Abstract
Extramedullary plasmacytoma (EMP) accounts for only 3% of plasma cell malignancies; others include multiple myeloma, plasma cell leukemia and solitary plasmacytoma of bone. The majority of EMPs are found in the upper respiratory tract. Other sites include the GI tract, bladder, CNS, thyroid, breast, testes, parotid gland, lymph nodes and skin. There are eight cases in the literature of adrenal plasmacytoma, however, only two were bilateral. We describe our recent experience of bilateral adrenal plasmacytoma and review of the literature. While EMP may present as aggressive locally destructive lesions, excellent local control can be achieved in a majority of cases. Follow-up should be lifelong due to risk of progression to multiple myeloma.
KEYWORDS : adrenal, plasmacytoma, surgery
Practise points.
Adrenal plasmacytomas are extremely rare, however, should be considered a differential diagnosis for an unusual nonfunctioning adrenal mass.
If plasmacytoma is suspected preoperatively on serum electrophoresis and urinary Bence Jones proteins, then complete investigation to exclude systemic plasma cell disease is required. Bone marrow examination and careful skeletal imaging should be performed to exclude multiple myeloma (MM), as surgery in this case would not be advised.
If plasmacytoma is diagnosed postoperatively then a bone marrow examination and careful skeletal imaging should be performed to exclude MM. Surgery can be considered for suspected cases of solitary or bilateral adrenal plasmacytoma with good results.
Radiotherapy (RT) could be considered as adjuvant therapy for unilateral adrenal extramedullary plasmacytoma.
Surgery alone as the initial management for bilateral adrenal extramedullary plasmacytoma might be the best option due to the potential harmful effects of RT to both kidneys. RT may then be used for local recurrence.
Chemotherapy is not as effective as surgery or RT but can be considered as second-line treatment though this may change with the advent of more effective drugs in plasma cell disease.
All solitary or bilateral adrenal plasmacytoma patients should undergo surveillance with serum electrophoresis, urinary Bence Jones protein analysis and serial imaging, with consideration of bone marrow examination.
Recurrence could be either local or seen as progression to MM.
Case report
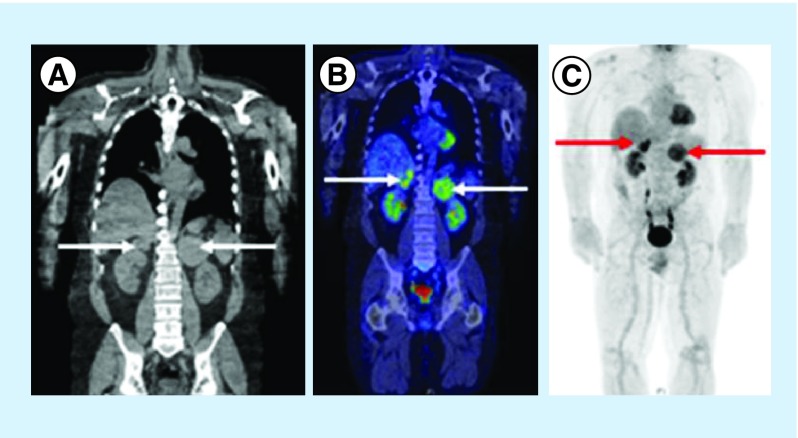
We report a 57‐year-old male with 1-month history of cramping and abdominal pain. As part of his investigations he received an abdominal ultrasound that showed incidental bilateral adrenal masses. He had a background of having a low-risk melanoma excised from his back but was otherwise reasonably healthy with no other medical issues or relevant family history. There were no complaints of back pain or other symptoms. He subsequently had a PET CT scan given his history of melanoma. This revealed high standardized uptake value in both adrenal glands, with the largest mass involving the left adrenal gland of 9 cm and right side measuring 5.5 cm (Figure 1). Biochemistry showed no abnormal hormonal activity. Core biopsy of the left adrenal mass showed a poorly differentiated cancer negative for melanin A, pan cytokeratin, S100, SOX‐10, synaptophysin, CD58, CD138, kappa, lamba, cyclin D1 and CD20, which ruled out diagnoses including melanoma, lymphoma, adrenal cortical carcinoma, pheochromocytoma, neuroendocrine tumor and myeloma. On tertiary review, a plasmacytoma was suspected by the reviewing pathologist but no tissue was available for further immunohistochemistry.
Figure 1. . Sagittal images of the thorax, abdomen and pelvis from preoperative noncontrast CT (A), FDG PET CT (B) and FDG PET (C) scans.
The arrows indicate bilateral adrenal masses. PET-CT scan and PET scan both show high SUV in the adrenal glands bilaterally.
FDG: Fludeoxyglucose; SUV: Standardized unit uptake.
Given the possibility of a plasma cell neoplasm, further evidence of plasma cell disease was sought. Serum protein electrophoresis showed no paraprotein and immunoglobulin levels were normal, a free light chain assay showed a mild increase in kappa at 37.2 mg/l (3.3–19.4 mg/l), which leads to a marginally abnormal free light chain ratio of 2.66 (0.26–1.65). There was also a trace of kappa Bence Jones proteinuria (detected by immunoelectropheresis only and not quantifiable). A bone marrow aspirate, trephine and flow cytometry did not show any abnormal plasma cell infiltrate either numerically or morphologically. His bone marrow cytogenetics were normal. A computed tomography (CT) skeletal survey and PET did not demonstrate any lytic bone disease. These findings ruled out multiple myeloma.
The patient was discussed in a multidisciplinary team setting and it was decided that he should have a bilateral adrenalectomy as it was thought that it was either metastatic disease or extramedullary plasmacytoma. Our patient underwent a bilateral adrenalectomy through a bilateral subcostal incision. No incidental metastatic deposits were identified during the operation. Postoperative oral corticosteroids were administered for adrenal deficiency. Although treatment for plasma cell malignancies can sometimes involve corticosteroids, this was not considered a part of the treatment at that time for the plasmacytoma. Further recovery was uneventful and he was discharged 7 days after the operation.
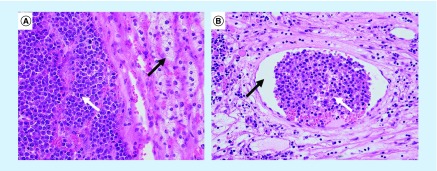
The histopathology of the resected specimens showed bilateral adrenal plasmacytoma with invasion on both sides into periadrenal fat (Figure 2A). There was also abundant mitosis in both specimens and evidence of vascular invasion (Figure 2B). Both tumors showed capsular invasion, but were nevertheless completely resected. Based on these findings, a postoperative multidisciplinary decision was to do active surveillance with PET CT without adjuvant treatment. Radiotherapy was discussed and was decided against due to risk of bilateral kidney damage.
Figure 2. . Histopathology slides (hematoxylin and eosin stain) of adrenal plasmacytoma.
(A) 400× magnification showing the adrenal plasmacytoma with an abundance of plasma cells (white arrow) with normal adrenal cortex (black arrow). (B) 200× magnification showing lymphovascular invasion with an abundance of plasma cells (white arrow) found in a blood vessel (black arrow).
Discussion & review
Plasma cell tumors can be divided into four categories: multiple myeloma (MM), plasma cell leukemia, solitary bone plasmacytoma (SBP) and extramedullary plasmacytoma (EMP). Solitary plasmacytomas most frequently occur in bone (SBP), but when found outside the bone are referred to as EMP [1]. EMP is the most uncommon of plasma cell malignancies and is a malignant neoplasm arising from the monoclonal proliferation of atypical plasma cells that develop outside the bone.
EMP accounts for 3% of all plasma cell malignancies and approximately 80% of these cases are found in the aerodigestive tract of the head and neck but may also occur in the GI tract, urinary bladder, CNS, thyroid, breast, testes, parotid gland, lymph nodes and skin [1]. Males are three-times more likely than females to develop EMP and it is typically found in adults in their 50s and 60s with a median age of 55 [1,2]. There are no typical clinical manifestations of adrenal plasmacytomas. Intermittent subcostal, abdominal and lumbar pain have all been reported but usually these lesions are found as incidentalomas on imaging (Table 1). An adrenal EMP is a rare clinical entity with only eight cases being reported in the literature. Interestingly, two of these cases have been bilateral (Table 2). This is the ninth reported case of adrenal plasmacytoma and the third reported case involving bilateral glands.
Table 1. . Patient characteristics and treatment.
| Study | Country | Study date | Age (years) | Gender | Symptoms | Incidentaloma | Pre-op diagnosis confirmed | Primary treatment | Radiotherapy | Chemotherapy | Follow-up (months) | Recurrence | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kahara et al. |
Japan |
2001 |
52 |
Male |
Nil |
Yes |
No |
Laparoscopic adrenalectomy |
40 Gy |
Yes |
12 |
No |
[28] |
| Asahi et al. |
Japan |
2001 |
52 |
Male |
Nil |
Yes |
No |
Laparoscopic adrenalectomy |
Yes |
Yes |
NP |
? |
[29] |
| Fujikata et al. |
Japan |
2002 |
77 |
Male |
Right back pain |
No |
No |
Open adrenalectomy, nephrectomy, IVC resection, right hepatic lobectomy and cholecystectomy |
45 Gy |
No |
12 |
No |
[11] |
| Rogers et al. |
America |
2004 |
75 |
Female |
R abdo pain and fatigue |
Yes – CT done for microscopic haematuria |
No |
Laparoscopic adrenalectomy |
Yes |
No |
NP |
? |
[30] |
| Li et al. |
China |
2007 |
64 |
Female |
Back pain |
No |
No |
Open adrenalectomy (bilateral) |
No |
No |
NP |
? |
[5] |
| Ahmed et al. |
Saudi Arabia |
2009 |
47 |
Male |
R hip pain/synovitis |
Yes |
Yes (FNB and core) |
Chemo and autologous stem cell transplant |
No |
Yes |
47 |
No |
[14] |
| Antona et al. |
Spain |
2011 |
76 |
Female |
? |
? |
No |
Adrenalectomy |
Yes |
No |
40 |
No |
[31] |
| Cao et al. |
China |
2014 |
26 |
Male |
Right flank pain |
No |
No |
Retroperitoneal laparoscopic adrenalectomy |
No |
No |
72 |
No |
[1] |
| Townend/Graus | Australia | 2016 | 57 | Male | Abdominal cramping | Yes | Yes | Open adrenalectomy (bilateral) | No | No | NP | ? | Current paper |
Table 2. . Tumor characteristics and pathology.
| Study | Year | Side | Functional | Tumor size (cm) | Bone marrow aspirate | Urinary Bense Jones | Serum protein electrophoresis | Kappa | Lambda | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Kahara et al. |
2001 |
Right |
No |
4 |
Negative |
– |
Positive |
No |
Specimen |
[28] |
| Asahi et al. |
2001 |
Right |
No |
4 |
Negative |
– |
Positive |
No |
Specimen |
[29] |
| Fujikata et al. |
2002 |
Right |
Yes |
10 |
Negative |
Negative |
Positive |
Specimen |
Blood |
[11] |
| Rogers |
2004 |
Right |
No |
3.5 |
Negative |
Negative |
Normal |
No |
Specimen |
[30] |
| Li |
2007 |
Bilateral |
No |
R = 6 L = 4 |
Negative |
Negative |
Normal |
No |
Specimen |
[5] |
| Ahmed et al. |
2009 |
Bilateral |
No |
R = 8 L = 8 |
Negative |
Positive |
Positive |
Specimen + urine |
No |
[14] |
| Blanco et al. |
2011 |
Left |
No |
6 |
– |
– |
– |
– |
– |
[31] |
| Cao et al. |
2014 |
Right |
No |
3.4 |
Negative |
Negative |
Positive |
Specimen |
No |
[1] |
| Townend/Graus | 2016 | Bilateral | No | R = 5.5 L = 9.5 |
Negative | Positive | Positive | Specimen + serum + urine | No |
L: Left; R: Right.
Solitary plasmacytoma of both bone and extramedullary type can progress to MM. As opposed to SPB which progresses to MM in 50–60% cases and has a median survival of 10 years, EMP progresses to MM in 11–30% and has an overall survival rate at 10 years of 70% [3]. Local recurrence occurs in 30% of the cases and distant metastasis is seen with transformation to MM [4]. Before making the diagnosis of an EMP or undertaking any therapeutic action, MM must be ruled out. A bone marrow biopsy should be done to look for any evidence of MM and evidence of clonal plasma cell expansion [5].
EMP of the adrenal glands has mostly been found on imaging as adrenal incidentalomas (AIs) (Table 1). Our case was initially detected on abdominal ultrasound while examining for biliary pathology. Other cases have been detected on CT showing an adrenal mass or an MRI with nonspecific signs demonstrating homogenous or heterogenous lesions on T1/T2 weighted imaging [5,6].
The exact pathogenesis of EMP remains unclear – one theory is that repeated trauma may act as a trigger to plasma cells to proliferate and lead to clonal infiltration [7]. The reason why some patients develop MM and others a single plasmacytoma is not understood, but might be related to differences in cellular adhesion molecules or chemokine receptor expression profiles of the malignant plasma cells [8].
Patient characteristics & clinical features
Of the nine reported cases between 2001 and 2016 (including our case), there were six males and three females. The age range was 26–77 years, with a median age of 57 years (Table 1). This is consistent with our knowledge of EMP presenting elsewhere in the body; males in the sixth and seventh decades are more likely to develop EMP than females [1,2].
The symptoms of EMP may vary according to the location of the mass. Eighty percent of EMP involves the head and neck region and upper respiratory tract mucosa and may present as epistaxis, rhinorrhea, nasal obstruction or a neck lump. Less common sites such as the GI tract may present with gastrointestinal bleeding. A primary plasmacytoma of the lung often presents as a pulmonary nodule or hilar mass with or without hemoptysis. Regional lymph nodes may be involved and patients may present with lymphadenopathy [8,9]. Of the nine known cases of EMP of the adrenal glands, six of the patients complained of symptoms including back pain, abdominal pain and fatigue. Three of the nine patients were asymptomatic. In regard to their location, there were five right sided, three bilateral and only one left-sided tumor (Table 1 & 2).
Diagnosis
All reported adrenal plasmacytomas have been found initially on imaging either specifically for symptoms of pain or more commonly found incidentally while imaging was performed for another reason. AIs are found in approximately 1–5% of abdominal CT scans [10]. These AIs then need to be diagnosed as either functioning or nonfunctioning based on blood and urine tests which can include plasma metanephrines, 24‐h urinary catecholamines, a dexamethasone suppression test, renin/aldosterone ratio, 24‐h urinary cortisol, ACTH (adrenocorticotropic hormone) and DHEA (dehydroepiandrosterone) levels. There has been only one reported case of an adrenal plasmacytoma with a hyperfunctional component, all other cases including ours were not hyperfunctional [11]. Further imaging should be performed to ascertain the likelihood of malignancy. A dedicated CT scan of the adrenal glands following strict contrast protocol is performed and the density of the adrenal mass is measured using Hounsfield units and contrast washout is measured. Benign tumors have a Hounsfield density of <10 U, while atypical or malignant lesions show a much higher noncontrast Hounsfield density with less than 50% washout of contrast at 15 min [12,13].
Seven of the nine reported cases of adrenal EMP were diagnosed only on histopathology once the specimen was resected (Table 1). We report the first case that was suspected prior to an operation. Another bilateral case was diagnosed via core biopsy and subsequently successfully treated with chemotherapy and autologous stem cell transplant as opposed to surgery [14].
MM may be differentiated from EMP and SPB by CRAB features (raised serum calcium, renal impairment, anemia and multiple bone lesions) and bone marrow biopsy.
Extramedullary plasmacytoma must also be distinguished from lymphoma. This can be done on biopsy of the primary suspected lesion; demonstrating that the infiltrate consists entirely of plasma cells and that there is no B-cell component (however in our case report biopsy was not useful). In this regard CD138, MUM1/IRF4, CD20 and PAX5 are the most useful markers for lymphoma however it should be recognized that CD20 and PAX5 sometimes express in plasma cell malignancies. Monoclonality and/or an aberrant plasma cell phenotype should be demonstrated with different markers, such as CD19, CD56, CD27, CD117 and cyclin D1 [15]. The diagnosis is made after obtaining a full history of the patient, physical examination, complete blood count, a bone marrow biopsy, serum protein electrophoresis, urine testing for Bence Jones proteinuria and a skeletal survey to detect osteoblastic response to bone destruction [16]. Bone imaging may be done by skeletal survey, CT skeletal survey, whole-body bone MRI or CT-PET. A PET CT or MRI may be more helpful to detect the extent of bone destruction, and may show no lytic lesions and a discrete mass. Diagnostic criteria of EMP are tissue biopsy revealing monoclonal plasma cells, bone marrow plasma cell infiltration which is less than 5% of all nucleated cells, absence of osteolytic bone lesions or other tissue involvement to rule out myeloma [17,18].
Treatment
The treatment of choice for EMP is traditionally radiotherapy (RT) given with a curative intent with usual dose of 40–50 Gy for a 4-week period [19,20]. Five year local disease control is expected to be 90% for patients who received ≥40 Gy compared with 40% for those who received <40 Gy [15]. However in our case with bilateral adrenal involvement, it was decided that the patient should undergo a bilateral adrenalectomy to avoid radiation damage to both kidneys. The role of RT in an adjuvant setting is not clear unless there is a suspicion of residual disease.
In an extended review of literature, surgery alone with negative margins yielded good results and may be enough without the need of combined RT therapy. Adjuvant RT is recommended when the pathology report or margins could not be obtained macroscopically or there is an evidence of lymph node metastasis [18].
Chemotherapy has no beneficial effects on EMP for disease control or progression to MM. Routine adjuvant chemotherapy does not appear to improve recurrence or increase disease-free survival [21,22]. However, for patients with large tumors (>5 cm) and a high-grade histology, adjuvant chemotherapy may be considered as for residual tumors that are unresponsive to RT. It has been suggested that early exposure to chemotherapy may speed the progression of resistant subclones to MM so it is advised to limit the use of chemotherapy itself for a later therapeutic option [18].
Prognostic factors
There are three factors that may determine the prognosis of EMP, these are progression to MM, local recurrence and development of new bone lesion with or without MM. SPB has a poor prognosis when compared with EMP [23,24]. SPB has a higher risk for progression to MM from 65 to 100% in 15 years; the median time for progression to MM is 2–3 years [25]. The 10-year overall survival ratio for EMP is 70% and 5-year survival approaching 100% is expected even when it progresses to MM as compared with 33% for SPB [16].
Surveillance, Epidemiology and End Results database included 1185 patients with EMP and reported a superior median survival among patients with involvement of the head and neck when compared with patients with other sites (13 vs 4 years). The patients with EMP that were included in the Surveillance, Epidemiology and End Results database were more likely to be treated with combined therapy (surgery and RT) than surgery alone [26,27]. Of the five reported cases that have published follow-up findings on adrenal EMP, there have been no documented recurrences, with follow-up ranging from 12 to 72 months despite massive variability in treatment (Table 1).
Follow-up
After the completion of the therapy, patients must be seen at periodic intervals to monitor the possible complications, recurrence and progression of the disease. In general, we advise follow-ups every 3–6 months. During this time patients are re-evaluated with urine and serum protein electrophoresis, M-protein, complete blood count, serum creatinine and serum calcium [27]. In regard to imaging surveillance, ultrasound, CT and MRI may be of use. We have decided to use PET CT for ongoing surveillance as we have demonstrated that adrenal plasmacytoma can be FDG avid.
Conclusion
Adrenal EMP is extremely rare, with only nine reported cases worldwide. EMP can either recur locally or progress to MM. Adrenal EMP is most likely going to be detected incidentally by imaging. In this report, we wish to emphasize the importance of excluding differential diagnoses before undertaking surgery and the importance of multidisciplinary input in the management process.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Cao D, Li L, Xiao W, et al. Solitary extramedullary plasmacytoma of the adrenal gland: a rare case report with review of the literature. Int. J. Clin. Exp. Path. 2014;7(12):9072–9075. [PMC free article] [PubMed] [Google Scholar]
- 2.Husarić S. Solitary extramedullary plasmocytoma of the liver. Acta Med. Academ. 2013;42(1):85–86. doi: 10.5644/ama2006-124.76. [DOI] [PubMed] [Google Scholar]
- 3.Soutar R, Lucraft H, Jackson G, et al. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Clin. Oncol. 2004;16(6):405–413. doi: 10.1016/j.clon.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez L, Couban S, Sy R, Miller R. An unusual presentation of extramedullary plasmacytoma occurring sequentially in the testis, subcutaneous tissue, and heart. Am. J. Hematol. 2001;67(3):194–196. doi: 10.1002/ajh.1106. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Guo Y, Yang Z, Ma E, Min P. Extramedullary plasmacytoma involving the bilateral adrenal glands on MR imaging. Korean J. Radiol. 2007;8(3):246–248. doi: 10.3348/kjr.2007.8.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawashima A, Sandler C, Fishman E, et al. Spectrum of CT findings in nonmalignant disease of the adrenal gland. Radiographics. 1998;18(2):393–412. doi: 10.1148/radiographics.18.2.9536486. [DOI] [PubMed] [Google Scholar]
- 7.Pasch W, Zhao X, Pezk S. Solitary plasmacytoma of the bone involving young individuals, is there a role for preceding trauma? Int. J. Clin. Exp. Path. 2012;5(1):463–467. [PMC free article] [PubMed] [Google Scholar]
- 8.Galieni P, Cavo M, Avvisati G, et al. Solitary plasmacytoma of bone and extramedullary plasmacytoma: two different entities? Ann. Oncol. 1995;6(7):687–691. doi: 10.1093/oxfordjournals.annonc.a059285. [DOI] [PubMed] [Google Scholar]
- 9.Rajkumar SV, Dispenzieri A, Kyle RA. Monoclonal gammopathy of undetermined significance, Waldenström macroglobulinemia, AL amyloidosis, and related plasma cell disorders: diagnosis and treatment. Mayo Clin. Proc. 2006;81(5):693–703. doi: 10.4065/81.5.693. [DOI] [PubMed] [Google Scholar]
- 10.Bovio S, Cataldi A, Reimondo G, et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J. Endocrinol. Invest. 2006;29(4):298–302. doi: 10.1007/BF03344099. [DOI] [PubMed] [Google Scholar]
- 11.Fujikata S, Tanji N, Aoki K, Ohoka H, Hojo N, Yokoyama M. Extramedullary plasmacytoma arising from an adrenal gland. Urology. 2002;60(3):514. doi: 10.1016/s0090-4295(02)01833-2. [DOI] [PubMed] [Google Scholar]
- 12.Boland GW, Lee MJ, Gazelle GS, Halpern EF, McNichols MM, Mueller PR. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR. Am. J. Roentgenol. 1998;171(1):201–204. doi: 10.2214/ajr.171.1.9648789. [DOI] [PubMed] [Google Scholar]
- 13.Korobkin M, Brodeur FJ, Francis IR, Quint LE, Dunnick NR, Goodsitt M. Delayed enhanced CT for differentiation of benign from malignant adrenal masses. Radiology. 1998;200(3):737–742. doi: 10.1148/radiology.200.3.8756924. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed M, Al-Ghambdi A, Al-Omari M, Alijurf M, Al-khadi Y. Autologous bone marrow transplantation for extramedullary plasmacytoma presenting as adrenal incidentaloma. Ann. Saudi Med. 2009;29(3):219–222. doi: 10.4103/0256-4947.51785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes M, Doig A, Soutar R. Solitary plasmacytoma and multiple myeloma: adhesion molecule and chemokine receptor expression patterns. Br. J. Haematol. 2007;137(5):486–487. doi: 10.1111/j.1365-2141.2007.06599.x. [DOI] [PubMed] [Google Scholar]
- 16.Hughes M, Soutar R, Lucraft H, Owen R, Bird J. Guidelines on the diagnosis and management of solitary plasmacytoma of bone, extramedullary plasmacytoma and multiple solitary plasmacytomas: 2009 update. UKMF Guidelines Working Group. British Committee for Standards in Haematology. 2009 [Google Scholar]
- 17.Liebross RH, Ha CS, Cox JD, Weber D, Delasalle K, Alexanian R. Clinical course of solitary extramedullary plasmacytoma. Radiother. Oncol. 1999;52(3):245–249. doi: 10.1016/s0167-8140(99)00114-0. [DOI] [PubMed] [Google Scholar]
- 18.Galieni P, Cavo M, Pulsoni A, et al. Clinical outcome of extramedullary plasmacytoma. Haematology. 2000;85(1):47–51. [PubMed] [Google Scholar]
- 19.Kilciksiz S, Karakoyun-Celik O, Agaoglu F, Haydaroglu A. A review for solitary plasmacytoma of bone and extramedullary plasmacytoma. ScientificWorldJournal. 2012 doi: 10.1100/2012/895765. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Creach K, Foote R, Neben-Wittich M. Radiotherapy for extramedullary plasmacytoma of the head and neck. Int. J. Rad. Oncol. Biol. Phys. 2007;69(3):S540–S541. doi: 10.1016/j.ijrobp.2008.04.077. [DOI] [PubMed] [Google Scholar]
- 21.Tournier-Rangeard L, Lapeyre M, Graff-Caillaud P, et al. Radiotherapy for solitary extramedullary plasmacytoma in the head-and-neck region: a dose greater than 45 Gy to the target volume improves the local control. Int. J. Rad. Oncol. Biol. Phys. 2006;64(4):1013–1017. doi: 10.1016/j.ijrobp.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Galieni P, Cavo M, Avvisati G, et al. Solitary plasmacytoma of bone and extramedullary plasmacytoma: two differena. Ann. Oncol. 1995;6(7):687–691. doi: 10.1093/oxfordjournals.annonc.a059285. [DOI] [PubMed] [Google Scholar]
- 23.Dimopoulos MA, Moulopoulos LA, Maniatis A, Alexanian R. Solitary plasmacytoma of bone and asymptomatic multiple myeloma. Blood. 2000;96(6):2037–2044. [PubMed] [Google Scholar]
- 24.Dores GM, Landgren O, McGlynn KA, Curtis RE, Linet MS, Devesa SS. Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: incidence and survival in the United States, 1992–2004. Br. J. Haemat. 2009;144(1):86–94. doi: 10.1111/j.1365-2141.2008.07421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyle RA. Monoclonal gammopathy of undetermined significance and solitary plasmacytoma: implications for progression to overt multiple myeloma. Hematology. 1997;11(1):71–87. doi: 10.1016/s0889-8588(05)70416-0. [DOI] [PubMed] [Google Scholar]
- 26.Knobel, Zhouhair A, Tsang RW, et al. Prognostic factors in solitary plasmacytoma of the bone: a multicenter Rare Cancer Network study. BMC Cancer. 2006;6:118. doi: 10.1186/1471-2407-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerry D, Lentsch EJ. Epidemiologic evidence of superior outcomes for extramedullary plasmacytoma of the head and neck. Otolaryngol. Head Neck Surg. 2013;148(6):974–981. doi: 10.1177/0194599813481334. [DOI] [PubMed] [Google Scholar]
- 28.Kahara T, Nagai Y, Yamashita H, Nohara E, Kobayashi K, Takamura T. Extramedullary plasmacytoma in the adrenal incidentaloma. Clin. Endocrinol. 2001;55(2):267–270. doi: 10.1046/j.1365-2265.2001.01191.x. [DOI] [PubMed] [Google Scholar]
- 29.Asahi H, Iwasa Y, Komatsu K, et al. A case of plasmacytoma involving the adrenal gland. Hinyokika Kiyo. 2001;47(9):629–631. [PubMed] [Google Scholar]
- 30.Rogers CG, Pinto PA, Weir EG. Extraosseous (extramedullary) plasmacytoma of the adrenal gland. Arch. Pathol. Lab. Med. 2004;128(7):e86–e88. doi: 10.5858/2004-128-e86-EEPOTA. [DOI] [PubMed] [Google Scholar]
- 31.Blanco AF, Bahamonde CS, Blanco AL, Marín Pérez-Tabernero A. Adrenal extramedullary plasmacytoma. Cir Esp. 2011;89:690–691. doi: 10.1016/j.ciresp.2010.06.013. [DOI] [PubMed] [Google Scholar]