐3′),
VH3‐FR3 (5′‐GGTCCGCCAGGCTCCAGGGAA‐3′),
VH4‐FR2 (5′‐TGGATCCGCCAGCCCCCAGGGAAGG‐3′),
VH5‐FR2 (5′‐GGGTGCGCCAGATGCCCGGGAAAGG‐3′),
VH6‐FR2 (5′‐TGGATCAGGCAGTCCCCATCGAGAG‐3′),
VH7‐FR2 (5′‐TTGGGTGCGACAGGCCCCTGGACAA‐3′),
and JH consensus primer (5′‐CTTACCTGAGGAGACGGTGACC‐3′). The JH consensus primer was fluorescently labeled. All primers were purchased from Sigma‐Aldrich (Sigma‐Aldrich Japan, Tokyo, Japan). PCR products were analyzed using an ABI PRISM 310 Genetic Analyzer with GeneScan Analysis and GeneMapper software (Applied Biosystems, Inc.). IGH gene rearrangements were analyzed and evaluated using the BIOMED‐2 protocol.8 If the waveform of the PCR product could be confirmed, even by a small amount, this finding was interpreted as polyclonal expression. If a peak was not visible (e.g., due to sample condition) then these samples were deemed to have undetectable expression.
Statistical analyses
Differences between DLBCL‐type and CHL‐type MTX‐LPD were determined using Student's t and χ2 tests. A P < 0.05 was considered statistically significant. Overall survival was defined as the time from the date of diagnosis of a MTX‐LPD to the date of death or last follow‐up. Progression‐free survival was defined as the time from the date of diagnosis of a MTX‐LPD to the date of commencing chemotherapy or the date of death or last follow‐up. The follow‐up duration was calculated as the time from the date of diagnosis of a MTX‐LPD to the date of death from any cause or last follow‐up. The duration from the date of diagnosis of a MTX‐LPD to the date of commencing chemotherapy was determined. Survival curves were generated using the Kaplan‐Meier method. All statistical analyses were conducted using SPSS for Windows software version 14.0 (SPSS Inc., Chicago, IL, USA).
Results
Comparison of the clinicopathological features between patients with DLBCL‐type and CHL‐type MTX‐LPD
Of the 51 patients with a MTX‐LPD, 34 patients (67%) had a DLBCL‐type MTX‐LPD and 17 patients (33%) had a CHL‐type MTX‐LPD. The median age of the entire cohort was 67 (range, 45–84) years, and the median age did not differ between the MTX‐LPD subtypes (Table 1). The male‐to‐female ratio of the entire cohort was 14:37 and the proportion of female patients was high for both MTX‐LPD subtypes.
Table 1.
Clinical findings of patients with rheumatoid arthritis who developed methotrexate‐associated lymphoproliferative disorders, compared between diffuse large B‐cell lymphoma‐type and classical Hodgkin lymphoma‐type
| All patients (n = 51) | DLBCL‐type (n = 34) | CHL‐type (n = 17) | P‐value | |
|---|---|---|---|---|
| Age | 67 (45–84) | 70 (55–82) | 64 (45–84) | 0.09 |
| Sex (male:female) | 14:37 | 10:24 | 4:13 | 0.46 |
| PS ≥ 2 | 37% (17/46) | 41% (12/29) | 29% (5/17) | 0.46 |
| Clinical stage ≥3 | 75% (38/51) | 74% (25/34) | 76% (13/17) | 0.55 |
| High LDH levels | 67% (33/49) | 72% (23/32) | 59% (10/17) | 0.37 |
| Extranodal disease | 57% (29/51) | 65% (22/34) | 41% (7/17) | 0.10 |
| Extranodal disease ≥2 | 8 | 5 | 3 | 0.76 |
| B symptom positive | 53% (24/45) | 57% (17/30) | 47% (7/15) | 0.38 |
| EBER positive | 82% (42/51) | 82% (28/34) | 82% (14/17) | 0.66 |
| Died | 6 | 5 | 1 |
High lactate dehydrogenase (LDH) levels were defined as values equal to or greater than the reference value. DLBCL, diffuse large B‐cell lymphoma; CHL, classical Hodgkin lymphoma; EBER, Epstein‐Barr Virus‐encoded small RNA in situ hybridization; MTX, methotrexate; PS, Eastern Cooperative Oncology Group performance status.
Reduced hemoglobin levels of <10.5 g/dL were observed in 30% (9/30) patients with a DLBCL‐type MTX‐LPD and 46% (6/13) patients with a CHL‐type MTX‐LPD. A reduction in the total lymphocyte count was observed in 34% (10/29) patients with a DLBCL‐type MTX‐LPD and 23% (3/13) patients with a CHL‐type MTX‐LPD. The median soluble interleukin 2 receptor value was 2,748 IU/mL for the DLBCL‐type MTX‐LPD (n = 30) and 2,450 IU/mL for the CHL‐type MTX‐LPD (n = 15).
The Eastern Cooperative Oncology Group performance status was not significantly different between the MTX‐LPD subtypes (P > 0.05). Twelve patients with a DLBCL‐type MTX‐LPD and five patients with a CHL‐type MTX‐LPD had an Eastern Cooperative Oncology Group performance status of ≥2. CS was also not significantly different between the MTX‐LPD subtypes (P > 0.05). Twenty‐five patients with a DLBCL‐type MTX‐LPD and 13 patients with a CHL‐type MTX‐LPD had a CS of ≥3 (Table 1).
Of the 17 patients with a CHL‐type MTX‐LPD, seven patients (41%) had extranodal lesions in organs and tissues, such as the brain, lungs, kidneys, liver, and bone marrow.
Histopathological classification
DLBCL‐type MTX‐LPD
Atypical lymphoid cells were large and revealed monomorphic proliferation. The rates of positive immunohistochemical staining for CD3, CD5, CD10, and CD20 were 0% (0/34), 0% (0/26), 5% (1/22), and 88% (30/34), respectively (Fig. 1). A high Ki‐67 labeling index of >30% (i.e., a high Ki‐67 labeling index) was observed for all of the cases with a DLBCL‐type MTX‐LPD. The EBER‐positive rate was 82% (28/34; Table 1).
Figure 1.
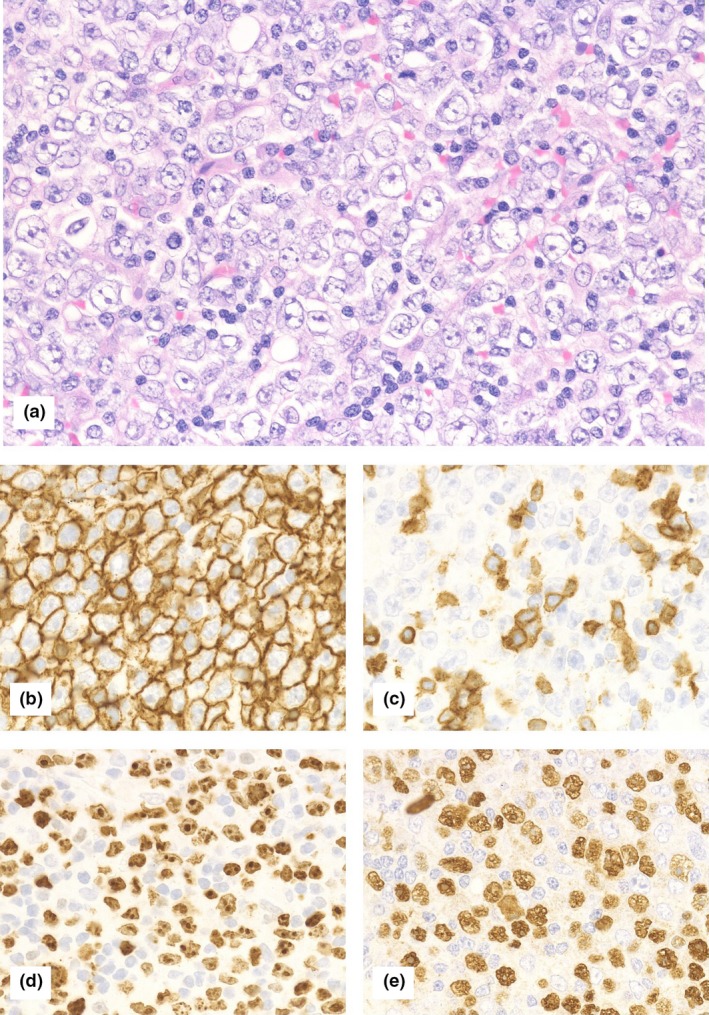
Histological findings of diffuse large B‐cell lymphoma‐type methotrexate‐associated lymphoproliferative disorders. (a) Hematoxylin and eosin staining at 400× magnification (high‐powered field). Large atypical lymphoid cells exhibited monomorphic proliferation. Tumor cells were (b) CD20‐positive, (c) CD3‐negative, and (d) had a high Ki‐67 labeling index. Tumor cells were also (e) positive for Epstein‐Barr Virus‐encoded small RNA (EBER) in situ hybridization.
CHL‐type MTX‐LPD
Hodgkin and Reed‐Sternberg cells were observed in a cellular background rich in non‐neoplastic small lymphocytes, histiocytes, and eosinophils. In the Hodgkin and Reed‐Sternberg cells, the rates of positive immunohistochemical staining for CD3, CD15, CD20, CD30, EBNA2, and LMP‐1 were 0% (0/16), 60% (9/15), 29% (5/17), 100% (17/17), 0% (0/11), and 62% (8/13), respectively (Fig. 2). The EBER‐positive rate was 82% (14/17; Table 1).
Figure 2.
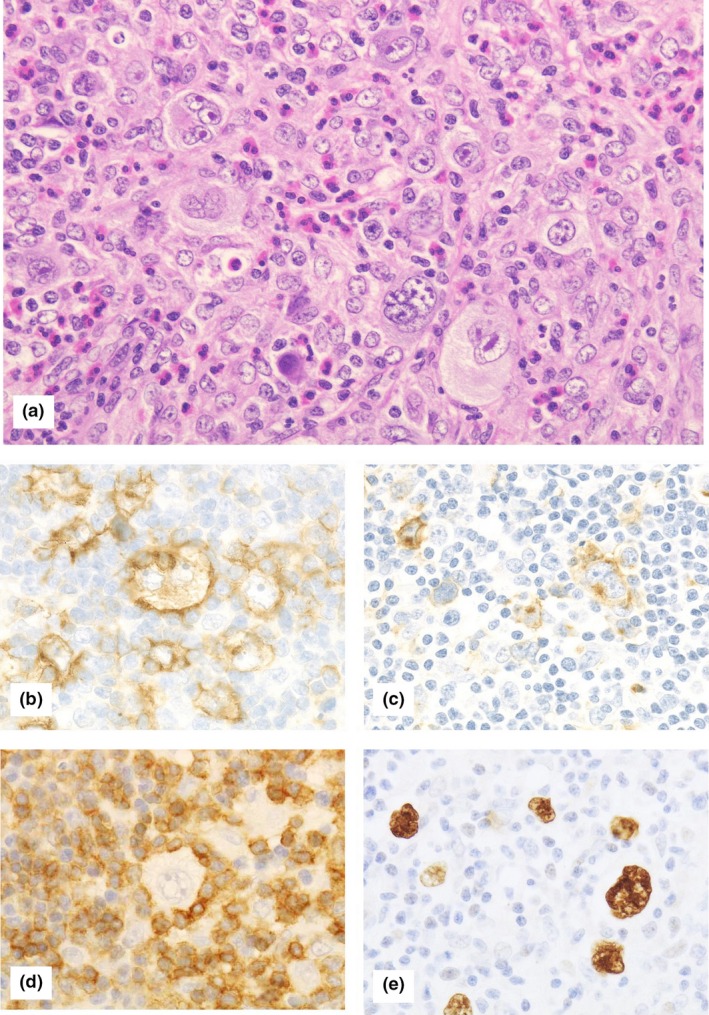
Histological findings of classical Hodgkin lymphoma‐type methotrexate‐associated lymphoproliferative disorders. (a) Hematoxylin and eosin staining at 400× magnification (high‐powered field). Hodgkin and Reed‐Sternberg cells were observed in a cellular background rich in lymphocytes, histiocytes, and eosinophils. Tumor cells were (b) CD30‐positive and (c) CD15‐positive. (d) CD3‐positive cells formed a rosette around the Hodgkin and Reed‐Sternberg cells. Tumor cells were also (e) positive for Epstein‐Barr Virus‐encoded small RNA (EBER) in situ hybridization.
The EBV latent infection type was analyzed in the CHL‐type MTX‐LPD cases. A type I infection (EBER‐positive, LMP‐1‐negative, and EBNA2‐negative) was observed in two cases, a type II infection (EBER‐positive, LMP‐1‐positive, and EBNA2‐negative) in six cases, and a type III infection (EBER‐positive, LMP‐1‐positive, and EBNA2‐positive) in 0 cases. The EBV latent infection type was not available for the remaining nine cases (Table 2).
Table 2.
Clinical and pathological findings of patients with classical Hodgkin lymphoma‐type methotrexate‐associated lymphoproliferative disorders
| Patient No. Age/Sex | Primary immune disease | Immunomodulator | Biopsy site | Extranodal involvement site | Clinical stage | EBER | LMP‐1 | EBNA2 | Immunophenotype | Response after MTX discontinuation | Chemo therapy | Outcome, follow‐up duration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1 45/F |
RA, SS | MTX, PSL | LN | Bone marrow | IV | + | + | − | CD30 (+), CD15 (NA), CD20 (−), CD79a (p+) | Exacerbation | Rituximab, ADR, CY | PD, 3 mo |
|
2 63/M |
RA | MTX, etanercept | LN | No | III | + | NA | − | CD30 (+), CD15 (+), CD20 (−), CD79a (NA) | Regrowth | ABVD | CR, 4.6 y |
|
3 69/F |
RA | MTX | Cerebellum | Cerebellum | I | + | + | NA | CD30 (+), CD15 (+), CD20 (p+), CD79a (NA) | Exacerbation | ABVD | CR, 6.5 y |
|
4 79/F |
RA | MTX | LN | No | III | + | + | − | CD30 (+), CD15 (+), CD20 (p+), CD79a (NA) | Regrowth | ABVD | CR, 1.7 y |
|
5 65/F |
RA | MTX, PSL | LN | No | III | + | + | − | CD30 (+), CD15 (+), CD20 (−), CD79a (NA) | No change | ABVD | CR, 3.1 y |
|
6 63/M |
RA | MTX, bucillamine, PSL | LN | No | I | + | + | − | CD30 (+), CD15 (+), CD20 (−), CD79a (NA) | No change | ABVD | CR, 5.8 y |
|
7 63/F |
RA | MTX, PSL | LN | Liver, Lung | IV | + | NA | NA | CD30 (+), CD15 (+), CD20 (p+), CD79a (−) | Regrowth | ABVD | CR, 4.7 y |
|
8 84/M |
RA | MTX, PSL | LN | No | III | − | − | − | CD30 (+), CD15 (+), CD20 (−), CD79a (NA) | No change | ABVD | CR, 1.9 mo |
|
9 53/F |
RA | MTX | LN | No | III | + | − | − | CD30 (+), CD15 (NA), CD20 (−), CD79a (−) | Regrowth | ABVD | CR, 1.7 y |
|
10 64/F |
RA | MTX, adalimumab, PSL | LN | Brain, Lung | IV | + | − | NA | CD30 (+), CD15 (−), CD20 (−), CD79a (−) | Reduction | − | DF, 1.7 y |
|
11 63/F |
RA | MTX, infliximab | LN | No | III | + | + | NA | CD30 (+), CD15 (−), CD20 (−), CD79a (p+) | Regrowth | ABVD | C R, 3.4 y |
|
12 73/F |
RA | MTX | Oral cavity | Gingiva | I | + | + | − | CD30 (+), CD15 (−), CD20 (+), CD79a (NA) | Regrowth | ABVD | CR, 3 y |
|
13 81/M |
RA | MTX | LN | No | III | + | − | − | CD30 (+), CD15 (−), CD20 (p+), CD79a (NA) | Reduction | − | DF, 6 mo |
|
14 48/F |
RA | MTX, PSL | LN | No | III | − | − | − | CD30 (+), CD15 (+), CD20 (−), CD79a (−) | No change | ABVD | CR, 9 y |
|
15 64/F |
RA | MTX | LN | Kidney, adrenal gland | IV | + | + | − | CD30 (+), CD15 (−), CD20 (−), CD79a (NA) | Reduction | − | DF, 3.4 y |
|
16 73/F |
RA | MTX | Tonsils | No | I | + | NA | NA | CD30 (+), CD15 (+), CD20 (−), CD79a (NA) | Reduction | − | DF, 1 mo |
|
17 56/F |
RA | MTX | Liver | Liver | IV | − | NA | NA | CD30 (+), CD15 (−), CD20 (−), CD79a (−) | Regrowth | ABVD |
ABVD, adriamycin + bleomycin + vinblastine + dacarbazine; CR, complete response after chemotherapy; DF, disease free after remission with discontinuation of MTX; EBER, Epstein‐Barr Virus‐encoded small RNA in situ hybridization; F, female; LN, lymph node; M, male; mo, month; MTX, methotrexate; NA, not available; p+, partial positive; PD, progressive disease; PSL, prednisolone; RA, rheumatoid arthritis; SS, Sjögren's syndrome; y, year; +, positive; −, negative.
IGH gene rearrangement
IGH gene rearrangements were analyzed in 36 cases (DLBCL‐type MTX‐LPD [n = 27] and CHL‐type MTX‐LPD [n = 9]). In the DLBCL‐type MTX‐LPD, monoclonal rearrangements were detected in two cases (7%) and polyclonal rearrangements were detected in 17 cases (63%). In the CHL‐type MTX‐LPD, polyclonal rearrangements were detected in five cases (56%). IGH gene rearrangements were undetectable in the remaining cases.
Clinical course of patients with MTX‐LPD after discontinuing MTX treatment
CHL‐type MTX‐LPD
In all patients, the administration of MTX had been discontinued at the time of MTX‐LPD diagnosis (Table 2). In four patients, the lesions were reduced and were controllable after the discontinuation of MTX treatment. Another seven patients showed regrowth after reduction of the lesions and additional chemotherapy was required. Relapse occurred in seven patients with a CHL‐type MTX‐LPD. The median duration until relapse was 6.4 months. Discontinuation of MTX treatment was ineffective in six patients. These patients received chemotherapy for the CHL. Therefore, additional chemotherapy was required in a total of 13 patients (76%). Of the 13 patients who required additional chemotherapy, 12 patients exhibited complete remission after chemotherapy; one patient died of bacterial pneumonia during chemotherapy (Table 3).
Table 3.
Clinical course of classical Hodgkin lymphoma‐type methotrexate‐associated lymphoproliferative disorders after discontinuation of methotrexate
| Patient No. | Response after MTX discontinuation | Regrowth after MTX discontinuation | Period from MTX‐LPD diagnosis to initiation of chemotherapy (months) | Response to chemotherapy | Final state | Final outcome |
|---|---|---|---|---|---|---|
| 1 | Exacerbation | — | 0.7 | Progressive disease | Bacterial pneumonia during chemotherapy | Dead |
| 2 | Reduction | Regrowth | 7.3 | Complete response | CR state after the chemotherapy | Alive |
| 3 | Exacerbation | — | 2.4 | Complete response | CR state after the chemotherapy | Alive |
| 4 | Reduction | Regrowth | 5.7 | Complete response | CR state after the chemotherapy | Alive |
| 5 | No change | — | 0.7 | Complete response | CR state after the chemotherapy | Alive |
| 6 | No change | — | 0.5 | Complete response | CR state after the chemotherapy | Alive |
| 7 | Reduction | Regrowth | 23.5 | Complete response | CR state after the chemotherapy | Alive |
| 8 | No change | — | 0.5 | Complete response | CR state after the chemotherapy | Alive |
| 9 | Reduction | Regrowth | 21.5 | Complete response | CR state after the chemotherapy | Alive |
| 10 | Reduction | None | None | — | Spontaneous remission | Alive |
| 11 | Reduction | Regrowth | 15.1 | Complete response | CR state after the chemotherapy | Alive |
| 12 | Reduction | Regrowth | 9.5 | Complete response | CR state after the chemotherapy | Alive |
| 13 | Reduction | None | None | — | Spontaneous remission | Alive |
| 14 | No change | — | 0.9 | Complete response | CR state after the chemotherapy | Alive |
| 15 | Reduction | None | None | — | Spontaneous remission | Alive |
| 16 | Reduction | None | None | — | Spontaneous remission | Alive |
| 17 | Reduction | Regrowth | 7.6 | Complete response | CR state after the chemotherapy | Alive |
CR, complete response; MTX, methotrexate; MTX‐LPD, methotrexate‐associated lymphoproliferative disorders.
DLBCL‐type MTX‐LPD
Among the 34 patients with a DLBCL‐type MTX‐LPD, MTX was discontinued in 27 patients at the time of MTX‐LPD diagnosis. In 22 patients (81%), remission of the lesions occurred after MTX was discontinued and additional chemotherapy was unnecessary. Only one patient exhibited regrowth after reduction of the lesion. The duration until relapse was 10.7 months. This patient died without receiving chemotherapy after relapse. Four patients required additional chemotherapy after the discontinuation of MTX. Of these, three patients exhibited complete remission after chemotherapy and one patient died of therapy‐related acute myeloid leukemia. The attending physician initiated chemotherapy using the R‐CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone) regimen or similar.
For five patients, chemotherapy was initiated at the same time as the MTX‐LPD was diagnosed. Of these, four patients exhibited complete remission after chemotherapy and one patient died of the MTX‐LPD.
Two patients with a MTX‐LPD were diagnosed at autopsy. The cause of death was the MTX‐LPD and gastrointestinal bleeding (Table 4).
Table 4.
Clinical and pathological findings of patients with diffuse large B‐cell lymphoma‐type methotrexate‐associated lymphoproliferative disorders
| Patient No. | Age/Sex | Primary immune disease | Immunomodulator | Biopsy site | Extranodal involvement site | Clinical stage | EBER | Outcome after MTX discontinuation | Chemotherapy | Outcome, follow‐up duration |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 71/M | RA | MTX | Tonsil | Adrenal gland | IV | + | Reduction | None | DF, 6.5 y |
| 2 | 61/F | RA | MTX, etanercept, PSL | Skin | Skin | IV | − | No change | R‐CHOP | Dead by therapy‐related leukemia after complete response, 1.8 y |
| 3 | 55/F | RA | MTX | LN | None | II | + | Reduction | None | DF, 6.3 y |
| 4 | 67/M | RA | MTX | Tonsil | None | I | + | Reduction | None | DF, 4.6 y |
| 5 | 61/F | RA | MTX, bucillamine | LN | Liver | IV | + | Reduction | None | DF, 3.4 y |
| 6 | 67/F | RA | MTX, PSL | LN | None | III | + | Start chemotherapy at the same time as diagnosis | R‐CHOP | CR, 3.3 mo |
| 7 | 67/F | RA | MTX | Tonsil | Bone marrow | IV | + | Reduction | None | DF, 1.4 y |
| 8 | 72/F | RA | MTX, infliximab, PSL | LN | Liver | IV | + | No change | R‐THP‐COP | CR, 6 mo |
| 9 | 71/M | RA | MTX | LN | None | III | + | Reduction | None | DF, 3.5 y |
| 10 | 67/F | RA | MTX, etanercept | LN | None | II | + | Reduction | None | DF, 3.4 y |
| 11 | 60/F | RA | MTX | LN | Lung | IV | + | Reduction | None | DF, 5 mo |
| 12 | 64/F | RA | MTX | Skin | Skin | IV | + | Exacerbation | R‐CHOP | CR, 2.5 y |
| 13 | 80/F | RA | MTX | LN | None | I | + | Start chemotherapy at the same time as diagnosis | R‐THP‐COP | CR, 9.9 mo |
| 14 | 71/F | RA | MTX | LN | Kidney, adrenal gland | IV | + | Reduction | None | DF, 2.7 y |
| 15 | 82/M | RA | MTX, bucillamine | LN | Liver, Lung, adrenal gland | IV | + | Start chemotherapy at the same time as diagnosis | R‐CHOP | CR, 4 mo |
| 16 | 59/F | RA | MTX | LN | Lung | IV | + | Start chemotherapy at the same time as diagnosis | R‐CHOP | CR, 3.6 mo |
| 17 | 72/F | RA | MTX | LN | None | II | − | Reduction | None | DF, 2.4 y |
| 18 | 75/F | RA | MTX | Skin | Skin | IV | + | Reduction | None | DF, 1.4 y |
| 19 | 77/F | RA | MTX, PSL | Bone marrow | Bone marrow | IV | + | Reduction | None | DF, 3 y |
| 20 | 60/F | RA | MTX | LN | None | I | + | Reduction | None | DF, 2.9 mo |
| 21 | 69/M | RA | MTX | LN | Lung, Kidney | IV | − | Reduction | None | DF, 1.8 mo |
| 22 | 64/M | RA | MTX | Liver | Lung, Liver | IV | − | No change | CHOP | CR, 10 mo |
| 23 | 81/M | RA | MTX | LN | None | II | + | Reduction | None | DF, 1.2 y |
| 24 | 73/F | RA | MTX | LN | Liver | IV | + | Reduction | None | DF, 1.3 y |
| 25 | 64/F | RA | MTX | Lung | Lung, Kidney | IV | + | Reduction | None | DF, 9.6 mo |
| 26 | 73/M | RA | MTX, PSL | LN | Lung | IV | + | Reduction | None | DF, 2.9 mo |
| 27 | 72/F | RA | MTX | LN | Lung | IV | + | Reduction | None | DF, 7.8 mo |
| 28 | 58/F | RA | MTX | LN | Bone marrow | IV | − | Start chemotherapy at the same time as diagnosis | R‐CHOP | Dead by MTX‐LPD after complete response, 1.6 y |
| 29 | 55/F | RA | MTX, PSL | LN | None | II | + | Reduction | None | DF, 5 y |
| 30 | 67M | RA | MTX, PSL | LN | None | I | + | Reduction | None | DF, 2.3 y |
| 31 | 77/F | RA | MTX, PSL | LN | None | III | + | Reduction | None | DF, 3 y |
| 32 | 72/F | RA | MTX, PSL | Lung | Lung | IV | − | NA (Diagnosed by autopsy) | None | Dead by gastrointestinal bleeding |
| 33 | 74/F | RA | MTX | LN | Liver | IV | + | NA (Diagnosed by autopsy) | None | Dead by MTX‐LPD |
| 34 | 74/M | RA | MTX, PSL | LN | Liver | IV | + | Regrowth | None | Dead by cerebral hemorrhage after regrowth of MTX‐LPD, 5.2 mo |
CHOP, cyclophosphamide + doxorubicin + vincristine + prednisolone; CR, complete response after chemotherapy; DF, disease free after remission with discontinuation of MTX; EBER, Epstein‐Barr Virus‐encoded small RNA in situ hybridization; LN, lymph node; MTX, methotrexate; NA, not available; PSL, prednisolone; RA, rheumatoid arthritis; R‐CHOP, rituximab‐CHOP; M, male; F, female; y, year; mo, month; +, positive; −, negative.
Progression‐free survival analysis of patients with MTX‐LPD
The progression‐free survival differed significantly between the two MTX‐LPD subtypes (12.1 vs 6.4 months for the DLBCL‐type and CHL‐type, respectively; P = 0.001) (Fig. 3). The two patients diagnosed at autopsy and the five patients who commenced chemotherapy at the time the MTX‐LPD was diagnosed were excluded from this analysis.
Figure 3.
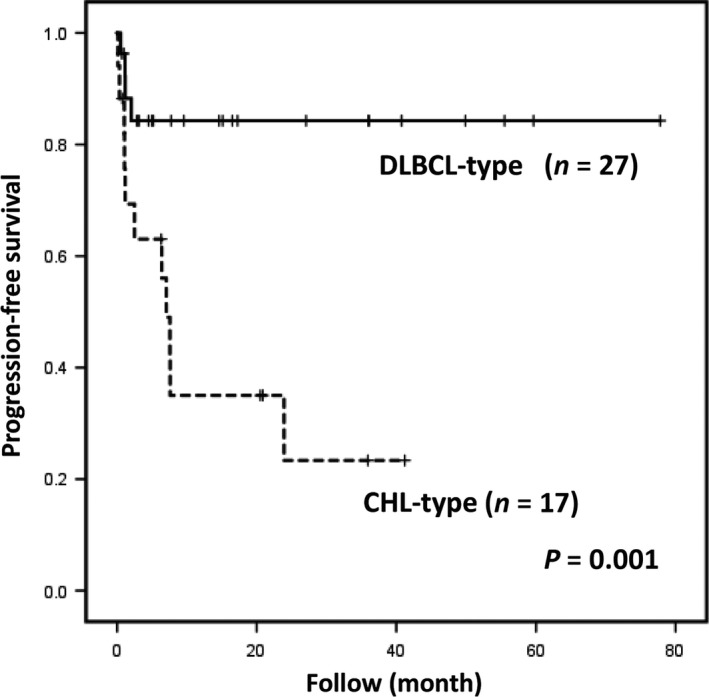
Progression‐free survival of patients with diffuse large B‐cell lymphoma‐type or classical Hodgkin lymphoma (CHL)‐type methotrexate‐associated lymphoproliferative disorders (MTX‐LPD). Patients with CHL‐type MTX‐LPD required additional chemotherapy because of no response to the discontinuation of methotrexate.
Overall survival analysis of patients with MTX‐LPD
The median overall survival did not differ significantly between the two MTX‐LPD subtypes (P > 0.05; Fig. 4). The two patients diagnosed at autopsy were also excluded from this analysis.
Figure 4.
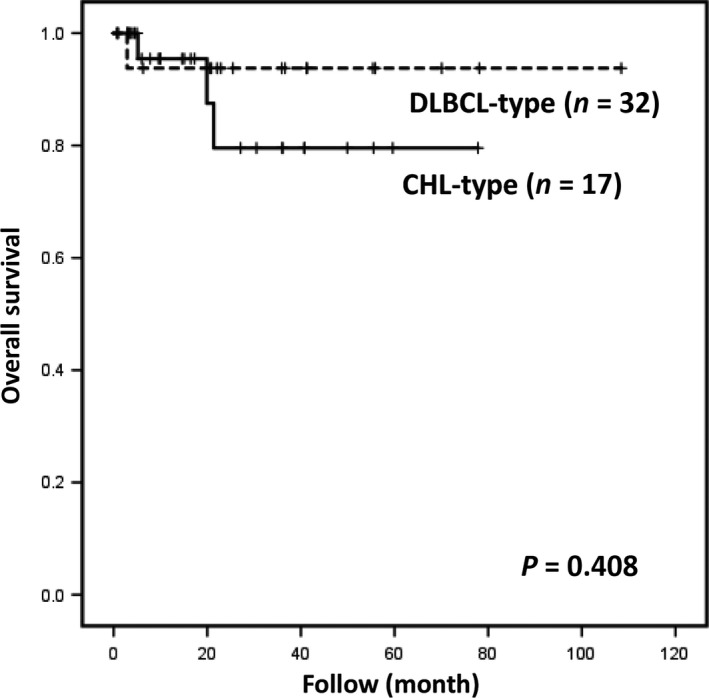
Overall survival of patients with diffuse large B‐cell lymphoma‐type or classical Hodgkin lymphoma‐type methotrexate‐associated lymphoproliferative disorders. The difference in overall survival was not statistically significant (P = 0.408).
The median follow‐up durations were 16.9 and 36.0 months for patients with a DLBCL‐type and CHL‐type MTX‐LPD, respectively.
Discussion
In this study, we investigated the clinicopathological differences between patients with DLBCL‐type (n = 34) and CHL‐type (n = 17) MTX‐LPD. Overall, more female patients developed MTX‐LPD, which might be related to the fact that RA is more common among women.
In previous studies,2, 3, 7, 9, 10 lesion reduction was observed in 25–60% of the total number of patients after MTX was discontinued, and subsequent regrowth was reported in 18–45% of patients. Comparatively, lesion reduction was observed in 34 patients (77%) in the present study, and regrowth was confirmed in eight patients (24%). Of the eight patients with tumor regrowth, only the patient with a DLBCL‐type MTX‐LPD died. The overall survival rates were comparable between the DLBCL‐type and CHL‐type MTX‐LPD and despite a high CS the overall survival rates were good. These results were consistent with those of previous reports.7, 9, 11 The immune deficiency owing to MTX administration helps to explain these findings, and the clinical course of the DLBCL‐type MTX‐LPD differs from that of non‐MTX‐associated DLBCL. In the present study, the median follow‐up durations of the DLBCL‐type and CHL‐type MTX‐LPD were 16.9 and 36.0 months, respectively. In several previously published reports,10 the mean follow‐up duration of MTX‐LPD patients was 24.3 (range, 8–60) months. Given that there were patients who suffered a relapse after remission was achieved by the discontinuation of MTX, we think that further long‐term observations are required.
In this study, the majority of the 51 patients had a high CS; the CS ≥ 3 in 25 patients (74%) with DLBCL‐type MTX‐LPD and 13 patients (76%) with CHL‐type MTX‐LPD. In addition, a large proportion of patients had extranodal lesions. Of the 17 patients with CHL‐type MTX‐LPD, seven patients (41%) had extranodal lesions in organs and tissues, such as the brain, lungs, kidneys, liver, and bone marrow. Non‐MTX‐associated CHL is usually localized, most frequently involving the lymph nodes of the cervical and mediastinal regions, and primary extranodal involvement is rare.12 However, iatrogenic immunodeficiency‐associated CHL in patients with a high CS has frequently been reported.13 Because the high CS and extranodal lesions with CHL‐type MTX‐LPD differ from the clinical findings of non‐MTX‐associated CHL, they are considered characteristic of CHL‐type MTX‐LPD.
Lesion regression as a result of simply discontinuing MTX treatment occurs more frequently in EBER‐positive cases than EBER‐negative cases.4, 14, 15 Since the majority of both DLBCL‐type and CHL‐type MTX‐LPD cases were EBER‐positive, we were unable to compare the prognoses of EBER‐positive and EBER‐negative patients. However, the EBER‐positive rate for the DLBCL‐type MTX‐LPD was higher (82%) than that reported previously (40–60%).3, 4, 7, 9, 11, 15
EBV infection usually exhibits a type II latency pattern. Among the CHL‐type MTX‐LPD cases in the present study, six cases exhibited a type II latency pattern, while two cases exhibited a type I latency pattern. Although we cannot explain the reason for the type I latency pattern, similar results were reported by Loo et al.13, and the cause may be related to the MTX‐LPD. We could not analyze the type of EBV latent infection in DLBCL‐type MTX‐LPD patients because of sample limitations. However, according to previous reports,6, 7 the EBV latency pattern of the DLBCL‐type MTX‐LPD may be identical to that of the CHL‐type MTX‐LPD.
According to the 4th edition of the World Health Organization classification system,2 the immunophenotype of iatrogenic immunodeficiency‐associated LPD does not appear to differ from that of comparable histological subtypes of lymphoma in non‐immunosuppressed hosts.2 Five patients with CHL‐type MTX‐LPD (29%) in the present study were CD20‐positive. This finding is frequently observed in CHL‐type MTX‐LPD.2, 16
Regarding IGH gene rearrangements, monoclonality of IGH is frequently observed in DLBCL‐type MTX‐LPD, and MTX‐LPD patients with monoclonality had an unfavorable prognosis in a previous study.7 Interestingly, however, although all DLBCL‐type MTX‐LPD cases exhibited monomorphic proliferation in the present study, of the 36 patients for whom IGH gene rearrangements were analyzed, only two DLBCL‐type MTX‐LPD cases exhibited monoclonality, with the majority of cases exhibiting polyclonal multiplication. Therefore, although we were unable to compare prognoses, the lesions in the two cases with monoclonal rearrangement were not reduced after discontinuing MTX treatment. In the present study, the EBER‐positive rate was high and the IGH monoclonality rate was low. The prognoses may have been good because of the high EBER‐positive rate.
There was a significant difference in the number of patients who experienced remission after discontinuing MTX treatment between DLBCL‐type and CHL‐type MTX‐LPD. Immunomodulatory agents were administered in combination with MTX in nine patients (DLBCL‐type MTX‐LPD [n = 5] and CHL‐type MTX‐LPD [n = 4]). The difference between the frequencies of administration of immunomodulatory agents was not significant (P > 0.05). Of the 27 DLBCL‐type MTX‐LPD patients who discontinued MTX, 22 patients (81%) showed remission. In contrast, discontinuing MTX treatment for CHL‐type MTX‐LPD patients was ineffective for disease control in 13 patients (76%) who then required additional chemotherapy. Chemotherapy resulted in a good response. Additional chemotherapy was required for iatrogenic immunodeficiency‐associated Hodgkin lymphoma patients in previous case reports.3, 13, 15, 16, 17, 18, 19, 20, 21 In addition, the progression‐free survival was significantly shorter in CHL‐type MTX‐LPD patients than DLBCL‐type MTX‐LPD patients.
A CHL‐type MTX‐LPD patient presented with a localized gingival lesion. Although this lesion may be considered a mucocutaneous ulcer, this patient exhibited tumor regrowth after lesion reduction and required additional chemotherapy.
In conclusion, the histological subtype of MTX‐LPD (DLBCL‐type vs CHL‐type) may represent an important factor for spontaneous or non‐spontaneous regression after the discontinuation of MTX treatment.
Disclosure Statement
The authors have no potential conflicts of interest to report.
Acknowledgments
This work was partially supported by a Grant‐in‐Aid for Scientific Research (C) (JSPS KAKENHI Grant No.: JP16K08666) from the Japan Society for the Promotion of Science.
Cancer Sci 108 (2017) 1271–1280
Funding Information
This work was partially supported by a Grant‐in‐Aid for Scientific Research (C) (JSPS KAKENHI Grant No.: JP16K08666) from the Japan Society for the Promotion of Science.
References
- 1. Ellman MH, Hurwitz H, Thomas C, Kozloff M. Lymphoma developing in a patient with rheumatoid arthritis taking low dose weekly methotrexate. J Rheumatol 1991; 18: 1741–3. [PubMed] [Google Scholar]
- 2. Gaulard P, Swerdlow S, Harris N, Jaffe E, Sundström C. Other iatrogenic immunodeficiency‐associated lymphoproliferative disorders In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press, 2008; 350–1. [Google Scholar]
- 3. Hoshida Y, Xu JX, Fujita S et al Lymphoproliferative disorders in rheumatoid arthritis: clinicopathological analysis of 76 cases in relation to methotrexate medication. J Rheumatol 2007; 322–31. [PubMed] [Google Scholar]
- 4. Kojima M, Itoh H, Hirabayashi K et al Methtrexate‐associated lymphoproliferative disorders. A clinicopathological study of 13 Japanese cases. Pathol Res Pract 2006; 202: 679–85. [DOI] [PubMed] [Google Scholar]
- 5. Salloum E, Cooper DL, Howe G et al Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rheumatoid arthritis and other rheumatic diseases. J Clin Oncol 1996; 14: 1943–9. [DOI] [PubMed] [Google Scholar]
- 6. Miyazaki T, Fujimaki K, Shirasugi Y et al Remission of lymphoma after withdrawal of methotrexate in rheumatoid arthritis: relationship with type of latent Epstein‐Barr virus infection. Am J Hematol 2007; 82: 1106–9. [DOI] [PubMed] [Google Scholar]
- 7. Ichikawa A, Arakawa F, Kiyasu J et al Methotrexate/iatrogenic lymphoproliferative disorders in rheumatoid arthritis: histology, Epstein‐Barr virus, and clonality are important predictors of disease progression and regression. Eur J Haematol 2013; 91: 20–8. [DOI] [PubMed] [Google Scholar]
- 8. van Dongen JJ, Langerak AW, Bruggemann M et al Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T‐cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED‐2 Concerted Action BMH4‐CT98‐3936. Leukemia 2003; 17: 2257–317. [DOI] [PubMed] [Google Scholar]
- 9. Niitsu N, Okamoto M, Nakamine H, Hirano M. Clinicopathologic correlations of diffuse large B‐cell lymphoma in rheumatoid arthritis patients treated with methotrexate. Cancer Sci 2010; 101: 1309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rizzi R, Curci P, Delia M et al Spontaneous remission of “methotrexate‐associated lymphoproliferative disorders” after discontinuation of immunosuppressive treatment for autoimmune disease. Review of the literature. Med Oncol 2009; 26: 1–9. [DOI] [PubMed] [Google Scholar]
- 11. Tokuhira M, Watanabe R, Nemoto T et al Clinicopathological analyses in patients with other iatrogenic immunodeficiency‐associated lymphoproliferative diseases and rheumatoid arthritis. Leuk Lymphoma 2012; 53: 616–23. [DOI] [PubMed] [Google Scholar]
- 12. Stein H, Desol G, PiLeri SA, Weiss LM, Poppema S, Jaffe R. Classsical Hodgkin lymphoma In: Stein H, Desol G, Pileri SA, Weiss LM, Poppema S, Jaffe R, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press, 2008; 326–9. [Google Scholar]
- 13. Loo EY, Medeiros LJ, Aladily TN et al Classical Hodgkin lymphoma arising in the setting of iatrogenic immunodeficiency: a clinicopathologic study of 10 cases. Am J Surg Pathol 2013; 37: 1290–7. [DOI] [PubMed] [Google Scholar]
- 14. Yamakawa N, Fujimoto M, Kawabata D et al A clinical, pathological, and genetic characterization of methotrexate‐associated lymphoproliferative disorders. J Rheumatol 2014; 41: 293–9. [DOI] [PubMed] [Google Scholar]
- 15. Mariette X, Cazals‐Hatem D, Warszawki J, Liote F, Balandraud N, Sibilia J. Lymphomas in rheumatoid arthritis patients treated with methotrexate: a 3‐year prospective study in France. Blood 2002; 99: 3909–15. [DOI] [PubMed] [Google Scholar]
- 16. Kamel OW, Weiss LM, van de Rijn M, Colby TV, Kingma DW, Jaffe ES. Hodgkin's disease and lymphoproliferations resembling Hodgkin's disease in patients receiving long‐term low‐dose methotrexate therapy. Am J Surg Pathol 1996; 20: 1279–87. [DOI] [PubMed] [Google Scholar]
- 17. Aksu K, Donmez A, Ertan Y et al Hodgkin's lymphoma following treatment with etanercept in ankylosing spondylitis. Rheumatol Int 2007; 28: 185–7. [DOI] [PubMed] [Google Scholar]
- 18. Ferraccioli GF, Casatta L, Bartoli E et al Epstein‐Barr virus‐associated Hodgkin's lymphoma in a rheumatoid arthritis patient treated with methotrexate and cyclosporin A. Arthritis Rheum 1995; 38: 867–8. [DOI] [PubMed] [Google Scholar]
- 19. Hasserjian RP, Chen S, Perkins SL et al Immunomodulator agent‐related lymphoproliferative disorders. Mod Pathol 2009; 22: 1532–40. [DOI] [PubMed] [Google Scholar]
- 20. Imundo L. Hodgkin's lymphoma associated with anti‐TNF use in juvenile idiopathic arthritis: supplemental case report. J Rheumatol 2008; 38 (8): 1681. [PubMed] [Google Scholar]
- 21. Yildirim‐Toruner C, Kimura Y, Rabinovich E. Hodgkin's lymphoma and tumor necrosis factor inhibitors in juvenile idiopathic arthritis. J Rheumatol 2008; 38 (8): 1680–1. [PubMed] [Google Scholar]


