Abstract
Programmed death‐ligand 1 (PD‐L1) plays a crucial role in the host immune system in cancer progression. The gene promoter region of PD‐L1 also contains a binding site for ZEB1, a transcription factor related to epithelial‐mesenchymal transition (EMT). The purpose of this study was to clarify the relationship between PD‐L1 and EMT and its clinical importance in esophageal squamous cell carcinoma (ESCC). PD‐L1 and ZEB1 expression at the tumor invasive front was examined by immunohistochemistry in resected specimens from 90 patients with ESCC who underwent surgery without preoperative therapy, and their expression and clinicopathological factors were compared. ZEB1 and PD‐L1 expression was determined in TE8 cells, which demonstrate the EMT phenotype, following ZEB1 knockdown by siZEB1. TE5, TE6 and TE11 cells with non‐EMT phenotype were also used for studies of TGF‐β1‐dependent EMT induction and ZEB1 and PD‐L1 expression. In cases of high PD‐L1 expression at the invasive front, significantly greater depth of tumor invasion, EMT, and less CD8+ lymphocyte infiltration were observed. High PD‐L1 expression was also associated with worse overall and relapse‐free survival. A correlation was observed between PD‐L1 and ZEB1 expression. In TE8 cells, siZEB1 suppressed PD‐L1 and promoted E‐cadherin mRNA and protein expression. TGF‐β1 induced EMT and surface expression of PD‐L1 in TE5, TE6 and TE11 cell lines. PD‐L1 expression at the ESCC invasive front was related to ZEB1 expression, EMT and poor prognosis. We suggest that a cooperative mechanism bridging between tumor immune avoidance and EMT contributes to tumor malignancy in ESCC.
Keywords: Epithelial‐mesenchymal transition, esophageal cancer, immune system, malignant potential, tumor invasion
Esophageal cancer is the eighth most common cancer world‐wide and ranks sixth among all cancers in mortality.1 Esophageal cancer has two major histological types: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma. ESCC is the dominant type in Asia.2, 3 In Japan, ESCC remains the predominant type among all esophageal cancers; the ratio of squamous cell carcinoma to adenocarcinoma is 26:1.3
Programmed death 1 (PD‐1), an inhibitory costimulatory molecule, is induced on activated T cells, B cells, and NK cells, and plays a crucial role in regulating peripheral tolerance. Two PD‐1 ligands have been identified, programmed death ligand 1 (PD‐L1, also known as CD274) and programmed death ligand 2 (PD‐L2); both are members of the B7 family.4, 5, 6 PD‐L1 is expressed by various human tumors and plays an essential role in evasion of the host immune system in cancer.7, 8 A high level of PD‐L1 expression in severe malignancies has been associated with a poor prognosis.9, 10, 11, 12 Current clinical trials show that anti‐PD‐1 and anti‐PD‐L1 therapies that inhibit the interaction between PD‐1 and PD‐L1 show potential for the treatment of cancer patients by generating measurable clinical activity with minimal toxicities.13, 14
Epithelial‐mesenchymal transition (EMT), a process whereby epithelial cells lose their cell polarity and cell–cell adhesion ability and acquire migratory and invasive properties to gain mesenchymal phenotype, is important for invasion and metastasis of carcinoma.15 In the case of many cancers, EMT‐inducing signals emanating from the tumor‐associated stroma, including those mediated by HGF, EGF, PDGF, and TGF‐β, appear to be responsible for the induction or functional activation in cancer cells of a series of EMT‐inducing transcription factors, including Snail, Slug, zinc finger E‐box binding homeobox 1 (ZEB1), Twist, and FOXC2.16, 17, 18, 19, 20
We previously reported that PD‐L1 expression is correlated with malignancy and prognosis in ESCC.21 However, the mechanism and pattern of PD‐L1 expression in ESCC has not been fully revealed. A recent study reported that TGF‐β1 induced ZEB1.22, 23 Because the PD‐L1 gene promoter region contains a ZEB1 binding site, we speculated that PD‐L1 expression might be regulated by the ZEB1 transcription factor. The aim of this study was to clarify the relationships between PD‐L1 expression and EMT, and clinicopathological features in ESCC.
Materials and Methods
Patients
This study included 90 patients (mean age 62.7 years) who had undergone esophageal resection for ESCC without preoperative treatment at the Department of Surgery and Science, Kyushu University Hospital, between April 1997 and March 2005. The study was approved by the Ethics Committee of Kyushu University (Approval Number: 27‐397).
Immunohistochemistry and evaluation of staining
Tumor sections were assessed by immunohistochemistry (IHC) using rabbit polyclonal antibodies against PD‐L1 (1:200; Lifespan Bioscience, Seattle, WA, USA), IgG mouse monoclonal antibodies against ZEB1 (1:150; Origene, Rockville, MD, USA), CD8 (1:100; DAKO, Santa Clara, CA, USA), and E‐cadherin (1:1000; TAKARA BIO, Siga, Japan). Briefly, 4‐μm sections were deparaffinized in xylene and dehydrated in an ethanol series. For antigen retrieval, the specimens were pretreated in an autoclave for PD‐L1 (121°C, 10 min, in 0.01 M citrate buffer; pH 6.0) and ZEB1 (121°C, 20 min, in Target EDTA, pH 9.0), and CD8 (121°C, 20 min, in 0.01 M citrate buffer; pH 6.0), E‐cadherin (121°C, 15 min, in 0.01 M citrate buffer; pH 6.0). The sections were incubated for 30 min in 0.3% hydrogen peroxidase in absolute methanol to deactivate endogenous peroxidases. After blocking nonspecific binding of antibodies, the specimens were incubated at room temperature with primary antibodies against PD‐L1, ZEB1, CD8, and E‐cadherin overnight. IHC staining was performed using an EnVision system and DAB kits (DAKO). The levels of expression of PD‐L1, ZEB1, CD8, and E‐cadherin were evaluated by two investigators, including one general pathologist. The expression of PD‐L1 was evaluated according the staining of the cell membrane and/or in the cytoplasm of tumor cells (TCs) at tumor invasive front as previously reported.10 We evaluated the tumor invasive front at the deepest layer of tumor, based on findings of hematoxylin‐eosin staining. In this study, PD‐L1 positivity was defined as ≥5% of TCs staining for PD‐L1 by IHC, as described in several previous reports.24, 25, 26, 27 The expression of ZEB1 was evaluated by counting positive staining of the cell nucleus of TCs at the same invasive front area as PD‐L1. The total numbers of ZEB1‐positive TCs were counted in three high power fields (3HPFs) in the hot spot areas and we calculated ZEB1 positive TCs/all TCs. The number of CD8+ tumor‐infiltrating lymphocytes were counted in 3HPFs and scored as low or high. In this study, the cut‐off value for ZEB1 and CD8 was set using the average of expression for each; 6.9% for ZEB1 and 50 CD8+ cells per typical HPF for CD8. Although previous studies have used immunohistochemistry to examine the expression levels of ZEB128, 29 and CD8,30, 31, 32 precise cutoffs for expression analyses have not been found to be constant. In our report, we decided to set the average of expression in the 90 total cases as the cutoff values. E‐cadherin was evaluated as followed: TCs with membranes that stained as strongly as normal epithelial cells membrane were considered to have preserved expression, whereas those that exhibited weaker staining patterns than normal epithelial cells or did not stain at all were considered to have reduced expression, as previously described.33
Cell culture and transfection assay
We used TE5, TE6, TE8, and TE11 human cell lines derived from poorly, well, moderately, and moderately ESCC, respectively. Cells were maintained in RPMI‐1640 medium plus 20% fetal bovine serum (FBS) in a humidified atmosphere containing 5% CO2 at 37°C. TE5, TE6 and TE11 cell lines had epithelial characteristics and the TE8 cell line had EMT characteristics.
For siRNA transfections, TE8 cells were seeded in 6‐well plate (0.25 × 106 cells per well) and transfected with 30 pmol of siRNA using Lipofectamine RNAimax reagent (Thermo Fisher Scientific, Waltham, MA, USA) for 48 h, according to the procedure provided by the manufacturer. The siRNAs specific for ZEB1 were Stealth siRNAs named HSS110548 and HSS186235 (referred as ZEB1‐1 and ZEB1‐2, respectively) (Thermo Fisher Scientific). The siRNAs specific for Twist and Snail were HSS144372 and HSS186975 (referred as Twist‐1 and Twist‐2, respectively) and HSS143995 and HSS143996 (referred as Snail‐1 and Snail‐2, respectively). Stealth RNAi siRNA negative control duplex was used as nontargeting (NT) siRNA. In TE8 cells, IFN‐γ (R&D Systems, Minneapolis, MN, USA) was used at 5 ng/mL.
For TGF‐β1 treatment, TE5, TE6, and TE11 cells were seeded in a 6‐well plate (0.25 × 106 cells per well) and cells were treated for 96 h with recombinant TGF‐β1 (Invitrogen, Carlsbad, CA, USA) at a final concentration of 20 ng/mL.
Quantitative real‐time PCR
We determined mRNA expression levels with TaqMan qPCR. Total RNA were synthesized into cDNA using the SuperScript III First‐strand Synthesis SuperMix (Invitrogen) according to the manufacturer's instructions. Real‐time PCR was performed using the StepOnePlus (Applied Biosystems, Foster City, CA, USA). Reactions were run in three independent experiments. The geometric mean of the housekeeping gene β‐actin was used as an internal control to normalize the variability in expression levels.
Western blot analysis
Cells cultured in 6‐well dishes were scraped into 300 μL ice‐cold RIPA buffer (Nacalai Tesque, Kyoto, Japan). Samples were clarified by centrifugation at 12 000 g for 30 min at 4°C. We used the iBind Western System (Invitrogen) and performed imaging using the Amersham Imager600 (GE Healthcare, Little Chalfont, UK). Primary antibodies included antibodies against β‐actin (Cell Signaling Technologies, Danvers, MA, USA, 1:1000), ZEB1 (Origene, clone 3G6, 1:1000), E‐cadherin (Cell Signaling Technologies, 1:1000), and Vimentin (Cell Signaling Technologies, 1:1000).
Flow cytometry analysis
Cells were stained with mouse monoclonal antibodies to human PD‐L1 (Biolegend, San Diego, CA, USA) and ZEB1 (Novusbio, Littleton, CO, USA), and mouse igG2b, κ (Biolegend) and IgG1 (Novusbio) were used as isotype controls, respectively. For cell membrane staining, cells were incubated with PD‐L1 antibody and isotype control for 30 min at 4°C. We added 7‐aminoactinomycin D (Thermo Fisher Scientific) before analysis. For intracellular staining, cells were fixed with Fixation and Permeabilization Solution (BD Biosciences, Franklin, MA, USA) and treated according to the manufacturer's instructions. Briefly, cells were fixed for 20 min at 4°C, washed two times with Perm/Wash buffer (BD Biosciences). Cells were stained with ZEB1 antibody and isotype control for 30 min at 4°C. Cells were washed with Perm/Wash buffer and re‐suspended with FACS buffer. The fluorescence data were collected using the Cell Sorter SH800 (Sony, Tokyo, Japan), and were analyzed using FlowJo software (Tree Star Inc., Ashland, OR, USA).
Statistical analysis
For in vitro studies, quantitative data are presented as means SD (unless indicated otherwise). Differences between two groups were estimated using Student's t‐test and the χ2 test. Kaplan–Meier curves were generated for overall survival and relapse free survival, and statistical significance was determined using the log‐rank test. A probability value of P < 0.05 was considered significant. All statistical analyses were performed using JMP9.0 software (SAS Institute Inc., Cary, NC, USA).
Results
Expression of PD‐L1 at invasive front and correlation with clinicopathological findings and prognostic outcomes
IHC evaluation showed that PD‐L1 was expressed in the cell membrane and cytoplasm of TCs in ESCC specimens (Fig. 1a,b). Among the 90 ESCC cases, positive expression of PD‐L1 at the invasive front was observed in 57 cases (63.3%). High expression of CD8+ lymphocyte infiltration was observed in 40 cases (44%) (Fig. 1c,d). E‐cadherin positive case is shown in Figure 1(e) and ZEB1 positive case is shown in Figure 1(f). In this report, we defined EMT when mesenchymal morphological changes and low E‐cadherin expression were found at the invasive front of tumor. A comparison of clinicopathological features according to PD‐L1 expression is shown in Table 1. In the PD‐L1‐positive expression group, lymph node metastasis and lymphatic invasion tended to be more frequent (P = 0.0906 and P = 0.0815, respectively). Greater depth of tumor invasion (P = 0.0021), EMT and less CD8+ lymphocyte infiltration were also more frequent (P = 0.0013 and P = 0.0053, respectively) in the PD‐L1‐positive group (Table 1).
Figure 1.
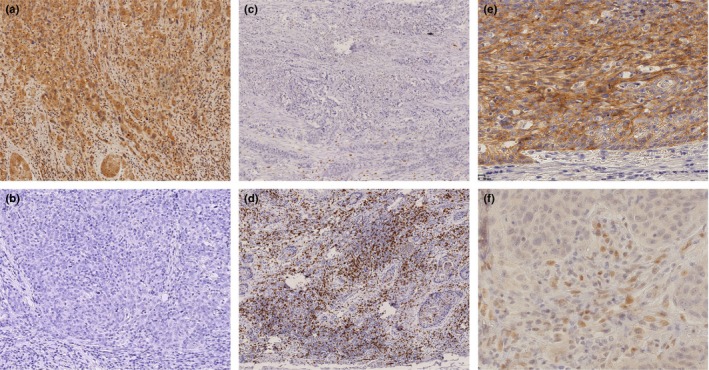
Expression of PD‐L1, ZEB1, E‐cadherin, and CD8 at the invasive front of esophageal squamous cell carcinoma. Representative images of immunohistochemical staining with anti‐PD‐L1 (a: positive, b: negative), anti‐CD8 (c: low, d: high), anti‐E‐cadherin (e: high), and anti‐ZEB1 (f: high) at the tumor invasive front. Magnification: (a, b, c, d) ×100, (e, f) × 400.
Table 1.
Clinicopathological features according to PD‐L1 expression in patients with esophageal squamous cell carcinoma who underwent esophagectomy
| Factor | PD‐L1 negative (n = 33) | PD‐L1 positive (n = 57) | P‐value | |
|---|---|---|---|---|
| Age (mean) | 62.1 | 63.0 | 0.4382 | |
| Sex (male/female) | ||||
| Male | 31 (93.9) | 49 (85.9) | 0.2460 | |
| Female | 2 (6.1) | 8 (14.1) | ||
| Differentiation of squamous cell carcinoma | ||||
| Well/moderately | 27 (81.8) | 46 (80.7) | 0.8960 | |
| Poorly | 6 (18.2) | 11 (19.3) | ||
| Pathological depth of tumor invasion | ||||
| T < 3 | 26 (78.7) | 26 (45.6) | 0.0021 | |
| T ≥ 3 | 7 (21.3) | 31 (54.3) | ||
| Pathological lymph node metastasis | ||||
| pN(−) | 20 (60.6) | 24 (42.1) | 0.0906 | |
| pN(+) | 13 (39.4) | 33 (57.9) | ||
| ly | (−) | 19 (57.5) | 22 (38.5) | 0.0815 |
| (+) | 14 (42.5) | 35 (61.5) | ||
| v | (−) | 24 (72.7) | 37 (65.0) | 0.4446 |
| (+) | 9 (27.3) | 20 (35.0) | ||
| EMT | (−) | 26 (78.7) | 25 (43.8) | 0.0013 |
| (+) | 7 (21.3) | 32 (56.2) | ||
| CD8 | Low | 12 (36.4) | 38 (66.7) | 0.0053 |
| High | 21 (63.6) | 19 (33.3) | ||
| ZEB1 | Low | 24 (72.7) | 29 (50.9) | 0.0397 |
| High | 9 (27.3) | 28 (49.1) | ||
The numbers in parentheses indicate percentages.
EMT, epithelial mesenchymal transition; ly, lymphatic permeation; PD‐L1, programmed death‐ligand 1; v, venous permeation.
In prognostic analysis, PD‐L1‐positive cases were associated with worse overall survival rate (OS) and relapse‐free survival (RFS) rates. The 5‐year OS rate in the patients with PD‐L1 positive expression was significantly poorer than those with negative expression (39.2% vs 67.0%, P = 0.0112). The 5‐year RFS rate in the patients with PD‐L1 positive expression was also worse than those with negative expression (22.4% vs 57.8%, P = 0.0040) (Fig. 2a,b). Univariate and multivariate analyses of RFS and OS in all patients are shown in Table S1. In multivariate analyses, ly (P = 0.032) and PD‐L1 (P = 0.015) were multivariate prognostic factors in RFS, and pathological lymph node metastasis (P = 0.028) was a multivariate prognostic factor in OS.
Figure 2.

Prognosis according to PD‐L1 and ZEB1 expression in patients with esophageal squamous cell carcinoma. (a) Overall and (b) relapse free survival rates of patients with esophageal squamous cell carcinoma in relation to PD‐L1 expression status. (c) Overall and (d) relapse free survival rates of patients in relation to PD‐L1 and ZEB1 expression status.
Correlation between ZEB1 expression and PD‐L1 expression
ZEB1 expression was evaluated by counting positive staining of the cell nucleus of TCs at the same invasive front area as PD‐L1 (Fig. 1f). High expression of ZEB1 was observed in 37 cases (41.1%). A comparison of clinicopathological features according to ZEB1 is shown in Table S2. ZEB1 high expression was related to depth of tumor invasion (P = 0.005) and EMT (P = 0.0006). In prognostic analysis, ZEB1 high expression was associated with worse OS and tended to be associated with worse RFS. The 5‐year OS rate in the patients with ZEB1 high and low expression was 36.2% and 60.1%, respectively (P = 0.0271). The 5‐year RFS rate in patients with ZEB1 high and low expression was 32.0% and 38.3%, respectively (P = 0.1836) (Fig. S1). In the ZEB1 high expression group, PD‐L1 positive and negative expression was observed in 76% and 24% of patients, respectively, indicating a positive correlation between ZEB1 and PD‐L1 expression (P = 0.0397) (Table 1). We divided patients into four groups according to expression of PD‐L1 and ZEB1, and the patient group with PD‐L1 positive and ZEB1 high expression showed the worst prognosis in OS (P = 0.0240) and RFS (P = 0.0328) (Fig. 2c,d).
Suppression of PD‐L1 expression by siRNA for ZEB1
We measured PD‐L1, ZEB1, E‐cadherin, Vimentin, and TGF‐β1 mRNA expression and protein expression in TE5, TE6, TE8 and TE11 cell lines (Fig. 3a–e). We also measured ZEB1, E‐cadherin, and Vimentin protein expression by WB (Fig. 3f). The levels of PD‐L1 mRNA expression were almost same in all cell lines (data not shown). ZEB1 and TGF‐β1 mRNA expression were higher in TE8 cells than the other cell lines. TE8 cells exhibited an EMT characteristic, spindle‐like mesenchymal morphology with high expression of the mesenchymal marker Vimentin and low expression of the epithelial marker E‐cadherin. In contrast, TE5, TE6, and TE11 cells exhibited epithelial characteristic, cobblestone‐like epithelial morphology with high expression of the epithelial marker E‐cadherin and low expression of the mesenchymal marker Vimentin.
Figure 3.
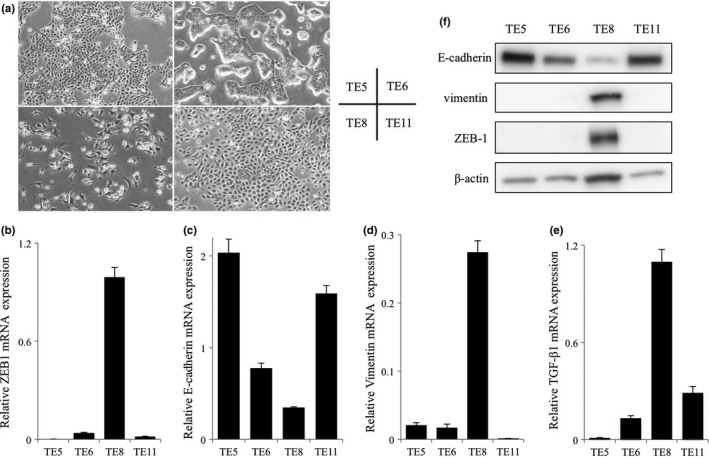
Characteristics of the esophageal squamous cell carcinoma cell lines. (a) Morphological features of TE5, TE6, TE8 and TE11 cells. TE5, TE6 and TE11 exhibited mesenchymal‐epithelial transition features; TE8 exhibited epithelial‐mesenchymal transition features. (b–e) Analysis of ZEB1, E‐cadherin, Vimentin and TGF‐β1 mRNA expression in TE5, TE6, TE8 and TE11 cells by quantitative real‐time PCR. (f) Western blotting analysis of ZEB1, E‐cadherin and Vimentin in TE5, TE6, TE8 and TE11 cells.
We next tested whether mRNA expressions of PD‐L1 and E‐cadherin were affected by EMT inducing transcriptional factors ZEB1, Twist and Snail by q‐PCR in the TE8 cell line. We used siRNAs to target ZEB1 (siZEB1‐1, siZEB1‐2), Twist (siTwist‐1, siTwist‐2) and Snail (siSnail1, siSnail‐2). In TE8 cells, siZEB1 effectively suppressed ZEB1 and PD‐L1 mRNA expression and promoted E‐cadherin mRNA expression compared with non‐targeting (NT) siRNA (Fig. 4a–c). On the other hand, siTwist and siSnail suppressed Twist and Snail mRNA expression (*P < 0.001), respectively, but did not suppress PD‐L1 mRNA expression (Fig. S2a–d).
Figure 4.
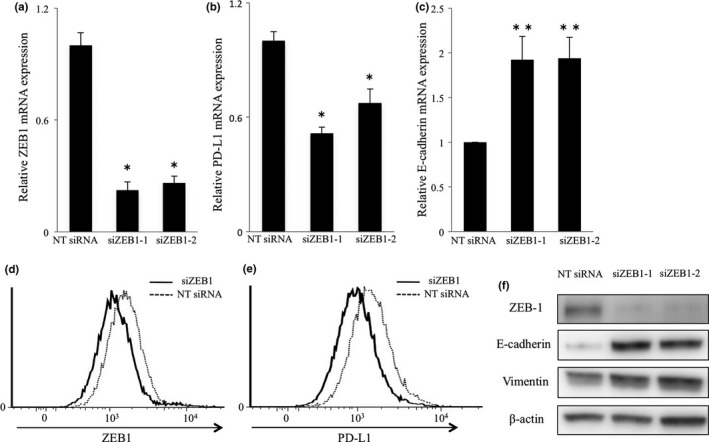
Regulation of PD‐L1 expression by ZEB1 in the TE8 cell line. TE8 cells were transfected by two different siRNAs directed against ZEB1 or nontargeting (NT) siRNA for 48 h and (a) ZEB1, (b) PD‐L1 and (c) E‐cadherin mRNA expression levels were examined by quantitative real‐time PCR. The results are expressed as fold mRNA expression levels of NT siRNA treated cells (arbitrarily defined as 1) (*P < 0.001, **P < 0.05). (d, e) Intracellular expression of ZEB1 (d) and surface expression of PD‐L1 (e) in cells transfected with NT siRNA or siZEB1 and subjected to IFN‐γ stimulation. (f) Western blotting analysis of the indicated proteins in cells transfected with NT siRNA or siRNA targeting ZEB1 and subjected to IFN‐γ stimulation.
We next measured the surface and intracellular expression of PD‐L1 and ZEB1 by FACS. The PD‐L1 surface expression in TE8 cells was low; thus to investigate the influence of ZEB1 on PD‐L1 expression more clearly, TE8 cells transfected by siZEB1 were treated with INF‐γ (5 ng/mL). FACS analysis confirmed that siZEB1 suppressed intracellular expression of ZEB1 (Fig. 4d). Surface expression of PD‐L1 was also suppressed by siZEB1 (Fig. 4e). siZEB1 promoted E‐cadherin expression and did not change vimentin expression (Fig. 4f). These results suggest that ZEB1 regulates PD‐L1 expression in ESCC cell lines.
EMT and PD‐L1 expression induced by TGF‐β1
Transforming growth factor‐β is a potent inducer of EMT in epithelial cancers.34, 35 As mentioned above, TE5, TE6 and TE11 cells show epithelial characteristics. We treated these cells with TGF‐β1 for 96 h and confirmed that their cobblestone‐like epithelial morphology changed to a spindle‐like mesenchymal morphology (Fig. 5a). WB analysis revealed low expression of E‐cadherin, high expression of Vimentin and ZEB1 (Fig. 5b). We measured the surface expression of PD‐L1 by FACS and found that PD‐L1 expression was clearly increased in TE5, TE6 and TE11 cells (Fig. 5c–e). These results revealed that TGF‐β1‐induced ZEB1 expression resulted in EMT phenotype and high surface expression of PD‐L1 in ESCC cell lines having cobblestone‐like epithelial phenotype.
Figure 5.
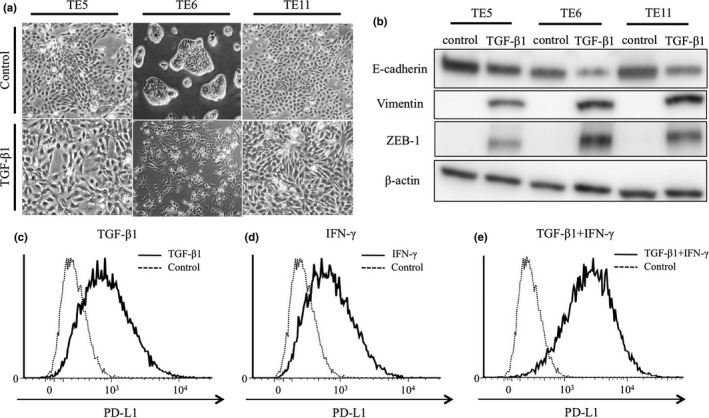
ZEB1 and PD‐L1 expression and morphological changes in esophageal squamous cell carcinoma cell lines induced by TGF‐β1. TE5, TE6 and TE11 cells were treated by TGF‐β1 for 96 h. (a) Morphological changes of TE5, TE6, and TE11 treated by TGF‐β1 for 96 h. (b) Western blot analysis of the indicated proteins in cells treated by TGF‐β1 for 96 h. (c–e) FACS analysis of surface expression of PD‐L1 in TE6 cells co‐treated with TGF‐β1 and IFN‐γ as indicated.
Discussion
Many reports have demonstrated PD‐L1 expression in many types of human cancer.7, 8 However, the mechanism and pattern of PD‐L1 expression in ESCC has been poorly understood. In the present study, patients with PD‐L1 positive expression had significantly poorer prognosis than those with negative expression. A previous study reported that prognosis was not related to PD‐L2 expression in ESCC.36 Our previous study reported that among patients with high expression of HLA class I, high PD‐L1 expression was correlated with significantly poorer RFS and OS in ESCC.21 These results implied that compared with patients with low HLA class I expression, patients with high HLA class I expression might be more likely to be affected by the PD‐1/PD‐L1 interaction that inhibits the CD8 T cell response and permits tumor progression. In the current study, we focused on PD‐L1 expression at the invasive front of ESCC. Our data showed that overexpression at the invasive front was associated with advanced stage and poor survival in ESCC, suggesting that PD‐L1 expression status at the deep invasive site might be a critical predictor of malignant potential of ESCC.
For patients in whom overexpression of tumor PD‐L1 was observed prior to treatment, anti‐PD‐1/PD‐L1 directed therapy could lead to improved clinical outcomes.13 Thus, high PD‐L1 expression may be a predictive marker for efficacy of PD‐1/PD‐L1 directed immune therapy. We evaluated CD8 expression to evaluate the immune response at the invasive front of ESCC. One mechanism that limits the host immune response in cancer tissues is via upregulation of PD‐L1 and the combining of PD‐L1 to PD‐1 on antigen‐specific CD8+ T cells, which are controlled by two mechanisms: innate immune resistance and adaptive immune resistance.7, 37, 38 Several reports showed that the relationships between CD8+ T cell and PD‐L1 expression were either an equilateral correlation or inverse correlation. This controversy may be a result of the differences in fields focused on the histological specimens. In this report, CD8+ T cell infiltration was reversely correlated with PD‐L1 expression, because we focused on the invasive front of cancer tissues. Besides these statistical results, in some cases, high PD‐L1 expression on TCs without EMT changes were also observed to be associated with CD8+ lymphocyte infiltration (data not shown). These findings suggest at least two different pathways for PD‐L1 expression under the control of adaptive immune resistance in ESCC7, 39: one is infiltrating CD8+ lymphocyte‐mediated IFN‐γ‐induced PD‐L1 upregulation, and the other is TGF‐β‐mediated PD‐L1 expression associated with EMT. Our in vitro data showing that both IFN‐γ and TGF‐β upregulated PD‐L1 expression of TE cell lines support this hypothesis. Therefore, adaptive immune resistance around PD‐L1 expression in cancer cells might accelerate tumor progression at the invasive front of ESCC.
Epithelial‐mesenchymal transition is related with metastasis and invasion of cancer.15 In ESCC, EMT status was significantly associated with invasion, metastasis and prognosis.40 We previously reported that the transcriptional factor Ets‐1 upregulates the expression of c‐Met, and consequently confers on cells a highly motile phenotype leading to an EMT‐like form.41, 42 Some studies have reported a relationship between PD‐L1 expression and EMT in various cancers.43, 44, 45 Ock et al. reported that EMT with PD‐L1 expression was an independent upstream pathway distinct from human papilloma virus/p16 association in head and neck squamous cell carcinoma.43 PD‐L1 was reported to function in the promotion of EMT via downregulation of E‐cadherin and upregulation of Slug and Twist in skin epithelial cells.44 Another report showed that the bidirectional effect between EMT status and PD‐L1 expression, especially in the Claudin‐low subtype of breast cancer cells, was mainly dependent on the activation of the PI3K/AKT pathway.45 In this study, we proposed a novel ZEB1‐PD‐L1 pathway and relationship with EMT at the invasive front of ESCC. The ZEB1 transcription factor strongly induces EMT in cancer metastasis, acting through transcriptional repression of E‐cadherin.46, 47 Because the PD‐L1 promoter region contains a ZEB1 binding site, we hypothesized that ZEB1 possibly affected PD‐L1 expression (UCSC Genome Browser).48 We herein report that ZEB1 expression was correlated with PD‐L1 expression at the invasive front of ESCC. In ESCC cell lines, siZEB1 suppressed PD‐L1 expression through the ZEB1‐PD‐L1 pathway and TGF‐β1 inducing EMT. Our data implied that ZEB1 transcription factor is upstream of the PD‐L1 signal pathway and regulates PD‐L1 expression, which simultaneously induces EMT and avoidance of the immune system. Chen et al. reported that microRNA‐200 (miR‐200) targeted PD‐L1 and ZEB1 relieved miR‐200 repression and PD‐L1 on tumor cells leading to CD8+ T‐cell immunosuppression and metastasis in lung cancer.49 Similarly, Noman et al. reported that the selective upregulation of PD‐L1 was dependent on the ZEB1/miR‐200 axis in breast cancer.50 In this article, we designed our study focus based on the PD‐L1 gene promoter region containing a binding site for ZEB1. No report has previously investigated the PD‐L1 gene promoter region for ZEB1 binding so far. We first showed relationship of PD‐L1 and ZEB1 by focusing on the gene promoter region. However, we consider that ZEB1 might have two signal pathways in the tumor microenvironment: one by direct regulation through the gene promoter region, and another by indirect regulation through miR‐200.
Several factors, such as IFN‐γ, TLR, JAK/STAT and viruses, regulate PD‐L1 expression.51 PD‐L1 is induced under inflammatory conditions, triggered by several cytokines, especially IFN‐γ, and exogenous stimuli delivered by pathogen‐associated molecular patterns. Receptor‐mediated signaling molecules that affect the cell cycle, proliferation, apoptosis, and survival (including NF‐κB, MAPK, PI3K, mTOR, and JAK/STAT) are involved in PD‐L1 induction.51 Upregulation of PD‐L1 in immune cells and several cancer cells is heavily dependent on TLR‐ or IFN‐γ‐mediated signaling pathways.51, 52, 53, 54 Several reports have shown that TGF‐β1 induces ZEB1 expression22, 23 and EMT.34, 35 Our data showed that TGF‐β1 induced ZEB1, and subsequently induced PD‐L1 expression in ESCC, which is consistent with several reports showing PD‐L1 induction by TGF‐β.55, 56, 57
In conclusion, PD‐L1 expression at the invasive front was related to ZEB1 expression, EMT and poor prognosis in ESCC. We suggest that a cooperative mechanism between tumor immune avoidance and EMT contributes to tumor malignancy. Whether the ZEB1‐PD‐L1 signal pathway could be a target in treatment for ESCC requires further investigation.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. Prognosis according to ZEB1 expression in the patients with esophageal squamous cell carcinoma. (a) Overall and (b) relapse free survival rates of patients with esophageal squamous cell carcinoma in relation to ZEB1 expression status.
Fig. S2. PD‐L1 expression after siTwist and siSnail transfection. TE8 cells were transfected by siTwist (a, b) and siSnail (c, d) or nontargeting (NT) siRNA for 48 h. Two different siRNAs directed against Twist and Snail were used. (a) Twist, (b) PD‐L1, (c) Snail, and (d) PD‐L1 mRNA expression levels were analyzed by quantitative real‐time PCR. The results are expressed as fold mRNA expression levels of NT siRNA treated cells (arbitrarily defined as 1) (*P < 0.001).
Table S1. Univariate and multivariate analyses of RFS and OS in patients with esophageal squamous cell carcinoma who underwent esophagectomy.
Table S2. Clinicopathological features according to ZEB1 expression in patients with esophageal squamous cell carcinoma who underwent esophagectomy.
Acknowledgments
The authors thank fellowship researcher Koji Teraishi and are grateful for technical assistance from Saori Tsurumaru and Yuko Kubota.
Cancer Sci 108 (2017) 1119–1127
Funding Information
None.
References
- 1. Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol 2013; 19: 5598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst 2008; 100: 1184–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shibata A, Matsuda T, Ajiki W, Sobue T. Trend in incidence of adenocarcinoma of the esophagus in Japan, 1993–2001. Jpn J Clin Oncol 2008; 38: 464–8. [DOI] [PubMed] [Google Scholar]
- 4. Riley JL. PD‐1 signaling in primary T cells. Immunol Rev 2009; 229: 114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okazaki T, Honjo T. PD‐1 and PD‐1 ligands: from discovery to clinical application. Int Immunol 2007; 19: 813–24. [DOI] [PubMed] [Google Scholar]
- 6. Ribas A. Tumor immunotherapy directed at PD‐1. N Engl J Med 2012; 366: 2517–9. [DOI] [PubMed] [Google Scholar]
- 7. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen DS, Irving BA, Hodi FS. Molecular pathways: next‐generation immunotherapy–inhibiting programmed death‐ligand 1 and programmed death‐1. Clin Cancer Res 2012; 18: 6580–7. [DOI] [PubMed] [Google Scholar]
- 9. Ohigashi Y, Sho M, Yamada Y et al Clinical significance of programmed death‐1 ligand‐1 and programmed death‐1 ligand‐2 expression in human esophageal cancer. Clin Cancer Res 2005; 11: 2947–53. [DOI] [PubMed] [Google Scholar]
- 10. Umemoto Y, Okano S, Matsumoto Y et al Prognostic impact of programmed cell death 1 ligand 1 expression in human leukocyte antigen class I‐positive hepatocellular carcinoma after curative hepatectomy. J Gastroenterol 2015; 50: 65–75. [DOI] [PubMed] [Google Scholar]
- 11. Thompson RH, Kuntz SM, Leibovich BC et al Tumor B7‐H1 is associated with poor prognosis in renal cell carcinoma patients with long‐term follow‐up. Cancer Res 2006; 66: 3381–5. [DOI] [PubMed] [Google Scholar]
- 12. Hino R, Kabashima K, Kato Y et al Tumor cell expression of programmed cell death‐1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 2010; 116: 1757–66. [DOI] [PubMed] [Google Scholar]
- 13. Topalian SL, Hodi FS, Brahmer JR et al Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med 2012; 366: 2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brahmer JR, Tykodi SS, Chow LQ et al Safety and activity of anti‐PD‐L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366: 2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thiery JP. Epithelial‐mesenchymal transitions in tumour progression. Nat Rev Cancer 2002; 2: 442–54. [DOI] [PubMed] [Google Scholar]
- 16. Kalluri R, Weinberg RA. The basics of epithelial‐mesenchymal transition. J Clin Invest 2009; 119: 1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Usami Y, Satake S, Nakayama F et al Snail‐associated epithelial‐mesenchymal transition promotes oesophageal squamous cell carcinoma motility and progression. J Pathol 2008; 215: 330–9. [DOI] [PubMed] [Google Scholar]
- 18. Korpal M, Lee ES, Hu G, Kang Y. The miR‐200 family inhibits epithelial‐mesenchymal transition and cancer cell migration by direct targeting of E‐cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem 2008; 283: 14910–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang J, Mani SA, Donaher JL et al Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004; 117: 927–39. [DOI] [PubMed] [Google Scholar]
- 20. Syed V. TGF‐beta signaling in cancer. J Cell Biochem 2016; 117: 1279–87. [DOI] [PubMed] [Google Scholar]
- 21. Ito S, Okano S, Morita M et al Expression of PD‐L1 and HLA class I in esophageal squamous cell carcinoma: prognostic factors for patient outcome. Ann Surg Oncol 2016; 23: 508–15. [DOI] [PubMed] [Google Scholar]
- 22. Joseph JV, Conroy S, Tomar T et al TGF‐beta is an inducer of ZEB1‐dependent mesenchymal transdifferentiation in glioblastoma that is associated with tumor invasion. Cell Death Dis 2014; 5: e1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shirakihara T, Saitoh M, Miyazono K. Differential regulation of epithelial and mesenchymal markers by deltaEF1 proteins in epithelial mesenchymal transition induced by TGF‐beta. Mol Biol Cell 2007; 18: 3533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herbst RS, Soria JC, Kowanetz M et al Predictive correlates of response to the anti‐PD‐L1 antibody MPDL3280A in cancer patients. Nature 2014; 515: 563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brahmer JR, Drake CG, Wollner I et al Phase I study of single‐agent anti‐programmed death‐1 (MDX‐1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28: 3167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taube JM, Anders RA, Young GD et al Colocalization of inflammatory response with B7‐h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4: 127ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ishii H, Azuma K, Kawahara A et al Significance of programmed cell death‐ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thorac Oncol 2015; 10: 426–30. [DOI] [PubMed] [Google Scholar]
- 28. Zhang Y, Liu G, Wu S, Jiang F, Xie J, Wang Y. Zinc finger E‐box‐binding homeobox 1: its clinical significance and functional role in human thyroid cancer. Onco Targets Ther 2016; 9: 1303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Galvan JA, Zlobec I, Wartenberg M et al Expression of E‐cadherin repressors SNAIL, ZEB1 and ZEB2 by tumour and stromal cells influences tumour‐budding phenotype and suggests heterogeneity of stromal cells in pancreatic cancer. Br J Cancer 2015; 112: 1944–50. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30. Soini Y, Tuhkanen H, Sironen R et al Transcription factors zeb1, twist and snai1 in breast carcinoma. BMC Cancer 2011; 11: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mella M, Kauppila JH, Karihtala P et al Tumor infiltrating CD8+ T lymphocyte count is independent of tumor TLR9 status in treatment naive triple negative breast cancer and renal cell carcinoma. Oncoimmunology 2015; 4: e1002726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Faraj SF, Munari E, Guner G et al Assessment of tumoral PD‐L1 expression and intratumoral CD8+ T cells in urothelial carcinoma. Urology 2015; 85: 703. e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Uchikado Y, Natsugoe S, Okumura H et al Slug expression in the E‐cadherin preserved tumors is related to prognosis in patients with esophageal squamous cell carcinoma. Clin Cancer Res 2005; 11: 1174–80. [PubMed] [Google Scholar]
- 34. Pakala SB, Singh K, Reddy SD et al TGF‐beta1 signaling targets metastasis‐associated protein 1, a new effector in epithelial cells. Oncogene 2011; 30: 2230–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu J, Lamouille S, Derynck R. TGF‐beta‐induced epithelial to mesenchymal transition. Cell Res 2009; 19: 156–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leng C, Li Y, Qin J et al Relationship between expression of PD‐L1 and PD‐L2 on esophageal squamous cell carcinoma and the antitumor effects of CD8+ T cells. Oncol Rep 2016; 35: 699–708. [DOI] [PubMed] [Google Scholar]
- 37. Spranger S, Spaapen RM, Zha Y et al Up‐regulation of PD‐L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 2013; 5: 200ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tumeh PC, Harview CL, Yearley JH et al PD‐1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515: 568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ribas A. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov 2015; 5: 915–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu J, Chen L, Deng H et al Epithelial‐to‐mesenchymal transition in human esophageal cancer associates with tumor progression and patient's survival. Int J Clin Exp Pathol 2014; 7: 6943–9. [PMC free article] [PubMed] [Google Scholar]
- 41. Saeki H, Oda S, Kawaguchi H et al Concurrent overexpression of Ets‐1 and c‐Met correlates with a phenotype of high cellular motility in human esophageal cancer. Int J Cancer 2002; 98: 8–13. [DOI] [PubMed] [Google Scholar]
- 42. Saeki H, Kuwano H, Kawaguchi H, Ohno S, Sugimachi K. Expression of ets‐1 transcription factor is correlated with penetrating tumor progression in patients with squamous cell carcinoma of the esophagus. Cancer 2000; 89: 1670–6. [DOI] [PubMed] [Google Scholar]
- 43. Ock CY, Kim S, Keam B et al PD‐L1 expression is associated with epithelial‐mesenchymal transition in head and neck squamous cell carcinoma. Oncotarget 2016; 7: 15901–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cao Y, Zhang L, Kamimura Y et al B7‐H1 overexpression regulates epithelial‐mesenchymal transition and accelerates carcinogenesis in skin. Cancer Res 2011; 71: 1235–43. [DOI] [PubMed] [Google Scholar]
- 45. Alsuliman A, Colak D, Al‐Harazi O et al Bidirectional crosstalk between PD‐L1 expression and epithelial to mesenchymal transition: significance in claudin‐low breast cancer cells. Mol Cancer 2015; 14: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vannier C, Mock K, Brabletz T, Driever W. Zeb1 regulates E‐cadherin and Epcam (epithelial cell adhesion molecule) expression to control cell behavior in early zebrafish development. J Biol Chem 2013; 288: 18643–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sanchez‐Tillo E, Lazaro A, Torrent R et al ZEB1 represses E‐cadherin and induces an EMT by recruiting the SWI/SNF chromatin‐remodeling protein BRG1. Oncogene 2010; 29: 3490–500. [DOI] [PubMed] [Google Scholar]
- 48. Rosenbloom KR, Armstrong J, Barber GP et al The UCSC Genome Browser database: 2015 update. Nucleic Acids Res 2015; 43: D670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen L, Gibbons DL, Goswami S et al Metastasis is regulated via microRNA‐200/ZEB1 axis control of tumour cell PD‐L1 expression and intratumoral immunosuppression. Nat Commun 2014; 5: 5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Noman MZ, Janji B, Abdou A et al The immune checkpoint ligand PD‐L1 is upregulated in EMT‐activated human breast cancer cells by a mechanism involving ZEB‐1 and miR‐200. Oncoimmunology 2017; 6: e1263412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ritprajak P, Azuma M. Intrinsic and extrinsic control of expression of the immunoregulatory molecule PD‐L1 in epithelial cells and squamous cell carcinoma. Oral Oncol 2015; 51: 221–8. [DOI] [PubMed] [Google Scholar]
- 52. Loke P, Allison JP. PD‐L1 and PD‐L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci USA 2003; 100: 5336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu J, Hamrouni A, Wolowiec D et al Plasma cells from multiple myeloma patients express B7‐H1 (PD‐L1) and increase expression after stimulation with IFN‐{gamma} and TLR ligands via a MyD88‐, TRAF6‐, and MEK‐dependent pathway. Blood 2007; 110: 296–304. [DOI] [PubMed] [Google Scholar]
- 54. Qian Y, Deng J, Geng L et al TLR4 signaling induces B7‐H1 expression through MAPK pathways in bladder cancer cells. Cancer Invest 2008; 26: 816–21. [DOI] [PubMed] [Google Scholar]
- 55. Baas M, Besancon A, Goncalves T et al TGFbeta‐dependent expression of PD‐1 and PD‐L1 controls CD8(+) T cell anergy in transplant tolerance. Elife 2016; 5: e08133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Song S, Yuan P, Wu H et al Dendritic cells with an increased PD‐L1 by TGF‐beta induce T cell anergy for the cytotoxicity of hepatocellular carcinoma cells. Int Immunopharmacol 2014; 20: 117–23. [DOI] [PubMed] [Google Scholar]
- 57. Ou JN, Wiedeman AE, Stevens AM. TNF‐alpha and TGF‐beta counter‐regulate PD‐L1 expression on monocytes in systemic lupus erythematosus. Sci Rep 2012; 2: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Prognosis according to ZEB1 expression in the patients with esophageal squamous cell carcinoma. (a) Overall and (b) relapse free survival rates of patients with esophageal squamous cell carcinoma in relation to ZEB1 expression status.
Fig. S2. PD‐L1 expression after siTwist and siSnail transfection. TE8 cells were transfected by siTwist (a, b) and siSnail (c, d) or nontargeting (NT) siRNA for 48 h. Two different siRNAs directed against Twist and Snail were used. (a) Twist, (b) PD‐L1, (c) Snail, and (d) PD‐L1 mRNA expression levels were analyzed by quantitative real‐time PCR. The results are expressed as fold mRNA expression levels of NT siRNA treated cells (arbitrarily defined as 1) (*P < 0.001).
Table S1. Univariate and multivariate analyses of RFS and OS in patients with esophageal squamous cell carcinoma who underwent esophagectomy.
Table S2. Clinicopathological features according to ZEB1 expression in patients with esophageal squamous cell carcinoma who underwent esophagectomy.


