Abstract
Reprogramming of glucose metabolism in tumor cells is referred to as the Warburg effect and results in increased lactic acid secretion into the tumor microenvironment. We have previously shown that lactic acid has important roles as a pro‐inflammatory and immunosuppressive mediator and promotes tumor progression. In this study, we examined the relationship between the lactic acid concentration and expression of LDHA and GLUT1, which are related to the Warburg effect, in human head and neck squamous cell carcinoma (HNSCC). Tumors expressing lower levels of LDHA and GLUT1 had a higher concentration of lactic acid than those with higher LDHA and GLUT1 expression. Lactic acid also suppressed the expression of LDHA and GLUT1 in vitro. We previously reported that lactic acid enhances expression of an M2 macrophage marker, ARG1, in murine macrophages. Therefore, we investigated the relationship between the lactic acid concentration and polarization of M2 macrophages in HNSCC by measuring the expression of M2 macrophage markers, CSF1R and CD163, normalized using a pan‐macrophage marker, CD68. Tumors with lower levels of CD68 showed a higher concentration of lactic acid, whereas those with higher levels of CSF1R showed a significantly higher concentration of lactic acid. A similar tendency was observed for CD163. These results suggest that tumor‐secreted lactic acid is linked to the reduction of macrophages in tumors and promotes induction of M2‐like macrophage polarization in human HNSCC.
Keywords: Head and neck squamous cell carcinoma, lactic acid, macrophage polarization, tumor microenvironment, Warburg effect
Reprogramming of energy metabolism is recognized as a hallmark of cancer.1 Glucose is imported into the cytoplasm by glucose transporters and metabolized to pyruvate. Under aerobic conditions, normal cells convert pyruvate to acetyl‐CoA in the mitochondria and metabolize it to CO2, H2O, and ATP through the tricarboxylic acid cycle. Tumor cells exhibit increased glucose uptake and convert pyruvate to lactate under aerobic conditions, resulting in a state referred to as the Warburg effect, or aerobic glycolysis.2, 3 Hence, tumor cells express metabolic enzymes and transporters involved in glycolysis at higher levels than normal cells.4 Glucose transporter 1, which transports glucose into the cytoplasm, is commonly overexpressed in human malignancies,4, 5 and tumor cells are thereby able to import large amounts of glucose. Lactate dehydrogenase, a tetramer composed of two subunits, LDHA and LDHB, catalyzes the interconversion of pyruvate and lactate. Lactate dehydrogenase A promotes conversion of pyruvate to lactate and is the predominant isoform in highly glycolytic tissues, especially in tumors.6, 7 The enhanced glycolysis in tumor cells produces large amounts of lactic acid, and higher levels of lactic acid are correlated with distant metastasis and poor prognosis in head and neck cancer and cervical cancer.8
Tumors are infiltrated by many types of innate and adaptive immune cells. These immune cells were classically thought to function to eliminate tumors, but recent studies have indicated that they induce inflammation in tumors and promote tumor progression through induction of angiogenesis and tissue remodeling in the tumor microenvironment, resulting in increased tumor invasion and metastasis.9, 10 Macrophages are typically classified into two polarization states: M1 and M2 phenotypes. M1 macrophages are potent effector cells that kill target cells and produce inflammatory cytokines such as IL‐12, IL‐1β, tumor necrosis factor‐α, and IL‐6. In contrast, M2 macrophages reduce inflammatory responses and adaptive Th1 immunity, and promote angiogenesis and tissue remodeling.11, 12, 13 Tumor‐associated macrophages polarize into the M2 phenotype and suppress the host anticancer immune response, leading to tumor progression. M2 macrophages are characterized by secreted cytokines (IL‐4, IL‐13, IL‐10, and CSF1), Toll‐like receptor ligands, and glucocorticoids.12, 14
We previously showed that lactic acid secreted by tumor cells enhances expression of ARG1, an enzyme that metabolizes l‐arginine into l‐ornithine and urea and converts macrophages to inhibit T cell proliferation and activation in mice.15 Depletion of l‐arginine from the tumor microenvironment through ARG1‐dependent consumption causes suppression of T cell activation and proliferation, and lactic acid secreted by tumor cells plays an important role as an inducer of M2‐like macrophages or immunosuppressive macrophage polarization in mice.16, 17 However, upregulation of ARG1 occurs in murine M2 macrophages, but not in human M2 macrophages; thus, human M2 macrophages cannot suppress T cells in an ARG1‐dependent manner, and it is unknown whether lactic acid secreted by tumors induces M2 macrophage polarization in humans.18 Therefore, to investigate the relationship between lactic acid and M2 macrophage polarization in human tumors, we measured the concentration of lactic acid and the expression of M2 macrophage markers in HNSCC.
Materials and Methods
Patients
Tumor tissue samples were obtained from 20 patients who underwent primary surgical resection of HNSCC between 2013 and 2015 at Gifu University Hospital (Gifu, Japan). The backgrounds of the patients are shown in Table S1. Normal pharyngeal tissues were obtained from four patients who underwent tonsillectomy for recurrent tonsillitis or IgA nephropathy and from one patient who underwent total laryngectomy for dysphagia at our hospital. Normal pharyngeal tissues were isolated from surrounding tonsillar or laryngeal tissue. Surgical tissue specimens were immediately put into a vial containing RNAlater Stabilization Reagent (Qiagen, Valencia, CA, USA) and then stored at −80°C for isolation of total RNA or homogenized in Dulbecco's PBS for measurement of the lactic acid concentration. All patients who provided HNSCC tissue or normal pharyngeal tissue gave informed consent in accordance with the institutional ethics committee requirements and the Declaration of Helsinki. This study was approved by the Institutional Review Board of Gifu University Graduate School of Medicine (approval no. 26‐181).
Cell lines and cell culture
Human HNSCC cell lines, HSC‐2 and HSC‐4, were shown to be derived from the JCRB0622 and JCRB0624 cell lines, respectively, in the JCRB cell bank using short tandem repeat analysis (Fig. S1). HSC‐2 and HSC‐4 cells were cultured in RPMI‐1640 supplemented with 10% heat‐inactivated FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C under a 5% CO2 atmosphere. l‐lactic acid was purchased from Sigma‐Aldrich (St. Louis, MO, USA). For treatment with l‐lactic acid, 2 × 105 cells were cultured in 0.5 mL culture medium/well (24‐well plate) or 1 × 105 cells were cultured in 5 mL culture medium/well (6‐well plate). In deacidification experiments, 1 × 105 cells were cultured in 5 mL culture medium/well (6‐well plate) in the presence of 0, 20, or 30 mM lactic acid for 24 h and further cultured in 5 mL fresh culture medium without lactic acid for 24 h.
Real‐time RT‐PCR
Total RNA was isolated from tissues or cells using a RNeasy Mini kit (Qiagen). Complementary DNA was synthesized at 37°C for 15 min using a PrimeScript RT reagent kit (Takara Bio, Otsu, Japan). Real‐time RT‐PCR was carried out using a TaqMan gene expression assay system (Applied Biosystems, Foster City, CA, USA), Premix Ex Taq and a Thermal Cycler Dice Real Time System TP800 (Takara Bio). The following TaqMan probes and primer sets were used: CD68, Hs02836816_g1; CD163, Hs00174705_m1; ARG1, Hs00968979_m1; CSF1R, Hs00911250_m1; LDHA, Hs00855332_g1; SLC2A1 (GLUT1 is named SLC2A1 in the HUGO gene nomenclature), Hs00892681_m1, and 18S rRNA, 4352930E. The relative expression of each gene was normalized to that of 18S rRNA or CD68 and calculated using the ∆∆Ct method.19
Measurement of lactic acid concentration in tumors
Tissue specimens were homogenized in Dulbecco's PBS (1 mL per 100 mg tumor) and centrifuged for 5 min at 440 × g. The supernatant was mixed with an equal volume of 0.8 N perchloric acid and centrifuged for 5 min at 3000 rpm. The concentrations of lactate in the obtained supernatants were measured using a Determiner LA Kit (Kyowa Medical Corp., Shizuoka, Japan).
Statistical analysis
Gene expression levels and lactate concentrations are shown as mean values ± SD. Differences between groups were analyzed by unpaired Student's t‐test. Relationships between two continuous variables were evaluated by Spearman's rank correlation coefficient. P < 0.05 was considered significant in all analyses.
Results
Increases in lactic acid and expression of LDHA and GLUT1 in HNSCC
Aerobic glycolysis is enhanced in tumor cells, and therefore these cells are likely to secrete a large amount of lactic acid. Consistent with this expectation, the lactic acid concentration in HNSCC tissues was significantly higher than that in normal pharyngeal tissues (Fig. 1a; P = 0.002). Expression of LDHA and GLUT1 determined by real‐time RT‐PCR was also significantly higher in HNSCC tissue (Fig. 1b; both P < 0.001). These results suggest that tumor cells secrete a large amount of lactic acid due to upregulation of glycolysis, compared to normal cells.
Figure 1.
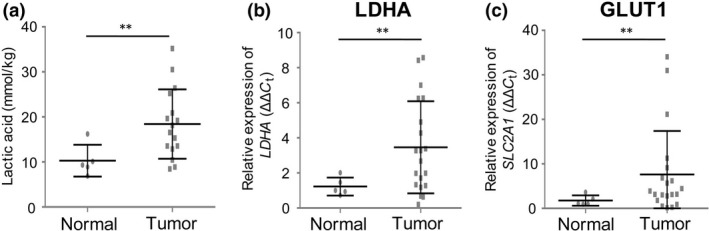
Increased concentration of lactic acid and expression of LDHA and GLUT1 in head and neck squamous cell carcinoma (HNSCC). (a) Concentrations of lactic acid in normal pharyngeal tissue (n = 5) and HNSCC tissue (n = 16). (b, c) Relative expression levels of LDHA (b) and GLUT1 (c) in normal pharyngeal tissue (n = 5) and HNSCC tissue (n = 20) measured by real‐time RT‐PCR. GLUT1 is named SLC2A1 in the HUGO gene nomenclature. **P < 0.01 by Student's t‐test.
Lactic acid is decreased in HNSCC expressing higher levels of LDHA or GLUT1
Expression levels of LDHA and GLUT1 in each HNSCC were strongly positively correlated (Fig. 2a; r s = 0.815, P < 0.001). To investigate the relationship between the concentration of lactic acid and expression of LDHA or GLUT1 in HNSCC, the samples were divided into groups (n = 8 in each) expressing lower and higher levels, using median values of 3.0 for LDHA and 4.2 for GLUT1. The lower expression LDHA and GLUT1 groups had significantly higher levels of lactic acid (Fig. 2b; P = 0.017 for LDHA, P = 0.045 for GLUT1). However, there was no significant correlation between the lactic acid level and expression of LDHA (r s = −0.364, P = 0.166) or GLUT1 (r s = −0.396, P = 0.129). These results suggest that a higher concentration of tumor‐secreted lactic acid might suppress the expression of LDHA and GLUT1.
Figure 2.
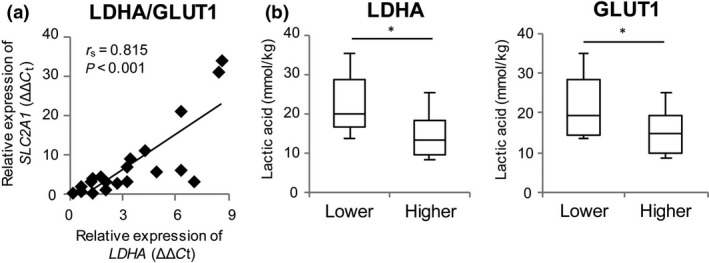
Expression of LDHA and GLUT1 in head and neck squamous cell carcinoma. (a) Correlation between expression of LDHA and GLUT1 mRNA in head and neck squamous cell carcinoma (n = 20). r s is the Spearman rank correlation coefficient. (b) Concentrations of lactic acid in tissues expressing lower and higher levels of LHDA (low, <3, n = 8; high, ≥3, n = 8) or GLUT1 (low, <4.2, n = 8; high, ≥4.2, n = 8). mRNA of these genes was measured by real‐time RT‐PCR. *P < 0.05 by Student's t‐test.
High lactic acid suppresses expression of LDHA and GLUT1 in vitro
The results in Figure 2(b) are the opposite of our original view that higher expression of LDHA and GLUT1 would lead to an increase in lactic acid in tumors. Therefore, we examined whether a higher concentration of lactic acid affects the expression of LDHA and GLUT1 in human HNSCC cell lines, HSC‐2 and HSC‐4, in vitro. HSC‐2 and HSC‐4 cells were cultured for 24 h, after which expression of LDHA and GLUT1 and the lactic acid level in culture supernatants were measured. The concentration of lactic acid was significantly higher in the supernatant of HSC‐2 cells compared with that of HSC‐4 cells (Fig. 3a; P < 0.001). Compared with HSC‐4 cells, LDHA expression was significantly elevated and GLUT1 expression was significantly reduced in HSC‐2 cells (Fig. 3b; P = 0.015 for LDHA, P = 0.033 for GLUT1).
Figure 3.
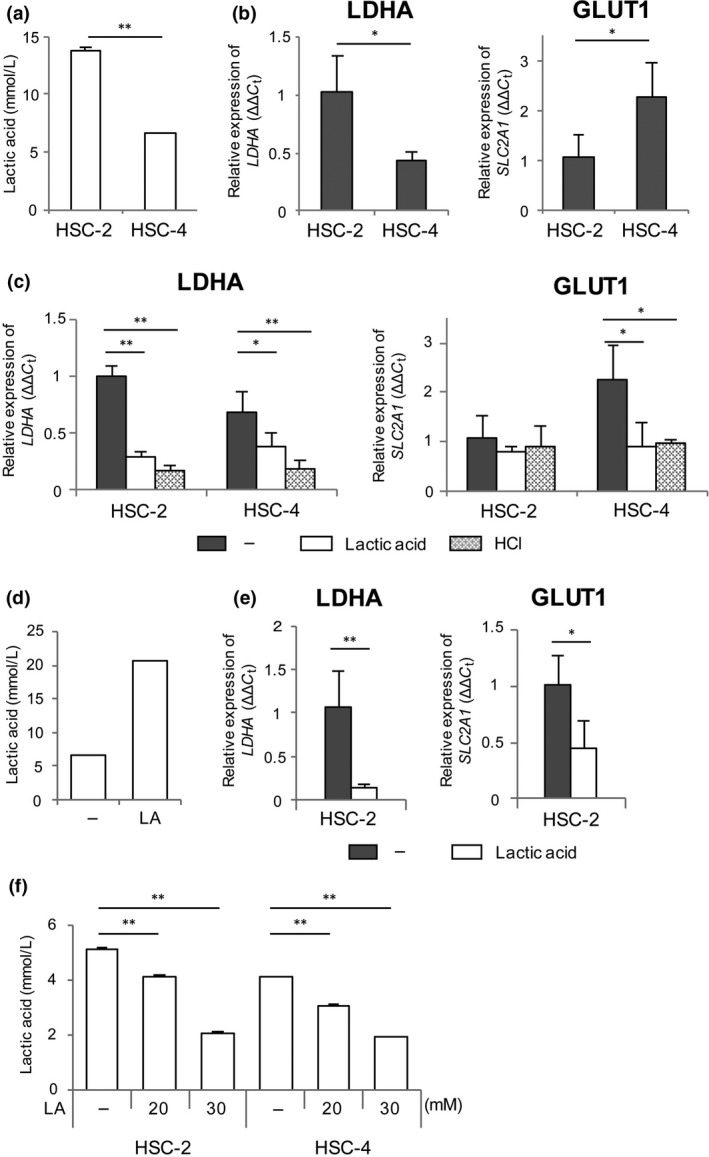
Expression of LDHA and GLUT1 in head and neck squamous cell carcinoma cells. (a, b) HSC‐2 and HSC‐4 cells (2 × 105 cells in 0.5 mL medium) were cultured for 24 h and the concentration of lactic acid was measured in the culture supernatant (a). LDHA and GLUT1 expression was measured in HSC‐2 and HSC‐4 cells by real‐time RT‐PCR (b). (c) HSC‐2 and HSC‐4 cells were cultured with 15 mM lactic acid or 10 mM HCl for 24 h, and LDHA and GLUT1 expression was measured by real‐time RT‐PCR. (d, e) HSC‐2 cells (1 × 105 cells in 5 mL medium) were cultured in the presence of 20 mM lactic acid (LA) for 48 h, and the concentration of lactic acid was measured in the culture supernatant (d). LDHA and GLUT1 expression was measured in HSC‐2 cells by real‐time RT‐PCR (e). (f) HSC‐2 and HSC‐4 cells (1 × 105 cells in 5 mL medium) were cultured with lactic acid (LA) for 24 h and then without lactic acid for 24 h. The concentration of lactic acid was measured in the culture supernatant after deacidification. Data are shown as the mean ± SD (n = 3) for at least two independent experiments. *P < 0.05; **P < 0.01. –, water.
To explore the effect of lactic acid on expression of these genes, HSC‐2 and HSC‐4 cells (2 × 105 cells in 0.5 mL/well) were cultured in the presence of 15 mM lactic acid. Lactic acid suppressed expression of LDHA in HSC‐2 (P < 0.001) and HSC‐4 cells (P = 0.037) and expression of GLUT1 in HSC‐4 cells (Fig. 3c; P = 0.024). The concentration of lactic acid in the HSC‐2 culture supernatant was 13.84 ± 0.17 mM, which might have been high enough for HSC‐2 cells to suppress GLUT1 expression without additional lactic acid (Fig. 3a). To remove the influence of lactic acid secreted by tumor cells, HSC‐2 cells (1 × 105 cells in 5 mL/well) were cultured in a larger amount of medium. The concentration of lactic acid in the supernatant was not increased after 24 h of incubation (6.48 ± 0.16 mM) and additional lactic acid significantly suppressed expression of LDHA and GLUT1 (Fig. 3d,e; P = 0.009 for LDHA, P = 0.013 for GLUT1). Moreover, results similar to those with lactic acid were observed after treatment with 10 mM HCl (Fig. 3c).
Next, we investigated whether tumor cells treated with lactic acid can secrete a large amount of lactic acid. HSC‐2 and HSC‐4 cells were pretreated with lactic acid for 24 h, and then the medium was changed to fresh medium without lactic acid. After 24 h, lactic acid levels in the culture supernatants were measured. The concentration of lactic acid was significantly suppressed in a dose‐dependent manner in HSC‐2 and HSC‐4 cells pretreated with lactic acid (Fig. 3f). These results suggest that extracellular acidification with lactic acid suppresses expression of LDHA and GLUT1 in tumor cells, resulting in reduced production of lactic acid from tumor cells.
Increased expression of CD68, CD163, and CSF1R in HNSCC
To examine accumulation and polarization of macrophage in tumors, expression of CD68, CD163, and CSF1R was measured by real‐time RT‐PCR. Expression of CD68, a pan‐macrophage marker, was normalized using that of 18S rRNA, and expression of CD163 and CSF1R, which are human M2 macrophage markers, was normalized against that of CD68. Compared with normal pharyngeal tissue, HNSCC tissue had significantly higher expression of CD68, CD163, and CSF1R (Fig. 4; P = 0.031, P = 0.014, P = 0.023, respectively). These results suggest that macrophages accumulated in the tumor and are polarized to the M2‐like phenotype in the tumor. We also measured the expression of ARG1 mRNA, a murine M2 macrophage marker, because we have previously shown that its expression in murine macrophages is increased by treatment with lactic acid.15 However, ARG1 showed the opposite result in human tumors: HNSCC tissue had a significantly lower expression of ARG1 than that in normal pharyngeal tissue (Fig. S2). This result suggests that ARG1 is not an M2 macrophage marker in human tumor tissue.18
Figure 4.
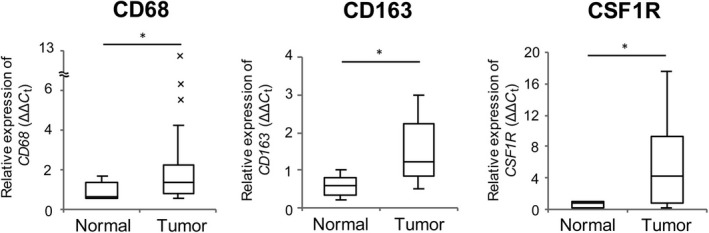
Upregulation of M2 macrophage markers in head and neck squamous cell carcinoma. Expression levels of CD68 (normalized to expression of 18S rRNA), CD163, and CSF1R (normalized to expression of CD68) in normal pharyngeal tissue (n = 5) and head and neck squamous cell carcinoma tissue (n = 20) were measured by real‐time RT‐PCR. *P < 0.05; **P < 0.01, by Student's t‐test.
M2 like‐macrophage polarization is promoted in HNSCC containing higher concentrations of lactic acid
The HNSCC cases were divided into groups expressing lower and higher levels of CD68, CD163, or CSF1R, and lactic acid levels were compared between each pair of groups (Fig. 5a). There were significantly higher levels of lactic acid in the groups with lower CD68 expression (P = 0.018) and higher CSF1R expression (P = 0.017), and a tendency for a higher level of lactic acid in the group with higher CD163 expression (P = 0.053). The concentration of lactic acid showed a significant negative correlation with CD68 expression (Fig. 5b; r s = −0.500, P = 0.048) and a significant positive correlation with CSFR1 expression (Fig. 5b; r s = 0.534, P = 0.037). These results suggest that lactic acid negatively regulates the accumulation of macrophages at a tumor site, but promotes M2‐like macrophage polarization, as observed in our mouse experiments.15 Finally, we examined the relationship between the lactic acid level and ARG1 expression, but we did not find a correlation between these parameters in HNSCC tumors (Fig. S3; r s = 0.128, P = 0.633).
Figure 5.
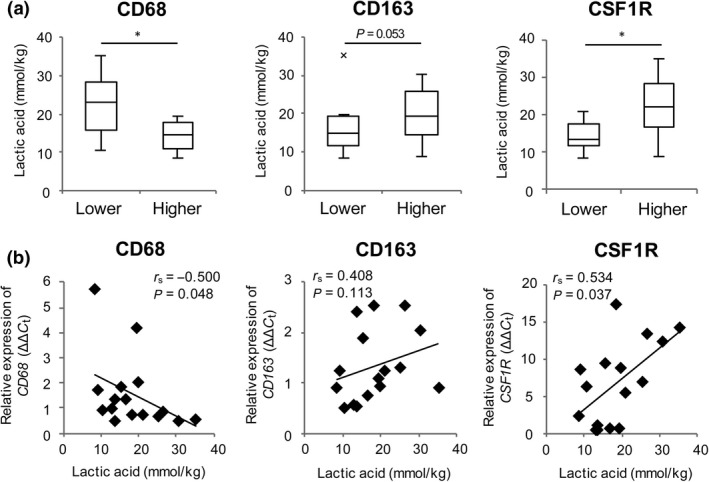
Accumulation of M2‐like macrophages is correlated with a high concentration of lactic acid in head and neck squamous cell carcinoma. (a) Concentrations of lactic acid in cells with different levels of CD68 (low, <0.95, n = 8; high, ≥0.95, n = 8), CD163 (low, <1.2, n = 8; high, ≥1.2, n = 8), or CSF1R (low, <6.6, n = 8; high, ≥6.6, n = 8). (b) Correlations between lactic acid concentration and expression levels of CD68,CD163, and CSF1R in head and neck squamous cell carcinoma (n = 20). Expression of these genes was measured by real‐time RT‐PCR. r s is Spearman's rank correlation coefficient. *P < 0.05 by Student's t‐test.
Discussion
The Warburg effect is caused by tumor cells adapting their metabolism to the demand for and limited supply of oxygen20 and by the required production of large amounts of nucleotides, amino acids, and lipids for tumor cell proliferation.21 The Warburg effect results in increased production of lactic acid, as the final product of glycolysis. In this study, we showed that the concentration of lactic acid in tumors is higher than that in normal tissue. We have shown that lactic acid secreted by tumor cells functions as a pro‐inflammatory and immunosuppressive mediator, promotes tumor progression,22, 23, 24 and converts macrophages into immunosuppressive macrophages and induces ARG1 expression in mice.15 Lactic acid secreted by tumor cells also inhibits the functions of natural killer cells, cytotoxic T cells, and dendritic cells, and induces myeloid‐derived suppressor cells to form an immunosuppressive environment in tumors.25, 26, 27
In this study, we found that the intratumoral concentration of lactic acid was linked to M2‐like macrophage polarization in the tumor microenvironment. However, unlike murine macrophages, ARG1 expression in human macrophages was not related to the intratumoral concentration of lactic acid. Expression levels of CD163, a member of the scavenger receptor cysteine‐rich family, and CSF1R, a receptor for macrophage colony stimulating factor, normalized by the pan‐macrophage marker CD68, were examined as M2 macrophage markers that reflect the rate of M2‐like macrophage polarization in tumors. We note that CD163 and CSF1R are expressed in breast and ovarian cancer cells, as well as in normal monocytes and macrophages.19, 28, 29, 30, 31, 32 Thus, it cannot be excluded that some HNSCC cells might express CD163 and CSF1R, despite there being no reports of expression of these genes in these cells. Indeed, CD163 was not detected, but CSF1R was slightly detected in small fractions of HSC‐2 and HSC‐4 cells (Fig. S4). Analysis of the expression of these genes in isolated tumor‐infiltrating macrophages may more clearly show that lactic acid is linked to M2‐like macrophage polarization.
Our results indicate that macrophages infiltrate tumors more effectively than normal tissues. Chronic inflammation in the tumor microenvironment may cause macrophages to accumulate within the tumor. However, interestingly, more macrophages are likely to accumulate at a tumor site when the intratumoral concentration of lactic acid is lower. Consistent with this finding, a high concentration of lactic acid inhibits monocyte migration in vitro.33 These results show that macrophages do not infiltrate well into tumors that produce a large amount of lactic acid, but can polarize toward the M2‐like phenotype in tumors that produce a large amount of lactic acid.
Increased expression of LDHA and GLUT1, which are mainly activated by oncogenes, is important in the Warburg effect.34 It is also well known that expression of LDHA and GLUT1 is increased in response to hypoxia and activation of hypoxia inducible factor‐1α.20 To evaluate induction of the Warburg effect, we measured the concentration of lactic acid and expression of these genes in tumors and normal tissues. In tumor tissues, the concentration of lactic acid was higher, and LDHA and GLUT1 expression was significantly increased, compared with normal tissues. GLUT1 expression was strongly correlated with LDHA expression. However, a lower intratumoral concentration of lactic acid was linked to the higher expression of LDHA and GLUT1. As upregulation of these genes directly causes the Warburg effect, whereas the accumulation of lactic acid is the result of the Warburg effect, it is not unexpected that the concentration of lactic acid and the expression levels of genes involved in glycolysis do not change synchronously. We suggest that aerobic glycolysis may be mainly promoted by oncogenes and hypoxia in tumors, and may be suppressed by negative feedback under higher lactic acid conditions. In fact, we showed that a high concentration of lactic acid suppressed the expression of LDHA and GLUT1 in HNSCC cells. Acidification of the culture medium gave the same results. Because lactate anions are co‐transported with protons into cells by monocarboxylate transporters, extracellular acidification would suppress efflux of both protons and lactate anions from cells and promote accumulation of lactic acid, resulting in suppression of aerobic glycolysis by negative feedback. Protons also activate G‐protein coupled receptors and upregulate cAMP, and this signaling pathway may also suppress the expression of genes involved in glycolysis.35, 36 Thus, our results suggest that expression of LDHA and GLUT1 is not a predictive marker for the intratumoral concentration of lactic acid.
We have previously shown that lactic acid secreted by tumors induces immunosuppressive M2‐like macrophages in a murine system.15 In addition, blocking of the lactic acid signal pathway directly improves the immunosuppressive function of murine macrophages, even if the intratumoral concentration of lactic acid is not decreased.15 In this study, we showed that a higher concentration of lactic acid was also linked to M2‐like macrophage polarization in human HNSCCs. Furthermore, lactic acid‐primed tumor cells did not necessarily recover the suppressed production of lactic acid after removal of lactic acid. Therefore, we predict that suppression of lactic acid production and the lactic acid signal pathway that induces M2‐like macrophages should improve the immune status of patients with cancer. However, the molecular mechanism underlying this effect in human tumors is unknown. Lactic acid secreted by tumor cells suppresses antitumor immunity and increases tumor angiogenesis through endothelial cells,24, 29, 37 and increasing evidence suggests that lactic acid is a tumor‐derived mediator that modulates the tumor microenvironment to promote tumor progression.5, 24, 38, 39, 40 Thus, antitumor therapies targeting lactic acid production and the lactic acid signaling pathway are of increasing interest. It will be important to determine the details of this signaling pathway.
Disclosure Statement
Norimitsu Inoue received speaker honoraria from Alexion Pharmaceuticals Inc. and research funding from Takeda Science Foundation. The other authors have no conflict of interest.
Abbreviations
- ARG1
arginase I
- CSF1
colony stimulating factor 1
- CSF1R
colony stimulating factor 1 receptor
- GLUT
glucose transporter
- HNSCC
head and neck squamous cell carcinoma
- IL
interleukin
- LDH
lactate dehydrogenase
Supporting information
Fig. S1. Short tandem repeat analyses were carried out by Takara Bio (Otsu, Japan) using the GenePrint 10 system (Promega, Fitchburg, WI, USA). Nine microsatellite loci and the sex‐typing marker Amelogenin were analyzed in HSC‐2 (b) and HSC‐4 (d) cell lines. The short tandem repeat profiles of eight microsatellite loci completely matched between our cells and cells registered in the JCRB cell bank (a, c; http://cellbank.nibioh5n.go.jp/english/cellsearch_e/). Although Y chromosome was detected in our HSC‐2 cell but not JACR cell, the HSC‐2 cell is originally derived from a man and should include both X and Y chromosomes.
Fig. S2. Expression of ARG1 in head and neck squamous cell carcinoma. ARG1 expression was measured in normal pharyngeal tissue (n = 5) and head and neck squamous cell carcinoma tissue (n = 20) by real‐time RT‐PCR. Expression of ARG1 was normalized using expression of CD68 and calculated using the ΔΔCt method. **P < 0.01 by Mann–Whitney U‐test.
Fig. S3. Expression of ARG1 was not correlated with a high concentration of lactic acid in head and neck squamous cell carcinoma. (a) Concentrations of lactic acid in cells expressing higher and lower levels of ARG1 (low, <0.18, n = 8; high, ≥0.18, n = 8). **P < 0.01 by Student's t‐test. (b) Correlation between the lactic acid concentration and ARG1 expression in head and neck squamous cell carcinoma (n = 20). r s is Spearman's rank correlation coefficient.
Fig. S4. Expression of CD163 and CSF1R in head and neck squamous cell carcinoma cell lines. HSC‐2 and HSC‐4 cells were stained with APC‐conjugated anti‐human CD163 antibody (GHI/61; BioLegend, San Diego, CA, USA) (a) or APC‐conjugated anti‐human CD115 (CSF1R) antibody (9‐4D2‐1E4; BioLegend) (b), and examined using a FACSCalibur system (BD Biosciences, San Jose, CA). APC‐conjugated mouse IgG1κ (MOPC‐21; BioLegend) and APC‐conjugated rat IgG1κ (RTK2071; BioLegend) isotype controls were used as anti‐human CD163 and anti‐human CD115 antibody controls, respectively. The human peripheral monocyte fraction, gated based on forward scatter and side scatter, was used as a positive control. Small fractions of both HSCC cell lines expressed CD115 (HSC‐2, 1.14%; HSC4, 2.34%), but not CD163.
Table S1. Background of patients with head and neck squamous cell carcinoma.
Acknowledgments
We thank S. Yoshida and T. Yasuda for technical assistance. This work was supported by the Japan Society for the Promotion of Science, KAKENHI Grant Numbers JP25893085 (to T.O.) and JP16H04703 (to N.I.).
Cancer Sci 108 (2017) 1128–1134
Funding Information
Japan Society for the Promotion of Science, KAKENHI Grant Numbers JP25893085 and JP16H04703.
Contributor Information
Toshimitsu Ohashi, Email: o_1043_toshi32_dragons@yahoo.co.jp.
Norimitsu Inoue, Email: inoue-no@mc.pref.osaka.jp.
References
- 1. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011; 144: 646–74. [DOI] [PubMed] [Google Scholar]
- 2. Kim JW, Dang CV. Cancer's molecular sweet tooth and the Warburg effect. Cancer Res 2006; 66: 8927–30. [DOI] [PubMed] [Google Scholar]
- 3. Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004; 4: 891–9. [DOI] [PubMed] [Google Scholar]
- 4. Amann T, Maegdefrau U, Hartmann A et al GLUT1 expression is increased in hepatocellular carcinoma and promotes tumorigenesis. Am J Pathol 2009; 174: 1544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gottfried E, Kreutz M, Mackensen A. Tumor metabolism as modulator of immune response and tumor progression. Semin Cancer Biol 2012; 22: 335–41. [DOI] [PubMed] [Google Scholar]
- 6. Yuan C, Li Z, Wang Y et al Overexpression of metabolic markers PKM2 and LDH5 correlates with aggressive clinicopathological features and adverse patient prognosis in tongue cancer. Histopathology 2014; 65: 595–605. [DOI] [PubMed] [Google Scholar]
- 7. Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest 2013; 123: 3685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walenta S, Mueller‐Klieser WF. Lactate: Mirror and motor of tumor malignancy. Semin Radiat Oncol 2004; 14: 267–74. [DOI] [PubMed] [Google Scholar]
- 9. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer‐related inflammation. Nature 2008; 454: 436–44. [DOI] [PubMed] [Google Scholar]
- 10. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140: 883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: Tumor‐associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002; 23: 549–55. [DOI] [PubMed] [Google Scholar]
- 12. Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med 2011; 9: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murray PJ, Allen JE, Biswas SK et al Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014; 41(1): 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sica A, Schioppa T, Mantovani A, Allavena P. Tumour‐associated macrophages are a distinct M2 polarised population promoting tumour progression: Potential targets of anti‐cancer therapy. Eur J Cancer 2006; 42: 717–27. [DOI] [PubMed] [Google Scholar]
- 15. Ohashi T, Akazawa T, Aoki M et al Dichloroacetate improves immune dysfunction caused by tumor‐secreted lactic acid and increases antitumor immunoreactivity. Int J Cancer 2013; 133: 1107–18. [DOI] [PubMed] [Google Scholar]
- 16. Gabrilovich DI, Ostrand‐Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12: 253–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: Mechanisms and therapeutic perspectives. Immunol Rev 2008; 222: 180–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Raes G, Van den Bergh R, De Baetselier P et al Arginase‐1 and Ym1 are markers for murine, but not human, alternatively activated myeloid cells. J Immunol 2005; 174: 6561; author reply ‐2. [DOI] [PubMed] [Google Scholar]
- 19. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods 2001; 25: 402–8. [DOI] [PubMed] [Google Scholar]
- 20. Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer 2008; 8: 705–13. [DOI] [PubMed] [Google Scholar]
- 21. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009; 324: 1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shime H, Yabu M, Akazawa T et al Tumor‐secreted lactic acid promotes IL‐23/IL‐17 proinflammatory pathway. J Immunol 2008; 180: 7175–83. [DOI] [PubMed] [Google Scholar]
- 23. Yabu M, Shime H, Hara H et al IL‐23‐dependent and ‐independent enhancement pathways of IL‐17A production by lactic acid. Int Immunol 2011; 23(1): 29–41. [DOI] [PubMed] [Google Scholar]
- 24. Hirschhaeuser F, Sattler UG, Mueller‐Klieser W. Lactate: A metabolic key player in cancer. Cancer Res 2011; 71: 6921–5. [DOI] [PubMed] [Google Scholar]
- 25. Gottfried E, Kunz‐Schughart LA, Ebner S et al Tumor‐derived lactic acid modulates dendritic cell activation and antigen expression. Blood 2006; 107: 2013–21. [DOI] [PubMed] [Google Scholar]
- 26. Fischer K, Hoffmann P, Voelkl S et al Inhibitory effect of tumor cell‐derived lactic acid on human T cells. Blood 2007; 109: 3812–9. [DOI] [PubMed] [Google Scholar]
- 27. Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor‐derived lactate modifies antitumor immune response: Effect on myeloid‐derived suppressor cells and NK cells. J Immunol 2013; 191: 1486–95. [DOI] [PubMed] [Google Scholar]
- 28. Kacinski BM, Carter D, Mittal K et al High level expression of fms proto‐oncogene mRNA is observed in clinically aggressive human endometrial adenocarcinomas. Int J Radiat Oncol Biol Phys 1988; 15: 823–9. [DOI] [PubMed] [Google Scholar]
- 29. Kacinski BM, Carter D, Mittal K et al Ovarian adenocarcinomas express fms‐complementary transcripts and fms antigen, often with coexpression of CSF‐1. Am J Pathol 1990; 137(1): 135–47. [PMC free article] [PubMed] [Google Scholar]
- 30. Kacinski BM, Scata KA, Carter D et al FMS (CSF‐1 receptor) and CSF‐1 transcripts and protein are expressed by human breast carcinomas in vivo and in vitro. Oncogene 1991; 6: 941–52. [PubMed] [Google Scholar]
- 31. Shabo I, Svanvik J. Expression of macrophage antigens by tumor cells. Adv Exp Med Biol 2011; 714: 141–50. [DOI] [PubMed] [Google Scholar]
- 32. Shabo I, Olsson H, Sun XF, Svanvik J. Expression of the macrophage antigen CD163 in rectal cancer cells is associated with early local recurrence and reduced survival time. Int J Cancer 2009; 125: 1826–31. [DOI] [PubMed] [Google Scholar]
- 33. Goetze K, Walenta S, Ksiazkiewicz M, Kunz‐Schughart LA, Mueller‐Klieser W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int J Oncol 2011; 39: 453–63. [DOI] [PubMed] [Google Scholar]
- 34. Palsson‐McDermott EM, O'Neill LA. The Warburg effect then and now: From cancer to inflammatory diseases. BioEssays 2013; 35: 965–73. [DOI] [PubMed] [Google Scholar]
- 35. Mogi C, Tobo M, Tomura H et al Involvement of proton‐sensing TDAG8 in extracellular acidification‐induced inhibition of proinflammatory cytokine production in peritoneal macrophages. J Immunol 2009; 182: 3243–51. [DOI] [PubMed] [Google Scholar]
- 36. Isom DG, Sridharan V, Baker R, Clement ST, Smalley DM, Dohlman HG. Protons as second messenger regulators of G protein signaling. Mol Cell 2013; 51: 531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beckert S, Farrahi F, Aslam RS et al Lactate stimulates endothelial cell migration. Wound Repair Regen 2006; 14: 321–4. [DOI] [PubMed] [Google Scholar]
- 38. Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF‐kappaB/IL‐8 pathway that drives tumor angiogenesis. Cancer Res 2011; 71: 2550–60. [DOI] [PubMed] [Google Scholar]
- 39. Dhup S, Dadhich RK, Porporato PE, Sonveaux P. Multiple biological activities of lactic acid in cancer: Influences on tumor growth, angiogenesis and metastasis. Curr Pharm Des 2012; 18: 1319–30. [DOI] [PubMed] [Google Scholar]
- 40. Choi SY, Collins CC, Gout PW, Wang Y. Cancer‐generated lactic acid: A regulatory, immunosuppressive metabolite? J Pathol 2013; 230: 350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Short tandem repeat analyses were carried out by Takara Bio (Otsu, Japan) using the GenePrint 10 system (Promega, Fitchburg, WI, USA). Nine microsatellite loci and the sex‐typing marker Amelogenin were analyzed in HSC‐2 (b) and HSC‐4 (d) cell lines. The short tandem repeat profiles of eight microsatellite loci completely matched between our cells and cells registered in the JCRB cell bank (a, c; http://cellbank.nibioh5n.go.jp/english/cellsearch_e/). Although Y chromosome was detected in our HSC‐2 cell but not JACR cell, the HSC‐2 cell is originally derived from a man and should include both X and Y chromosomes.
Fig. S2. Expression of ARG1 in head and neck squamous cell carcinoma. ARG1 expression was measured in normal pharyngeal tissue (n = 5) and head and neck squamous cell carcinoma tissue (n = 20) by real‐time RT‐PCR. Expression of ARG1 was normalized using expression of CD68 and calculated using the ΔΔCt method. **P < 0.01 by Mann–Whitney U‐test.
Fig. S3. Expression of ARG1 was not correlated with a high concentration of lactic acid in head and neck squamous cell carcinoma. (a) Concentrations of lactic acid in cells expressing higher and lower levels of ARG1 (low, <0.18, n = 8; high, ≥0.18, n = 8). **P < 0.01 by Student's t‐test. (b) Correlation between the lactic acid concentration and ARG1 expression in head and neck squamous cell carcinoma (n = 20). r s is Spearman's rank correlation coefficient.
Fig. S4. Expression of CD163 and CSF1R in head and neck squamous cell carcinoma cell lines. HSC‐2 and HSC‐4 cells were stained with APC‐conjugated anti‐human CD163 antibody (GHI/61; BioLegend, San Diego, CA, USA) (a) or APC‐conjugated anti‐human CD115 (CSF1R) antibody (9‐4D2‐1E4; BioLegend) (b), and examined using a FACSCalibur system (BD Biosciences, San Jose, CA). APC‐conjugated mouse IgG1κ (MOPC‐21; BioLegend) and APC‐conjugated rat IgG1κ (RTK2071; BioLegend) isotype controls were used as anti‐human CD163 and anti‐human CD115 antibody controls, respectively. The human peripheral monocyte fraction, gated based on forward scatter and side scatter, was used as a positive control. Small fractions of both HSCC cell lines expressed CD115 (HSC‐2, 1.14%; HSC4, 2.34%), but not CD163.
Table S1. Background of patients with head and neck squamous cell carcinoma.


