Abstract
Background
β2-adrenoceptor agonists have been shown to reduce the lipopolysaccharide (LPS)-induced cytokine release by human monocyte-derived macrophages (MDMs). We compare the expression of β2-adrenoceptors and the inhibitory effect of formoterol and salmeterol on the LPS-induced release of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and a range of chemokines (CCL2, 3, 4, and IL-8) by human lung macrophages (LMs) and MDMs.
Methods
LMs were isolated from patients undergoing resection and MDMs were obtained from blood monocytes in the presence of GM-CSF. LMs and MDMs were incubated in the absence or presence of formoterol or salmeterol prior to stimulation with LPS. The effects of formoterol were also assessed in the presence of the phosphodiesterase inhibitor roflumilast.
Results
LPS-induced cytokine production was higher in LMs than in MDMs. Salmeterol and formoterol exerted an inhibitory effect on the LPS-induced production of TNF-α, IL-6, CCL2, CCL3, and CCL4 in MDMs. In contrast, the β2-adrenoceptor agonists were devoid of any effect on LMs - even in the presence of roflumilast. The expression of β2-adrenergic receptors was detected on Western blots in MDMs but not in LMs.
Conclusions
Concentrations of β2-adrenoceptor agonists that cause relaxation of the human bronchus can inhibit cytokine production by LPS-stimulated MDMs but not by LMs.
Electronic supplementary material
The online version of this article (doi:10.1186/s12931-017-0613-y) contains supplementary material, which is available to authorized users.
Keywords: β2-adrenoceptor, Cytokines, Lipopolysaccharide, Lung macrophage, Monocyte-derived macrophage
Background
Pollens, house dust mites (HDMs), and cat dander are major triggers in allergic respiratory diseases such as asthma [1–3]. Air pollution is also associated with the acute worsening of pre-existing asthma and chronic obstructive pulmonary disease (COPD) and with progression from asthma to COPD [4, 5].
In addition to its well-characterized involvement in the response to lipopolysaccharide (LPS), toll-like receptor 4 (TLR4) is involved in the airways’ response to various allergens (e.g. ragweed pollen, house dust extract, and cat dander) and many air pollutants including particulate matter and their components other than allergens and LPS, such as viruses and fungal spores [6–9]. Particles that are less than 5 μm in size may gain access to the lower airways and alveoli, where they encounter macrophages (which account for more than 80% of the leukocyte population) [9]. LPS-mediated activation of macrophages causes the release of cytokines (tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and chemokines such as CCL2, CCL3, CCL4, and CXCL8 (IL-8)). This release contributes to airway inflammation by increasing the recruitment of inflammatory cells [10]. Recent research has highlighted the role of neutrophil recruitment in the response to allergen exposure and the subsequent development of allergen sensitization and inflammation [11].
In murine models of LPS-induced lung inflammation, formoterol and salmeterol reduce the recruitment of neutrophils to the lung and inhibit the release of pro-inflammatory mediators [12, 13]. We recently described the anti-inflammatory effect of the long-acting β2-adrenoceptor agonist (LABA) olodaterol on (i) murine and guinea pig models of cigarette smoke- and LPS-induced lung inflammation, and (ii) LPS-induced cytokine release from explants of human lung parenchyma [14]. In a clinical setting, salmeterol also reduces neutrophil influx, neutrophil degranulation and TNF-α release after LPS inhalation by healthy individuals [15].
β2-adrenoceptors are widely expressed throughout the lung [16, 17], and are found on epithelial and bronchial smooth muscle cells, endothelial and vascular smooth muscle cells, and pneumocytes [18, 19]. The β2-adrenoceptors expressed by airway smooth muscle are involved in the relaxant effect of β2-adrenoceptor agonists. Moreover, β2-adrenoceptors are expressed on inflammatory cells, such as neutrophils, monocytes/macrophages and lymphocytes [20–23]. With respect to the monocyte/macrophage lineage, β2-adrenoceptor activation was shown to variably reduce the LPS-stimulated release of leukotriene B4 (LTB4), TNF-α, IL-1β, IL-8 and CCL3 from human peripheral blood mononuclear cells [24–29]. Formoterol and salmeterol suppressed the LPS-induced release of TNF-α by monocyte-derived macrophages (MDMs) [30]. Clenbuterol and terbutaline suppressed the LPS-induced TNF-α and IL-6 release by phorbol-myristate-acetate-differentiated U937 human macrophages [31]. Furthermore, salmeterol reduced the cigarette-smoke-extract-induced release of IL-8 by MDMs [32].
Human monocytes, MDMs and U937 macrophages are all surrogate cell models that do not adequately recapitulate the biology of primary tissue macrophages. Previous studies have identified a large number of differentially expressed proteins [33, 34] and genes [35, 36] when comparing unstimulated monocytes, MDMs and human lung macrophages (LMs). It is noteworthy that the scarce data on the anti-inflammatory effects of β2-adrenoceptor agonists are much less clear for LMs than for MDMs or monocytes. The non-selective β2-adrenergic agonist isoprenaline did not alter the zymosan- and IgE-triggered release of the eicosanoids LTB4 and thromboxane B2 (TXB2) [37], whereas high concentrations of salmeterol inhibited the release of TXB2 in LMs [38]. Neither the short-acting β2-adrenergic agonists salbutamol and terbutaline nor the LABAs salmeterol and formoterol inhibit the LPS-stimulated release of IL-1β [39]. However, treatment with isoprenaline was associated with an increase in levels of cyclic AMP (cAMP) in LMs via the activation of β2-adrenergic receptors [22, 40, 41]. Furthermore, other cAMP-elevating agents (adenosine receptor agonists, phosphodiesterase 4 (PDE4) inhibitors, PGE1/2/4 and forskolin) either increased the cAMP content [22, 40] or had inhibitory effects on LPS-induced cytokine release by LMs [41–44]. During the preparation of the present manuscript, Gill et al. reported on the inhibitory effects of high concentrations of β2-adrenoceptor agonists on the LPS-induced production of TNF-α and IL-6 by LMs [45].
Hence, the present study was designed to assess and compare the effects of the LABAs formoterol and salmeterol on LPS-stimulated cytokine production and the expression of β2-adrenoceptors by LMs and MDMs. We selected a LABA concentration range (10−11 to 10−7 M) that relaxes isolated human bronchus [46, 47], and we used an LPS preparation that is selective for TLR4. We assessed the production of TNF-α, IL-6 and three CC chemokines (CCL2, CCL3, and CCL4), levels of which are markedly increased by LPS exposure and inhibited by cAMP-elevating agents [30, 41–45]. Furthermore, we assessed the LABAs’ effects on the LPS-induced production of IL-1β and IL-8, which is only weakly or not altered by various cAMP-elevating agents [30, 39, 42–44].
Methods
Reagents
Penicillin-streptomycin, dimethyl sulfoxide (DMSO), fetal calf serum (FCS), LPS from Escherichia coli (serotype 0111:B4), trypan blue dye, indomethacin, PGE2, salmeterol xinafoate, and formoterol fumarate were purchased from Sigma (St. Louis, MO, USA). Acrylamide, SDS, Tris, HEPES, RPMI 1640 medium, phosphate-buffered saline (PBS) and bovine serum albumin (BSA) were obtained from Eurobio Biotechnology (Les Ulis, France). Roflumilast was synthesized by Nycomed GmbH (Konstanz, Germany; a gift from Dr. H. Tenor). Recombinant human GM-CSF (rhGM-CSF) was purchased from R&D Systems Europe (Lille, France). All cell culture plastics were from CML (Nemours, France). Specific antibodies against β2-adrenoceptors and β-actin were obtained from Thermo Scientific (Vilnius, Lithuania) and Cytoskeleton (Denver, CO, USA), respectively. A Bradford protein assay and Precision Plus Protein Dual Color Standards were purchased from Bio-Rad (Hercules, CA, USA). Stock solutions of roflumilast and indomethacin were prepared in DMSO. A PGE2 stock solution (10 mM) was prepared in ethanol. All subsequent dilutions were prepared daily in complete medium. The DMSO concentration applied to cells in culture never exceeded 0.1%. Neither the vehicle nor any of the compounds used in this study altered cell viability. All wells were run in duplicate for each series of experiments performed with LMs or MDMs obtained from a single patient’s sample.
Isolation and culture of human LMs and MDMs
Experiments on human tissues had been approved by the regional independent ethics committee (Comité de Protection des Personnes Ile de France VIII, Boulogne-Billancourt, France).
Lung tissue was obtained from 15 patients (mean ± standard error mean (SEM) age: 67 ± 4 years; gender (M:F): 10:5; FEV1/FVC ratio: 0.83 ± 0.04; 9 smokers and 6 ex-smokers; pack years: 47 ± 7) undergoing surgical resection for lung carcinoma and who had not received chemotherapy or radiotherapy. Only one donor was treated on a daily basis with a β2-adrenergic agonist. The LMs were isolated from lung parenchyma, as previously described [44]. The mean ± SEM adherent macrophage count was 191 ± 13 × 103 per well in 24-well plates. More than 95% of the adherent cells were macrophages, as determined by May–Grünwald–Giemsa staining and CD68 immunocytochemistry. Cell viability exceeded 90%, as assessed by trypan blue dye exclusion.
The monocytes were isolated from blood, as previously described [48]. Briefly, peripheral blood mononuclear cells from nine healthy blood donors were harvested from human buffy-coat (Etablissement Français du Sang, Ivry-sur-Seine, France) by differential centrifugation on UNI-SEP® U-10 (Novamed, Jerusalem, Israel). The experiments were performed in compliance with the French legislation on blood donation and blood product use. Cells were resuspended in RPMI 1640 medium supplemented with penicillin 100 IU.ml−1-streptomycin 100 μg.ml−1, L-glutamine 2 mM and 10% (v/v) FCS and seeded into 24-well plates at a density of 106 cells/well. Monocytes were isolated by adherence on cell culture plates for 1.5 h. Non-adherent cells were removed by aspiration, and the remaining monocytes were incubated with 50 ng.ml−1 rhGM-CSF for 8 days to obtain MDMs [48].
Treatment of LMs and MDMs with salmeterol and formoterol
The experiments were performed in RPMI medium supplemented with 1% FCS. The 24-well plates containing either LMs or MDMs were washed and pre-incubated with vehicle, salmeterol or formoterol for 1 h before stimulation with LPS. Following a 24 h incubation period, supernatants were collected and stored at −80 °C for later analysis of the cytokine concentration. The submaximal LPS concentration (10 ng.ml−1) was selected on the basis of previous data [43, 44] [see Additional file 1].
To explore the LMs’ responsiveness to a β2-adrenoceptor agonist, the effect of formoterol (10 nM) was also tested in the presence (1 or 100 nM) or absence of roflumilast. Roflumilast acts as a selective PDE4 inhibitor up to a concentration of 1 μM [49]. This compound has been shown to enhance the inhibitory effect of cAMP-inducing agents (such as PGE2) on the LPS-induced release of cytokine by LMs [44]. In this series of experiments, PGE2 (10 nM) was used as an internal control for the LMs’ responsiveness to a cAMP-elevating agent [42, 44]. In order to avoid any interference of the LPS-induced production of endogenous prostanoids on the response to formoterol and PGE2, the experiments were performed in presence of indomethacin (1 μM) [44].
Measurement of cytokine production
The levels of cytokine in the supernatants were measured using the Duoset® ELISA kit (R&D Systems Europe). The optical density was determined at 450 nm (MRX II, Dynex Technologies, Saint-Cloud, France). Cytokine levels were expressed in ng per 106 cells. The detection limits of these assays were 4 pg.ml−1 for CCL3 and IL-1β, 8 pg.ml−1 for TNF-α, CCL2 and CCL4, 9 pg.ml−1 for IL-6, and 32 pg.ml−1 for IL-8.
Expression of β2-adrenoceptors on LMs and MDMs
For real-time quantitative-PCR (RT-qPCR) analysis, LMs or MDMs (stimulated or not with LPS for 24 h) were harvested in TRIzol® reagent (Life Technologies, Saint Aubin, France). The RNA’s intactness was determined by running an aliquot of each sample on an ExperionTM automated electrophoresis station (Bio-Rad, Marnes-la-Coquette, France). Next, 1 μg of total RNA was reverse-transcribed using SuperScript® III First-strand SuperMix kit (Life Technologies). Specific TaqMan® arrays based on predesigned reagents (Life Technologies) were used for the analysis of β2-adrenoceptor transcripts (ADRB2). RT-qPCR was performed using Gene Expression Master Mix (Life Technologies) with 20 ng of cDNA in a StepOnePlus thermocycler (Life Technologies). The thermal cycling conditions were as follows: initial denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The housekeeping genes coding for hypoxanthine phosphoribosyltransferase (HPRT1) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used for signal normalization. The relative expression of mRNAs was calculated according to the 2(−∆Ct) method [50].
For Western blotting, LMs and MDMs were incubated with medium alone or LPS for 24 h. The cells were then washed with PBS and lysed for 15 min in an appropriate buffer (Cytobuster, Novagen, San Diego, CA, USA) containing protease inhibitor cocktail and phosphatase inhibitor cocktail (Roche, Mannheim, Germany) on ice. Equal amounts of cell lysate (30 μg) were separated on 10% SDS-PAGE gels and then transferred onto nitrocellulose membranes. The membranes were blocked for 1 h with 5% w/v non-fat powdered milk in Tris base containing 0.1% Tween 20. Next, the membranes were incubated with a mouse monoclonal antibody specific for human β2-adrenoceptors (Thermo Scientific, Vilnius, Lithuania) and diluted (1/1000) for 2 h at room temperature. After washing, the membranes were incubated for 2 h with a horseradish-peroxidase-conjugated anti-mouse antibody (Dako, Glostrup, Denmark). The membranes were then incubated with an enhanced chemiluminescence solution for 1 min and quantified with QuantityOne 4.2.1 (Bio-Rad, Marnes-La-Coquette, France).
Statistical analysis
Data were expressed as the mean ± SEM; n represents the number of patients from whom MDM or LM preparations were obtained. Wilcoxon’s test or a one-way ANOVA for repeated measures was followed by Dunnett’s post-tests, as appropriate. The threshold for statistical significance was set to p ≤ 0.05.
Results
Effects of LPS on cytokine production by MDMs and LMs
There was no significant difference between unstimulated LMs and MDMs in terms of the production of TNF-α, IL-1β, and the chemokines (IL-8, CCL2, CCL3, and CCL4). However, IL-6 production was higher in MDMs. Following incubation with LPS, the release of all cytokines other than TNF-α and CCL4 was greater for LMs than for MDMs (Table 1).
Table 1.
The effect of LPS on cytokine levels in the supernatants of MDM and LM cultures
| Monocyte-derived macrophages | Lung macrophages | |||
|---|---|---|---|---|
| Cytokine | LPS - | LPS + | LPS - | LPS + |
| TNF-α | 1.8 ± 0.5 | 16.4 ± 6.5 | 0.7 ± 0.1 | 33.8 ± 7.1 |
| IL-1ß | 0.05 ± 0.01 | 0.09 ± 0.02 | 0.07 ± 0.03 | 0.54 ± 0.18* |
| IL-6 | 1.3 ± 0.5 | 13.2 ± 3.6 | 0.2 ± 0.1* | 229.2 ± 80.3* |
| CCL2 | 2.2 ± 0.6 | 9.7 ± 2.4 | 3.1 ± 0.9 | 19.6 ± 3.9* |
| CCL3 | 4.5 ± 2.5 | 22.2 ± 8.1 | 3.1 ± 0.8 | 148.7 ± 41.2* |
| CCL4 | 6.5 ± 2.3 | 86.5 ± 31.4 | 6.8 ± 2.3 | 264.2 ± 78.6 |
| IL-8 | 5.5 ± 2.3 | 93.2 ± 32.1 | 39.3 ± 14.3 | 1411.4 ± 383.1* |
Macrophages were incubated with medium only (LPS-) or 10 ng.ml−1 LPS in medium (LPS+) for 24 h. Cell culture supernatants were collected and analyzed using an ELISA. Data are expressed as the mean ± SEM ng.10−6 cells from 5 to 8 experiments. Asterisks indicate significant differences (Wilcoxon’s t test) between MDM and LM experiments
Effects of formoterol and salmeterol on LPS-induced cytokine release by MDMs and LMs
We next investigated the effects of serial increases in the concentration (10−11 to 10−7 M) of formoterol and salmeterol on LPS-induced cytokine release. In MDMs, formoterol and salmeterol inhibited the LPS-induced production of TNF-α, IL-6 and the three CCL chemokines at concentrations greater than or equal to 10−10 M (Figs. 1 and 2). The respective effects of formoterol and salmeterol on the (weak) production of IL-1β were highly variable from one preparation to another. The production of IL-8 was not altered by the two LABAs. In sharp contrast to the results for MDMs, formoterol and salmeterol did not alter the LPS-induced production of any of the seven cytokines by LMs (Figs. 1 and 2). To definitively establish that the LMs’ lack of response is not restricted to these two LABAs, we performed additional experiments on four preparations of MDMs and LMs with salbutamol at 1 μM (a concentration that causes maximal relaxation of isolated human bronchus and is equipotent to the concentrations used in the present study with the LABAs (0.01 μM for formoterol and 0.1 μM for salmeterol)). Our results confirmed that this short-acting β2-adrenoceptor agonist inhibited MDMs (to much the same extent as in the work by Gill et al. [45]) but had no effect on LMs [see Additional file 2].
Fig. 1.
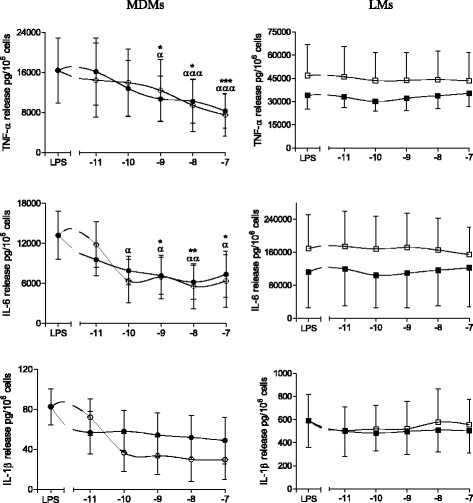
Effects of formoterol and salmeterol on LPS-induced release of TNF-α, IL-6 and IL-1β by monocyte-derived macrophages (MDMs) and lung macrophages (LMs). MDMs (left-hand column) and LMs (right-hand column) were incubated with formoterol (○,□) and salmeterol (●,■) for 1 h prior to stimulation with LPS (10 ng.ml−1) for 24 h. The culture supernatants were collected, and the cytokine concentrations were measured using an ELISA. The data represent the mean ± SEM of 5 to 8 independent experiments..*p < 0.05, **p < 0.01, ***p < 0.001 for salmeterol vs. LPS, α p < 0.05, αα p < 0.01, ααα p < 0.001 for formoterol vs. LPS
Fig. 2.
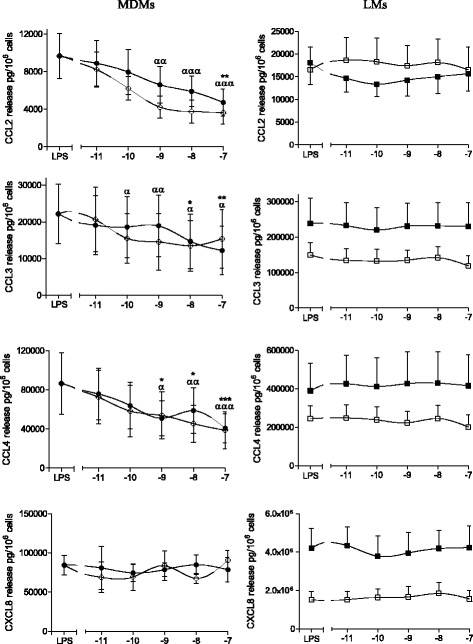
Effects of formoterol and salmeterol on LPS-induced release of chemokines by monocyte-derived macrophages (MDMs) and lung macrophages (LMs). MDMs (left-hand column) and LMs (right-hand column) were incubated with formoterol (○,□) and salmeterol (●,■) for 1 h prior to stimulation with LPS (10 ng.ml−1) for 24 h. The culture supernatants were collected, and the cytokine concentrations were measured using an ELISA. The data represent the mean ± SEM of 5 to 8 independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 for salmeterol vs. LPS, α p < 0.05, αα p < 0.01, ααα p < 0.001 for formoterol vs. LPS
Effect of formoterol on LPS-induced cytokine release in the presence of roflumilast
Since formoterol was more potent than salmeterol in altering LPS-induced cytokine production by MDMs, we also looked at whether the presence of roflumilast could unmask an effect of 10−8 M formoterol on the LPS-induced release of TNF-α and the CCL chemokines by LMs. In this series of experiments, formoterol has no effect alone or in the presence of roflumilast on LPS-induced release of TNF-α, CCL2, CCL3 and CCL4 (Fig. 3). In contrast, the greater inhibitory effect of PGE2 on production of the four cytokines in the presence of roflumilast evidences the latter drug’s effect on a cAMP-elevator other than the LABAs in LMs (Fig. 3). In addition, formoterol did not increase significantly the inhibitory effect of PGE2 (data not shown).
Fig. 3.
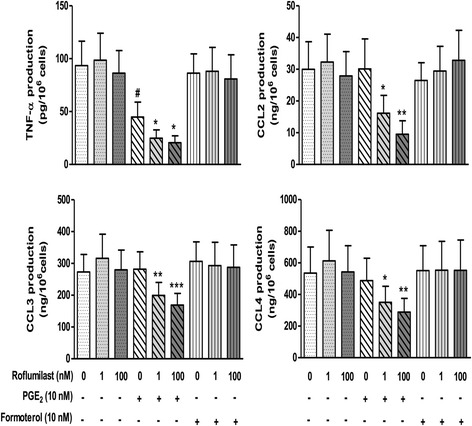
Effects of formoterol, PGE2 and roflumilast on LPS-induced TNF-α, CCL2, CCL3 and CCL4 release from LMs. Cells were pre-incubated with indomethacin (1 μM) for 30 min, followed by incubation with roflumilast (1 nM or 100 nM), PGE2 (10 nM), formoterol (10 nM) or vehicle for another 30 min prior to stimulation with LPS (10 ng.ml−1) for 24 h). The data represent the mean ± SEM of 6 different experiments, *p < 0.05, **p < 0.01, ***p < 0.001 vs. LPS + PGE2 treatment; #p < 0.05 vs. LPS
Expression of β2-adrenoceptors on MDMs and LMs in presence and absence of LPS
Levels of ß2-adrenoceptor transcript expression were similar in MDMs and LMs (Table 2), whereas β1- and β3-adrenoceptor transcripts were only found in macrophages from two and three patients, respectively (data not shown). Strikingly, incubation of LMs with LPS for 24 h induced an approximately 7-fold decrease in β2-adrenoceptor expression (Table 2).
Table 2.
Expression of β 2-adrenoreceptor mRNA transcripts (ADRB2) in human MDMs and LMs
| Relative expression in control (LPS-) | Relative expression after LPS exposure | Fold-change for LPS versus control | |
|---|---|---|---|
| MDMs | 172.6 [134.2, 228.7] | 108.6 [76.2, 158.4] | −1.6 |
| LMs | 310.8 [187.1, 546.0] | 44.7 [23.6, 75.3] | −7.5 |
The quoted result is the median [min, max] × 1000 of 3 to 5 independent experiments
To determine whether the LMs’ absence of response to the β2-adrenoceptor agonists was related to a loss of β2-adrenoceptors relative to MDMs, we performed a Western blot analysis. As shown in Fig. 4, MDMs (but not LMs) expressed β2-adrenoceptors. LPS treatment for 24 h did not alter the expression of the β2-adrenoceptors in MDMs.
Fig. 4.
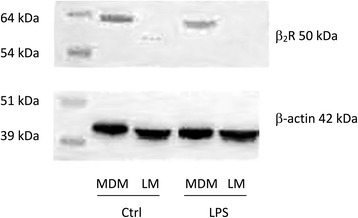
Western blot analysis of the expression of β2-adrenoceptors on MDMs and LMs. MDMs and LMs were incubated with medium alone (control) or LPS (10 ng.ml−1) for 24 h. Cell lysates were immunoblotted with a β2-adrenoceptor-specific antibody
Discussion
Our present results notably showed that (i) cytokine production in response to LPS differs in MDMs and LMs, (ii) salmeterol and formoterol exert an inhibitory effect on the LPS-induced production of TNF-α, IL-6, CCL2, CCL3, and CCL4 by MDMs, (iii) the two LABAs were strikingly devoid of any effect on LMs, and (iv) Western blots revealed β2-adrenergic receptor in MDMs but not in LMs.
We confirmed the recent report in which formoterol and salmeterol can inhibit the LPS-induced release of TNF-α and IL-6 from MDMs [30]. We extended these findings to three CCL chemokines (CCL2, CCL3 and CCL4) involved in the recruitment of monocytes, immature dendritic cells and T cells [51–53]. The range of concentrations at which these two LABAs influence LPS-induced cytokine production is suggestive of a β2-adrenoceptor-dependent mechanism; this is also suggested by the attenuating effect of a β2-adrenoceptor antagonist on formoterol’s inhibitory action [30]. We also confirmed that the two LABAs did not alter the LPS-induced production of IL-1β and IL-8.
In a very recent study [45], salmeterol and indacaterol were found to inhibit the LPS-induced production of TNF-α and IL-6 by human LMs. However, four other β2-adrenoceptor agonists (formoterol, salbutamol, terbutaline and isoprenaline) were inactive, and the inhibitory effect of the two LABAs was only observed at a concentration (10−5 M) that is at least 100-fold higher than those used in the present study and caused maximum relaxation of isolated human bronchi [46, 47]. These differences in the inhibitory activities of the various β2-adrenoceptor agonists and the high concentration of the two active LABAs used in Gill et al.’s study calls into question both the clinical relevance of these results and the involvement of a β2-adrenoceptor-mediated effect. It should be noted that the inhibitory effect of indacaterol was only partly reversed by a selective β2-adrenoceptor antagonist, and the inhibitory effect of salmeterol was not reversed [45]. Moreover, the production of TXB2 by LMs stimulated with either zymosan or the calcium ionophore A23187 was not inhibited by salbutamol (at concentrations up to 10−5 M), and the inhibitory effect of salmeterol was not blocked by propranolol - further suggesting that the effects of high concentrations of LABAs are not mediated by β2-adrenoceptors in LMs [38], as also reported for human monocytes [27, 38]. Furthermore, four β2-adrenoceptor agonists (salmeterol, formoterol, salbutamol, and terbutaline) did not alter LPS- or zymosan-induced LTB4 release from LMs at concentrations up to 10−5 M [39]. Taken as a whole, these results suggest that the inhibitory effects of β2-adrenoceptor agonists on LMs is weak or even null, and might only be produced at very high concentrations via a β2-adrenoceptor-independent mechanism. Given that macrophages express membrane-associated CD14, activation of the TLR4/MD-2 complex by LPS does not therefore require dimerization of the complex induced by LPS binding protein (LBP) [54]. Nevertheless, LBP (if required) was present in the FCS added to the culture medium. Hence, LBP and CD14 do not account for the results and conclusions of the present work.
MDMs differentiated by treatment with GM-CSF have been typically considered to be phenotypically and “behaviorally” similar to LMs [30, 32, 55]. However, recent high-throughput analyses have revealed remarkable differences in gene expression between MDMs and LMs; these differences include the transcripts for G-protein-coupled receptors [35, 36]. These results call into question the use of macrophage surrogates (such as MDMs) to mimic the behavior of LMs. Although β2-adrenoceptor transcript levels were similar in MDMs and LMs ([35] and the present study), we found that protein levels of these receptors (as assessed by Western blotting) were much lower in LMs than in MDMs. Western blotting based on a peroxidase-conjugated secondary antibody is probably less sensitive than radioligand binding methods for detecting receptors. Human macrophages isolated from bronchoalveolar lavages (either by elutriation or adherence to culture dishes) were found to express a moderate density of β2-adrenoceptors in radioligand binding studies [21, 22]. In both of the latter studies [21, 22], the β2-adrenoceptors appear to be functionally coupled to adenylate cyclase; exposure to high concentrations of isoprenaline (≥10−7 M) in the presence of the PDE inhibitor isobutylmethylxanthine resulted in increased cAMP accumulation. However, the increase in cAMP was much lower in the absence of isobutylmethylxanthine, and the two studies did not determine whether the increase in cAMP levels impacted macrophage function. It is noteworthy that in a subsequent study by one of the research groups, β2-adrenoceptor agonists did not alter the LPS- or zymosan-induced release of LTB4 from LMs [39] suggesting that the signal induced by the agonists was not strong enough to inhibit the effect of either LPS or zymosan. Furthermore, in the presence of roflumilast at a concentration that enhances the inhibitory influence of PGE2 on LPS-induced TNF-α and chemokine production by LMs, we found that formoterol also remained inactive. This finding suggests that stimulation of the β2-adrenoceptors did not increase the cAMP level enough to inhibit the production of the four cytokines. In line with these results, isoprenaline alone or in combination with a PDE inhibitor had any inhibitory effect on the release of eicosanoids induced by zymosan or IgE/anti-IgE complexes by LMs. These results for LMs contrast with the additive effects of formoterol and a PDE inhibitor (roflumilast or rolipram) in human monocytes and MDMs [30, 56].
However, forskolin inhibits TXB2 release [37] and PGE2 ([41, 42, 44] and the present study), NECA and roflumilast [43, 44] inhibit the LPS-induced production of cytokines by LMs - demonstrating that other cAMP elevators than LABAs are able to curb the production of eicosanoids or cytokines from LMs. Since the adenylyl cyclase/cAMP/cAMP-dependent protein kinase A axis stimulated by PGE2 reduces the LPS-induced cytokine production [42], the β2-adrenoceptor agonists’ lack of effect in LMs is probably due to the low expression of β2-adrenoceptors in these cells and thus insufficient stimulation of the pathway. The use of ten-fold lower concentrations of LPS (to markedly reduce the strength of the stimulus) unmasked a modest inhibitory effect of salbutamol on the release of TNF-α by LMs [45] - suggesting that the β2-adrenoceptor-dependent rise in cAMP content might be only sufficient to counteract a relatively weak inflammatory stimulus. Lastly, the absence of an inhibitory effect of formoterol in LMs (evidenced in the present study) rules out the involvement of this cell type in the inhibitory effect of this LABA [57] and olodaterol [14] on LPS-induced cytokine release by human lung explants. Macrophages have been implicated in the pathophysiology of COPD and (to a lesser degree) in the inflammatory load in asthma. However, given the absence of LABAs’ effects on LMs in vitro, macrophages are unlikely to account for these compounds’ anti-inflammatory effects.
Conclusion
Our present results showed that concentrations of β2-adrenoceptor agonists that cause the relaxation of isolated human bronchus can inhibit cytokine production by LPS-stimulated MDMs but not by LPS-stimulated LMs - even in the presence of a PDE inhibitor. The LMs’ lack of response could be due to low β2-adrenoceptor expression and thus an insufficiently strong cAMP-dependent trigger for the LPS-induced inflammatory response, since other cAMP elevators were able to inhibit the LPS-induced responses. The present results highlighted the lack of a clinically relevant, anti-inflammatory effect of β2-adrenoceptor agonists on LMs.
Additional files
LPS concentration-response data for MDMs and LMs, and time-course experiments in LMs (figures). (PDF 94 kb)
Effect of salbutamol (1 μM) on LPS-induced cytokine production by MDMs and LMs (figures). (PDF 52 kb)
Acknowledgments
The authors thank CAPES–COFECUB for additional, bilateral funding (Brazil–France) and David Fraser PhD (Biotech Communication SARL, Ploudalmezeau, France) for copy-editing assistance. CA received a doctoral grant from Foch Hospital.
Funding
none.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
EN, SG-D, MB, LCSP, TV and CA performed the research. VL and PD designed the research. TV, SG-D, MB and PD analyzed the data. TV and PD wrote the paper. All authors read and approved the final manuscript.
Competing interests
The authors have no conflicts of interest with regard to the present study, which was not sponsored by a company. PD and EN have received research funding from Boehringer-Ingelheim in the field of respiratory research. PD has received consulting fees, honoraria for lectures and/or participation in scientific advisory boards from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline and Novartis. TV, CA, MB, VL, SGD, HS and LP have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The use of human lung tissue for in vitro experiments was approved by the local independent ethics committee (Comité de Protection des Personnes Ile de France VIII, Boulogne-Billancourt, France). In line with the French legislation on clinical research (and as approved by the independent ethics committee), only verbal informed consent (rather than written consent) was required. A brief note from the investigating physician (stating that consent had been requested and verbally granted at the time of the consultation) was included in the patient’s medical records. If the patients disagreed (by ticking a box and signing the information sheet [available on request]), their lung tissue (if any) was not made available to our laboratory.
Hence, informed consent was obtained from each patient.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- BSA
Bovine serum albumin
- cAMP
Cyclic AMP
- COPD
Chronic obstructive pulmonary disease
- DMSO
Dimethyl sulfoxide
- FCS
Fetal calf serum
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- HDMs
House dust mites
- IL
Interleukin
- LMs
Human lung macrophages
- LPS
Lipopolysaccharide
- LTB4
Leukotriene B4
- MDMs
Human monocyte-derived macrophages
- PBS
Phosphate-buffered saline
- PDE4
Phosphodiesterase 4
- rhGM-CSF
Recombinant human GM-CSF
- TLR4
Toll-like receptor 4
- TNF
Tumor necrosis factor
- TXB2
Thromboxane B2
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12931-017-0613-y) contains supplementary material, which is available to authorized users.
Contributor Information
Tatiana Victoni, Email: tatianavictoni@hotmail.com.
Hélène Salvator, Email: h.salvator@hopital-foch.com.
Charlotte Abrial, Email: abrial.charlotte@gmail.com.
Marion Brollo, Email: m.brollo@hopital-foch.org.
Luis Cristovão Sobrino Porto, Email: luis.cristovaoporto@gmail.com.
Vincent Lagente, Email: vincent.lagente@univ-rennes1.fr.
Emmanuel Naline, Email: upresea220@orange.fr.
Stanislas Grassin-Delyle, Email: s.grassindelyle@hopital-foch.org.
Philippe Devillier, Phone: +33-1-4625-2791, Email: p.devillier@hopital-foch.com.
References
- 1.Nelson RP, Jr, DiNicolo R, Fernandez-Caldas E, Seleznick MJ, Lockey RF, Good RA. Allergen-specific IgE levels and mite allergen exposure in children with acute asthma first seen in an emergency department and in nonasthmatic control subjects. J Allergy Clin Immunol. 1996;98:258–63. doi: 10.1016/S0091-6749(96)70148-3. [DOI] [PubMed] [Google Scholar]
- 2.Salo PM, Calatroni A, Gergen PJ, Hoppin JA, Sever ML, Jaramillo R, Arbes SJ, Jr, Zeldin DC. Allergy-related outcomes in relation to serum IgE: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2011;127:1226–35. doi: 10.1016/j.jaci.2010.12.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terreehorst I, Oosting AJ, Tempels-Pavlica Z, de Monchy JG, Bruijnzeel-Koomen CA, Hak E, van Wijk RG. Prevalence and severity of allergic rhinitis in house dust mite-allergic patients with bronchial asthma or atopic dermatitis. Clin Exp Allergy. 2002;32:1160–5. doi: 10.1046/j.1365-2745.2002.01461.x. [DOI] [PubMed] [Google Scholar]
- 4.To T, Feldman L, Simatovic J, Gershon AS, Dell S, Su J, Foty R, Licskai C. Health risk of air pollution on people living with major chronic diseases: a Canadian population-based study. BMJ Open. 2015;5:e009075. doi: 10.1136/bmjopen-2015-009075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.To T, Zhu J, Larsen K, Simatovic J, Feldman L, Ryckman K, Gershon A, Lougheed MD, Licskai C, Chen H, et al. Progression from asthma to chronic obstructive pulmonary disease. Is air pollution a risk factor? Am J Respir Crit Care Med. 2016;194:429–38. doi: 10.1164/rccm.201510-1932OC. [DOI] [PubMed] [Google Scholar]
- 6.Bauer RN, Diaz-Sanchez D, Jaspers I. Effects of air pollutants on innate immunity: the role of Toll-like receptors and nucleotide-binding oligomerization domain-like receptors. J Allergy Clin Immunol. 2012;129:14–24. doi: 10.1016/j.jaci.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregory LG, Lloyd CM. Orchestrating house dust mite-associated allergy in the lung. Trends Immunol. 2011;32:402–11. doi: 10.1016/j.it.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam D, Ng N, Lee S, Batzer G, Horner AA. Airway house dust extract exposures modify allergen-induced airway hypersensitivity responses by TLR4-dependent and independent pathways. J Immunol. 2008;181:2925–32. doi: 10.4049/jimmunol.181.4.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minnicozzi M, Sawyer RT, Fenton MJ. Innate immunity in allergic disease. Immunol Rev. 2011;242:106–27. doi: 10.1111/j.1600-065X.2011.01025.x. [DOI] [PubMed] [Google Scholar]
- 10.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118:3546–56. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosoki K, Itazawa T, Boldogh I, Sur S. Neutrophil recruitment by allergens contribute to allergic sensitization and allergic inflammation. Curr Opin Allergy Clin Immunol. 2016;16:45–50. doi: 10.1097/ACI.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosmann M, Grailer JJ, Zhu K, Matthay MA, Sarma JV, Zetoune FS, Ward PA. Anti-inflammatory effects of beta2 adrenergic receptor agonists in experimental acute lung injury. FASEB J. 2012;26:2137–44. doi: 10.1096/fj.11-201640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maris NA, van der Sluijs KF, Florquin S, de Vos AF, Pater JM, Jansen HM, van der Poll T. Salmeterol, a beta2-receptor agonist, attenuates lipopolysaccharide-induced lung inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1122–8. doi: 10.1152/ajplung.00125.2003. [DOI] [PubMed] [Google Scholar]
- 14.Wex E, Kollak I, Duechs MJ, Naline E, Wollin L, Devillier P. The long-acting beta2 -adrenoceptor agonist olodaterol attenuates pulmonary inflammation. Br J Pharmacol. 2015;172:3537–47. doi: 10.1111/bph.13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maris NA, de Vos AF, Dessing MC, Spek CA, Lutter R, Jansen HM, van der Zee JS, Bresser P, van der Poll T. Antiinflammatory effects of salmeterol after inhalation of lipopolysaccharide by healthy volunteers. Am J Respir Crit Care Med. 2005;172:878–84. doi: 10.1164/rccm.200503-451OC. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda T, Anisuzzaman AS, Yoshiki H, Sasaki M, Koshiji T, Uwada J, Nishimune A, Itoh H, Muramatsu I. Regional quantification of muscarinic acetylcholine receptors and beta-adrenoceptors in human airways. Br J Pharmacol. 2012;166:1804–14. doi: 10.1111/j.1476-5381.2012.01881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spina D, Rigby PJ, Paterson JW, Goldie RG. Autoradiographic localization of beta-adrenoceptors in asthmatic human lung. Am Rev Respir Dis. 1989;140:1410–5. doi: 10.1164/ajrccm/140.5.1410. [DOI] [PubMed] [Google Scholar]
- 18.Barnes PJ, Basbaum CB, Nadel JA, Roberts JM. Localization of beta-adrenoreceptors in mammalian lung by light microscopic autoradiography. Nature. 1982;299:444–7. doi: 10.1038/299444a0. [DOI] [PubMed] [Google Scholar]
- 19.Carstairs JR, Nimmo AJ, Barnes PJ. Autoradiographic visualization of beta-adrenoceptor subtypes in human lung. Am Rev Respir Dis. 1985;132:541–7. doi: 10.1164/arrd.1985.132.3.541. [DOI] [PubMed] [Google Scholar]
- 20.Barnes PJ. Effect of beta-agonists on inflammatory cells. J Allergy Clin Immunol. 1999;104:S10–7. doi: 10.1016/S0091-6749(99)70269-1. [DOI] [PubMed] [Google Scholar]
- 21.Hjemdahl P, Larsson K, Johansson MC, Zetterlund A, Eklund A. Beta-adrenoceptors in human alveolar macrophages isolated by elutriation. Br J Clin Pharmacol. 1990;30:673–82. doi: 10.1111/j.1365-2125.1990.tb03835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liggett SB. Identification and characterization of a homogeneous population of beta 2-adrenergic receptors on human alveolar macrophages. Am Rev Respir Dis. 1989;139:552–5. doi: 10.1164/ajrccm/139.2.552. [DOI] [PubMed] [Google Scholar]
- 23.Anderson R, Theron AJ, Steel HC, Durandt C, Tintinger GR, Feldman C. The beta-2-adrenoreceptor agonists, formoterol and indacaterol, but not salbutamol, effectively suppress the reactivity of human neutrophils in vitro. Mediators Inflamm. 2014;2014:105420. doi: 10.1155/2014/105420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ezeamuzie CI, Shihab PK, Al-Radwan R. Loss of surface beta-2 adrenoceptors accounts for the insensitivity of cultured human monocytes to beta-2 adrenoceptor agonists. Int Immunopharmacol. 2011;11:1189–94. doi: 10.1016/j.intimp.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Li CY, Tsai CS, Chueh SH, Hsu PC, Wang JY, Wong CS, Ho ST. Dobutamine inhibits monocyte chemoattractant protein-1 production and chemotaxis in human monocytes. Anesth Analg. 2003;97:205–209. doi: 10.1213/01.ANE.0000066013.34263.54. [DOI] [PubMed] [Google Scholar]
- 26.Li CY, Tsai CS, Hsu PC, Wu CT, Wong CS, Ho ST. Dobutamine modulates lipopolysaccharide-induced macrophage inflammatory protein-1alpha and interleukin-8 production in human monocytes. Anesth Analg. 2003;97:210–215. doi: 10.1213/01.ANE.0000066257.38180.04. [DOI] [PubMed] [Google Scholar]
- 27.Linden M. The effects of beta 2-adrenoceptor agonists and a corticosteroid, budesonide, on the secretion of inflammatory mediators from monocytes. Br J Pharmacol. 1992;107:156–60. doi: 10.1111/j.1476-5381.1992.tb14479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshimura T, Kurita C, Nagao T, Usami E, Nakao T, Watanabe S, Kobayashi J, Yamazaki F, Tanaka H, Inagaki N, Nagai H. Inhibition of tumor necrosis factor-alpha and interleukin-1-beta production by beta-adrenoceptor agonists from lipopolysaccharide-stimulated human peripheral blood mononuclear cells. Pharmacology. 1997;54:144–52. doi: 10.1159/000139481. [DOI] [PubMed] [Google Scholar]
- 29.Yoshimura T, Kurita C, Nagao T, Usami E, Nakao T, Watanabe S, Kobayashi J, Yamazaki F, Tanaka H, Nagai H. Effects of cAMP-phosphodiesterase isozyme inhibitor on cytokine production by lipopolysaccharide-stimulated human peripheral blood mononuclear cells. Gen Pharmacol. 1997;29:633–8. doi: 10.1016/S0306-3623(96)00580-0. [DOI] [PubMed] [Google Scholar]
- 30.Donnelly LE, Tudhope SJ, Fenwick PS, Barnes PJ. Effects of formoterol and salmeterol on cytokine release from monocyte-derived macrophages. Eur Respir J. 2010;36:178–86. doi: 10.1183/09031936.00158008. [DOI] [PubMed] [Google Scholar]
- 31.Izeboud CA, Monshouwer M, van Miert AS, Witkamp RF. The beta-adrenoceptor agonist clenbuterol is a potent inhibitor of the LPS-induced production of TNF-alpha and IL-6 in vitro and in vivo. Inflamm Res. 1999;48:497–502. doi: 10.1007/s000110050493. [DOI] [PubMed] [Google Scholar]
- 32.Sarir H, Mortaz E, Karimi K, Johnson M, Nijkamp FP, Folkerts G. Combination of fluticasone propionate and salmeterol potentiates the suppression of cigarette smoke-induced IL-8 production by macrophages. Eur J Pharmacol. 2007;571:55–61. doi: 10.1016/j.ejphar.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 33.Jin M, Opalek JM, Marsh CB, Wu HM. Proteome comparison of alveolar macrophages with monocytes reveals distinct protein characteristics. Am J Respir Cell Mol Biol. 2004;31:322–9. doi: 10.1165/rcmb.2004-0080OC. [DOI] [PubMed] [Google Scholar]
- 34.Tomechko SE, Lundberg KC, Jarvela J, Bebek G, Chesnokov NG, Schlatzer D, Ewing RM, Boom WH, Chance MR, Silver RF. Proteomic and bioinformatics profile of paired human alveolar macrophages and peripheral blood monocytes. Proteomics. 2015;15:3797–805. doi: 10.1002/pmic.201400496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groot-Kormelink PJ, Fawcett L, Wright PD, Gosling M, Kent TC. Quantitative GPCR and ion channel transcriptomics in primary alveolar macrophages and macrophage surrogates. BMC Immunol. 2012;13:57. doi: 10.1186/1471-2172-13-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Pritchard DK, Wang X, Park DR, Bumgarner RE, Schwartz SM, Liles WC. cDNA microarray analysis reveals fundamental differences in the expression profiles of primary human monocytes, monocyte-derived macrophages, and alveolar macrophages. J Leukoc Biol. 2007;81:328–35. doi: 10.1189/jlb.0206124. [DOI] [PubMed] [Google Scholar]
- 37.Fuller RW, O’Malley G, Baker AJ, MacDermot J. Human alveolar macrophage activation: inhibition by forskolin but not beta-adrenoceptor stimulation or phosphodiesterase inhibition. Pulm Pharmacol. 1988;1:101–6. doi: 10.1016/S0952-0600(88)80006-1. [DOI] [PubMed] [Google Scholar]
- 38.Baker AJ, Palmer J, Johnson M, Fuller RW. Inhibitory actions of salmeterol on human airway macrophages and blood monocytes. Eur J Pharmacol. 1994;264:301–6. doi: 10.1016/0014-2999(94)00480-3. [DOI] [PubMed] [Google Scholar]
- 39.Zetterlund A, Linden M, Larsson K. Effects of beta2-agonists and budesonide on interleukin-1beta and leukotriene B4 secretion: studies of human monocytes and alveolar macrophages. J Asthma. 1998;35:565–73. doi: 10.3109/02770909809048959. [DOI] [PubMed] [Google Scholar]
- 40.Zetterlund A, Hjemdahl P, Larsson K. beta2-Adrenoceptor desensitization in human alveolar macrophages induced by inhaled terbutaline in vivo is not counteracted by budesonide. Clin Sci (Lond) 2001;100:451–7. doi: 10.1042/cs1000451. [DOI] [PubMed] [Google Scholar]
- 41.Gill SK, Yao Y, Kay LJ, Bewley MA, Marriott HM, Peachell PT. The anti-inflammatory effects of PGE2 on human lung macrophages are mediated by the EP4 receptor. Br J Pharmacol. 2016;173:3099–109. doi: 10.1111/bph.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Birrell MA, Maher SA, Dekkak B, Jones V, Wong S, Brook P, Belvisi MG. Anti-inflammatory effects of PGE2 in the lung: role of the EP4 receptor subtype. Thorax. 2015;70:740–7. doi: 10.1136/thoraxjnl-2014-206592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buenestado A, Grassin Delyle S, Arnould I, Besnard F, Naline E, Blouquit-Laye S, Chapelier A, Bellamy JF, Devillier P. The role of adenosine receptors in regulating production of tumour necrosis factor-alpha and chemokines by human lung macrophages. Br J Pharmacol. 2010;159:1304–11. doi: 10.1111/j.1476-5381.2009.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buenestado A, Grassin-Delyle S, Guitard F, Naline E, Faisy C, Israel-Biet D, Sage E, Bellamy JF, Tenor H, Devillier P. Roflumilast inhibits the release of chemokines and TNF-alpha from human lung macrophages stimulated with lipopolysaccharide. Br J Pharmacol. 2012;165:1877–90. doi: 10.1111/j.1476-5381.2011.01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gill SK, Marriott HM, Suvarna SK, Peachell PT. Evaluation of the anti-inflammatory effects of beta-adrenoceptor agonists on human lung macrophages. Eur J Pharmacol. 2016;793:49–55. [DOI] [PubMed]
- 46.Bouyssou T, Casarosa P, Naline E, Pestel S, Konetzki I, Devillier P, Schnapp A. Pharmacological characterization of olodaterol, a novel inhaled beta2-adrenoceptor agonist exerting a 24-hour-long duration of action in preclinical models. J Pharmacol Exp Ther. 2010;334:53–62. doi: 10.1124/jpet.110.167007. [DOI] [PubMed] [Google Scholar]
- 47.Naline E, Trifilieff A, Fairhurst RA, Advenier C, Molimard M. Effect of indacaterol, a novel long-acting beta2-agonist, on isolated human bronchi. Eur Respir J. 2007;29:575–81. doi: 10.1183/09031936.00032806. [DOI] [PubMed] [Google Scholar]
- 48.Gicquel T, Victoni T, Fautrel A, Robert S, Gleonnec F, Guezingar M, Couillin I, Catros V, Boichot E, Lagente V. Involvement of purinergic receptors and NOD-like receptor-family protein 3-inflammasome pathway in the adenosine triphosphate-induced cytokine release from macrophages. Clin Exp Pharmacol Physiol. 2014;41:279–86. doi: 10.1111/1440-1681.12214. [DOI] [PubMed] [Google Scholar]
- 49.Hatzelmann A, Schudt C. Anti-inflammatory and immunomodulatory potential of the novel PDE4 inhibitor roflumilast in vitro. J Pharmacol Exp Ther. 2001;297:267–79. [PubMed] [Google Scholar]
- 50.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 51.Bachelerie F, Ben-Baruch A, Burkhardt AM, Combadiere C, Farber JM, Graham GJ, Horuk R, Sparre-Ulrich AH, Locati M, Luster AD, et al. International Union of Basic and Clinical Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev. 2014;66:1–79. doi: 10.1124/pr.113.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. 2011;378:1015–26. doi: 10.1016/S0140-6736(11)60988-4. [DOI] [PubMed] [Google Scholar]
- 53.Ravi AK, Khurana S, Lemon J, Plumb J, Booth G, Healy L, Catley M, Vestbo J, Singh D. Increased levels of soluble interleukin-6 receptor and CCL3 in COPD sputum. Respir Res. 2014;15:103. doi: 10.1186/s12931-014-0103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsukamoto H, Fukudome K, Takao S, Tsuneyoshi N, Kimoto M. Lipopolysaccharide-binding protein-mediated Toll-like receptor 4 dimerization enables rapid signal transduction against lipopolysaccharide stimulation on membrane-associated CD14-expressing cells. Int Immunol. 2010;22:271–80. doi: 10.1093/intimm/dxq005. [DOI] [PubMed] [Google Scholar]
- 55.Komuro I, Keicho N, Iwamoto A, Akagawa KS. Human alveolar macrophages and granulocyte-macrophage colony-stimulating factor-induced monocyte-derived macrophages are resistant to H2O2 via their high basal and inducible levels of catalase activity. J Biol Chem. 2001;276:24360–4. doi: 10.1074/jbc.M102081200. [DOI] [PubMed] [Google Scholar]
- 56.Tannheimer SL, Sorensen EA, Haran AC, Mansfield CN, Wright CD, Salmon M. Additive anti-inflammatory effects of beta 2 adrenoceptor agonists or glucocorticosteroid with roflumilast in human peripheral blood mononuclear cells. Pulm Pharmacol Ther. 2012;25:178–84. doi: 10.1016/j.pupt.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 57.Buenestado A, Chaumais MC, Grassin-Delyle S, Risse PA, Naline E, Longchampt E, Tenor H, Devillier P. Roflumilast inhibits lipopolysaccharide-induced tumor necrosis factor-alpha and chemokine production by human lung parenchyma. PLoS One. 2013;8:e74640. doi: 10.1371/journal.pone.0074640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
LPS concentration-response data for MDMs and LMs, and time-course experiments in LMs (figures). (PDF 94 kb)
Effect of salbutamol (1 μM) on LPS-induced cytokine production by MDMs and LMs (figures). (PDF 52 kb)
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


