Abstract
Melanoma is usually apparent on the skin and readily detected by trained medical providers using a routine total body skin examination, yet this malignancy is responsible for the majority of skin cancer-related deaths. Currently, there is no national consensus on skin cancer screening in the USA, but dermatologists and primary care providers are routinely confronted with making the decision about when to recommend total body skin examinations and at what interval. The objectives of this paper are: to propose rational, risk-based, data-driven guidelines commensurate with the US Preventive Services Task Force screening guidelines for other disorders; to compare our proposed guidelines to recommendations made by other national and international organizations; and to review the US Preventive Services Task Force's 2016 Draft Recommendation Statement on skin cancer screening.
KEYWORDS : early detection, guidelines, keratinocyte carcinoma, melanoma, melanoma odds ratio, melanoma relative risk, melanoma risk factors, screening, skin cancer, USPSTF
Practice points.
Background
Melanoma is a potentially deadly form of skin cancer and is most often evident on the skin's surface.
Skin cancer screening with a total body skin examination (TBSE) is arguably the safest, easiest and possibly the most cost-effective screening test in medicine, yet there is no national consensus regarding its benefit or implementation.
The purpose of this paper is to: propose rational, risk-based, data-driven guidelines for skin cancer screening; compare our proposed guidelines to recommendations made by other national and international organizations; and critique the US Preventive Service's Task Force's (USPSTF) 2016 Draft Recommendation Statement on skin cancer screening in an effort to initiate discourse regarding the USPSTF's conclusions.
Recent melanoma epidemiology: a large & growing problem
Melanoma is now the fifth most common invasive cancer in men and the seventh in women, with an estimated 76,380 new cases in the USA in 2016.
Melanoma-related deaths, estimated to be around 10,130 in 2016, account for the majority of skin cancer-related deaths.
The average 5-year survival rate is 91.5%, but it varies significantly based on the stage of disease, ranging from 98.4% for stage IA to 17.9% for stage IV disease.
Current skin cancer screening practice in primary care: much room for improvement
Total body skin examination is not usually part of the general physical examination performed by primary care providers or nondermatology specialties.
Based on National Health Interview Survey data, only 8% of patients who had seen either a primary care provider or obstetrician/gynecologist within the prior 12 months had received a skin examination.
Our proposed data-driven skin cancer screening guidelines
A target age range of 35–75 years was established based on several trends observed among the various USPSTF-derived age ranges for malignancies that received a grade A or B recommendation, including colorectal, cervical, breast and lung.
Target risk groups were established by comparing the relative risks/odds ratios of melanoma risk factors with the relative risks/odds ratios associated with risk factors for developing other common diseases and malignancies.
Full recommendations are provided.
Review of national skin cancer screening guidelines: what do we have?
Aside from the USPSTF, only a few professional organizations offer specific statements or recommendations about skin cancer screening; these include the American Academy of Dermatology, the American Cancer Society, the American Academy of Family Physicians and the Skin Cancer Foundation.
International screening guidelines: what can we learn from other countries?
Australia, New Zealand, Germany, the Netherlands and the UK recommend screening certain subsets of patients at increased risk for melanoma.
Our screening recommendations were modeled after international guidelines from countries with similarly elevated risk.
Recommendations of national & international organizations: what potential biases exist?
Inevitably, bias exists in all organizations and likely impacts the screening recommendations made by these organizations.
USPSTF recommendation: rationale & critique
USPSTF's 2016 Draft Recommendation on adult skin cancer screening reports that there is insufficient evidence to make a clear statement on the benefit of skin cancer screening.
- Several critical questions regarding the USPSTF draft are reviewed, including:
- Is it appropriate to consider the detection of keratinocyte carcinoma a harm of screening rather than a benefit?
- Why was the morbidity associated with a delayed diagnosis of basal and squamous cell carcinoma and melanoma omitted in the USPSTF risk estimates?
- Is the number of excisions needed to treat basal cell carcinoma (one out of nine) and melanoma (one out of 28) too high, particularly given the morbidity increases with delayed diagnosis?
- In the USA, are the majority of biopsies for keratinocyte carcinoma excisional, and are the majority of excisional biopsies for melanoma the same type reported in the German article?
- Is it valid to extrapolate satisfaction of results from a cosmetic procedure to results from a diagnostic procedure for cancer?
- How were the inclusion and exclusion criteria applied to the worldwide publications that ultimately formed the rationale for the decision statement?
Melanoma is a potentially deadly form of skin cancer that is most often evident on the skin's surface. The vast majority of melanomas are curable if caught early, yet over 10,000 people in the USA will die of melanoma in 2016 [1] (the age-adjusted mortality rate was 2.7 deaths per 100,000 adults based on cases and deaths from 2009 to 2013) [2]. Exploring opportunities to decrease mortality from this largely visible cancer are warranted. Screening for melanoma involves a total body skin examination (TBSE), a relatively quick, inexpensive and noninvasive process compared with screening for internal malignancies, such as colorectal, lung and breast cancer.
In fact, skin cancer screening through TBSE is arguably the safest, easiest and possibly most cost-effective screening test in medicine [3], but there is no consensus regarding its benefit or implementation and no randomized controlled trial (RCT) proving that screening reduces melanoma mortality. To complicate this issue, dermatologists and other healthcare providers already routinely perform skin cancer screening examinations, as part of routine clinical care and through nationwide public health initiatives such as the SPOTMe® program sponsored by the American Academy of Dermatology (AAD) [4]. Proponents of skin cancer screening believe that routine TBSE identifies early-stage melanomas and other invasive cutaneous malignancies that might otherwise progress to life-threatening advanced stages. However, proving this case is not straightforward.
Conducting RCTs to evaluate skin cancer screening is fraught with challenges. Comparing mortality due to melanoma in screened versus nonscreened individuals would be difficult and costly, requiring a large population (stratified by clearly delineated melanoma risk factors) and a particularly long follow-up interval to demonstrate a correlation [5]. In addition, identifying a control population might be considered unethical, as a subset of individuals with elevated melanoma risk may be randomized into a nonscreening arm. Also, the potential exists for bias or erroneous comparisons between screened and controlled groups if uneven opportunistic screening occurs. Thus, the absence of a large prospective RCT should not preclude the development of skin cancer screening recommendations in the USA.
Given the unavoidable limitation in the quality of evidence for skin cancer screening benefits, it is not surprising that routine screening recommendations remain inconsistent among professional and advocacy organizations such as the US Preventive Services Task Force (USPSTF), the AAD, the American Cancer Society (ACS), the American Academy of Family Physicians (AAFP) and the Skin Cancer Foundation (SCF). Herein, we propose rational, risk-based, data-driven guidelines for skin cancer screening that are internally consistent with USPSTF guidelines for other cancers and diseases. We then compare our proposed recommendations to those of other national and international organizations. Finally, we critique the USPSTF's 2016 Draft Recommendation Statement on skin cancer screening with respect to six observed deficiencies in an effort to initiate discourse regarding the USPSTF's current conclusion. To ensure broad applicability of our recommendations, we included input from a variety of melanoma experts, spanning several disciplines (e.g., social psychology, epidemiology, clinical research and practitioners) and medical subspecialties (e.g., dermatology, dermatopathology, cutaneous oncology, surgical oncology and medical oncology).
Recent melanoma epidemiology: a large & growing problem
Over the past four decades, melanoma incidence has increased by nearly 200% [2]. It is now the fifth most common invasive cancer in men and the seventh in women, with an estimated 76,380 new cases in the USA in 2016 [1,6]. About one in 33 men and one in 52 women in the USA will develop melanoma during their lifetime [1]. Incidence and mortality rates are highest among older adults (aged 55–74 years and 75–84 years, respectively) [2]; however, melanoma is the most common cancer in young adults aged 25–29 years, with females being disproportionately affected in this age group [7]. It has been postulated that higher rates of melanoma in young women compared with young men is due to hormonal differences, physiologic effects of pregnancy and/or increased indoor tanning exposure [8,9]. Melanoma deaths account for the majority of skin cancer-related deaths, estimated to be around 10,130 in 2016 [2]. The average 5-year survival rate is 91.5% overall but varies significantly based on the stage of disease, ranging from 98.4% for localized disease, to 17.9% for distantly metastatic disease [2]. While advances in the treatment of metastatic melanoma will likely improve these statistics substantially, a diagnosis of metastatic melanoma remains grave, with several new treatment regimens resulting in significant contributions to healthcare costs. Moreover, there is no sign that the rise in melanoma incidence is slowing; melanoma incidence rates are predicted to increase roughly 50% over 2010 levels by 2020 and 100% over 2010 levels by 2030 [10].
Current skin cancer screening practice in primary care: much room for improvement
TBSE is not usually part of the general physical examination performed by primary care providers (PCPs) or nondermatology specialists [11]. More often, only exposed areas relevant to the physical exam are evaluated. While over half of PCPs feel that skin cancer screening is ‘extremely’ important [12], skin cancer screening is not common in the primary care setting in the USA, likely due to time constraints as well as the lack of emphasis and training in medical school and residency. Two surveys found that roughly two-thirds of medical students and three-quarters of primary care residents felt that they had inadequate training in performing a TBSE [13,14]. As a result, a stronger emphasis on skin cancer screening education should be implemented in US medical schools, as well as continued medical education courses, to ensure quality TBSEs are performed in the primary care setting.
According to a study analyzing National Health Interview Survey (NHIS) data, only 8% (1070 of 13,381) of patients who had seen either a PCP or obstetrician/gynecologist within the prior 12 months had received a skin examination [11]. Based on a similar study incorporating NHIS data, only 24% of ‘high-risk’ individuals reported having undergone a TBSE once in their lifetime, and only about 11% (11,988,052 of 104,671,157 participants) had a TBSE within a year [15]. ‘High-risk’ is a nonspecific term in melanoma literature and was defined in this study as non-Hispanic, white men and women aged >65 years; individuals with a history of sunburn; and/or individuals with a family history of skin cancer [15]. Despite the technical simplicity of TBSE, a third study using NHIS data reported remarkably low skin cancer screening rates (16% in men and 13% in women) compared with the screening rates for colorectal (51%), breast (54%) and prostate (43%) cancers [16].
In summary, skin cancer screening rates remain low in the USA, rendering high-risk populations vulnerable to a delay in melanoma diagnosis. Clarification of ‘high-risk’ groups and the risk-stratified screening recommendations outlined here may encourage PCPs to incorporate TBSE into routine wellness exams (which generally involve conducting age-appropriate risk assessments and screening tests supported by the USPSTF) [17]. Skin is the only fully accessible organ for clinical examination by visual inspection; therefore, TBSE provides the unique secondary benefit of visually screening for numerous systemic diseases simultaneously [18,19].
Our proposed data-driven skin cancer-screening guidelines
Despite a lack of national consensus on skin cancer screening guidelines for asymptomatic patients, clinical dermatologists and PCPs are routinely confronted with making a decision about when to recommend TBSE and at what time interval. Currently, recommendations are diverse, with no unifying rationale for screening. This paper aims to propose evidence-based screening guidelines, commensurate with other USPSTF screening guidelines, by identifying a subset of patients that fall into a risk category consistent with other diseases. As with USPSTF guidelines, the primary goals for developing skin cancer screening recommendations included identification of a target age range of individuals to screen (based on incidence and mortality rates) and identification of a high-risk group of individuals (based on relative risk and odds ratio data) that could most benefit from skin cancer screening (Box 1).
Box 1. . Proposed skin cancer screening guidelines.
Adults aged 35–75 years with one or more of the following risk factors should be screened at least annually with a total body skin examination†:
-
Personal history:
History of melanoma, AK or KC
CDKN2A (or other high-penetrance gene‡) mutation carrier
Immunocompromised§
-
Family history:
Melanoma in one or more family members
Family history suggestive of a hereditary predisposition to melanoma
-
Physical features:
Light skin (Fitzpatrick I-III¶)
Blonde or red hair
>40 total nevi
Two or more atypical nevi#
Many freckles
Severely sun-damaged skin
-
UVR overexposure:
History of blistering or peeling sunburns
History of indoor tanning
†Total body skin exam includes evaluation of entire skin surface (scalp, face, ears, neck, chest, abdomen, back, buttocks, genitals, upper and lower extremities, hands, feet), eyes (iris and sclera), oral mucosa, hair and nails.
‡High penetrance genes: CDKN2A, CDK4, MITF, BAP1, p14 ARF, TERT, POT1, ACD, TERF2IP, BRCA2, PTEN [20–22].
§Immunocompromised includes patients with suppressed immune systems due to a disease (e.g., HIV/AIDS) or medication (e.g., anti-rejection medications for organ transplants, chemotherapies or immunosuppresants for autoimmune disorders).
¶Fitzpatrick skin types [23].
#Atypical nevi clinical criteria is based on International Agency for Research on Cancer [24]. Consideration should be given to individuals that have a combination of risk factors, potentially elevating them to a higher risk category.
AK: Actinic keratosis; KC: Keratinocyte carcinoma; UVR: Ultraviolet radiation.
Clearly, guidelines are not absolute, and more or less stringent screening can be appropriate in individual circumstances. These guidelines are meant to serve as a starting point for further discussion and may be refined as additional data become available.
Target age group for skin cancer screening
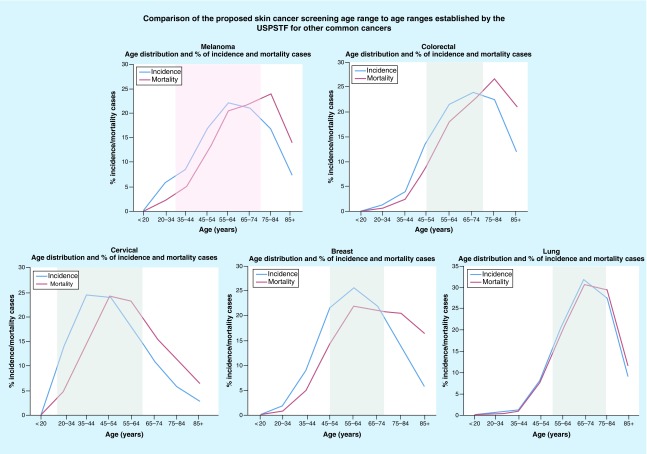
Initially, we compared melanoma to other cancers that have received a USPSTF grade A or B rating, with the goal of defining a comparable age range to screen. Screening methods categorized as grade A and B are recommended by the USPSTF and are differentiated based on the degree of certainty that the net benefit is either substantial or moderately substantial [25]. The rationale used by the USPSTF to define screening ages for other cancers is unclear, however. Therefore, we examined the recommended target age ranges for malignancies that received a grade A or B recommendation (including colorectal, cervical, breast and lung). We then determined the number of affected individuals falling within these age ranges [25]. More specifically, we associated the screening age ranges of these cancers to age-stratified incidence and mortality rates, median age at diagnosis and the total percent sum of incidence rates falling within an age range. Next, the numbers of affected individuals in each category across the different cancer types were determined. These values were remarkably similar among the grade A and B cancers and provided a reproducible approach to defining a recommended age range for skin cancer screening.
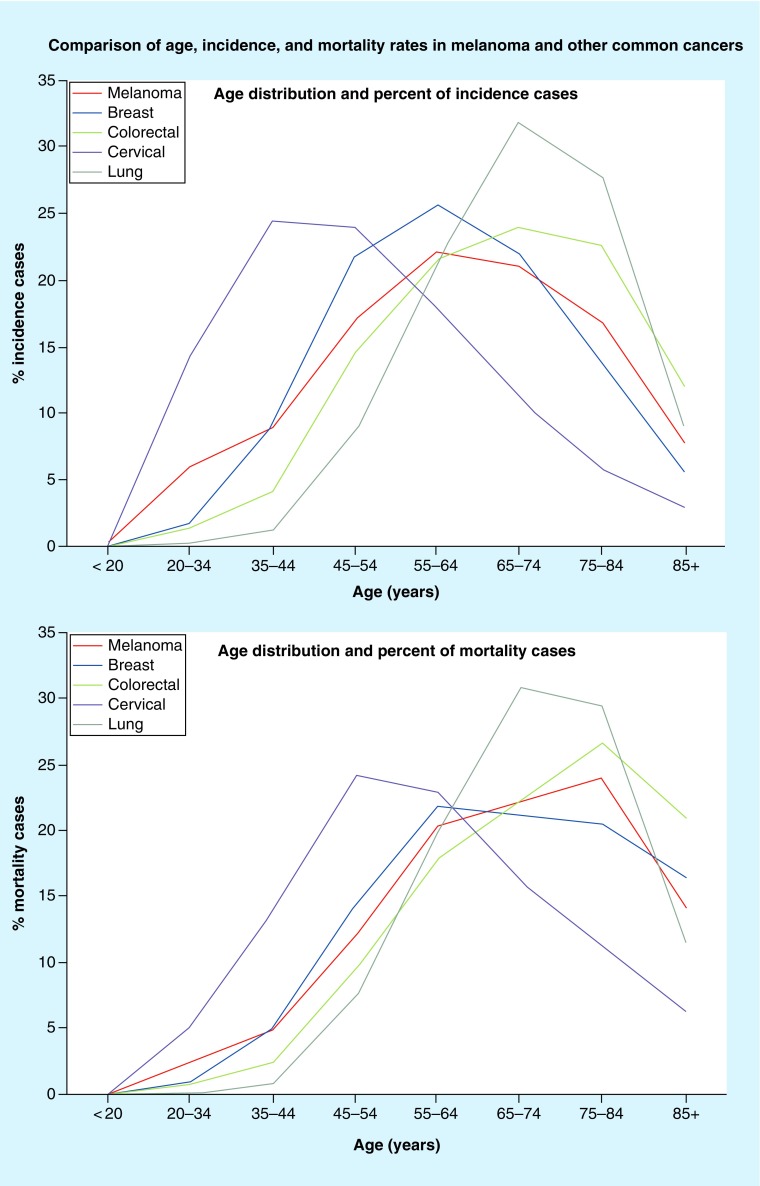
Data from the National Cancer Institute Surveillance, Epidemiology and End Results (SEER) Registry were used to evaluate USPSTF-recommended screening age ranges relative to the percent of incidence and mortality rates at the associated ages for each of the colorectal, cervical, breast and lung cancer types (Figures 1 & 2) [26,27]. Based on these data, the ages recommended by the USPSTF for screening initiation and termination for grade A and B cancers, lie at or near the steepest positive and negative slopes of the incidence and mortality curves. In other words, initiation of screening occurs at an age in which the slope of the incidence and mortality curves are at or near the steepest incline and screening ends at an age in which the incidence and mortality curves are at or near the steepest decline. Based on the slope of the incidence and mortality curves for melanoma, the steepest incline is near age 35 years, and the steepest decline is near age 77 years. In addition to the slope of the incidence and mortality curves, we also compared the percentage of cases falling into the age ranges adopted for each cancer. From 2008 to 2012, the percentage of cancer cases that occurred within the recommended age ranges was 60% for colorectal (45–74 years), 69% for breast (45–74 years), 80% for cervical (20–64 years) and 81% for lung cancer (55–84 years). For melanoma, 70% of cases fell within the 35–74-year age range, 60% fell within 45–74 years and 86% fell within 35–84 years.
Figure 1. . Surveillance, Epidemiology and End Results derived data, 2008–2012; comparison of the percentage of incidence and percentage mortality cases based on age.
Figure 2. . Surveillance, Epidemiology and End Results derived data, 2008–2012; comparison of the percent of incidence and percent mortality cases based on age.
Data include all races and both sexes [26,27]. USPSTF screening guidelines for the above cancers include: melanoma – grade I; breast cancer – grade B; colorectal cancer – grade A; cervical cancer – grade A; lung cancer – grade B. Areas shaded in gray indicate the target screening age group based on USPSTF guidelines. Area shaded in pink indicates our proposed skin cancer screening age group.
USPSTF: US Preventive Sevices Task Force.
Finally, the median age at diagnosis for each cancer was compared with the USPSTF screening initiation recommendations for colorectal, cervical, breast and lung cancer [2,28–31]. In these grade A and B cancers, the recommended age of initiation of screening was between 12 and 28 years prior to the median age at diagnosis. For example, the recommendation for initiation of breast cancer screening was age 50 years (median age 62 years), and the recommendation for initiation of cervical cancer screening was age 21 years (median age 49 years) [2,26,28–31]. The median age at diagnosis for melanoma is 63 years, suggesting that the range of possible initiation of screening should be somewhere between ages 35 and 51 years [2].
Taken together, it seems reasonable to propose a screening age of 35–75 years for melanoma. Although the declining slope of the incidence and mortality curves suggests a slightly older termination of screening (age 77 years), when taken in the context of the percentage of cases within the various age ranges and the median age at diagnosis, a slightly lower age of termination seems more reasonable and better aligned with the other cancer types. Furthermore, given the additional risk factors outlined below, individuals with elevated risk that fall outside of these age ranges could still be screened outside the guidelines.
Target risk group for skin cancer screening
After defining a target age range for the skin cancer screening population (see above), we sought to better refine the recommendations by incorporating risk factors into the assessment. Well-established, published melanoma risk factors were identified, along with their relative risks, and were used to determine subpopulations at elevated risk of developing cutaneous melanoma. In this way, individuals at low risk of developing melanoma were eliminated from the recommendations. Fortunately, risk factors for melanoma and keratinocyte carcinomas (KC), (i.e., nonmelanoma skin cancer) such as basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (SCC) [32], overlap substantially, making the recommendations applicable for skin cancer in general. To identify the most appropriate risk factors to include in screening recommendations, the relative risks/odds ratios (RRs/ORs) associated with melanoma risk factors were compared with the RRs/ORs associated with other common diseases and malignancies that have received a USPSTF grade A or B screening recommendation based on risk (Tables 1 & 2). Colorectal and cervical cancers were excluded from these tables, as USPSTF guidelines recommend whole-population screening based on age and do not specify alternative-screening regimens based on risk factors.
Table 1. . Risk levels of other disorders resulting in grade A and B US Preventive Service's Task Force recommendations.
| Study (year) | USPSTF screening recommendations based on risk factors | Risk factors | Comparison | RR/OR (95% CI) | Ref. |
|---|---|---|---|---|---|
| Wang (2006) | High blood pressure in adults: – Grade A: Screening should begin with all adults age 18 years or older. Adults aged 18–39 years should be screened every 3–5 years if BP is normal with no risk factors. Adults aged <40 years with risk factors or ≥40 years without risk factors should be screened annually Risk factors include high-normal BP, overweight or obese, or African–American |
High-normal BP (130–139/85–89 mmHg) Overweight (BMI 25–29.9) Obese (BMI ≥30 |
High-normal BP vs normal BP Overweight vs normal BMI Obese vs normal BMI |
†3.5 (3.0–4.1) †1.5 (1.1–1.8) †1.9 (1.5– 2.4) |
[33] |
| Mokdad (2003) | Lipid disorders in adults (cholesterol, dyslipidemia): – Grade A: Men aged ≥35 years and women aged ≥45 years should be routinely screened. Interval uncertain, but every 5 years is reasonable – Grade B: Men aged 20–35 years and women aged 20–45 years should be screened if they are at increased risk for coronary artery disease. Interval uncertain, but every 5 years is reasonable |
Obesity BMI 30–39.9 Morbid obesity BMI 40–49.8 |
Obese vs normal BMI Morbidly obese vs normal BMI |
†1.9 (1.8– 2.0) †1.9 (1.7– 2.1) |
[34] |
| Kent (2010) Vardulaki (2000) |
AAA: – Grade B: Men aged 65–75 years who have ever smoked should undergo one-time screening for AAA with ultrasonography |
Gender Smoking history |
Male vs female Ever smoker vs never smoker |
†5.7 (5.6–5.9) †3.1 (2.1– 4.5) |
[35] [36] |
| Mokdad (2003) | Abnormal blood glucose and Type 2 diabetes screening: – Grade B: Adults aged 40–70 years who are overweight or obese should be screened for abnormal blood glucose as part of cardiovascular risk assessment every 3 years |
Overweight or obese | Overweight (BMI 25–29.9) vs normal BMI Obese (BMI 30–39.9) versus normal BMI Morbidly obese (BMI ≥ 40) vs normal BMI |
†1.6 (1.5–1.7) †3.4 (3.2– 3.7) †7.4 (6.4– 8.5) |
[34] |
| Brose (2002) Pharoah (1997) |
Breast cancer: – Grade B: Women with a family history associated with an increased risk of BRCA1 and BRCA2 mutations should be referred for genetic counseling and evaluation for BRCA testing |
BRCA1 mutations Family history |
BRCA1 mutation carrier vs none carrier First-degree relative vs no relative |
6.1 (lifetime risk to age 70 years) 2.1 (2.0–2.2) |
[37] [38] |
| Pesch (2012) | Lung cancer: – Grade B: Adults aged 55–80 years with a 30-pack year history who currently smoke or quit smoking within 15 years should be screened annually for lung cancer with low-dose CT |
Smoking history | Men: current vs never Women: current vs never Men: 30–40 pack-years vs never smokers Women: 30–40 pack-years vs never smokers |
†23.6 (20.4–27.2) †7.8 (6.8–9.0) †24.6 (20.8–29.0) †12.9 (9.9–16.9) |
[39] |
USPSTF screening guidelines that require risk stratification, the relative risks associated with these diseases and how they compare with melanoma relative risk.
†Values that are odd ratios (instead of relative risk).
AAA: Abdominal aortic aneurysm; BP: Blood pressure; CT: Computed tomography; OR: Odds ratio; RR: Relative risk; USPSTF: US Preventive Services Task Force.
Table 2. . Relative risk of developing melanoma compared with relative risk of developing other US Preventive Services Task Force grade A/B diseases.
| Melanoma risk factors | Melanoma RR/†OR | Comparable disease (risk factor) | Comparable disease RR/OR | Comparable screening modality |
|---|---|---|---|---|
| One atypical nevus vs 0* | 1.5 [24] | High BP (overweight vs normal BMI) | †1.5 [33] | BP cuff |
| Total common nevi 16–40 vs <15* | 1.5 [24] | High BP (overweight vs normal BMI) | †1.5 [33] | BP cuff |
| Blue eye color vs dark* | 1.5 [40] | High BP (overweight vs normal BMI) | †1.5 [33] | BP cuff |
| Hazel eye color vs dark* | 1.5 [40] | High BP (overweight vs normal BMI) | †1.5 [33] | BP cuff |
| Green eye color vs dark* | 1.6 [40] | Type II diabetes (overweight vs normal BMI) | †1.6 [34] | Blood test |
| Light brown hair vs dark* | 1.6 [40] | Type II diabetes (overweight vs normal BMI) | †1.6 [34] | Blood test |
| Indoor tanning ever use in men/women vs never use* | †1.7 [41] | Type II diabetes (overweight vs normal BMI) | †1.6 [34] | Blood test |
| Fitzpatrick II vs IV*§ | 1.8 [40] | High BP (obese vs normal BMI) | †1.9 [33] | BP cuff |
| Fitzpatrick III vs IV* | 1.8 [40] | |||
| History of sunburn vs no history* | 2.0 [42] | Lipid disorders (obese vs normal BMI) | †1.9 [34] | Blood test |
| Blond hair vs dark* | 2.0 [40] | Lipid disorders (morbidly obese vs normal BMI) | †1.9 [34] | Blood test |
| 2 atypical nevi vs 0* | 2.1 [24] | Lipid disorders (morbidly obese vs normal BMI | †1.9 [34] | Blood test |
| Fitzpatrick I vs IV* | 2.1 [40] | Lipid disorders (morbidly obese vs normal BMI | †1.9 [34] | Blood test |
| High density of freckles vs low* | 2.1 [40] | Breast cancer (first degree relative vs no relative) | 2.1 [38] | Mammogram |
| Total common nevi 41–60 vs <15* | 2.2 [24] | Breast cancer (first degree relative vs no relative) | 2.1 [38] | Mammogram |
| Indoor tanning ever use in women aged 40–49 vs never use* | †2.3 [8] | Breast cancer (first-degree relative vs no relative) | 2.1 [38] | Mammogram |
| Family history of melanoma in 1 or more 1st degree relative¶ | 1.7–3.0 [40,43–44] | AAA (ever smoker vs never smoker) | †3.1 [36] | Ultrasound |
| 3 atypical nevi vs 0¶ | 3.0 [24] | AAA (ever smoker vs never smoker) | †3.1 [36] | Ultrasound |
| Total common nevi 61–80 vs <15¶ | 3.3 [24] | Type II diabetes (obese vs normal BMI) | †3.4 [34] | Blood test |
| Red hair vs dark¶ | 3.6 [40] | High blood pressure (high-normal BP vs normal BP) | †3.5 [33] | BP cuff |
| History of AK and/or KC vs no history¶ | 4.3 [40] | High blood pressure (high-normal BP vs normal BP) | †3.5 [33] | BP cuff |
| Indoor tanning ever use in women aged 30–39 years vs never use¶ | †4.3 [8] | AAA (male vs female) | 5.7 [35] | Ultrasound |
| 4 atypical nevi vs 0¶ | 4.4 [24] | AAA (male vs female) | 5.7 [35] | Ultrasound |
| Indoor tanning ever use in women aged <30 years vs never use# | †6.0 [8] | Breast cancer (BRCA1 mutation carriers) | 6.1 [37] | Mammogram |
| 5 atypical nevi vs 0# | 6.4 [24] | Type II diabetes (morbidly obese vs normal BMI) | †7.4 [34] | Blood test |
| Total common nevi 101–120 vs <15# | 6.9 [24] | Lung cancer (current female smoker vs never smoker) | †7.8 [39] | CT scan |
| Personal history of melanoma# | ‡8.2–13.4 [45] | Lung cancer (30–40 pack-year smoking history in woman) | †12.9 [39] | CT scan |
| CDKN2A mutation carrier# | §14–28 [46] | Lung cancer (current male smoker vs never) Lung Cancer (30–40 pack-year smoking history in males) |
†23.6 [39] †24.6 [39] |
CT scan CT scan |
The diseases and malignancies chosen for comparisons have received US Preventive Services Task Force grade A or B screening recommendations based on risk. Comparable risk factors are listed in order of increasing risk (* signifies minimally increased risk, ¶ signifies moderately increased risk, # signifies greatly increased risk).
†Odds ratio.
‡Risk estimate ranges based on risk at age ≥50 years and risk at age <30 years.
§Risk estimate range based on risk at age 50 years and risk at age 80 years.
AAA: Abdominal aortic aneurysm; AK: Actinic keratosis; BP: Blood pressure; BMI: Body mass index; KC: Keratinocyte carcinoma.
The data contained in Tables 1 & 2 were condensed and simplified to create the final guidelines (Box 1). Of note, these guidelines are not intended for individuals diagnosed with melanoma in the past 5 years. These melanoma patients should be followed according to established melanoma guidelines, such as those produced by the National Comprehensive Cancer Network (NCCN) or the AAD [47,48]. Additionally, there is no reason to avoid examining the skin in any area being examined for other purposes, also known as opportunistic screening (e.g., evaluation of the skin on the chest when auscultating the lungs).
In reviewing the USPSTF grade A and B screening recommendations that have been based on risk factors (e.g., hypertension and aortic aneurysm), we determined that most recommendations were associated with RRs of at least 1.8–2.0 (Table 1). We identified melanoma risk factors that reached or exceeded these RR levels (Table 3). For example, ‘ever smokers’ face a risk (OR: 3.1) [36] of developing an abdominal aortic aneurysm, which is comparable to the risk an individual with three atypical nevi has of developing melanoma (RR: 3.0) [24]. Using this rationale, we combined the risk factors that met the criteria of similarity to other recommended USPSTF screening methods (RR/OR of ≥1.8–2.0) into simple categories that would be easy to remember and apply to everyday practice (Box 1). In sum, we recommend that any asymptomatic individual in the USA between the ages of 35 and 75 years who has one or more of the following risk factors in any of the four categories be screened at least annually: a personal history of melanoma, BCC, SCC, actinic keratosis or ongoing immunocompromise; a family history of melanoma in one or more first-degree relatives or a family history suggestive of a hereditary predisposition to melanoma [49,50]; one or more physical features suggestive of high-risk, including lightly colored skin (Fitzpatrick skin types I–III), blonde or red hair, greater than 40 moles, greater than two atypical moles, freckles or severely sun-damaged skin; and ultraviolet radiation overexposure, including a history of sunburn or indoor tanning. We do not recommend screening for patients without risk factors. These guidelines obviously need to be applied in the context of individual circumstances. There may be individuals with greater risk that require more frequent screening (e.g., those with a CDKN2A mutation or a personal history of multiple melanomas in the setting of a large number of nevi and/or atypical nevi [51]) or screening outside of the 35–75 years old age range (e.g., a child with a giant congenital nevus [52]).
Table 3. . Comparison of US national skin cancer screening and counseling guidelines.
| US professional organization | Screening and counseling recommendations |
|---|---|
| US Preventive Services Task Force | Screening: – Published statement 2009: insufficient evidence to assess the balance of benefits and harms of screening for skin cancer by primary care providers or by patient skin self-examination [53]. Grade I† – Draft statement recommendation 2016: a clear statement cannot be made about the benefit of skin cancer screening for melanoma mortality and all-cause mortality or association with thinner lesions [54] Counseling: – Published statement 2012: it is recommended that children, adolescents and young adults aged 10 to 24 years who have fair skin be counseled about minimizing their exposure to UV radiation to reduce the risk for skin cancer [55]. Grade B† – Published statement 2012: there is insufficient evidence to assess the balance of benefits and harms of counseling adults older than age 24 years about minimizing risks to prevent skin cancer [55]. Grade I† |
| American Academy of Family Physicians | Screening: – Published statement 2009: current evidence is insufficient to assess the balance of benefits and harms of using a whole-body skin examination by a primary care provider or patient skin self-examination for the early detection of cutaneous melanoma, basal cell carcinoma or squamous cell carcinoma in the adult general population [56]. Grade I† Counseling: – Published statement 2012: it is recommended that children, adolescents and young adults ages 10 to 24 years who have fair skin be counseled about minimizing their exposure to UV radiation to reduce the risk for skin cancer [56]. Grade B† – Published statement 2012: There is insufficient evidence to assess the balance of benefits and harms of counseling adults older than age 24 years about minimizing risks to prevent skin cancer [56]. Grade I† Updated statement for 2016 pending. |
| American Cancer Society | Screening: – Published statement 2015: for people aged 20 or older who get periodic health examinations, a cancer-related check-up should include health counseling and, depending on a person's age and gender, examinations for cancers of the thyroid, oral cavity, skin, lymph nodes, testes and ovaries, as well as for other diseases besides cancer (i.e., tobacco, diet and nutrition, sexual practices, risk factors and environmental and occupational exposures [57] – Published statement 2016: the Society recommends periodic cancer-related checkups to examine thyroid, oral cavity, skin, lymph nodes, testicles and ovaries [1]. Recommendations no longer include a specified age group |
| American Academy of Dermatologists | Screening: – Published statement 2015: the Academy encourages all members of the public to serve as their own health advocates by regularly conducting skin self-examinations. If an unusual lesion is detected, or if any lesions are changing, itching or bleeding, it is recommended that individuals seek evaluation by a board-certified dermatologist. It is also recommended that people with either a history of skin cancer or an increased risk of skin cancer discuss routine screening increments with a doctor [58] |
| Skin Cancer Foundation | Screening: – Recommend annual skin examinations with a physician [59] |
†Current skin cancer screening and counseling guidelines based on several US medical organizations.
Grade B and Grade I are based on US Preventive Services Task Force grading definitions. Grade B: High certainty that the net benefit is moderate or moderate certainty that the net benefit is moderate to substantial. The service is recommended by the US Preventive Services Task Force and should be offered or provided to the patient. Grade I: The current evidence is insufficient to assess the balance of benefits and harms of the service.
The rationale we used above to establish our guidelines for skin cancer screening is in good alignment with what is already being done in many dermatologic practices. For example, the recommendation of an annual skin exam is consistent with the frequency of screening recommended by the SCF. It is also in accord with most international recommendations (see section on International Guidelines, Table 4).
Table 4. . Comparison of international skin cancer screening and counseling guidelines .
| Organization | Guidelines | Population | Frequency | Counseling | Ref. |
|---|---|---|---|---|---|
| Australia & New Zealand | |||||
| CCA and Australasian College of Dermatologists | Routine TBSE | ‘High-risk’ patients, defined as: – Fair skin, light eyes, light or red hair – Tendency to burn – Freckles – Increased number of dysplastic nevi – Immunosuppression – FH of melanoma in first degree relative – PH of melanoma or KC |
Every 3–12 months | How to detect lesions suspicious for melanoma and when to seek advice from medical practitioner | [60] |
| NHMRC (with the CCA and the New Zealand Ministry of Health) | Routine TBSE (grade B†) | ‘High-risk’ patients, assessed by: – Skin/hair color – Sun sensitivity – Number of common and atypical nevi – Chronic actinic skin damage – FH of melanoma – PH of melanoma or KC – Age and gender |
Every 6 months | How to detect lesions that are suspicious for melanoma | [61] |
| Royal Australian College of General Practitioners | Routine TBSE | ‘High-risk’ patients, defined as: – >6-fold increased risk of melanoma: – Multiple dysplastic nevi – FH of melanoma in first-degree relative – PH of melanoma |
Every 3–12 months | How to detect lesions suspicious for skin cancer and how to prevent skin cancer | [62] |
| Opportunistic skin examinations | ‘Average or increased risk’ patients, defined as two- to fivefold increased risk of melanoma | Opportunistically | |||
| The UK | |||||
| British Association of Dermatologists | Routine TBSE (grade B†) | ‘Moderately increased risk’ patients: – Clinically atypical nevi – Large number of nevi – PH of melanoma |
Interval undefined Refer to specialist |
[63] | |
| ‘Greatly increased risk’ patients: – Giant congenital nevi |
Interval undefined Lifetime monitoring |
||||
| FH of melanoma in 3 or more members or FH of pancreatic cancer | Interval undefined Refer to specialist |
||||
| The Netherlands | |||||
| Dutch Working Group on Melanoma | Routine TBSE | At risk patients: – 5 or more atypical nevi – 100 or more common nevi |
Annually | How to perform an SSE and identify risk factors | [64] |
| First-degree relatives with diagnosis of familiar melanoma/FAMMM syndrome or CDKN2A mutation | 1–2-times per year (starting at 12 years) | ||||
| Second-degree relatives with known CDKN2A mutation | 1–2-times per year (starting at 20 years) | ||||
| Germany | |||||
| Germany | Routine TBSE | All adults age 35 years or older with health insurance | Every 2 years | [65] | |
| German Guideline Program in Oncology | Routine TBSE (EC) | At risk patients, assessed by: – Skin type – Chronic actinic skin damage – Number of acquired or atypical nevi – Large congenital nevus – Immunosuppression – FH of melanoma – PH of melanoma, AK or KC |
Determined by physician and patient | How to detect lesions suspicious for skin cancer and how to perform SSE | [66] |
†Most organizations recommend screening only at-risk individuals. Notice how most of the above recommendations are based risk factors. Germany is the only country that offers whole-population screening.
Grade B: Body of evidence can be trusted to guide practice in most situations [61].
AK: Actinic keratosis; CCA: Cancer Council Australia; EC: Expert opinion; FAMMM: Familial Atypical Multiple Mole Melanoma Syndrome; FH: Family history; KC: Keratinocyte carcinoma; NHMRC: National Health and Medical Research Council; PH: Personal history; SSE: Self-skin exam; TBSE: Total body skin examination.
Other screening considerations
In adults ≥18 years old with fair skin, TBSE can be considered at the time of their first wellness exam, if this occurs before age 35 years. This is consistent with the USPSTF grade B recommendation that children and young adults ages 10–24 years be counseled regarding the desirability of protecting their skin from ultraviolet radiation [55]. A special appointment for skin cancer screening does not need to be scheduled; the baseline TBSE can instead be performed opportunistically, that is, at a time that is convenient for the provider and amenable to the patient, such as a wellness exam. At this time, patients should also be educated about risk factors, sun protection and self-skin exams. By counseling and performing TBSE to demonstrate a model of skin examination, providers may capitalize upon a teachable moment to encourage positive behavior change. If one or more risk factor is identified during this introductory exam, the practitioner and patient may then decide what screening interval is suitable until annual skin exams begin at 35 years. As data become more available, we may continue to refine the interval.
A TBSE involves inspection of the entire skin surface, including the scalp, hair, nails, oral mucosa, eyes, genitals and anus [67,68]. Much of this examination can be accomplished during a thorough physical examination when the patient is undressed to evaluate other organ systems such as cardiac, gastrointestinal, musculoskeletal and pulmonary systems. A self-skin exam involves a systematic evaluation of most areas of the skin with either a partner or a mirror to assist in visualizing the scalp, back and buttocks [69].
Review of US national skin cancer screening guidelines: what are the recommendations?
In the development of skin cancer screening guidelines, it is important to evaluate the recommendations presented here in the context of existing US national guidelines. Aside from the USPSTF, few professional organizations offer specific statements or recommendations about skin cancer screening. These organizations include the AAFP, the AAD, the ACS and the SCF. The AAFP echoes the USPSTF, reporting insufficient evidence to recommend skin cancer screening [54,56]. The ACS includes skin cancer screening as part of a generalized, periodic cancer-related check-up, but does not specify target population, age range or frequency [1]. The AAD recommends that physicians assess patient risk factors to devise individualized screening recommendations [58]. Lastly, the SCF recommends annual TBSE without specifying a target population or age range. The American College of Preventive Medicine, the American College of Physicians, the American Joint Committee on Cancer, the NCCN, the American Society of Clinical Oncology and the National Cancer Institute offer no official guidance regarding the performance of skin examinations by physicians [70–75]. Recommendations from the above organizations are summarized in Table 3.
International screening guidelines: what can we learn from other countries?
The various approaches to screening recommendations around the world may be divided into four categories: no recommendations due to insufficient evidence, opportunistic screening for high-risk populations, routine screening in high-risk populations and routine whole population screening.
Most countries evaluated in this review, including Australia, New Zealand, Germany, the Netherlands and the UK, recommend screening certain subsets of patients at increased risk for melanoma (variably defined) [60–66,76,77]. Many of these countries include specific recommendations for screening intervals as well. Germany is the only country that offers whole-population skin cancer screening (for adults aged 35 years and older) [65] in addition to high-risk population guidelines. In Australia, the Royal Australian College of General Practitioners recommends opportunistic screening for patients at average or mildly increased risk of melanoma. For more detailed recommendations, see Table 4.
The USA is arguably behind, as no evidence-based skin cancer screening guidelines have been established. Admittedly, the AAD, the ACS and the SCF have made statements on skin cancer screening, but these recommendations lack a specified target population and screening interval and, aside from those from the ACS, are not evidence based. Meanwhile, the US white population has the third highest cumulative risk (0–74 years) for melanoma in the world, second only to Australia and New Zealand [78]. Therefore, in addition to developing a data-driven rationale for our guidelines, we modeled our screening recommendations on international guidelines from countries with similarly elevated risk. Our end product is a set of comprehensive, risk-based, data-driven guidelines comparable to guidelines in Australia, New Zealand, The Netherlands and the UK.
Recommendations of national & international organizations: what potential biases exist?
In addition to comparing the different skin cancer screening recommendations of various organizations, it is important to evaluate potential biases or conflicts of interest that could lead a group to make certain recommendations. For example, provider-centric organizations could have a bias toward overestimating the power of a screening program to impact disease or may be less sensitive to cost-effective interventions. Patient advocacy organizations may have a bias toward screening for reasons such as personal experiences that may not be supported by data-driven evidence. In critiquing the USPSTF recommendations, it is important to note that the USPSTF may have a predilection against screening if it increases the complexity and duration of PCP examinations without unequivocal patient benefit. It is also important to note that many of the expert members of the USPSTF bring necessary epidemiological expertise to the group but are not engaged in direct patient care, which may result in assumption biases such as the technicalities of clinical procedures. The USPSTF's 2016 Draft Recommendation document on adult skin cancer screening included only one physician [54], who specializes in general preventive medicine and public health. While a background in clinical preventive medicine is valuable in the development of evidence-based screening guidelines, it may also be beneficial in the future to add task force members with clinical or research expertise in skin cancer.
USPSTF recommendation: rationale & critique
As mentioned previously, with respect to the development and implementation of screening guidelines, the USPSTF is perhaps the most influential organization in the nation. Under the Affordable Care Act, all recent health insurance plans and policies must cover preventive services that receive a grade A or B recommendation [79]. Therefore, the USPSTF's recommendation on skin cancer screening may significantly impact the extent of examination performed at the time of routine medical care, as well as the public's perception regarding the importance of skin cancer screenings. Currently, the USPSTF's 2016 Draft Recommendation reports that, “a clear statement cannot be made about the benefit of skin cancer screening for melanoma mortality” due to insufficient evidence [54].
The rationale and the data selected for inclusion in the USPSTF analysis (that ultimately produced the final determination of ‘insufficient evidence’) merits further review. Earlier in this article, we summarize several reasons why an RCT is not possible – and probably not appropriate – for melanoma screening. The requirement for demonstrating a reduction of mortality before any recommendation can be made about skin cancer screening is a high bar that may unnecessarily delay or prohibit skin screening of millions of Americans. While the USPSTF draft details a thorough review of the literature, several critical questions remain regarding the validity of the conclusions reached:
The USPSTF warns, “an important consideration for the 2.1 million Medicare enrollees diagnosed with nonmelanoma skin cancer annually is the increase in the detection and treatment of basal cell carcinoma in adults that likely has limited impact on life expectancy [54].” Is it appropriate to consider the detection of basal cell carcinoma a harm of screening rather than a benefit?
One aim of the USPSTF's literature review was to investigate, “the association between earlier detection of skin cancer and skin cancer morbidity,” as well as the effects on quality of life [54]. Why was the morbidity associated with a delayed diagnosis of basal and squamous cell carcinoma, and melanoma omitted in the USPSTF risk estimates?
The USPSTF draft cited two German articles in review of the risks of skin cancer screening [80,81] and concluded that the numbers of excisions needed to treat skin cancer were too high. Is the estimated number of excisions needed to treat BCC (one out of nine excisions) and melanoma (one out of 28 excisions) [80] too high, particularly given the morbidity associated with delayed diagnosis?
The USPSTF assumed that the standards of care for skin biopsy techniques are the same in the USA and Germany. The term ‘excision’ implies a full-thickness fusiform-type excision with closure. The USPSTF based their assessment of risk for complications and cosmetic outcome on the assumption that all potential skin cancers are biopsied in an excisional fashion. In the USA, are the majority of biopsies for KC excisional, and are the majority of excisional biopsies for melanoma the same type reported in the German article?
The USPSTF concluded that excisions for skin cancer result in risk of cosmetically displeasing scars, based on a German study examining removal of benign nevi for cosmetic purposes [81]. Is it valid to extrapolate satisfaction of results from a cosmetic procedure to results from a diagnostic procedure for cancer?
The USPSTF applied rigorous criteria to the articles that were included in the analysis. How were the inclusion and exclusion criteria applied to the worldwide publications that ultimately formed the rationale for the decision statement?
Question 1: is it appropriate to consider the detection of basal cell carcinoma a harm of screening rather than a benefit?
One of the five key questions addressed in the USPSTF's Draft Recommendation is the harm associated with skin cancer screenings and diagnostic follow-up (Box 2) [54]. This factor is critical because it would be inappropriate to recommend screening if more harm than good was accomplished by the process. Ultimately, the USPSTF concludes screening will result in an ‘increase in the detection and treatment of basal cell carcinoma in adults (that) likely has limited impact on life expectancy [54]’. However, KCs are not trivial with respect to morbidity and mortality. Importantly, even though BCCs are typically less aggressive than SCCs, these two entities often appear clinically similar and must be biopsied to confirm the diagnosis. KCs are the most common cancers in the USA, with an estimated prevalence of over 5.4 million cases in 3.3 million individuals in 2012 [82]. Furthermore, KCs are rising in incidence and severity as immunosuppressive therapies increase [83,84]. The ACS estimates that KCs, namely SCCs, are responsible for around 2000 deaths each year in the USA [85]. Some authors even suggest that KC death rates may be underestimated in national statistics [86]; nonetheless, 2000 preventable deaths per year is substantial. Although a subset of elderly patients with comorbidities and limited life expectancy might be adversely impacted by an excision for skin cancer, detection of a skin cancer does not mandate removal (or even biopsy) if individual circumstances suggest this would negatively impact the patient. We contend that overall, early identification of both BCC and SCC should be considered a valuable potential benefit of TBSE rather than a potential harm.
Box 2. . US Preventive Services Task Force's five key questions.
What is the direct evidence that visual skin cancer screening by a primary care provider or dermatologist reduces skin cancer morbidity and mortality, and all-cause mortality?
What are the harms of skin cancer screening and diagnosis follow-up?
What are the test characteristics of visual skin cancer screening when performed by primary care providers versus dermatologists?
Does visual skin cancer screening lead to earlier detection of skin cancer compared with usual care?
What is the association between earlier detection of skin cancer and skin cancer morbidity and mortality, and all-cause mortality?
Sourced from [54].
Question 2: why was the morbidity associated with a delayed diagnosis of BCC, SCC & melanoma omitted in the USPSTF risk estimates?
One aim of the USPSTF's literature review was to investigate, “the association between earlier detection of skin cancer and skin cancer morbidity,” as well as the effects on quality of life [54], yet a review of morbidity associated with KC and melanoma was not included in the draft. The USPSTF concluded that no studies on morbidity met their inclusion criteria, and therefore consideration of this issue was omitted. This is a critical oversight.
In the case of melanoma, a delay in diagnosis can result in a thicker melanoma that requires wider local excision, staging with sentinel lymph node biopsy, potential lymph node dissection and/or systemic therapy, each of which is associated with increased morbidity. A study to compare the morbidity associated with a simple excision versus lymph node dissection or systemic therapy would not be practical, yet common sense suggests that patients identified prior to the need for staging procedures and more aggressive therapies will avoid that unnecessary morbidity. However, the USPSTF did not include an assessment of the increased morbidity associated with these more aggressive therapies or the decreased morbidity associated with earlier melanoma detection.
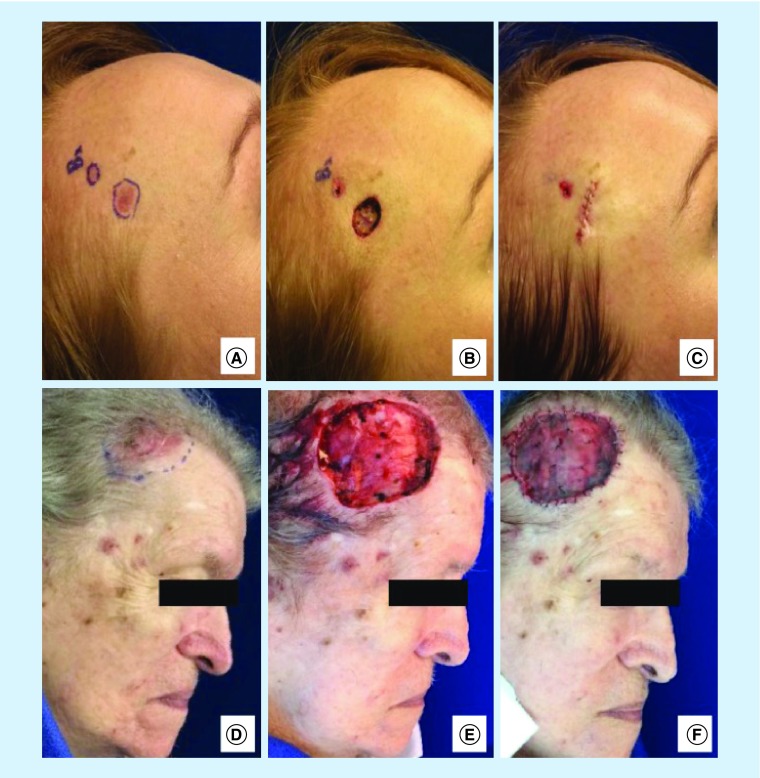
In addition to the morbidity from melanoma, KC treatment options are often associated with significant morbidity, including facial disfigurement and functional loss with decreased quality of life, especially when the subtype is aggressive or if it is diagnosed at a locally advanced stage (Figure 3) [87]. Procedures necessitated by diagnosis of both melanoma and KC at a more advanced stage are associated with notable morbidity and financial impact. If this morbidity and financial burden had been considered by the USPSTF, it is possible that a different conclusion might have been reached.
Figure 3. . Comparison of small and large basal cell carcinomas.
Larger basal cell carcinomas (BCCs) are often associated with higher morbidity due to more complicated surgical procedures. (A) Patient 1 with nodular BCC prior to Mohs micrographic surgery (MMS). (B) Final defect after one stage MMS. (C) Defect repaired with complex linear layered closure. (D) Patient 2 with nodular BCC in a similar location to Patient 1, prior to MMS. (E) Final defect after three stages of MMS. (F) Defect repaired with a split thickness skin graft.
Question 3: is the estimated number of excisions needed to treat BCC (one out of nine excisions) and melanoma (one out of 28 excisions) too high, particularly given the morbidity associated with delayed diagnosis?
The USPSTF draft cited a report of Germany's SCREEN program, conducted in the German state of Schleswig-Holstein, to substantiate the high numbers needed to treat for melanoma or KC [80] in a skin cancer screening program. In this statewide screening effort, 360,288 adults were screened for skin cancer, mainly by trained general practitioners, and 15,983 total excisions (in one of 23 people screened) were performed [80]. An estimated one per 28 excisions were needed to detect melanoma, and about one per nine excisions were needed for BCC detection, with more variable estimates for detecting SCC (one per 56 excisions in women and one per 28 excisions in men) [80]. These detection rates are relatively high and seem quite acceptable if the biopsy technique is associated with low risk and morbidity. The USPSTF's determination that the rates were too low was based on a questionable risk–benefit assumption. As mentioned above, we do not believe all of the risks and benefits relative to morbidity were considered, and furthermore, the harm of ‘excisions’ seems to have been overestimated in the USPSTF draft due to the assumption that skin cancer screening procedures in the USA and Germany are performed similarly (see Question 4 below).
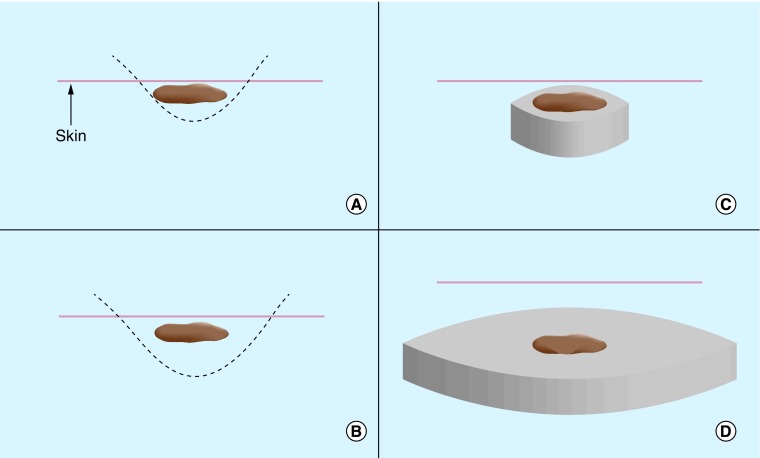
Question 4: in the USA, are the majority of biopsies for KC excisional, & are the majority of excisional biopsies for melanoma the same type reported in the German article?
In the various SCREEN-related publications, KCs were directly excised without prior shave biopsies, yet no discussion of differences in biopsy practices between Germany and the USA was included in the USPSTF's 2016 Draft Recommendation. Therefore, this procedural terminology was carried over into the USPSTF draft without clarifying an important nuance between an ‘excisional biopsy’ and an ‘excision’ in the USA. An ‘excisional biopsy’ is a general term meaning removal of an entire lesion and can be accomplished by any of the following techniques: shave, saucerization, punch or excision (Figure 4) [88]. The term ‘excision’ generally implies a fusiform/elliptical excision that requires closure with deep and superficial sutures. Excisional biopsy is recommended by the NCCN and AAD to diagnose melanoma with the intent to entirely remove the clinically apparent lesion for pathologic examination. Many excisional biopsies are now performed by a deep shave biopsy method (i.e., saucerization or scoop biopsy). This approach has the advantage of obtaining the entire lesion for histopathologic examination, while avoiding the time, cost and morbidity of a full fusiform excision, particularly if pathology demonstrates a benign process. The scars from a deep shave (saucerization) biopsy are much smaller in general than those of a full excision and should not be compared cosmetically to the excisions described in the German SCREEN effort. Furthermore, the majority of biopsies resulting from a TBSE would be done for KCs, due to their high incidence. In the case of KC, a shave biopsy is generally employed in the USA [89]. and may be performed in an excisional or incisional fashion. These biopsies are more superficial and usually heal with less scarring than an excisional biopsy with closure. If a cancer is histopathologically confirmed, then a subsequent excision with appropriate margins and closure is required. Alternatively, certain biopsy-proven KCs may be treated less invasively with electrodessication and curettage, if medically appropriate. The distinctions in biopsy type are important for this discussion, as the potential morbidity associated with shave, punch or saucerization biopsies is substantially less than that of fusiform/elliptical excisional biopsies. Moreover, excisional biopsies are infrequently performed on equivocal KCs and extrapolation of risk estimates from excisional procedure to KC biopsies is not valid. Omission of this distinction by the USPSTF has the effect of overestimating the harm due to negative biopsies and may have led the USPSTF to draw an incorrect conclusion regarding the safety and utility of skin cancer screening.
Figure 4. . The differences in biopsy type impact morbidity and scarring.
(A) Shave biopsies are the most superficial, with the fastest healing time, but run the risk of transecting the base of the tumor. The term ‘shave biopsy’ has variable meanings depending on the practitioner and can range from a superficial incisional biopsy to a complete excisional biopsy of a thin lesion. Shaves are appropriate for biopsying thin keratinocyte carcinomas but should not be utilized for biopsying suspicious pigmented lesions. The defect heals via secondary intention. (B) The saucerization (i.e., deep shave or scoop) biopsy is similar to a shave biopsy but is wider and deeper (involving reticular dermis). Saucerizations may be used for biopsying suspicious pigmented lesions. The defect heals via secondary intention. (C) The punch biopsy allows sampling of all skin layers and may be used for biopsying suspicious pigmented lesions or keratinocyte carcinomas. The defect is closed with sutures. (D) The fusiform/elliptical excision is the largest type of biopsy and requires placement of sutures, leaving the largest scar.
Question 5: is it valid to extrapolate satisfaction of results from a cosmetic procedure to results from a diagnostic procedure for cancer?
Regarding cosmesis, the USPSTF cited a German article that evaluated the cosmetic outcomes associated with using a saucerization technique with 0.5 mm of clear margins for removal of benign pigmented lesions [81]. Only macular nevi <15 mm were included in this study; lesions suspicious for melanoma were excluded. Six months after the shave excision was performed, the appearance of the resulting scars was evaluated. According to the report, 7.1% of patients and 16.1% of physicians rated the cosmetic outcome as ‘poor’ [81]. The inclusion of this manuscript in the USPSTF analysis is questionable: the study was not reporting nevi removed for diagnostic purposes but rather for cosmetic purposes (i.e., as opposed to the situation with skin cancer screening, the participant and physician expectation of outcome did not include a suspicion of cancer); the lesions were macular, so the removal resulted in a depressed scar that was not acceptable because the indication was not removing an unsightly elevated growth but rather a small, flat pigmented lesion. Furthermore, the choice of a deep saucerization technique for aesthetic removal of flat lesions was not appropriate. A superficial shave excision might have been more acceptable for aesthetic removal of benign nevi that did not require biopsy. An appropriate comparator would be the acceptability of the outcome of a biopsy to the patient when cancer was suspected. In addition, the USPSTF used this study of pigmented lesions to draw conclusions regarding the degree of KC morbidity experienced due to a biopsy, suggesting that the members of the USPSTF do not appreciate the difference between a partial biopsy for KC diagnosis versus that recommended for a potential melanoma as discussed above. In sum, the data used by the USPSTF to evaluate harms of biopsy procedures following skin cancer screening is not representative of the situation encountered in clinical practice.
Question 6: how were the inclusion & exclusion criteria applied to the worldwide publications that ultimately formed the rationale for the decision statement?
The USPSTF investigators performed a wide literature search to find relevant studies that could be used to answer their five key questions (Box 2). They reviewed 12,514 abstracts, which were limited broadly by relevance to 453 full text articles that were then subjected to further inclusion/exclusion criteria, yielding a final result of 15 articles (representing 13 studies) [54]. The overwhelming majority of reviewed articles were excluded based on whether or not the articles addressed at least one of five key questions (Box 2). Additional studies were excluded if they were not deemed relevant or generalizable to the primary care setting, if the study design did not meet quality criteria parameters, or if original research was not reported. Several significant and compelling screening studies were identified and summarized in a comprehensive 2014 review by Mayer et al., which was intended for consideration in the USPSTF review during preparation for release of the 2016 draft document [90,91]. However, this review article was ultimately excluded in the USPSTF draft because it did not fit the criteria of, ‘original research in a peer-reviewed journal’ [54]. While valid, all four of the key studies highlighted by Mayer et al. warranted inclusion in the USPSTF literature review; instead, only two out of four were included [77,92]. The other two studies were excluded due to ineligible study design [93] and ineligible setting (nongeneralizable to primary care) [94]. Swetter et al. reported a two-times higher likelihood of being diagnosed with a thinner T1 (≤1 mm) melanoma in patients who reported having a physician skin examination (PCP or dermatologist) in the year prior to diagnosis. The greatest benefit was observed in men over 60 years of age, who had a four-times higher likelihood of T1 melanoma following physician skin examination in the previous year [93]. This study was likely omitted due its design as a retrospective survey, with patient reported healthcare practices queried in the time period prior to melanoma diagnosis. However, given the difficulty in performing this type of study prospectively, the results remain valuable. Schneider et al. reported a 69% reduction in the crude incidence of thick (>0.75 mm) melanomas and decreased estimated melanoma mortality during a decade-long employee education, intervention and active screening program among employees at the Lawrence Livermore National Laboratory (LLNL) in Northern California. The study occurred from 1976–1996 among employees at LLNL, after implementation of a skin cancer screening and education campaign [94]. While the setting of this study was conducted within the LLNL workplace, we do not agree that this setting is nongeneralizable to primary care. Lastly, an additional article that warranted inclusion is a French population-based study performed by Grange et al., which supports the efficacy of PCP-centered skin cancer campaigns on secondary prevention of melanoma [95]. This study was excluded from USPSTF review due to ‘ineligible outcomes’, the details of which are unclear [54].
In summary, with respect to the USPSTF's 2016 Draft Recommendation, several concerns exist, including the implication that KC detection is a harm of skin cancer screening, as well as the omission of disease morbidity, misinterpretation of procedural data and cosmetic outcomes, and overly stringent inclusion criteria. The lack of a dermatology expert on the panel likely contributed to some of the omissions and misinterpretations. While the USPSTF is an esteemed organization with tremendous responsibility and capacity to provide evidence-based screening recommendations, we respectfully disagree with their conclusion that insufficient evidence exists to endorse skin cancer screening based on the rationale provided.
In conclusion, our proposed skin cancer screening recommendations (Box 1) have been developed with input from a diverse group of melanoma experts. These guidelines are based on consistently applied data and are in alignment with USPSTF recommendations for other cancers and diseases, as well as international skin cancer screening guidelines from Australia, New Zealand, Germany and the UK. While no large prospective RCT has been or is likely to be completed to show melanoma mortality reduction from skin cancer screening, this should not be a deterrent to identifying high-risk individuals and performing skin screening to improve patient outcomes in the USA.
Future perspective
The literature and data presented in this article suggest that risk-based skin cancer screening is warranted and justifiable. Screening could potentially impact early detection of melanoma, resulting in a reduction of morbidity, mortality and cost of treatment. Although an evaluation of cost was beyond the scope of this article, as costs climb with the use of novel systemic agents for advanced melanoma, a formal cost assessment would be valuable. A risk prediction model utilizing many of the risk factors we have included in our guidelines has been recently developed and validated by Vuong et al. [96]. In the future, it may be possible to use such a self-assessment tool as a means of identifying individuals in need of screening even more systematically. It may also be possible in the future to establish a skin cancer screening registry to standardize screening recommendations, implement these recommendations nationwide and monitor outcomes over time. Ultimately, melanoma risk assessment and screening will likely be more heavily based on melanoma susceptibility genes (CDKN2A, CDK4, MITF, BAP1, p14 ARF, TERT, POT1, ACD, TERF2IP, BRCA2, PTEN, among others.) [20–22] and molecular pathology tests and criteria. However, as these technologies are not yet widely available, our guidelines provide a foundation on which to base screening in the current era.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Open Access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.American Cancer Society. Cancer Facts & Figures 2016. https://cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/; •• Reports 2016 melanoma statistics and the American Cancer Society skin cancer screening recommendations.
- 2.National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER Stat Fact Sheets: Melanoma of the Skin. http://seer.cancer.gov/statfacts/html/melan.html
- 3.Losina E, Walensky RP, Geller A, et al. Visual screening for malignant melanoma: a cost–effectiveness analysis. Arch. Dermatol. 2007;143(1):21–28. doi: 10.1001/archderm.143.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Academy of Dermatology. SPOTme® Skin Cancer Screenings. https://aad.org/public/spot-skin-cancer/programs/screenings
- 5.Curiel-Lewandrowski C, Kim CC, Swetter SM, et al. Survival is not the only valuable end point in melanoma screening. J. Invest. Dermatol. 2012;132(5):1332–1337. doi: 10.1038/jid.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 7.National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER Cancer Statistics Review 1975–2011: Section 32: adolescent and young adult cancer by site, incidence, survival and mortality. http://seer.cancer.gov/archive/csr/1975_2011/results_merged/sect_32_aya.pdf
- 8.Lazovich D, Isaksson Vogel R, Weinstock MA, Nelson HH, Ahmed RL, Berwick M. Association between indoor tanning and melanoma in younger men and women. JAMA Dermatol. 2016;152(3):268–275. doi: 10.1001/jamadermatol.2015.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu F, Bessonova L, Taylor TH, Ziogas A, Meyskens FL, Jr, Anton-Culver H. A unique gender difference in early onset melanoma implies that in addition to ultraviolet light exposure other causative factors are important. Pigment Cell Melanoma Res. 2013;26(1):128–135. doi: 10.1111/pcmr.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 11.Leblanc WG, Vidal L, Kirsner RS, et al. Reported skin cancer screening of US adult workers. J. Am. Acad. Dermatol. 2008;59(1):55–63. doi: 10.1016/j.jaad.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altman JF, Oliveria SA, Christos PJ, Halpern AC. A survey of skin cancer screening in the primary care setting: a comparison with other cancer screenings. Arch. Fam. Med. 2000;9(10):1022–1027. doi: 10.1001/archfami.9.10.1022. [DOI] [PubMed] [Google Scholar]
- 13.Wise E, Singh D, Moore M, et al. Rates of skin cancer screening and prevention counseling by US medical residents. Arch. Dermatol. 2009;145(10):1131–1136. doi: 10.1001/archdermatol.2009.242. [DOI] [PubMed] [Google Scholar]
- 14.Moore MM, Geller AC, Zhang Z, et al. Skin cancer examination teaching in US medical education. Arch. Dermatol. 2006;142(4):439–444. doi: 10.1001/archderm.142.4.439. [DOI] [PubMed] [Google Scholar]
- 15.Lakhani NA, Saraiya M, Thompson TD, King SC, Guy GP., Jr Total body skin examination for skin cancer screening among U.S. adults from 2000 to 2010. Prev. Med. 2014;61:75–80. doi: 10.1016/j.ypmed.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Finds that only 24% of high-risk individuals have been screened with a total body skin examination (TBSE) once in their lifetime.
- 16.Coups EJ, Geller AC, Weinstock MA, Heckman CJ, Manne SL. Prevalence and correlates of skin cancer screening among middle-aged and older white adults in the United States. Am. J. Med. 2010;123(5):439–445. doi: 10.1016/j.amjmed.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Department of Health & Human Services, Centers for Medicare & Medicaid Services. The ABCs of the Annual Wellness Visit (AWV) https://cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/AWV_chart_ICN905706.pdf
- 18.Uliasz A, Lebwohl M. Cutaneous manifestations of cardiovascular diseases. Clin. Dermatol. 2008;26(3):243–254. doi: 10.1016/j.clindermatol.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Rigopoulos D, Larios G, Katsambas A. Skin signs of systemic diseases. Clin. Dermatol. 2011;29(5):531–540. doi: 10.1016/j.clindermatol.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Ribero S, Longo C, Glass D, Nathan P, Bataille V. What is new in melanoma genetics and treatment. Dermatology. 2016;232(3):259–264. doi: 10.1159/000445767. [DOI] [PubMed] [Google Scholar]
- 21.The Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J. Natl Cancer Inst. 1999;91(15):1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 22.Bubien V, Bonnet F, Brouste V, et al. High cumulative risks of cancer in patients with PTEN hamartoma tumour syndrome. J. Med. Genet. 2013;50(4):255–263. doi: 10.1136/jmedgenet-2012-101339. [DOI] [PubMed] [Google Scholar]
- 23.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch. Dermatol. 1988;124(6):869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 24.Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur. J. Cancer. 2005;41(1):28–44. doi: 10.1016/j.ejca.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Preventive Services Task Force. Grade Definitions. http://uspreventiveservicestaskforce.org/Page/Name/grade-definitions
- 26.National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER-derived Data Box 1.11. Age distribution (%) of incidence cases by site, 2008–2012, all races, both sexes. http://seer.cancer.gov/archive/csr/1975_2012/results_merged/sect_01_overview.pdf
- 27.National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER-derived Data Box 1.13. Age distribution (%) of deaths by site, 2008–2012, all races, both sexes. http://seer.cancer.gov/archive/csr/1975_2012/results_merged/sect_01_overview.pdf
- 28.National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER Stat fact sheets: lung and bronchus cancer. http://seer.cancer.gov/statfacts/html/lungb.html
- 29.National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER Stat fact sheets: female breast cancer. http://seer.cancer.gov/statfacts/html/breast.html
- 30.National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER Stat fact sheets: cervix uteri cancer. http://seer.cancer.gov/statfacts/html/cervix.html
- 31.National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER Stat fact sheets: colon and rectum cancer. http://seer.cancer.gov/statfacts/html/colorect.html
- 32.Karimkhani C, Boyers LN, Dellavalle RP, Weinstock MA. It's time for “keratinocyte carcinoma” to replace the term “nonmelanoma skin cancer”. J. Am. Acad. Dermatol. 2015;72(1):186–187. doi: 10.1016/j.jaad.2014.09.036. [DOI] [PubMed] [Google Scholar]; • Defines ‘keratinocyte carcinoma’ as a more accurate term than ‘non-melanoma skin cancer’, therefore, keratinocyte carcinoma was used throughout this article.
- 33.Wang W, Lee ET, Fabsitz RR, et al. A longitudinal study of hypertension risk factors and their relation to cardiovascular disease: the Strong Heart Study. Hypertension. 2006;47(3):403–409. doi: 10.1161/01.HYP.0000200710.29498.80. [DOI] [PubMed] [Google Scholar]
- 34.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 35.Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J. Vasc. Surg. 2010;52(3):539–548. doi: 10.1016/j.jvs.2010.05.090. [DOI] [PubMed] [Google Scholar]
- 36.Vardulaki KA, Walker NM, Day NE, Duffy SW, Ashton HA, Scott RA. Quantifying the risks of hypertension, age, sex and smoking in patients with abdominal aortic aneurysm. Br. J. Surg. 2000;87(2):195–200. doi: 10.1046/j.1365-2168.2000.01353.x. [DOI] [PubMed] [Google Scholar]
- 37.Brose MS, Rebbeck TR, Calzone KA, Stopfer JE, Nathanson KL, Weber BL. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J. Natl Cancer Inst. 2002;94(18):1365–1372. doi: 10.1093/jnci/94.18.1365. [DOI] [PubMed] [Google Scholar]
- 38.Pharoah PD, Day NE, Duffy S, Easton DF, Ponder BA. Family history and the risk of breast cancer: a systematic review and meta-analysis. Int. J. Cancer. 1997;71(5):800–809. doi: 10.1002/(sici)1097-0215(19970529)71:5<800::aid-ijc18>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 39.Pesch B, Kendzia B, Gustavsson P, et al. Cigarette smoking and lung cancer–relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int. J. Cancer. 2012;131(5):1210–1219. doi: 10.1002/ijc.27339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. Eur. J. Cancer. 2005;41(14):2040–2059. doi: 10.1016/j.ejca.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 41.Lazovich D, Vogel RI, Berwick M, Weinstock MA, Anderson KE, Warshaw EM. Indoor tanning and risk of melanoma: a case-control study in a highly exposed population. Cancer Epidemiol. Biomarkers Prev. 2010;19(6):1557–1568. doi: 10.1158/1055-9965.EPI-09-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur. J. Cancer. 2005;41(1):45–60. doi: 10.1016/j.ejca.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 43.Ford D, Bliss JM, Swerdlow AJ, et al. Risk of cutaneous melanoma associated with a family history of the disease. The International Melanoma Analysis Group (IMAGE) Int. J. Cancer. 1995;62(4):377–381. doi: 10.1002/ijc.2910620403. [DOI] [PubMed] [Google Scholar]
- 44.Kefford RF, Newton Bishop JA, Bergman W, Tucker MA. Counseling and DNA testing for individuals perceived to be genetically predisposed to melanoma: a consensus statement of the Melanoma Genetics Consortium. J. Clin. Oncol. 1999;17(10):3245–3251. doi: 10.1200/JCO.1999.17.10.3245. [DOI] [PubMed] [Google Scholar]
- 45.Bradford PT, Freedman DM, Goldstein AM, Tucker MA. Increased risk of second primary cancers after a diagnosis of melanoma. Arch. Dermatol. 2010;146(3):265–272. doi: 10.1001/archdermatol.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Begg CB, Orlow I, Hummer AJ, et al. Lifetime risk of melanoma in CDKN2A mutation carriers in a population-based sample. J. Natl Cancer Inst. 2005;97(20):1507–1515. doi: 10.1093/jnci/dji312. [DOI] [PubMed] [Google Scholar]
- 47.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): melanoma, Version 2.2016. https://nccn.org/professionals/physician_gls/f_guidelines_nojava.asp#melanoma [DOI] [PubMed]
- 48.Bichakjian CK, Halpern AC, Johnson TM, et al. Guidelines of care for the management of primary cutaneous melanoma. American Academy of Dermatology. J. Am. Acad. Dermatol. 2011;65(5):1032–1047. doi: 10.1016/j.jaad.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 49.Leachman SA, Carucci J, Kohlmann W, et al. Selection criteria for genetic assessment of patients with familial melanoma. J. Am. Acad. Dermatol. 2009;61(4):677.e1–677.e14. doi: 10.1016/j.jaad.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ransohoff KJ, Jaju PD, Tang JY, Carbone M, Leachman S, Sarin KY. Familial skin cancer syndromes: Increased melanoma risk. J. Am. Acad. Dermatol. 2016;74(3):423–434. doi: 10.1016/j.jaad.2015.09.070. [DOI] [PubMed] [Google Scholar]
- 51.Moloney FJ, Guitera P, Coates E, et al. Detection of primary melanoma in individuals at extreme high risk: a prospective 5-year follow-up study. JAMA Dermatol. 2014;150(8):819–827. doi: 10.1001/jamadermatol.2014.514. [DOI] [PubMed] [Google Scholar]
- 52.Viana AC, Gontijo B, Bittencourt FV. Giant congenital melanocytic nevus. An. Bras. Dermatol. 2013;88(6):863–878. doi: 10.1590/abd1806-4841.20132233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.U.S. Preventive Services Task Force. Final update summary: skin cancer: screening. http://uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-screening
- 54.Agency for Healthcare Research and Quality. Screening for skin cancer in adults: an updated systematic evidence review for the U.S. Preventive Services Task Force. http://uspreventiveservicestaskforce.org/Page/Document/draft-evidence-review159/skin-cancer-screening2 [PubMed]; •• Details the rationale behind the US Preventive Services Task Force's (USPSTF) 2016 Draft Recommendations.
- 55.U.S. Preventive Services Task Force. Final update summary: skin cancer: counseling. http://uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-counseling
- 56.American Academy of Family Physicians. Skin cancer – Clinical Preventive Service Recommendation. http://aafp.org/patient-care/clinical-recommendations/all/skin-cancer.html; • Details the American Academy of Family Physicians’ skin cancer screening recommendations.
- 57.American Cancer Society. Cancer facts & figures 2015. http://cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf
- 58.American Academy of Dermatology. AAD statement on skin cancer screening. https://www.aad.org/media/news-releases/aad-statement-on-skin-cancer-screening; • Details skin cancer screening recommendations.
- 59.Skin Cancer Foundation. Prevention guidelines. http://skincancer.org/prevention/sun-protection/prevention-guidelines; • Details the Skin Cancer Foundation's skin cancer screening recommendations.
- 60.Cancer Council Australia. Position statement – Screening and early detection of skin cancer. https://dermcoll.edu.au/wp-content/uploads/2014/05/PosStatEarlyDetectSkinCa.pdf; • Details Australian skin cancer screening recommendations.
- 61.Cancer Council Australia and Australian Cancer Network and New Zealand Guidelines Group. Clinical practice guidelines for the management of melanoma in Australia and New Zealand: evidence-based best practice guidelines. https://nhmrc.gov.au/_files_nhmrc/publications/attachments/cp111.pdf; • Details Australian and New Zealand skin cancer screening recommendations.
- 62.Royal Australian College of General Practitioners. Guidelines for preventive activities in general practice. http://racgp.org.au/your-practice/guidelines/redbook/early-detection-of-cancers/skin-cancer; • Details Australian skin cancer screening recommendations.
- 63.Marsden JR, Newton-Bishop JA, Burrows L, et al. Revised UK guidelines for the management of cutaneous melanoma 2010. J. Plast. Reconstr. Aesthet. Surg. 2010;63(9):1401–1419. doi: 10.1016/j.bjps.2010.07.006. [DOI] [PubMed] [Google Scholar]; • Details Dutch skin cancer screening recommendations.
- 64.Dutch Working Group on Melanoma. Melanoma Guideline 2012. 2013. http://oncoline.nl/uploaded/docs/melanoom/201208_vertaling%20Richtlijn%20melanoom%20def.pdf; • Details Dutch skin cancer screening recommendations.
- 65.Katalinic A, Eisemann N, Waldmann A. Skin cancer screening in Germany. Documenting melanoma incidence and mortality from 2008 to 2013. Dtsch. Arztebl. Int. 2015;112(38):629–634. doi: 10.3238/arztebl.2015.0629. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Details the unique, nation-wide screening program in Germany.
- 66.German Guideline Program in Oncology (GGPO) Evidence-based guideline on prevention of skin cancer (short version) http://leitlinienprogramm-onkologie.de/uploads/media/Short_version_-_Guideline_on_prevention_of_skin_cancer.pdf; • Details the risk-based skin cancer screening recommendations in Germany.
- 67.American Academy of Dermatology. Learning module: the skin exam. https://aad.org/education/basic-derm-curriculum/suggested-order-of-modules/the-skin-exam
- 68.Leachman SA, Cassidy PB, Chen SC, et al. Methods of melanoma detection. Cancer Treat. Res. 2016;167:51–105. doi: 10.1007/978-3-319-22539-5_3. [DOI] [PubMed] [Google Scholar]
- 69.American Academy of Dermatology. Detect skin cancer. https://aad.org/public/spot-skin-cancer/learn-about-skin-cancer/detect
- 70.American College of Preventive Medicine. ACPM Position Statements. http://acpm.org/?page=Position_Statements
- 71.American College of Physicians. ACP Clinical Guidelines and Recommendations. https://acponline.org/clinical-information/guidelines
- 72.National Comprehensive Cancer Network. NCCN Guidelines for detection, prevention, & risk reduction. https://nccn.org/professionals/physician_gls/f_guidelines.asp#detection
- 73.American Society of Clinical Oncology. ASCO Guidelines Wiki. https://pilotguidelines.atlassian.net/wiki
- 74.National Cancer Institute. Skin cancer screening (PDQ®)–patient version. http://cancer.gov/types/skin/patient/skin-screening-pdq#section/all
- 75.American Joint Committee on Cancer. AJCC – American Joint Committee on cancer. https://cancerstaging.org/pages/default.aspx
- 76.Negrier S, Saiag P, Guillot B, et al. [Clinical practice guideline: 2005 update of recommendations for the management of patients with cutaneous melanoma without distant metastases (summary report)] Bull. Cancer. 2006;93(4):371–384. [PubMed] [Google Scholar]
- 77.Katalinic A, Waldmann A, Weinstock MA, et al. Does skin cancer screening save lives?: an observational study comparing trends in melanoma mortality in regions with and without screening. Cancer. 2012;118(21):5395–5402. doi: 10.1002/cncr.27566. [DOI] [PubMed] [Google Scholar]; •• Also details the unique, nation-wide screening program in Germany. It was included in the USPSTF'S 2016 draft document.
- 78.Erdmann F, Lortet-Tieulent J, Schuz J, et al. International trends in the incidence of malignant melanoma 1953–2008 – are recent generations at higher or lower risk? Int. J. Cancer. 2013;132(2):385–400. doi: 10.1002/ijc.27616. [DOI] [PubMed] [Google Scholar]
- 79.Agency for Healthcare Research and Quality. Guide to Clinical Preventive Services, 2014: Preface. http://ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/guide/preface.html [PubMed]
- 80.Waldmann A, Nolte S, Geller AC, et al. Frequency of excisions and yields of malignant skin tumors in a population-based screening intervention of 360,288 whole-body examinations. Arch. Dermatol. 2012;148(8):903–910. doi: 10.1001/archdermatol.2012.893. [DOI] [PubMed] [Google Scholar]
- 81.Gambichler T, Senger E, Rapp S, Alamouti D, Altmeyer P, Hoffmann K. Deep shave excision of macular melanocytic nevi with the razor blade biopsy technique. Dermatol. Surg. 2000;26(7):662–666. doi: 10.1046/j.1524-4725.2000.00036.x. [DOI] [PubMed] [Google Scholar]
- 82.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151(10):1081–1086. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 83.Scott FI, Mamtani R, Brensinger CM, et al. Risk of nonmelanoma skin cancer associated with the use of immunosuppressant and biologic agents in patients with a history of autoimmune disease and nonmelanoma skin cancer. JAMA Dermatol. 2016;152(2):164–172. doi: 10.1001/jamadermatol.2015.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Athar M, Walsh SB, Kopelovich L, Elmets CA. Pathogenesis of nonmelanoma skin cancers in organ transplant recipients. Arch. Biochem. Biophys. 2011;508(2):159–163. doi: 10.1016/j.abb.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.American Cancer Society. Key statistics for basal and squamous cell skin cancers. http://cancer.org/cancer/skincancer-basalandsquamouscell/detailedguide/skin-cancer-basal-and-squamous-cell-key-statistics
- 86.Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J. Am. Acad. Dermatol. 2013;68(6):957–966. doi: 10.1016/j.jaad.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 87.Clayman GL, Lee JJ, Holsinger FC, et al. Mortality risk from squamous cell skin cancer. J. Clin. Oncol. 2005;23(4):759–765. doi: 10.1200/JCO.2005.02.155. [DOI] [PubMed] [Google Scholar]
- 88.Pickett H. Shave and punch biopsy for skin lesions. Am. Fam. Physician. 2011;84(9):995–1002. [PubMed] [Google Scholar]
- 89.Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases. J. Am. Acad. Dermatol. 2016;74(1):1–16. doi: 10.1016/j.jaad.2015.06.033. quiz 17–18. [DOI] [PubMed] [Google Scholar]
- 90.Mayer JE, Swetter SM, Fu T, Geller AC. Screening, early detection, education, and trends for melanoma: current status (2007–2013) and future directions: Part I. Epidemiology, high-risk groups, clinical strategies, and diagnostic technology. J. Am. Acad. Dermatol. 2014;71(4):599.e1–599.e2. doi: 10.1016/j.jaad.2014.05.046. [DOI] [PubMed] [Google Scholar]; •• Highlights the rising incidence and mortality rates of melanoma and emphasizes the importance of education and screening.
- 91.Mayer JE, Swetter SM, Fu T, Geller AC. Screening, early detection, education, and trends for melanoma: current status (2007–2013) and future directions: Part II. Screening, education, and future directions. J. Am. Acad. Dermatol. 2014;71(4):611.e1–611.e10. doi: 10.1016/j.jaad.2014.05.045. quiz 621–612. [DOI] [PubMed] [Google Scholar]; •• This comprehensive review article identifies several significant and compelling screening studies that were intended for consideration in the USPSTF review during preparation of the 2016 draft document
- 92.Aitken JF, Elwood M, Baade PD, Youl P, English D. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int. J. Cancer. 2010;126(2):450–458. doi: 10.1002/ijc.24747. [DOI] [PubMed] [Google Scholar]; •• Provides evidence that shows TBSEs reduce the thickness of melanomas. It was included in the USPSTF draft document.
- 93.Swetter SM, Pollitt RA, Johnson TM, Brooks DR, Geller AC. Behavioral determinants of successful early melanoma detection: role of self and physician skin examination. Cancer. 2012;118(15):3725–3734. doi: 10.1002/cncr.26707. [DOI] [PubMed] [Google Scholar]; •• Shows a two-times higher likelihood of being diagnosed with a thinner melanoma in patients who had a TBSE within 1 year prior to diagnosis.
- 94.Schneider JS, Moore DH, 2nd, Mendelsohn ML. Screening program reduced melanoma mortality at the Lawrence Livermore National Laboratory, 1984 to 1996. J. Am. Acad. Dermatol. 2008;58(5):741–749. doi: 10.1016/j.jaad.2007.10.648. [DOI] [PubMed] [Google Scholar]; •• Shows a 69% reduction in thick melanomas and decreased melanoma mortality during a decades-long employee education intervention and active skin cancer screening program.
- 95.Grange F, Woronoff AS, Bera R, et al. Efficacy of a general practitioner training campaign for early detection of melanoma in France. Br. J. Dermatol. 2014;170(1):123–129. doi: 10.1111/bjd.12585. [DOI] [PubMed] [Google Scholar]
- 96.Vuong K, Armstrong BK, Weiderpass E, et al. Development and external validation of a melanoma risk prediction model based on self-assessed risk factors. JAMA Dermatol. 2016;152(8):889–896. doi: 10.1001/jamadermatol.2016.0939. [DOI] [PubMed] [Google Scholar]