Abstract
Several studies have produced contradictory findings about the prognostic implications for inhibitor of apoptosis proteins (IAP) in different types of cancer. Cellular inhibitor of apoptosis 2 (cIAP2/BIRC) is one of the most extensively characterized human IAP. To date, no studies have focused on the expression level of cIAP2 in human gallbladder cancer (GBC), and the mechanism of cIAP2 in GBC invasion and lymphangiogenesis remains unclear. Therefore, in the present study, cIAP2 expression in GBC was detected using quantitative real‐time polymerase chain reaction and immunohistochemistry, and the relationship between cIAP2 levels in cancer tissues and the clinicopathological characteristics of patients was analyzed. The biological effect of cIAP2 in GBC cells was tested using the Cell Counting Kit‐8 Assay, Transwell assays and the ability of human dermal lymphatic endothelial cells (HDLEC) to undergo tube formation. The role of cIAP2 in activating the NF‐κB pathway was determined using a dual‐luciferase reporter assay, immunofluorescence staining, western blotting and ELISA. Finally, an animal model was used to further confirm the role of cIAP2 in lymphangiogenesis. We showed that cIAP2 expression was elevated in human GBC tissues and correlated with a negative prognosis for patients. Moreover, cIAP2 was identified as a lymphangiogenic factor of GBC cells and, thus, promoted lymph node metastasis in GBC cells. Our study is the first to suggest that cIAP2 can promote GBC invasion and lymphangiogenesis by activating the NF‐κB pathway.
Keywords: Cellular inhibitor of apoptosis 2, gallbladder cancer, invasion, lymphangiogenesis, nuclear factor‐κB
Gallbladder carcinoma (GBC) represents the most common and aggressive type of biliary tree cancers worldwide;1 however, approaches to the treatment of GBC still face enormous challenges, because the average survival of GBC patients from the time of diagnosis is only several months.2 This poor prognosis is partially due to the aggressive biological behavior of GBC and a lack of an efficient systemic therapy.3 Therefore, discovering an effective method to diagnose and treat GBC has become increasingly urgent.
Apoptosis, an important process of programmed cell death, plays a vital role in regulating the dynamic balance between cell survival and cell death to maintain homeostasis.4 Insufficient cell death may threaten tissue homeostasis and result in tumor formation and progression.5 Because tumors often possess the ability to suppress pro‐apoptotic signaling, there is a need to identify key molecules that participate in the process. The inhibitor of apoptosis proteins (IAP) are a family of proteins characterized by the presence of one or more baculoviral IAP repeat (BIR) domains in the N‐terminus6, 7 and, as their name suggests, were originally identified as modulators of caspase function to inhibit apoptosis.8, 9 However, recent literature suggests that IAP also perform a wider variety of functions, such as regulation of tumor migration and metastasis, which has garnered increasing attention.10, 11, 12, 13
Cellular inhibitor of apoptosis 2 (cIAP2) is one of the most extensively characterized of the human IAP, and several studies have described the overexpression of cIAP2 in different tumor types;14, 15, 16 however, no research has been conducted regarding the expression level of cIAP2 in human GBC. Currently, the mechanism of cIAP2 in tumor invasion and lymphangiogenesis is unclear; furthermore, several studies have offered contradictory findings about the prognostic implications for IAP in different types of cancer. There have been reports showing that in patients with non‐small cell lung cancer or renal cell carcinoma, elevated levels of IAP correspond to a positive prognosis;17, 18 however, other reports have shown that overexpression of IAP is associated with a negative prognosis in patients with breast cancer or cervical squamous cell carcinoma.19, 20 These conflicting reports strongly suggest that the prognostic implications of IAP are tumor type‐dependent. What remains unclear is the prognostic implication of cIAP2 in patients with GBC.
The nuclear factor‐kappaB (NF‐κB) pathway is involved in a broad range of biological activities, including cell inflammation, survival and apoptosis.21, 22 Moreover, NF‐κB activation can directly promote tumor survival and metastasis.23, 24 A recent study demonstrated a role of IAP‐mediated regulation of non‐canonical NF‐κB signaling in modulating the migration and invasion of glioblastoma cells in a preclinical tumor model.25 However, much uncertainty still exists about the relationship between cIAP2 and the NF‐κB pathway in human GBC.
In the present study, we showed that cIAP2 expression was elevated in human GBC and was correlated with a negative prognosis for patients. Moreover, cIAP2 was identified as a lymphangiogenic factor of GBC cells and, thus, promoted lymph node metastasis in GBC cells. Our study is the first to demonstrate that cIAP2 may participate in TNF‐α‐induced NF‐κB activation.
Material and Methods
Tissue samples and cell lines
In the present study, 44 GBC tissues and matched non‐tumor tissues were obtained from patients who were admitted to Fujian Medical University Union Hospital in China and provided written informed consent prior to surgical resection. The samples were collected based on a protocol approved by the Ethics Committee of the Medical Faculty of Fujian Medical University in accordance with the Declaration of Helsinki. All of the tumors were confirmed as GBC by the Clinicopathology Department. The human GBC cell lines NOZ (obtained from the Health Science Research Resources Bank in Japan) and SGC‐996 (provided by the Tumor Cytology Research Unit, Medical College, Tongji University, China) were maintained in DMEM (Gibco, USA) supplemented with 10% FBS (Gibco, GrandI Island, NY, USA). Human dermal lymphatic endothelial cells (HDLEC, purchased from ScienCell, San Diego, California, USA) were incubated in endothelial cell medium (ScienCell).
Quantitative real‐time polymerase chain reaction
Total RNA was extracted from the GBC tissues with TRIzol reagent (Life Technologies, Gaithersburg, MD, USA) and was reverse transcribed using the RevertAid First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland). PCR was performed with Fast Start Universal SYBR Green Master Mix (Roche, Basel, Switzerland), and the fluorescence was measured using an ABI 7500 Real‐Time System (Applied Biosystems, Life Technologies) following the manufacturer's instructions, with GAPDH as an internal control. The data were analyzed using the −ΔΔCt method.
Immunohistochemistry
Immunohistochemistry was performed as previously described.26 Primary antibodies specific for cIAP2 (1:400, R&D Systems) were incubated with the sections overnight at 4°C. Images of five random fields per section were obtained under a light microscope (Olympus, Tokyo, Japan). The expression level of cIAP2 was evaluated by semiquantitatively analyzing cIAP2 expression using the following equation: Mean Optical Density (MOD) = Integral Optical Density (IOD)/Positive Area. All image analyses were conducted using Image‐Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA).
Small interference RNA, short hairpin RNA and transfection
Two siRNA targeting the human cIAP2 sequence (GenBank accession number: NM‐001165) were purchased from GenePharma (Shanghai, China). Transfection of siRNA was performed in Opti‐MEM medium (Life Technologies) using Lipofectamine 2000 (Life Technologies).27 Then, synthesized short hairpin RNA oligonucleotides targeting cIAP2 (LV‐shcIAP2) and negative control short hairpin RNA oligonucleotides (LV‐shNC) were annealed and ligated into the pGLVH1/GFP/Puro plasmid. The lentiviral vectors were used to infect GBC cells, after which 2 weeks of puromycin treatment was used to select for stably infected cells.
Cell proliferation assay
The proliferative ability of the cells was assayed using the Cell Counting Kit‐8 (CCK‐8) assay according to the manufacturer's instructions (Dojindo Laboratories, Kumamoto, Japan). Briefly, cells were seeded at 2.5 × 103/well in 96‐well plates and medium was changed every other day. At 24, 36, 48, 60 and 72 h post‐transfection, the cells were treated with CCK‐8 solution reagent (10 μL) for 2 h, and the proliferative rate was measured using a plate reader at 450 nm (Bio‐Rad, Hercules, CA, USA). All conditions were performed in triplicate.
Cell invasion assay
The cell invasion ability was assayed using Transwells (24‐well format) with 8‐μm polycarbonate membranes (Millipore, Washington, DC, USA). Briefly, the sides of the membrane in the upper chamber were coated with Matrigel (20 μg/well) (BD Biosciences, Lake Franklin, NJ, USA) and then air‐dried for 2 h at 37°C. Cells that passed through the membranes were fixed and stained with crystal violet. The number of cells on the lower surface of the membrane was counted in five random fields (400×) using a bright field light microscope. Each condition was repeated in triplicate.
Tube formation assay
The tube formation assay was performed as previously described by Zeng et al.28 Briefly, 7.5 × 103/well of GBC cells and 7.5 × 103/well of HDLEC (labeled with DiI) were seeded into the same Matrigel‐coated well (10 μg/well) of a microwell‐plate (ibidi, Martinsried, Germany). Tube formation of HDLEC was observed using inverted fluorescence microscopy (Nikon, Japan), and images were digitally captured at 1, 3, 5, 8 and 24 h after cell seeding. The total number of tube‐like structures formed in each well was measured using AxioVision Rel 4.1 software (Carl Zeiss AG, Jena, Germany).
Dual‐luciferase reporter assay
Gallbladder cancer cell lines (3 × 105/well in 12‐well plates) were transfected with reporter plasmids encoding 5× NF‐κB‐luc (60 ng) and pEF‐Renilla‐luc (10 ng) either with or without the indicated siRNA using Lipofectamine 2000. After 20 h, the cells were treated with TNF‐α (20 ng/mL) for 15 min,29 cell lysates were prepared, and luciferase activity was measured using a Dual‐Luciferase Assay Kit (Promega, Madison, WI, USA).
Immunofluorescence staining
Cell lines were plated on coverslips in 6‐well culture plates and incubated for 24 h. The cells were then washed, fixed, permeabilized, blocked and stained using an antibody against NF‐κB (p65). Images were observed under a fluorescence microscope (Nikon, Japan).
Western blotting
Western blotting analysis was performed as previously described.30 Anti‐human cIAP2 was obtained from R&D Systems (Minneapolis, MN, USA), anti‐human IκBα was purchased from Santa Cruz (Santa Cruz, CA, USA), anti‐human NF‐κB (p65) was acquired from Cell Signaling Technology (Danvers, MA, USA), and anti‐human VEGF‐C was obtained from Abcam (Cambridge, UK). GAPDH and histone H3 were used as protein markers for the cytosolic and nuclear fractions, respectively.
ELISA
The concentration of VEGF‐C in the cell culture medium was assessed by using double antibody sandwich ELISA with a Quantikine ELISA Kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. The wells were read by a plate reader at 450 nm (Bio‐Rad, Hercules, CA, USA), and each condition was performed in triplicate.
Establishment of an orthotopic xenograft model
Male athymic BALB ⁄c nude mice (4–6 weeks old) were obtained from SLACCAS (Shanghai, China). Thirty‐six nude mice were randomized into 6 groups with 6 mice in each group: an untreated group (NOZ and SGC‐996 cells), the LV‐shNC group (NOZ and SGC‐996 cells) and the LV‐shcIAP2 group (NOZ and SGC‐996 cells). After anesthesia, approximately 4 × 105 GBC cells (10 μL) were mixed with Matrigel (10 μL). The gallbladder was exposed via abdominal midline incision, and the bile in the gallbladder was extruded. Then, 20 μL of the Matrigel cell suspension was slowly injected into the gallbladder with a 29G insulin syringe (BD, Research Triangle Park, North Carolina, USA). The syringe was withdrawn from the gallbladder when the cell suspension became white because of solidification. Four weeks later, the mice were killed by exposure to CO2 and primary tumors were dissected and excised.
Counting of lymphatic vessel
The lymph vessels of GBC specimens were detected by IHC. The primary antibody used in the nude mouse specimens was a goat anti‐mouse LYVE‐1 polyclonal antibody (R&D Systems, Minneapolis, MN, USA). Sections were first examined at low magnification (100×) to identify areas with the most intense staining and the highest density of microvessel (hotspot).31 Three areas of hotspots were selected by three pathologists who independently evaluated the slides for microvessel counting using 400× magnification, without the knowledge of section identities. In the absence of hotspots, five randomly‐selected areas were counted. The largest number of vessels counted was recorded and used in the statistical analysis.
Statistical analysis
The results were presented as the means ± SD. Statistical analyses were performed with SPSS software (version 17.0) (using Fisher's exact test for quantitative data, a t‐test for comparing means between two groups, and a one‐way analysis of variance for comparing means among multiple groups). P < 0.05 was considered statistically significant.
Results
cIAP2 expression is upregulated in gallbladder cancer tissues
To investigate whether the mRNA expression levels of cIAP2 were increased in GBC, we performed quantitative RT‐PCR (qRT‐PCR) in RNA extracted from GBC tissues and matched non‐tumor tissues from 44 GBC patients. The data are shown as the −ΔΔCt values (Fig. 1a) and the 2−ΔCt values (Fig. 1b) and indicate that the average expression levels of cIAP2 were significantly upregulated in GBC tissues compared with matched non‐tumor tissues. To further assess the protein expression of cIAP2 in GBC, IHC was performed and showed that cIAP2 was weakly present in the cytoplasm of normal gallbladder mucosa cells under microscopy (Fig. 1c); however, a much stronger signal was detected in the GBC tissues (Fig. 1d). The cIAP2 levels in the GBC tissues (0.2854 ± 0.0061) were significantly higher than those in the matched non‐tumor gallbladder tissues (0.1722 ± 0.0084, P < 0.001; Table 1). Together, these results provide strong evidence that cIAP2 is significantly upregulated in GBC tissues.
Figure 1.
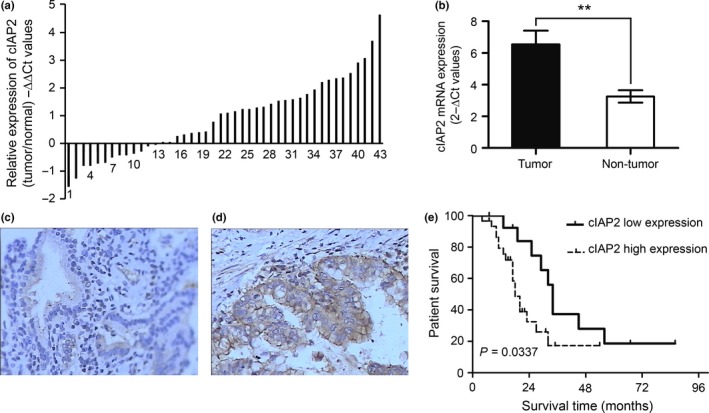
cIAP2 expression is elevated in human gallbladder cancer (GBC) tissues and correlates with a poor prognosis. (a) The relative mRNA expression levels of cIAP2 in GBC and matched non‐tumor surgical specimens were measured by quantitative RT‐PCR. The data are shown as the −ΔΔCt values. (b) The mean and standard deviation of the cIAP2 expression levels in GBC and matched non‐tumor gallbladder surgical specimens. The data are shown as the 2−ΔCt values. (c) Weak cIAP2 protein expression was detected in the cytoplasm of normal gallbladder mucosa cells (200×). (d) GBC tissues exhibited strong cIAP2 protein expression (200×). (e) The overall survival rate of patients with elevated cIAP2 expression was significantly lower than that of patients with low cIAP2 expression (**P < 0.01).
Table 1.
IHC of cIAP2 expression in gallbladder cancer (GBC) tissues and normal tissues
| N | cIAP2 expression (MOD ± SD) | t‐value | P | |
|---|---|---|---|---|
| GBC tissues | 40 | 0.2854 ± 0.0061 | 10.87 | <0.001 |
| Matched non‐tumor tissues | 40 | 0.1722 ± 0.0084 |
Correlation between cIAP2 expression and gallbladder cancer clinicopathological parameters
We next analyzed whether there was any association between cIAP2 expression and the clinicopathological characteristics of GBC patients. The correlations between the mRNA expression levels of cIAP2 and selected clinicopathological variables are summarized in Table 2. We found a significant relationship between high cIAP2 mRNA levels and lymph node metastasis (P < 0.05); otherwise, no significant correlations were observed between cIAP2 levels and the studied clinicopathological characteristics.
Table 2.
Correlation between the clinicopathological characteristics and mRNA expression of cIAP2 in 44 patients
| Clinicopathologic variables | n | cIAP2 expression (2‐ΔΔCT) | X 2‐value | P‐value | |
|---|---|---|---|---|---|
| Low | High | ||||
| Sex | |||||
| Male | 10 | 2 | 8 | 0.566 | 0.452 |
| Female | 34 | 11 | 23 | ||
| Age | |||||
| ≤65 years | 20 | 5 | 15 | 0.364 | 0.546 |
| >65 years | 24 | 8 | 16 | ||
| Tumor site | |||||
| Bottom | 25 | 9 | 16 | 1.159 | 0.282 |
| Corporis and other | 19 | 4 | 15 | ||
| Tumor size | |||||
| ≤2 cm | 24 | 7 | 17 | 0.004 | 0.952 |
| >2 cm | 20 | 6 | 14 | ||
| Pathological type | |||||
| Adenocarcinoma | 38 | 12 | 26 | 1.354 | 0.866 |
| Squamous carcinoma | 2 | 0 | 2 | ||
| Mucinous adenocarcinoma | 3 | 1 | 2 | ||
| Adenosquamous carcinoma | 1 | 0 | 1 | ||
| Differentiation degree | |||||
| G1 (high) | 10 | 3 | 7 | 0.730 | 0.768 |
| G2 (moderate) | 20 | 7 | 13 | ||
| G3 (poor) | 14 | 3 | 11 | ||
| Nevin stage | |||||
| S1–S2 | 18 | 5 | 13 | 0.046 | 0.831 |
| S3–S5 | 26 | 8 | 18 | ||
| Invasion depth | |||||
| Serosal invasion (−) | 17 | 6 | 11 | 0.440 | 0.507 |
| Serosal invasion (+) | 27 | 7 | 20 | ||
| Lymph node metastasis | |||||
| (−) | 21 | 7 | 14 | 13.227 | 0.001* |
| (+) | 23 | 6 | 17 | ||
| Liver invasion | |||||
| (−) | 30 | 9 | 21 | 0.009 | 0.923 |
| (+) | 14 | 4 | 10 | ||
Survival analysis and prognostic significance of cIAP2 expression in gallbladder cancer
A survival analysis was conducted using Kaplan–Meier curves for general survival. The overall survival rate of patients with elevated cIAP2 expression was significantly lower than that of patients with lower cIAP2 expression (P = 0.0377, Fig. 1e). Compared to the low‐expression group, the 2‐year overall survival rate of the high‐expression group was profoundly lower (64.3 vs 16.7%, respectively).
Knockdown of cIAP2 mRNA and protein expression in gallbladder cancer cell lines using RNAi
An RNAi‐mediated method was used to knock down cIAP2 and identify the biological effects of cIAP2 in GBC cell lines. DNA sequencing results verified that two cIAP2 siRNA plasmids (cIAP/siRNA‐1 and cIAP/siRNA‐2) were successful in knocking down cIAP2 mRNA levels. We used qRT‐PCR and western blotting to determine the cIAP2 levels at 48 and 72 h, respectively, after transfection of the siRNA plasmids into NOZ and SGC‐996 cells. qRT‐PCR revealed a decrease in cIAP2 mRNA levels in siRNA‐transfected cells, and the western blot results showed that both cIAP/siRNA‐1 and cIAP/siRNA‐2 could effectively knock down the protein expression of cIAP2 compared with the NC/siRNA and wild‐type (untreated) cells. As shown in Figure 2(a,b), cIAP/siRNA‐2 cells showed more effective knock down of cIAP2 protein expression than the cIAP/siRNA‐1 cells. Next, we used a lentiviral vector (pGPU6/GFP/Neo) expressing the cIAP/siRNA‐2/shRNA construct (LV‐shcIAP2) as well as a control vector containing a non‐targeting sequence (LV‐shNC) to establish cells that stably expressed shRNA for cIAP2 knockdown or a control shRNA, respectively (Fig. 2c). As shown in Figure 2(d,e), the protein levels of cIAP2 in the LV‐shcIAP2 cells were sharply decreased relative to those in the wild‐type and LV‐shNC cells (**P < 0.001). These stably transduced cells were used for subsequent pathway experiments.
Figure 2.
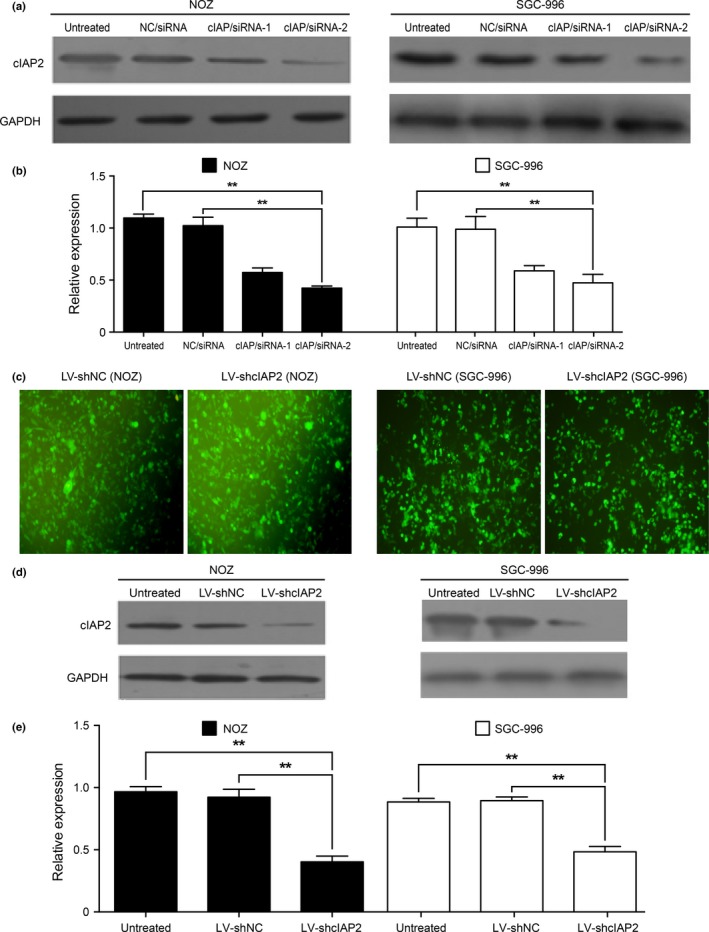
RNAi was used to knock down cIAP2 expression in GBC cells. (a) The expression of cIAP2 protein in NOZ and SGC‐996 cells was suppressed by siRNA targeted to cIAP2. (b) The relative expression of cIAP2 in NOZ and SGC‐996 cells transfected with cIAP/siRNA‐2 oligos was significantly reduced compared with untreated cells and cells transfected with NC/siRNA. (c) Construction of NOZ and SGC‐996 cells stably expressing green fluorescent protein and either shRNA targeting cIAP2 (LV‐shcIAP2; produces the cIAP/siRNA‐2 sequence) or a non‐specific shRNA (LV‐shNC). (d) cIAP2 expression in NOZ and SGC‐996 cells stably transduced with LV‐shcIAP2 or LV‐shNC was analyzed by western blotting. (e) The protein levels of cIAP2 in NOZ and SGC‐996 cells stably transduced with LV‐shcIAP2 were significantly decreased relative to the levels in untreated and LV‐shNC cells.
Knockdown of cIAP2 affects gallbladder cancer proliferation
To demonstrate whether cIAP2 promotes GBC cell proliferation, we first transfected NOZ and SGC‐996 cells with siRNA (cIAP/siRNA‐1 and cIAP/siRNA‐2) to induce the downregulation of cIAP2 gene expression with NC/siRNA and untreated cells used as controls. We tested the proliferative rate using the CCK‐8 assay and observed that cell proliferation in the cIAP/siRNA‐1 and cIAP/siRNA‐2 groups was slower compared to that of the NC/siRNA cells, which was the same as the growth of the untreated cells (Fig. 3a). These data support the notion that cIAP2 affects the proliferation of GBC cells.
Figure 3.
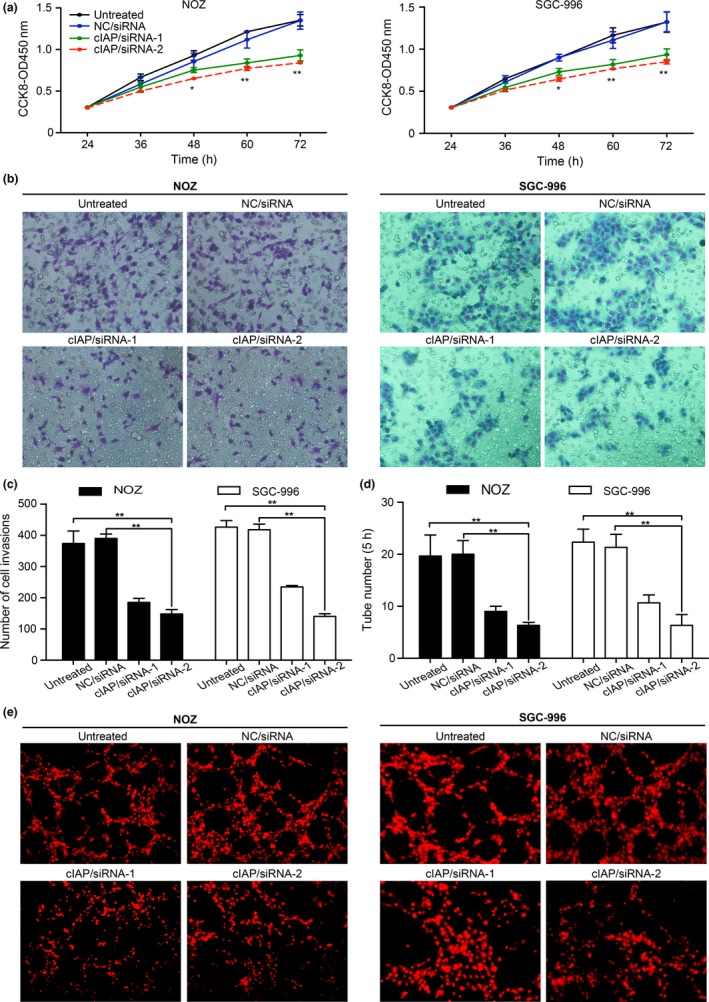
Knockdown of cIAP2 can strongly decrease the malignant biological characteristics of gallbladder cancer (GBC) cells. (a) Knockdown of cIAP2 inhibited the proliferation of GBC cells. NOZ and SGC‐996 cells were transfected with either siRNA targeting cIAP2 or NC/siRNA, and the cell growth rates were determined using the CCK‐8 assay. The results are presented as the means ± standard deviation (SD) of three independent experiments. (b,c) cIAP2 regulated the invasiveness of GBC cells. Crystal violet‐stained images show the number of cells that penetrated the porous membrane. The statistical results are based on three independent experiments. (d,e) cIAP2 regulated tube formation of human dermal lymphatic endothelial cells (HDLEC). DiI‐labelled HDLEC (red fluorescence) were co‐cultured with either NOZ or SGC‐996 cells and subsequently treated with TNF‐α (20 ng/mL) for 5 h. Tube formation was observed using fluorescence microscopy, and the number of tubes was counted (*P < 0.05; **P < 0.01).
Knockdown of cIAP2 influences gallbladder cancer invasiveness
To determine whether the invasiveness of GBC cells depends on cIAP2, we used a Transwell assay to determine the effects of cIAP2 gene knockdown in GBC cells. After staining the migratory cells with crystal violet, five different fields (400×) were counted to examine the numbers of invaded cells. The total number of cells in the cIAP/siRNA‐1 and cIAP/siRNA‐2 groups that invaded through the Transwell polycarbonate filter was markedly lower than the number of infiltrating cells in the untreated group and the NC/siRNA group (Fig. 3b,c), both of which were similar. These data suggest that cIAP2 plays a key role in the invasive ability of GBC cells.
Role of cIAP2 in tube formation of human dermal lymphatic endothelial cells in vitro
To observe the role of cIAP2 in the lymphangiogenesis of GBC, we used a 3‐D co‐culture system in which NOZ or SGC‐996 cells and HDLEC were cultured together to observe the role of cIAP2 in the ability of HDLEC to form tubes. As shown in Figure 3(d,e), the greatest number of tubes was observed at 5 h after cell seeding, and the tube formation ability of HDLEC was obviously decreased in the cIAP/siRNA‐1 and cIAP/siRNA‐2 groups of NOZ or SGC‐996 cells.
Knockdown of cIAP2 reduces TNF‐α‐induced NF‐κB activation
Previous studies have indicated that the NF‐κB signaling pathway plays a critical role in tumor cell progression.32 Currently, three recognizable pathways are known to NF‐κB activation: the canonical, non‐canonical and atypical IKK‐independent pathways.33 Our laboratory has reported that canonical TNF‐α‐induced NF‐κB activation promotes the lymphangiogenesis of GBC.34 These observations prompted us to examine whether cIAP2 is required for TNF‐α‐induced NF‐κB activation. First, we used a dual‐luciferase reporter system to measure the impact of cIAP2 on NF‐κB activation in response to TNF‐α stimulation (20 ng/mL for 5 h). Compared with the NC/siRNA and untreated groups, the cIAP/siRNA‐1 and cIAP/siRNA‐2 groups exhibited lower relative luciferase activities (Fig. 4a). This result is reminiscent of the data suggesting that cIAP2 may participate in TNF‐α‐induced NF‐κB activation. Furthermore, in most cells, NF‐κB is bound to IκB in a latent, inactive state in the cytoplasm. When activated by various stimuli, IKK can phosphorylate either IκB protein (IκBα or IκBβ) and lead to their degradation by the ubiquitin proteosome. Then, NF‐κB heterodimers enter the nucleus to promote the transcription of target genes.33 We utilized this pathway to further determine the relevance of cIAP2‐induced NF‐κB activation during gallbladder tumorigenesis using western blotting. Cell groups were treated with TNF‐α (20 ng/ml for 5 h), and nuclear and cytosolic fractions were prepared and subjected to western blotting using an anti‐IκBα or anti‐NF‐κB (p65) antibody. As shown in Figure 4(b,c), in the cytoplasm fraction, the LV‐shcIAP2 group showed significantly increased IκBα and NF‐κB (p65) levels compared with the untreated and control groups. However, in the nuclear fraction, the NF‐κB (p65) levels were decreased in the LV‐shcIAP2 group compared with those in the untreated and control groups (Fig. 4d,e). The statistical analysis was based on three independent experiments.
Figure 4.
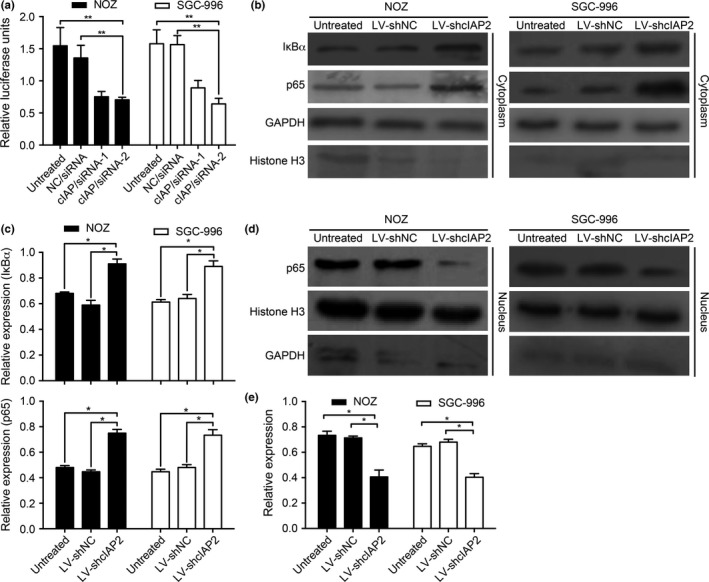
The impact of cIAP2 on NF‐κB activation in response to TNF‐α stimulation. (a) Knockdown of cIAP2 inhibited NF‐κB activity in gallbladder cancer (GBC) cells. NF‐κB promoter activity was measured using a dual‐luciferase reporter system. The data are expressed as the mean ± SD of three independent experiments. (b,c) In the cytoplasm protein lysates, NOZ and SGC‐996 cells transduced with LV‐shcIAP2 targeting cIAP2 showed significantly increased IκBα and p65 levels following TNF‐α stimulation with GAPDH as a protein marker. (d,e) In the nuclear protein lysates, NOZ and SGC‐996 cells transduced with LV‐shcIAP2 targeting cIAP2 showed significantly decreased p65 levels following TNF‐α stimulation with histone H3 as a protein marker.
Knockdown of cIAP2 prevents p65 translocation from the cytosol into the nucleus
Another key event leading to NF‐κB activation is the translocation of the NF‐κB heterodimer into the nucleus. Therefore, we observed whether knockdown of cIAP2 could restrain NF‐κB (p65) subunit translocation in GBC cell lines in response to TNF‐α stimulation. As shown in Figure 5, the cIAP/siRNA‐1 and cIAP/siRNA‐2 groups of NOZ or SGC‐996 had markedly reduced p65 translocation from the cytosol into the nucleus. The observation supports the concept that cIAP2 plays a role in NF‐κB activation.
Figure 5.
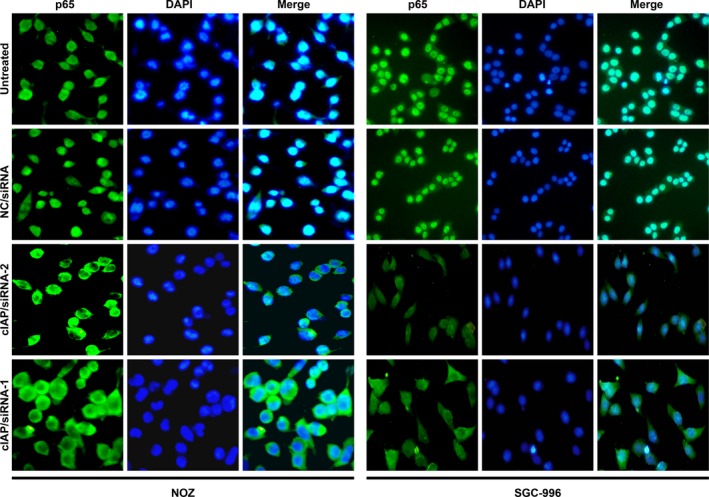
Knockdown of cIAP2 inhibits p65 translocation from the cytosol into the nucleus. The different cell types were treated with TNF‐α (20 ng/mL) for 5 h. The translocation of NF‐κB (p65) was observed using a fluorescence microscope. Compared to untreated and NC/siRNA cells, cells transfected with either cIAP/siRNA‐1 or cIAP/siRNA‐2 showed markedly reduced p65 translocation from the cytosol into the nucleus.
Knockdown of cIAP2 leads to VEGF‐C protein downregulation and decreases the concentration of VEGF‐C in cell culture media
Expression of the lymphangiogenic factor VEGF‐C has been detected in many tumor types.34, 35 Interestingly, the above data indicate that a significant relationship exists between high cIAP2 levels and lymph node metastasis. One possible mechanism is that cIAP2 influences NF‐κB and thereby indirectly influences VEGF‐C levels. In support of this hypothesis, knockdown of cIAP2 significantly decreased VEGF‐C levels following TNF‐α stimulation (Fig. 6a,b), which strongly suggests a role for cIAP2 in controlling VEGF‐C expression. Furthermore, we used Quantikine ELISA to assess the concentration of VEGF‐C in the cell culture media. As shown in Figure 6(c), the concentration of VEGF‐C in the culture medium from the LV‐shcIAP2 group was lower than that in the untreated and LV‐shNC groups. To directly verify that the reduction of VEGF‐C secretion by knockdown of cIAP2 in GBC cells is the cause of decreased lymphangiogenesis, it would be important to determine whether the addition of VEGF‐C protein to the culture medium could rescue the induction of tube formation by cIAP2 knockdown cells. As shown in Figure 6(d,e), adding VEGF‐C protein (50 ng/mL, BioVision, USA) to the culture medium of the LV‐shcIAP2 group rescued the induction of tube formation.
Figure 6.
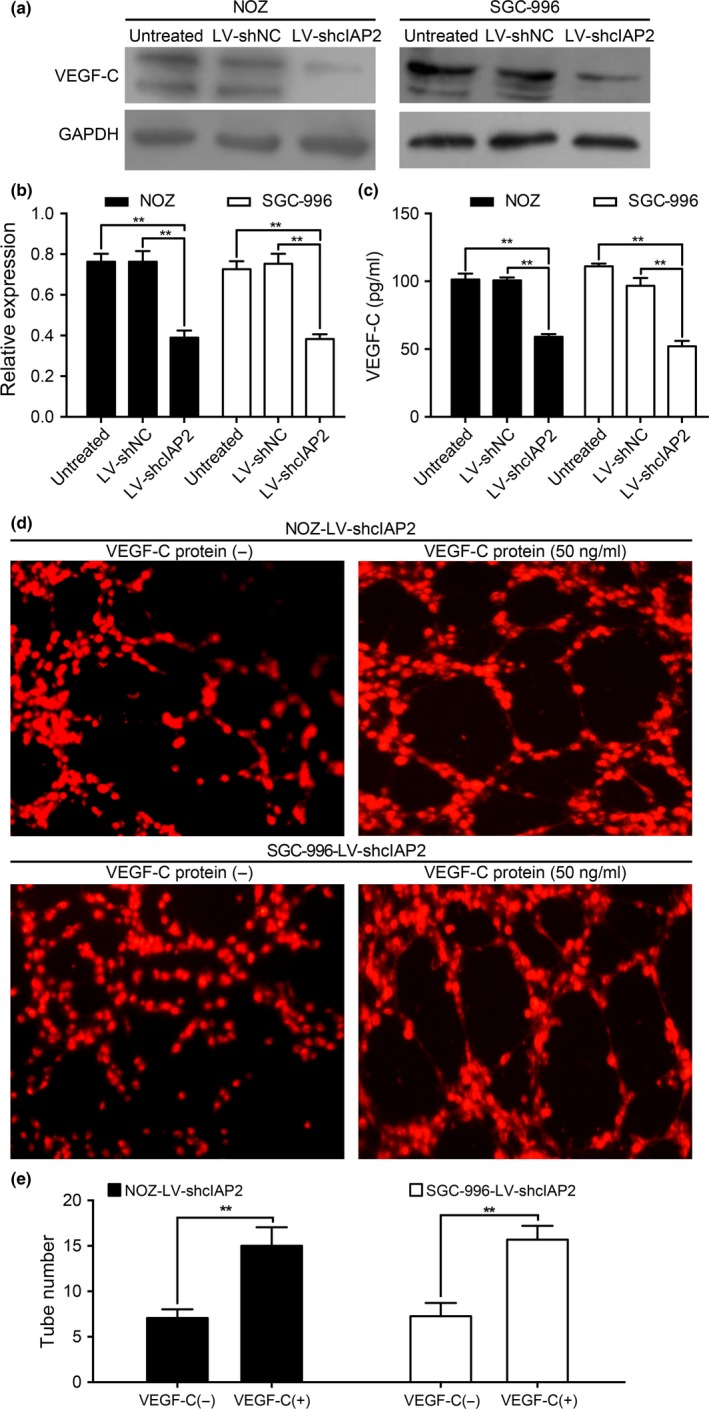
The impact of cIAP2 on VEGF‐C activation in response to TNF‐α stimulation (20 ng/mL). (a) Following TNF‐α stimulation, total protein lysates were prepared and subjected to western blotting with an anti‐VEGF‐C antibody. (b) Statistical results based on three independent experiments demonstrated that NOZ and SGC‐996 transduced with LV‐shcIAP2 showed significantly decreased VEGF‐C levels. (c) The concentration of VEGF‐C in the culture media from NOZ or SGC‐996 cells transduced with LV‐shcIAP2 was lower than that in the untreated and LV‐shNC groups. (d) Adding VEGF‐C protein (50 ng/mL) to the culture medium of the LV‐shcIAP2 group rescued the ability of tube formation induction. (e) Tube formation was observed using fluorescence microscopy, and the number of tubes was counted (**P < 0.01).
Knockdown of cIAP2 reduces lymphangiogenesis of gallbladder cancer in vivo
To study the effect of cIAP2 on lymphangiogenesis in vivo, we established orthotopic xenograft models of GBC in nude mice that were inoculated with one of the six aforementioned cell types: NOZ or SGC‐996 cells transfected with LV‐shcIAP2, LV‐shNC or neither (untreated) (Fig. 7a,b). Lymph node metastasis was confirmed by H‐E staining (Fig. 7c); invasive tumor cells (indicated by black arrows) could be observed in the lymphoid follicles (indicated by white arrows). The lymphatic vessels of the tumors were observed by IHC using LYVE‐1 (a marker for novel lymphatic vessels). As shown in Figure 7(d–f), mice injected with NOZ or SGC‐996 cells expressing LV‐shcIAP2 showed a decreased lymphatic vessel number (LVN) in the orthotopic xenograft tumors. Moreover, the lymph node metastasis rates of orthotopic xenograft tumors were decreased in the LV‐shcIAP2 groups (Table 3).
Figure 7.
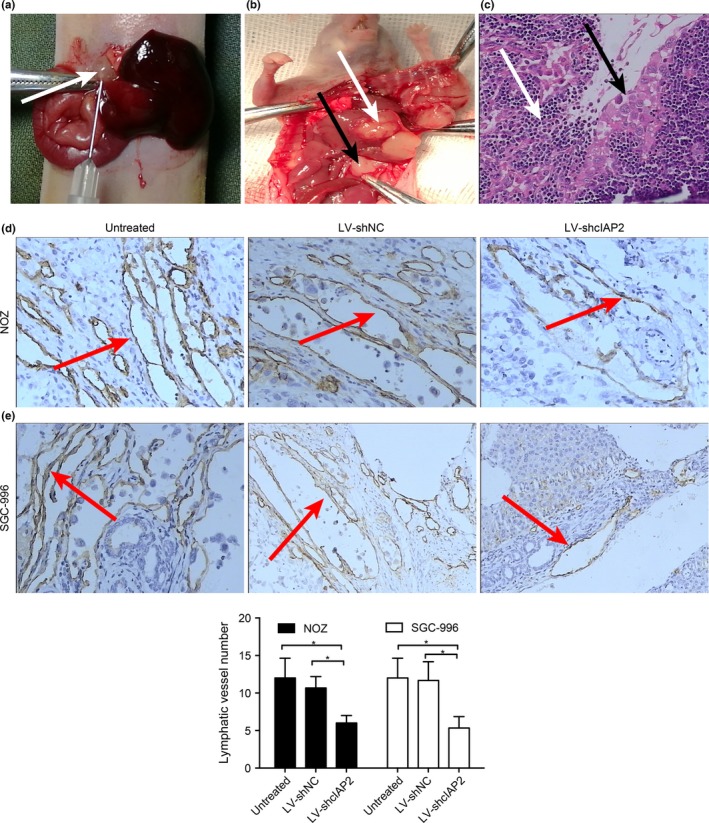
Knockdown of cIAP2 decreases lymphangiogenesis in an orthotopic xenograft model of gallbladder cancer (GBC). (a) After anesthesia and exposure of the gallbladder (white arrow), different cell types were injected into the gallbladder cavity. (b) Orthotopic xenograft model mice were successfully established. Lymph node metastases are indicated by black arrows, and the orthotopic gallbladder carcinoma is indicated by white arrows. (c) Lymph node metastasis was confirmed by H‐E staining (200×). Invasive tumor cells (black arrows) could be observed in the lymphoid follicles (white arrows). (d,e) Lymphatic vessels in the orthotopic xenograft tumors were detected by immunohistochemistry. The brown tubular structures (red arrows) are lymphatic vessels (400×). (e) Lymphatic vessel numbers of the orthotopic xenograft tumors. Knockdown of cIAP2 decreased the number of lymphatic vessels.
Table 3.
Lymph node metastasis rate (LMR) and lymphatic vessel number (LVN) of orthotopic xenograft tumors in nude mice
| NOZ | SGC‐996 | |||
|---|---|---|---|---|
| LMR | LVN | LMR | LVN | |
| Untreated | 4/6 | 12.00 ± 2.65 | 5/6 | 11.67 ± 3.06 |
| LV‐shNC | 5/6 | 10.67 ± 1.53 | 5/6 | 11.67 ± 2.52 |
| LV‐shcIAP2 | 1/6 | 6.00 ± 1.00a | 2/6 | 5.33 ± 1.23a |
P < 0.05.
Discussion
Inhibition of apoptotic programming is a key factor in the progression of tumors towards malignancy. This process provides tumor cells the ability to survive and metastasize in harsh microenvironments.36 IAP are critical proteins that regulate apoptosis.37 Here, we found that cIAP2 expression was elevated in human GBC tissues and correlated with poor prognosis, which is consistent with the findings of Nagata et al.15 and Kempkensteffen et al.38 in oral squamous cell carcinoma and renal cell carcinoma, respectively. Our study also discovered a relationship between elevated cIAP2 levels and lymph node metastasis in GBC patients. The subsequent in vitro experiments showed that knockdown of cIAP2 could strongly decrease the malignant biological characteristics of human GBC cells, such as tumor cell proliferation, cell invasiveness and tube formation. Furthermore, we confirmed that these activities caused by cIAP2 activity functioned through the NF‐κB pathway in GBC. In addition, the in vivo experiments demonstrated that knockdown of cIAP2 reduced the lymphangiogenesis of GBC in a xenograft nude mouse model.
Inhibitor of apoptosis proteins are a family of proteins characterized by the presence of a BIR domain. There are eight known mammalian IAP: cIAP1 (cellular IAP1), cIAP2 (cellular IAP2), XIAP (X‐linked IAP), Survivin, NAIP (neuronal IAP), BRUCE (BIR containing ubiquitin conjugating enzyme), ML‐IAP (melanoma IAP) and hILP2 (IAP‐like protein 2).7 Earlier reports revealed that IAP prevent apoptosis by directly inhibiting caspases, which contributes to the development of chemoresistance in cancer cells.4 Recent studies have demonstrated that several IAP have E3 ligase activity and activate various signaling pathway; therefore, cIAP also affect tumor cell proliferation, invasion and metastasis.5, 6, 7, 39 However, the mechanism of this process is far from clear. cIAP2 is one of the most extensively characterized human IAP and has been shown to exhibit both pro‐migratory and anti‐migratory functions in various tumor cells.8, 9, 10 Based on these observations, determining the specific role of cIAP2 in GBC has become particularly important.
Some studies have focused on the relationship between cIAP2 expression levels and cancer. For example, in 2003, Dai et al.14 and Hasegawa et al.16 reported that cIAP2 is overexpressed in human lung cancer and neutrophils, respectively, and Nagata et al.15 reported parallel results in oral squamous cell carcinoma in 2011. The present study revealed similar results in GBC. In addition, we demonstrated that cIAP2 levels are an indicator of poor prognosis in patients with GBC. We showed that cIAP2 can influence the malignant biological characteristics of human GBC cells, which supports the notion that cIAP2 is an indicator of poor prognosis in GBC patients.
NF‐κB activation can directly promote tumor invasion and metastasis.40, 41 However, more recent publications have presented contradictory findings concerning the role of IAP in NF‐κB signaling. Some studies have demonstrated that overexpression of IAP can either directly activate or cooperate in the activation of NF‐κB signaling.17, 18 In contrast, other studies have cast doubt on this mechanism.19, 20 The novelty of our study resides in identifying the canonical NF‐κB pathway as a critical mediator of cIAP2‐promoted GBC invasion and lymphangiogenesis. This conclusion is supported by several independent lines of evidence. First, the dual‐luciferase reporter system demonstrated that cIAP2 knockdown could reduce TNF‐α‐induced NF‐κB activation (Fig. 4a). Second, cIAP2 knockdown restricted the nuclear translocation of the NF‐κB heterodimer (Fig. 4b,d and 5). Third, the levels of the NF‐κB target gene VEGF‐C were decreased upon cIAP2 knockdown (Fig. 6a,c). Together, these data underscore the critical role of canonical NF‐κB signaling in cIAP2‐promoted GBC invasion and lymphangiogenesis.
GBC is a type of malignancy that tends to metastasize to the lymph nodes during the early stages. Lymphangiogenesis is thought to be an important step in cancer lymph node metastasis.42, 43 Interestingly, when combined with clinical data, our results revealed a relationship between elevated cIAP2 levels and lymph node metastasis in GBC patients. This finding suggests that cIAP2 may be a lymphangiogenic factor and promotes lymph node metastasis in GBC, which should be given considerable attention. Our previous study confirmed that VEGF‐C is an important protein that promotes lymphangiogenesis;34 similar results have been observed in other types of cancer, including breast cancer44, 45 and melanoma.46 Here, our experimental results demonstrated that cIAP2 knockdown in GBC cells could decrease the tube formation ability of HDLEC. Therefore, we hypothesized that cIAP2 may influence the expression of VEGF‐C in GBC cells, and our results showed that cIAP2 could affect the expression level of VEGF‐C (Fig. 6a). In addition, we found that cIAP2 could influence VEGF‐C secretion from GBC cells based on the concentration of VEGF‐C in the supernatant of GBC media as determined by ELISA (Fig. 6c). Moreover, adding VEGF‐C protein to the culture medium of the LV‐shcIAP2 group rescued the induction of tube formation. Therefore, we posited that one mechanism by which cIAP2 promotes lymphangiogenesis in GBC is by regulating the expression and secretion of VEGF‐C.
To further bolster our in vitro conclusions, we conducted in vivo experiments using an animal model of GBC. We observed that silencing cIAP2 expression suppressed the number of lymphatic vessels in a metastatic lymph node (Fig. 7), which was consistent with our in vitro results.
Our study suggests that cIAP2 is highly expressed in human GBC and correlates with poor prognosis; furthermore, the results show that cIAP2 can promote GBC invasion and lymphangiogenesis by activating the NF‐κB pathway. These data indicate that cIAP2 may be a potential treatment target to prevent the invasion and lymphatic metastasis of GBC.
Disclosure Statement
The authors have no conflict of interest to declare.
Acknowledgments
This study was supported by a grant from the National Natural Science Foundation of China (No. 81672468).
Cancer Sci 108 (2017) 1144–1156
Funding Information
The National Natural Science Foundation of China (No. 81672468).
Contributor Information
Feifei She, Email: shefeifei@yeah.net.
Yanling Chen, Email: drchenyl@126.com.
References
- 1. Gourgiotis S, Kocher HM, Solaini L, Yarollahi A, Tsiambas E, Salemis NS. Gallbladder cancer. Am J Surg 2008; 196: 252–64. [DOI] [PubMed] [Google Scholar]
- 2. Masior L, Krasnodebski M, Kobryn K, Grat M, Krawczyk M. Surgical treatment of gall‐bladder cancer. Pol Przegl Chir 2015; 87: 324–30. [DOI] [PubMed] [Google Scholar]
- 3. Kanthan R, Senger JL, Ahmed S, Kanthan SC. Gallbladder cancer in the 21st century. J Oncol 2015; 2015: 967472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fulda S. Inhibitor of apoptosis proteins as targets for anticancer therapy. Expert Rev Anticancer Ther 2007; 7: 1255–64. [DOI] [PubMed] [Google Scholar]
- 5. Hengartner MO. The biochemistry of apoptosis. Nature 2000; 407: 770–6. [DOI] [PubMed] [Google Scholar]
- 6. Crook NE, Clem RJ, Miller LK. An apoptosis‐inhibiting baculovirus gene with a zinc finger‐like motif. J Virol 1993; 67: 2168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Srinivasula SM, Ashwell JD. IAPs: what's in a name? Mol Cell 2008; 30: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC. The c‐IAP‐1 and c‐IAP‐2 proteins are direct inhibitors of specific caspases. EMBO J 1997; 16: 6914–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saleem M, Qadir MI, Perveen N et al Inhibitors of apoptotic proteins: New targets for anticancer therapy. Chem Biol Drug Des 2013; 82: 243–51. [DOI] [PubMed] [Google Scholar]
- 10. Samuel T, Okada K, Hyer M, Welsh K, Zapata JM, Reed JC. cIAP1 Localizes to the nuclear compartment and modulates the cell cycle. Cancer Res 2005; 65: 210–18. [PubMed] [Google Scholar]
- 11. Cheung CH, Cheng L, Chang KY, Chen HH, Chang JY. Investigations of surviving: the past, present and future. Front Biosci (Landmark Ed) 2011; 16: 952–61. [DOI] [PubMed] [Google Scholar]
- 12. Oberoi‐Khanuja TK, Murali A, Rajalingam K. IAPs on the move: role of inhibitors of apoptosis proteins in cell migration. Cell Death Dis 2013; 4: e784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kenneth NS, Duckett CS. IAP proteins: Regulators of cell migration and development. Curr Opin Cell Biol 2012; 24: 871–5. [DOI] [PubMed] [Google Scholar]
- 14. Dai Z, Zhu WG, Morrison CD et al A comprehensive search for DNA amplification in lung cancer identifies inhibitors of apoptosis cIAP1 and cIAP2 as candidate oncogenes. Hum Mol Genet 2003; 12: 791–801. [DOI] [PubMed] [Google Scholar]
- 15. Nagata M, Nakayama H, Tanaka T et al Overexpression of cIAP2 contributes to 5‐FU resistance and a poor prognosis in oral squamous cell carcinoma. Br J Cancer 2011; 105: 1322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hasegawa T, Suzuki K, Sakamoto C et al Expression of the inhibitor of apoptosis (IAP) family members in human neutrophils: Up‐regulation of cIAP2 by granulocyte colony‐stimulating factor and overexpression of cIAP2 in chronic neutrophilic leukemia. Blood 2003; 101: 1164–71. [DOI] [PubMed] [Google Scholar]
- 17. Ferreira CG, van der Valk P, Span SW et al Expression of X‐linked inhibitor of apoptosis as a novel prognostic marker in radically resected non‐small cell lung cancer patients. Clin Cancer Res 2001; 7: 2468–74. [PubMed] [Google Scholar]
- 18. Haferkamp A, Bedke J, Vetter C et al High nuclear livin expression is a favourable prognostic indicator in renal cell carcinoma. BJU Int 2008; 102: 1700–6. [DOI] [PubMed] [Google Scholar]
- 19. Zhang Y, Zhu J, Tang Y et al X‐linked inhibitor of apoptosis positive nuclear labeling: a new independent prognostic biomarker of breast invasive ductal carcinoma. Diagn Pathol 2011; 6: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Imoto I, Tsuda H, Hirasawa A et al Expression of cIAP1, a target for 11q22 amplification, correlates with resistance of cervical cancers to radiotherapy. Cancer Res 2002; 62: 4860–6. [PubMed] [Google Scholar]
- 21. Baldwin AS Jr. The NF‐kappa B and I kappa B proteins: New discoveries and insights. Annu Rev Immunol 1996; 14: 649–83. [DOI] [PubMed] [Google Scholar]
- 22. Skaug B, Jiang X, Chen ZJ. The role of ubiquitin in NF‐kappaB regulatory pathways. Annu Rev Biochem 2009; 78: 769–96. [DOI] [PubMed] [Google Scholar]
- 23. Shishodia S, Aggarwal BB. Nuclear factor‐kappaB activation mediates cellular transformation, proliferation, invasion angiogenesis and metastasis of cancer. Cancer Treat Res 2004; 119: 139–73. [DOI] [PubMed] [Google Scholar]
- 24. Hagemann T, Wilson J, Kulbe H et al Macrophages induce invasiveness of epithelial cancer cells via NF‐kappaB and JNK. J Immunol 2005; 175: 1197–205. [DOI] [PubMed] [Google Scholar]
- 25. Tchoghandjian A, Jennewein C, Eckhardt I, Rajalingam K, Fulda S. Identification of non‐canonical NF‐kappaB signaling as a critical mediator of Smac mimetic‐stimulated migration and invasion of glioblastoma cells. Cell Death Dis 2013; 4: e564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhu G, Chen X, Wang X et al Expression of the RIP‐1 gene and its role in growth and invasion of human gallbladder carcinoma. Cell Physiol Biochem 2014; 34: 1152–65. [DOI] [PubMed] [Google Scholar]
- 27. Ye Y, Jin L, Wilmott JS et al PI(4,5)P2 5‐phosphatase A regulates PI3K/Akt signalling and has a tumour suppressive role in human melanoma. Nat Commun 2013; 4: 1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zeng Y, Opeskin K, Goad J, Williams ED. Tumor‐induced activation of lymphatic endothelial cells via vascular endothelial growth factor receptor‐2 is critical for prostate cancer lymphatic metastasis. Cancer Res 2006; 66: 9566–75. [DOI] [PubMed] [Google Scholar]
- 29. Bertrand MJ, Milutinovic S, Dickson KM et al cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell 2008; 30: 689–700. [DOI] [PubMed] [Google Scholar]
- 30. Chen Y, Jiang L, She F et al Vascular endothelial growth factor‐C promotes the growth and invasion of gallbladder cancer via an autocrine mechanism. Mol Cell Biochem 2010; 345: 77–89. [DOI] [PubMed] [Google Scholar]
- 31. Weidner N, Semple JP, Welch WR et al Tumor angiogenesis and metastasis‐correlation in invasive breast carcinoma. N Engl J Med 1991; 324: 1–8. [DOI] [PubMed] [Google Scholar]
- 32. Bharti AC, Aggarwal BB. Nuclear factor‐kappa B and cancer: its role in prevention and therapy. Biochem Pharmacol 2002; 64: 883–8. [DOI] [PubMed] [Google Scholar]
- 33. Gilmore TD. Introduction to NF‐kappaB: Players, pathways, perspectives. Oncogene 2006; 25: 6680–4. [DOI] [PubMed] [Google Scholar]
- 34. Du Q, Jiang L, Wang X, Wang M, She F, Chen Y. Tumor necrosis factor‐alpha promotes the lymphangiogenesis of gallbladder carcinoma through nuclear factor‐kappaB‐mediated upregulation of vascular endothelial growth factor‐C. Cancer Sci 2014; 105: 1261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hirakawa S, Brown LF, Kodama S, Paavonen K, Alitalo K, Detmar M. VEGF‐C‐induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood 2007; 109: 1010–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science 1995; 267: 1456–62. [DOI] [PubMed] [Google Scholar]
- 37. Gyrd‐Hansen M, Meier P. IAPs: from caspase inhibitors to modulators of NF‐kappaB, inflammation and cancer. Nat Rev Cancer 2010; 10: 561–74. [DOI] [PubMed] [Google Scholar]
- 38. Kempkensteffen C, Hinz S, Christoph F et al Expression parameters of the inhibitors of apoptosis cIAP1 and cIAP2 in renal cell carcinomas and their prognostic relevance. Int J Cancer 2007; 120: 1081–6. [DOI] [PubMed] [Google Scholar]
- 39. Mehrotra S, Languino LR, Raskett CM, Mercurio AM, Dohi T, Altieri DC. IAP regulation of metastasis. Cancer Cell 2010; 17: 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yu HG, Yu LL, Yang Y et al Increased expression of RelA/nuclear factor‐kappa B protein correlates with colorectal tumorigenesis. Oncology 2003; 65: 37–45. [DOI] [PubMed] [Google Scholar]
- 41. Fujioka S, Sclabas GM, Schmidt C et al Function of nuclear factor kappaB in pancreatic cancer metastasis. Clin Cancer Res 2003; 9: 346–54. [PubMed] [Google Scholar]
- 42. Stacker SA, Williams SP, Karnezis T et al Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 2014; 14: 159–72. [DOI] [PubMed] [Google Scholar]
- 43. Muratore A, Polastri R, Capussotti L. Radical surgery for gallbladder cancer: current options. Eur J Surg Oncol 2000; 26: 438–43. [DOI] [PubMed] [Google Scholar]
- 44. Skobe M, Hawighorst T, Jackson DG et al Induction of tumor lymphangiogenesis by VEGF‐C promotes breast cancer metastasis. Nat Med 2001; 7: 192–8. [DOI] [PubMed] [Google Scholar]
- 45. Mattila MM, Ruohola JK, Karpanen T et al VEGF‐C induced lymphangiogenesis is associated with lymph node metastasis in orthotopic MCF‐7 tumours. Int J Cancer 2002; 98: 946–51. [DOI] [PubMed] [Google Scholar]
- 46. Schietroma C, Cianfarani F, Lacal PM et al Vascular endothelial growth factor‐C expression correlates with lymph node localization of human melanoma metastases. Cancer 2003; 98: 789–97. [DOI] [PubMed] [Google Scholar]


