Abstract
Background
Central nervous system tumours constitute 25% of all childhood cancers; more than half are located in the posterior fossa and surgery is usually part of therapy. One of the most disabling late effects of posterior fossa tumour surgery is the cerebellar mutism syndrome (CMS) which has been reported in up to 39% of the patients but the exact incidence is uncertain since milder cases may be unrecognized. Recovery is usually incomplete. Reported risk factors are tumour type, midline location and brainstem involvement, but the exact aetiology, surgical and other risk factors, the clinical course and strategies for prevention and treatment are yet to be determined.
Methods
This observational, prospective, multicentre study will include 500 children with posterior fossa tumours. It opened late 2014 with participation from 20 Nordic and Baltic centres. From 2016, five British centres and four Dutch centres will join with a total annual accrual of 130 patients. Three other major European centres are invited to join from 2016/17. Follow-up will run for 12 months after inclusion of the last patient. All patients are treated according to local practice. Clinical data are collected through standardized online registration at pre-determined time points pre- and postoperatively. Neurological status and speech functions are examined pre-operatively and postoperatively at 1–4 weeks, 2 and 12 months. Pre- and postoperative speech samples are recorded and analysed. Imaging will be reviewed centrally. Pathology is classified according to the 2007 WHO system. Germline DNA will be collected from all patients for associations between CMS characteristics and host genome variants including pathway profiles.
Discussion
Through prospective and detailed collection of information on 1) differences in incidence and clinical course of CMS for different patient and tumour characteristics, 2) standardized surgical data and their association with CMS, 3) diversities and results of other therapeutic interventions, and 4) the role of host genome variants, we aim to achieve a better understanding of risk factors for and the clinical course of CMS - with the ultimate goal of defining strategies for prevention and treatment of this severely disabling condition.
Trial registration
Clinicaltrials.gov: NCT02300766, date of registration: November 21, 2014.
Keywords: Cancer, Paediatric, Children, Cerebellar mutism, CMS, Brain tumour, Cerebellum, Posterior fossa syndrome, Genetics, Neurosurgery
Background
Incidence and definition of CMS
Central nervous system (CNS) tumours account for 25% of all cancers in children and over half of these are located in the posterior fossa [1]. For most of these patients, treatment includes surgery. Posterior fossa tumours in children are associated with high risk of chronic neurological and neurocognitive disability [2–6]. The cerebellar mutism syndrome (CMS) refers to the constellation of transient mutism, ataxia, hypotonia and irritability following surgery for cerebellar or fourth ventricle tumours in children and adolescents [7]. Although some patients may recover completely, recovery may be prolonged, and many are left with permanent disabling sequelae in the form of e.g. dysarthria, dysfluency, slowed speech rate and ataxia. Many may in addition be burdened by emotional problems and lower IQ [8–12].
The spectrum of CMS definitions varies greatly [7, 12–14], leading to differences in reported incidence and uncertainties about recovery. Incidence figures thus range from 8% [15, 16] to 32% [17] in children with any kind of cerebellar tumour when a variety of definitions are used, compared to 24% [12] to 39% [18] in patients with medulloblastomas using a more precise CMS definition. A recent study of 148 children with cerebellar tumours found that the overall incidence of the broader Posterior Fossa Syndrome was 28%, subdivided by tumour pathology into 40% for medulloblastoma, 16% for astrocytoma and 20% for ependymoma [19]. The CMS definition created by the Neurology Committee of the Children’s Cancer Group in USA in 1993 is currently the only one associated with a specific scoring scale [12] and is used in this project.
Risk factors and prevention
Cerebellar mutism is thought to be caused by bilateral disturbance of the dentate nuclei and/or their efferents [16, 20–24]. Ataxia and irritability together with other cognitive, affective and motor symptoms that are frequently observed in CMS patients are caused by damage to various parts of the cerebellum and cerebello-cerebral pathways passing through the brainstem [25–28]. This can result in secondary diaschisis of supratentorial brain areas due to lack of excitatory input from cerebellum [29–31].
Known risk factors are brainstem involvement by the tumour, midline location and tumour type; thus the incidence in children with medulloblastoma is two to three times higher than for astrocytoma or ependymoma but the biological mechanisms behind these associations are uncertain [12, 19, 22, 32–35]. Recently proposed risk factors include brainstem compression by the tumour, pre-operative language impairment, low socioeconomic level of the families and left-handedness [24, 36–38]; however, these remain to be verified on large patient cohorts. Tumour size, neurosurgical techniques and approaches, radical resection and younger age at diagnosis are uncertain risk factors, as previous studies have been inconclusive [12, 23, 32–35, 39, 40]. Gender, hydrocephalus, post-operative central nervous system infections, type of neurosurgeon (adult/paediatric), and oedema/swelling of the cerebellum have not been significantly correlated to CMS and are considered unlikely risk factors [12, 16, 34, 35, 41, 42].
In traumatic brain injury common host genomic variants are related to the severity of symptoms and degree of recovery [43–45]. Similar associations are likely for surgical brain injury, but such studies have not been performed for CMS.
Non-surgical treatment
Supportive speech and rehabilitation therapy is often offered to patients with CMS, but the benefit hereof has not been demonstrated. No publications exist on systematic approaches to pharmacological neuroprotection, and pharmacological interventions are only sporadically reported in the literature [46–50]. Glucocorticosteroids are routinely given to most patients pre-, intra- and post-operatively to reduce inflammation and edema [51] [52], but there is no consensus recommendation and the impact on the clinical course of CMS is undetermined.
Aims
The study focuses on the risk factors for development and severity of CMS including surgery (approaches, techniques and tissue and vascular damage, re-operation) and host genome variants. The aims of this study are thus to describe differences in incidence, severity and clinical course of CMS related to:
Clinical factors: gender, age, handedness, speech, language and neuropsychological abilities before and after surgery.
Tumour factors: histological tumour type and tumour location
Surgical factors: Surgical strategy and surgical trauma including access routes, removal technique, tissue and vascular injury, bleeding and primary surgery vs. re-operation
Non-surgical interventions: glucocorticosteroids, other symptomatic medication and chemo- and radiotherapy
Host genome variants
Methods/design
Design
This open observational study is registered at Clinicaltrials.gov (file NCT02300766) and EANS (http://www.sbns.org.uk/index.php/research/eu-multi-centre-trials). All children younger than 18 years with a tumour in the posterior fossa requiring surgery or open biopsy at one of the participating centres will be included following informed consent. Patients who have received surgery, chemotherapy and/or radiotherapy previously are also eligible. The study will run for five years with a targeted sample size of 500 patients. It opened late 2014 with participation from 20 Nordic and Baltic centres. From 2016, five British centres and four Dutch centres will join leading to an expected annual accrual of 130 patients. Three other major European centres are invited to join from 2016/17. The target of 500 patients is expected to be reached in 2018. Patients will be followed for 12 months after inclusion of the last patient, and the study will thus be completed during 2019.The participating centres provide surgery and supportive care according to local practice and register all study information in an online database developed specifically for this study. Consensus concerning the study aims, study design and data registration was achieved at three international planning meetings among the initiating centres during 2013. The annual enrolment from each country will be compared to the number of registered patients in the national cancer registries to document the inclusion rate and representativeness.
Primary endpoint
The primary endpoint is incidence and severity of CMS. Symptoms and severity are scored according to the CMS survey published by Robertsons et al. [12]. Our main focus is the impact of different surgical tumour approaches. We hypothesize that 1) minimally traumatic techniques and 2) sparing the dentate nuclei and their efferents will be associated with a 50% reduced risk of CMS when compared to more invasive tumour removal approaches. Furthermore, we hypothesize that the risk of developing CMS is higher after re-operation(s) compared to primary surgery.
Secondary endpoint
The secondary endpoint is incidence of “reduced speech output” defined as “severely reduced speech production limited to single words or short sentences which can only be elicited after vigorous stimulation” [19]. The risk of reduced speech output will be related to different surgical approaches with the underlying hypothesis that damage to the dentate nuclei and/or their efferents increases the risk.
Furthermore, we want to explore the following:
Genetics
We will analyse the role of host genome variants on development, severity and recovery from CMS by carrying out broad genetic pathway profiling of all study participants using both non-CMS cases from the study cohort and non-CNS tumour patients as controls. Genotyping will use single nucleotide polymorphism (SNP) exome enriched arrays (e.g. Illumina Omni2.5-exome platform). We will apply agnostic genome-wide association studies (GWAS) as well as more complex pathway analyses. Thus, we will interrogate combined effects of multiple SNPs acting in the same pathways or protein-protein interaction complexes using our validated non-linear machine learning algorithm (artificial neural networks approach) [53, 54], which allows testing of a large range of pathways from various databases. This approach will yield hypotheses easier to test across cohorts and also provide mechanistic insights. We hypothesize that host genome variants explain at least 50% of the variation in incidence of CMS and at least 40% of the variation in severity, duration and level of recovery from the CMS.
Other non-surgical treatments including glucocorticosteroids
The possible effects of chemo- and radiotherapy on recovery from CMS will be investigated. We hypothesize that chemo- and radiotherapy delay recovery from CMS. For descriptive documentation purposes we also ask for information on medications given specifically to treat the symptoms of CMS.
We hypothesize that glucocorticosteroids 1) given preoperatively protect against CMS due to reduced oedema; 2) given intraoperatively increase the risk of CMS due to worsening of acute neurological injury by hyperglycaemia; 3) given postoperatively negatively affect the course of CMS as earlier studies have shown a negative effect of glucocorticosteroids on the outcome of traumatic brain injury [55, 56]. It may be expected that most patients receive glucocorticosteroids at all 3 time points which would make it difficult to assess added positive and negative effect in the same patients.
Tumour type
The incidence of the CMS will be correlated to tumour histology using the 2007 WHO classification. We hypothesize that the risk of CMS is highest among patients with medulloblastoma. With increasing focus on subtyping of medulloblastoma [57] this additional classification may later be added to the risk factor analysis.
Neuroradiology
Tumour location, enhancement pattern, invasiveness and growth velocity may affect the risk and severity of CMS [12, 18, 58]. Accordingly, we hypothesize that a statistical risk of CMS may be predicted by defining specific neuroradiological features [59–61]. Likewise, postoperative neuroradiological features could give prognostic information about probable degree of recovery.
Handedness
We will determine whether the risk of the CMS varies according to handedness. We hypothesize that the risk of CMS is increased in left-handed patients, and possibly even more so in patients with medulloblastoma [24].
Language and speech
Our hypothesis that preoperative speech and language impairment increases the risk of postoperative speech and language deficits will be explored by recording pre- and postoperative speech (e.g. articulation, prosodic features and voice) and language (e.g. word finding difficulties, fluency and narrative ability) statuses and relating these to incidence and course of CMS. All speech recordings will be analysed nationally by speech therapists.
Registration of data
The following data will be registered at five time points by online standard registration forms:
1. Preoperatively
Hospital, country, patient related variables such as date of birth, handedness, comorbidities, bilingualism, gender and date of diagnosis, medical history and preoperative neurological status. A speech and language test will be performed and recorded. If the patient is younger than two years a bedside assessment of speech will be performed instead of a formalized test. A two millilitre blood sample for genetic analysis will be collected.
2. Postoperatively within 72 h of surgery
Surgery related variables such as date, patient position during surgery, surgical approach, tumour removal method (én bloc, piecemeal or ultrasonic aspiration), duration and course of operation, damage to non-tumour tissue, complications, technology employed (endoscopy, neuronavigation, electrophysiological monitoring etc.), surgeon’s estimate of tumour resection extent and presence of preoperative hydrocephalus.
3. Postoperatively within one to four weeks from surgery
Approximately one to two weeks post-operatively: neurological examination, postoperative speech and language status including speech and language recording or bedside assessment and medications used for treatment of CMS.
Approximately four weeks post-operatively: Development and treatment of postoperative intracranial haematoma and hydrocephalus, leakage of cerebrospinal fluid and need for ventilator. These complications are usually seen earlier but we wait until the fourth post-operative week to register these in order to ensure no complications are missed.
4. Postoperatively two months after surgery
Neurological examination, CMS-survey, speech and language recording or bedside assessment and any medications given to treat CMS since last registration.
5. Postoperatively twelve months after surgery
Neurological examination, speech and language status including speech and language recording or bedside assessment, medications given since last registration to treat CMS, chemo- and/or radiotherapy, neuropsychological assessment(s) if performed, final neuropathological classification of tumour, and additional neuroimaging performed since the first follow-up. Copies of the neuroimaging and descriptions performed pre- and postoperatively will be collected for central review.
Acute and repeated neurosurgery
In case of emergency surgery (e.g. due to risk of incarceration or coma) information about the study and invitation to participate can be given within seven days postoperatively. These patients will be included in all parts of the study except for the recording and analysis of preoperative speech and language status.
In cases with repeat tumour surgery during the twelve months follow-up, the patient can re-enter the study and start a new follow-up programme (Fig. 1, Repeated Surgeries). A new pre-operative registration is then performed corresponding to the re-operation. Post-operative registrations will be performed again, and used in the analysis of risk related to first versus further surgeries. If surgery is performed again after the twelve months follow-up period, the patient will be re-invited to participate in the study.
Fig. 1.
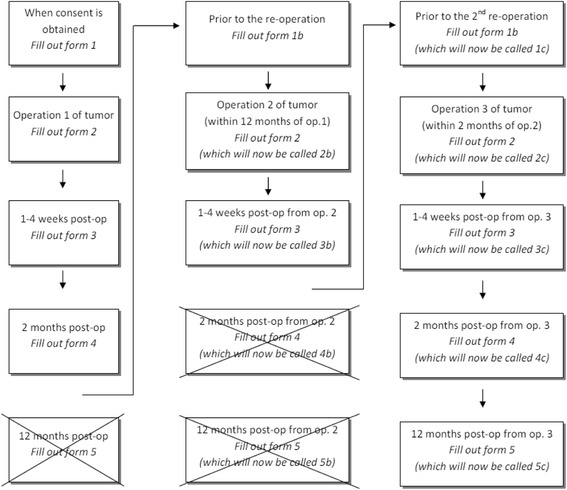
The follow up process in case of repeated surgeries
Statistical considerations
In accordance with our surgical hypothesis of 50% risk reduction by less traumatic techniques, and assuming that 35% of the patients are operated using an approach with a low risk of CMS (assumed to be 10%) and the remaining 65% of patients are operated using other approaches (assumed carrying a 20% risk), a total of 450 patients have to be included to identify a 5% significance level and 80% power. Based on a projected overall risk of CMS of 20%, an estimated frequency of a specific SNP of 30%, and a projected doubled risk of CMS with this particular SNP, we will need to include a total of 343 patients to identify such a genetic predisposition at a 5% significance level with 90% power.
Discussion
The study will be the largest prospective international study on CMS to date, and the first one to 1) systematically register surgery, use of steroids, standardized speech samples and 2) to investigate the influence of host genome. Detailed information on neuroradiological features, tumour and patient characteristics (incl. Handedness and pre-language impairment) will also be gathered, and may help further elucidate the incidence and clinical course of the syndrome for various patient and tumour types.
On-line registration compliance rates to Nordic/Baltic multicentre trials are in general above 95% [62]. Furthermore, we will implement an automated email reminder system at the four follow-up time points and the project coordinator and data manager will validate all data inputs, request clarifications and updates for unclear or missing data, and secure that DNA of sufficient quality is received, processed and stored for later host genome analyses.
Currently, a randomized intervention study is unrealistic due to limited data supporting any specific neurosurgical approach and given the diversity of tumour subtypes, localisation and invasiveness. However, such a randomisation may be realistic if the present study does not clearly identify surgical approaches with statistically significant reduced risks of CMS.
Acknowledgements
Peder Skov Wehner, and Steen Rosthøj (Denmark); Christoffer Ehrstedt, Peter Siesjö, Irene Devenney, Per Nyman, Magnus Sabel and Mattias Mattsson (Sweden); Einar Stensvold, Ingrid Torsvik and Tore Stokland (Norway); Mia Westerholm-Ormio, Kristiina Nordfors, Jouni Pesola, Päivi Lähteenmäki and Satu Lehtinen (Finland); Rosita Kiudeliene and Giedre Rutkauskiene (Lithuania) have all recruited patients.
Funding
This study has received financial support from the Danish Children’s Cancer Foundation, the Swedish Childhood Cancer Foundation the Dagmar Marshall foundation, The Danish Cancer Society and King Christian IX and Queen Louise’s anniversary grant. The funding bodies have not had any role in designing the study, collecting, analysing or interpreting data, in writing the manuscript or in the decision to submit the manuscript for publication.
Availability of data and materials
Access to the protocol is possible through the corresponding author. All study information is registered in an online database. Access to the data is restricted to participating centres until the planned study is completed. The database may be opened for additional studies following completion of this ongoing study.
Authors’ contributions
TG, AS, KN, KS, and MJ conceived the design of the study. TG performed literature research and drafted the primary protocol. MW subsequently made revisions, implemented the study and drafted the manuscript for this paper. CC, NC, PG, MH, SH, TL, RM, OR and HT contributed to the development of the pre- and postoperative registration forms except the neurosurgical registration form. JC, BG, AK and PN contributed to development of the neurosurgical registration form. CK contributed to the development of the strategies for neuroradiological analysis. RG contributed to the development of the strategies for genetic analysis. IO contributed to the development of the neuropsychological test strategy, BZ and KP developed the strategies for speech analysis. All authors have revised and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study has been approved by the Regional Ethics Committee for the
Capital Region in Denmark (file H-6-2014-002), the Ethics Committee in Sweden (file 2014/3:8), Finland (file 176/13/03/03/2014) and Lithuania (file BE-2-29). The project is approved by the Danish Data Protection Agency (file 2007–58-0015). All participants give written informed consent to participate prior to enrolment in the study.
Abbreviations
- CMS
Cerebellar mutism syndrome
- CNS
Central nervous system
- GWAS
Genome-wide association studies (GWAS)
- SNP
Single nucleotide polymorphism
Contributor Information
Morten Wibroe, Email: morten@wibroe.name.
Johan Cappelen, Email: johan.cappelen@stolav.no.
Charlotte Castor, Email: charlotte.castor@med.lu.se.
Niels Clausen, Email: nc@dadlnet.dk.
Pernilla Grillner, Email: pernilla.grillner@karolinska.se.
Thora Gudrunardottir, Email: info@posteriorfossa.org.
Ramneek Gupta, Email: ramneek@cbs.dtu.dk.
Bengt Gustavsson, Email: bengt.gustavsson@karolinska.se.
Mats Heyman, Email: mats.heyman@ki.se.
Stefan Holm, Email: stefan.holm@karolinska.se.
Atte Karppinen, Email: atte.karppinen@hus.fi.
Camilla Klausen, Email: camilla.klausen@regionh.dk.
Tuula Lönnqvist, Email: tuula.lonnqvist@hus.fi.
René Mathiasen, Email: rene.mathiasen@regionh.dk.
Pelle Nilsson, Email: pelle.nilsson@akademiska.se.
Karsten Nysom, Email: karsten.nysom@regionh.dk.
Karin Persson, Email: karin.persson@skane.se.
Olof Rask, Email: olof.rask@skane.se.
Kjeld Schmiegelow, Email: kjeld.schmiegelow@regionh.dk.
Astrid Sehested, Email: astrid.marie.sehested@regionh.dk.
Harald Thomassen, Email: harald.thomassen@stolav.no.
Ingrid Tonning-Olsson, Email: ingrid.tonning-olsson@skane.se.
Barbara Zetterqvist, Email: barbara.zetterqvist@karolinska.se.
Marianne Juhler, Email: marianne.juhler@gmail.com.
References
- 1.Rickert CH, Paulus W. Epidemiology of central nervous system tumors in childhood and adolescence based on the new WHO classification. Childs Nerv Syst. 2001;17(9):503–511. doi: 10.1007/s003810100496. [DOI] [PubMed] [Google Scholar]
- 2.Sonderkaer S, Schmiegelow M, Carstensen H, Nielsen LB, Muller J, Schmiegelow K. Long-term neurological outcome of childhood brain tumors treated by surgery only. J Clin Oncol. 2003;21(7):1347–1351. doi: 10.1200/JCO.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Radcliffe J, Packer RJ, Atkins TE, Bunin GR, Schut L, Goldwein JW, Sutton LN. Three- and four-year cognitive outcome in children with noncortical brain tumors treated with whole-brain radiotherapy. Ann Neurol. 1992;32(4):551–554. doi: 10.1002/ana.410320411. [DOI] [PubMed] [Google Scholar]
- 4.Ellenberg L, McComb JG, Siegel SE, Stowe S. Factors affecting intellectual outcome in pediatric brain tumor patients. Neurosurgery. 1987;21(5):638–644. doi: 10.1227/00006123-198711000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Livesey EA, Hindmarsh PC, Brook CG, Whitton AC, Bloom HJ, Tobias JS, Godlee JN, Britton J. Endocrine disorders following treatment of childhood brain tumours. Br J Cancer. 1990;61(4):622–625. doi: 10.1038/bjc.1990.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunn ME, Lahdesmaki T, Malila N, Arola M, Gronroos M, Matomaki J, et al. Late morbidity in long-term survivors of childhood brain tumors: a nationwide registry-based study in Finland. Neuro-Oncology. 2014; [DOI] [PMC free article] [PubMed]
- 7.Gudrunardottir T, Sehested A, Juhler M, Schmiegelow K. Cerebellar mutism: review of the literature. Childs Nerv Syst. 2011;27(3):355–363. doi: 10.1007/s00381-010-1328-2. [DOI] [PubMed] [Google Scholar]
- 8.Grill J, Viguier D, Kieffer V, Bulteau C, Sainte-Rose C, Hartmann O, Kalifa C, Dellatolas G. Critical risk factors for intellectual impairment in children with posterior fossa tumors: the role of cerebellar damage. J Neurosurg. 2004;101(2 Suppl):152–158. doi: 10.3171/ped.2004.101.2.0152. [DOI] [PubMed] [Google Scholar]
- 9.Puget S, Boddaert N, Viguier D, Kieffer V, Bulteau C, Garnett M, Callu D, Sainte-Rose C, Kalifa C, Dellatolas G, et al. Injuries to inferior vermis and dentate nuclei predict poor neurological and neuropsychological outcome in children with malignant posterior fossa tumors. Cancer. 2009;115(6):1338–1347. doi: 10.1002/cncr.24150. [DOI] [PubMed] [Google Scholar]
- 10.Huber JF, Bradley K, Spiegler BJ, Dennis M. Long-term effects of transient cerebellar mutism after cerebellar astrocytoma or medulloblastoma tumor resection in childhood. Childs Nerv Syst. 2006;22(2):132–138. doi: 10.1007/s00381-005-1223-4. [DOI] [PubMed] [Google Scholar]
- 11.Huber JF, Bradley K, Spiegler B, Dennis M. Long-term neuromotor speech deficits in survivors of childhood posterior fossa tumors: effects of tumor type, radiation, age at diagnosis, and survival years. J Child Neurol. 2007;22(7):848–854. doi: 10.1177/0883073807303995. [DOI] [PubMed] [Google Scholar]
- 12.Robertson PL, Muraszko KM, Holmes EJ, Sposto R, Packer RJ, Gajjar A, Dias MS, Allen JC, Children's Oncology G. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children's Oncology group. J Neurosurg. 2006;105(6 Suppl):444–451. doi: 10.3171/ped.2006.105.6.444. [DOI] [PubMed] [Google Scholar]
- 13.Gudrunardottir T, Sehested A, Juhler M, Grill J, Schmiegelow K. Cerebellar mutism: definitions, classification and grading of symptoms. Childs Nerv Syst. 2011;27(9):1361–1363. doi: 10.1007/s00381-011-1509-7. [DOI] [PubMed] [Google Scholar]
- 14.Gudrunardottir T, De Smet H, Bartha-Doering L, van Dun K, Verhoeven J, Paquier P, Mariën P: Posterior Fossa Syndrome (PFS) and Cerebellar Mutism. In: The linguistic cerebellum. edn. Edited by Mariën P, Manto M. Amsterdam: Academic Press; 2016: 257–313.
- 15.Van CF, Van de Laar A, Plets C, Goffin J, Casaer P. Transient cerebellar mutism after posterior fossa surgery in children. Neurosurgery. 1995;37(5):894–898. doi: 10.1227/00006123-199511000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Pollack IF, Polinko P, Albright AL, Towbin R, Fitz C. Mutism and pseudobulbar symptoms after resection of posterior fossa tumors in children: incidence and pathophysiology. Neurosurgery. 1995;37(5):885–893. doi: 10.1227/00006123-199511000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Kotil K, Eras M, Akcetin M, Bilge T. Cerebellar mutism following posterior fossa tumor resection in children. Turk Neurosurg. 2008;18(1):89–94. [PubMed] [Google Scholar]
- 18.Wells EM, Khademian ZP, Walsh KS, Vezina G, Sposto R, Keating RF, Packer RJ. Postoperative cerebellar mutism syndrome following treatment of medulloblastoma: neuroradiographic features and origin. J Neurosurg Pediatr. 2010;5(4):329–334. doi: 10.3171/2009.11.PEDS09131. [DOI] [PubMed] [Google Scholar]
- 19.Catsman-Berrevoets CE, Aarsen FK. The spectrum of neurobehavioural deficits in the posterior fossa syndrome in children after cerebellar tumour surgery. Cortex. 2010;46(7):933–946. doi: 10.1016/j.cortex.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Ersahin Y, Mutluer S, Cagli S, Duman Y. Cerebellar mutism: report of seven cases and review of the literature. Neurosurgery. 1996;38(1):60–65. doi: 10.1097/00006123-199601000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Morris EB, Phillips NS, Laningham FH, Patay Z, Gajjar A, Wallace D, Boop F, Sanford R, Ness KK, Ogg RJ. Proximal dentatothalamocortical tract involvement in posterior fossa syndrome. Brain. 2009;132(Pt 11):3087–3095. doi: 10.1093/brain/awp241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Baarsen KM, Grotenhuis JA. The anatomical substrate of cerebellar mutism. Med Hypotheses. 2014;82(6):774–780. doi: 10.1016/j.mehy.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 23.Wells EM, Walsh KS, Khademian ZP, Keating RF, Packer RJ. The cerebellar mutism syndrome and its relation to cerebellar cognitive function and the cerebellar cognitive affective disorder. Dev Disabil Res Rev. 2008;14(3):221–228. doi: 10.1002/ddrr.25. [DOI] [PubMed] [Google Scholar]
- 24.Law N, Greenberg M, Bouffet E, Taylor MD, Laughlin S, Strother D, Fryer C, McConnell D, Hukin J, Kaise C, et al. Clinical and neuroanatomical predictors of cerebellar mutism syndrome. Neuro-Oncology. 2012;14(10):1294–1303. doi: 10.1093/neuonc/nos160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 26.Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44(2):489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 27.Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baillieux H, De Smet HJ, Paquier PF, De Deyn PP, Marien P. Cerebellar neurocognition: insights into the bottom of the brain. Clin Neurol Neurosurg. 2008;110(8):763–773. doi: 10.1016/j.clineuro.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Marien P, Engelborghs S, Fabbro F, De Deyn PP. The lateralized linguistic cerebellum: a review and a new hypothesis. Brain Lang. 2001;79(3):580–600. doi: 10.1006/brln.2001.2569. [DOI] [PubMed] [Google Scholar]
- 30.Miller NG, Reddick WE, Kocak M, Glass JO, Lobel U, Morris B, Gajjar A, Patay Z. Cerebellocerebral diaschisis is the likely mechanism of postsurgical posterior fossa syndrome in pediatric patients with midline cerebellar tumors. AJNR Am J Neuroradiol. 2010;31(2):288–294. doi: 10.3174/ajnr.A1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Smet HJ, Baillieux H, Wackenier P, De Praeter M, Engelborghs S, Paquier PF, De Deyn PP, Marien P. Long-term cognitive deficits following posterior fossa tumor resection: a neuropsychological and functional neuroimaging follow-up study. Neuropsychology. 2009;23(6):694–704. doi: 10.1037/a0016106. [DOI] [PubMed] [Google Scholar]
- 32.Doxey D, Bruce D, Sklar F, Swift D, Shapiro K. Posterior fossa syndrome: identifiable risk factors and irreversible complications. Pediatr Neurosurg. 1999;31(3):131–136. doi: 10.1159/000028848. [DOI] [PubMed] [Google Scholar]
- 33.Korah MP, Esiashvili N, Mazewski CM, Hudgins RJ, Tighiouart M, Janss AJ, Schwaibold FP, Crocker IR, Curran WJ, Jr, Marcus RB., Jr Incidence, risks, and sequelae of posterior fossa syndrome in pediatric medulloblastoma. Int J Radiat Oncol Biol Phys. 2010;77(1):106–112. doi: 10.1016/j.ijrobp.2009.04.058. [DOI] [PubMed] [Google Scholar]
- 34.Catsman-Berrevoets CE, Van Dongen HR, Mulder PG, Geuze D, Paquier PF, Lequin MH. Tumour type and size are high risk factors for the syndrome of "cerebellar" mutism and subsequent dysarthria. J Neurol Neurosurg Psychiatry. 1999;67(6):755–757. doi: 10.1136/jnnp.67.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reed-Berendt R, Phillips B, Picton S, Chumas P, Warren D, Livingston JH, Hughes E, Morrall MC. Cause and outcome of cerebellar mutism: evidence from a systematic review. Childs Nerv Syst. 2014;30(3):375–385. doi: 10.1007/s00381-014-2356-0. [DOI] [PubMed] [Google Scholar]
- 36.McMillan HJ, Keene DL, Matzinger MA, Vassilyadi M, Nzau M, Ventureyra EC. Brainstem compression: a predictor of postoperative cerebellar mutism. Childs Nerv Syst. 2009;25(6):677–681. doi: 10.1007/s00381-008-0777-3. [DOI] [PubMed] [Google Scholar]
- 37.Di RC, Chieffo D, Frassanito P, Caldarelli M, Massimi L, Tamburrini G. Heralding cerebellar mutism: evidence for pre-surgical language impairment as primary risk factor in posterior fossa surgery. Cerebellum. 2011;10(3):551–562. doi: 10.1007/s12311-011-0273-2. [DOI] [PubMed] [Google Scholar]
- 38.Kupeli S, Yalcin B, Bilginer B, Akalan N, Haksal P, Buyukpamukcu M. Posterior fossa syndrome after posterior fossa surgery in children with brain tumors. Pediatr Blood Cancer. 2011;56(2):206–210. doi: 10.1002/pbc.22730. [DOI] [PubMed] [Google Scholar]
- 39.Zaheer SN, Wood M. Experiences with the telovelar approach to fourth ventricular tumors in children. Pediatr Neurosurg. 2010;46(5):340–343. doi: 10.1159/000321539. [DOI] [PubMed] [Google Scholar]
- 40.Avula S, Mallucci C, Kumar R, Pizer B. Posterior fossa syndrome following brain tumour resection: review of pathophysiology and a new hypothesis on its pathogenesis. Childs Nerv Syst. 2015;31(10):1859–1867. doi: 10.1007/s00381-015-2797-0. [DOI] [PubMed] [Google Scholar]
- 41.Gelabert-Gonzalez M, Fernandez-Villa J. Mutism after posterior fossa surgery. Review of the literature. Clin Neurol Neurosurg. 2001;103(2):111–114. doi: 10.1016/S0303-8467(01)00125-1. [DOI] [PubMed] [Google Scholar]
- 42.Pollack IF. Posterior fossa syndrome. Int Rev Neurobiol. 1997;41:411–432. doi: 10.1016/S0074-7742(08)60362-1. [DOI] [PubMed] [Google Scholar]
- 43.Zhou W, Xu D, Peng X, Zhang Q, Jia J, Crutcher KA. Meta-analysis of APOE4 allele and outcome after traumatic brain injury. J Neurotrauma. 2008;25(4):279–290. doi: 10.1089/neu.2007.0489. [DOI] [PubMed] [Google Scholar]
- 44.Dardiotis E, Fountas KN, Dardioti M, Xiromerisiou G, Kapsalaki E, Tasiou A, Hadjigeorgiou GM. Genetic association studies in patients with traumatic brain injury. Neurosurg Focus. 2010;28(1) doi: 10.3171/2009.10.FOCUS09215. [DOI] [PubMed] [Google Scholar]
- 45.Waters RJ, Nicoll JA. Genetic influences on outcome following acute neurological insults. Curr Opin Crit Care. 2005;11(2):105–110. doi: 10.1097/01.ccx.0000155354.78617.91. [DOI] [PubMed] [Google Scholar]
- 46.Caner H, Altinors N, Benli S, Calisaneller T, Albayrak A. Akinetic mutism after fourth ventricle choroid plexus papilloma: treatment with a dopamine agonist. Surg Neurol. 1999;51(2):181–184. doi: 10.1016/S0090-3019(98)00120-7. [DOI] [PubMed] [Google Scholar]
- 47.Shyu C, Burke K, Souweidane MM, Dunkel IJ, Gilheeney SW, Gershon T, Khakoo Y. Novel use of zolpidem in cerebellar mutism syndrome. J Pediatr Hematol Oncol. 2011;33(2):148–149. doi: 10.1097/MPH.0b013e3182053a1a. [DOI] [PubMed] [Google Scholar]
- 48.Akhaddar A, Salami M, El Asri AC, Boucetta M. Treatment of postoperative cerebellar mutism with fluoxetine. Childs Nerv Syst. 2012;28(4):507–508. doi: 10.1007/s00381-012-1719-7. [DOI] [PubMed] [Google Scholar]
- 49.Pitsika M, Tsitouras V. Cerebellar mutism. J Neurosurg Pediatr. 2013;12(6):604–614. doi: 10.3171/2013.8.PEDS13168. [DOI] [PubMed] [Google Scholar]
- 50.Kuper M, Timmann D. Cerebellar mutism. Brain Lang. 2013;127(3):327–333. doi: 10.1016/j.bandl.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 51.Hockey B, Leslie K, Williams D. Dexamethasone for intracranial neurosurgery and anaesthesia. J Clin Neurosci. 2009;16(11):1389–1393. doi: 10.1016/j.jocn.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Mekitarian FE, Carvalho WB, Cavalheiro S, Horigoshi NK, Freddi NA, Vieira GK. Hyperglycemia and postoperative outcomes in pediatric neurosurgery. Clinics (Sao Paulo) 2011;66(9):1637–1640. doi: 10.1590/S1807-59322011000900022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wesolowska A, Dalgaard MD, Borst L, Gautier L, Bak M, Weinhold N, Nielsen BF, Helt LR, Audouze K, Nersting J, et al. Cost-effective multiplexing before capture allows screening of 25 000 clinically relevant SNPs in childhood acute lymphoblastic leukemia. Leukemia. 2011;25(6):1001–1006. doi: 10.1038/leu.2011.32. [DOI] [PubMed] [Google Scholar]
- 54.Wesolowska-Andersen A, Borst L, Dalgaard MD, Yadav R, Rasmussen KK, Wehner PS, et al. Genomic profiling of thousands of candidate polymorphisms predicts risk of relapse in 778 Danish and German childhood acute lymphoblastic leukemia patients. Leukemia. 2014; [DOI] [PMC free article] [PubMed]
- 55.Edwards P, Arango M, Balica L, Cottingham R, El-Sayed H, Farrell B, Fernandes J, Gogichaisvili T, Golden N, Hartzenberg B, et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet. 2005;365(9475):1957–1959. doi: 10.1016/S0140-6736(05)66552-X. [DOI] [PubMed] [Google Scholar]
- 56.Roberts I, Yates D, Sandercock P, Farrell B, Wasserberg J, Lomas G, Cottingham R, Svoboda P, Brayley N, Mazairac G, et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet. 2004;364(9442):1321–1328. doi: 10.1016/S0140-6736(04)17188-2. [DOI] [PubMed] [Google Scholar]
- 57.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123(4):465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perreault S, Ramaswamy V, Achrol AS, Chao K, Liu TT, Shih D, Remke M, Schubert S, Bouffet E, Fisher PG, et al. MRI surrogates for molecular subgroups of medulloblastoma. AJNR Am J Neuroradiol. 2014;35(7):1263–1269. doi: 10.3174/ajnr.A3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raybaud C, Ramaswamy V, Taylor MD, Laughlin S. Posterior fossa tumors in children: developmental anatomy and diagnostic imaging. Childs Nerv Syst. 2015;31(10):1661–1676. doi: 10.1007/s00381-015-2834-z. [DOI] [PubMed] [Google Scholar]
- 60.Patay Z. Postoperative posterior fossa syndrome: unraveling the etiology and underlying pathophysiology by using magnetic resonance imaging. Childs Nerv Syst. 2015;31(10):1853–1858. doi: 10.1007/s00381-015-2796-1. [DOI] [PubMed] [Google Scholar]
- 61.Spiteri M, Windridge D, Avula S, Kumar R, Lewis E. Identifying quantitative imaging features of posterior fossa syndrome in longitudinal MRI. J Med Imag. 2015;2:044502. [DOI] [PMC free article] [PubMed]
- 62.Frandsen TL, Heyman M, Abrahamsson J, Vettenranta K, Asberg A, Vaitkeviciene G, Pruunsild K, Toft N, Birgens H, Hallbook H, et al. Complying with the European clinical trials directive while surviving the administrative pressure - an alternative approach to toxicity registration in a cancer trial. Eur J Cancer. 2014;50(2):251–259. doi: 10.1016/j.ejca.2013.09.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Access to the protocol is possible through the corresponding author. All study information is registered in an online database. Access to the data is restricted to participating centres until the planned study is completed. The database may be opened for additional studies following completion of this ongoing study.


