Abstract
Importance
Effective long-term treatments are needed to address the obesity epidemic. There are numerous wearable technologies specific to physical activity and diet, but it is unclear if these are effective at improving weight loss.
Objective
To test the hypothesis that, compared with a standard behavioral weight loss intervention (SBWI), a technology-enhanced intervention (EWLI) would result in greater weight loss.
Design, Setting, Participants
Randomized trial with participants enrolled between October 2010 and October 2012, with data collection completed by December 2014. The study was conducted at the University of Pittsburgh (Pittsburgh, PA). Participants were randomized to SBWI (N=233) or EWLI (N=237) interventions.
Interventions
Participants were placed on a low calorie diet, prescribed increases in physical activity, and had group counseling sessions. At 6 months the interventions added telephone counseling sessions, text message prompts, and access to study materials on a website. At 6 months SBWI initiated self-monitoring of diet and physical activity using a website, whereas EWLI was provided a wearable device and accompanying web interface to monitor diet and physical activity.
Main Outcome Measure
The primary outcome of weight was measured over 24 months at 6 months intervals, and the primary hypothesis tested the change in weight between two groups at 24 months. Secondary outcomes included body composition, fitness, physical activity, and dietary intake.
Results
Among the 470 participants randomized (BMI: 25 to <40 kg/m2; age: 18–35 years; 28.9% non-white, 77.2% female), 74.5% completed the study. Weight change at 24-months differed significantly by intervention groups [estimated mean weight loss of 3.5 kg (95% CI: 2.6, 4.5) in EWLI and 5.9 kg (95% CI: 5.0, 6.8) in SBWI, difference of 2.4 kg (95% CI 3.7, 1.0), p=0.002]. Both groups had significant improvements in body composition, fitness, physical activity, and diet, with no significant difference between groups.
Conclusions and Relevance
Among young adults with a BMI between 25 to <40 kg/m2, the addition of a wearable technology device to a standard behavioral intervention resulted in less weight loss over 24 months. Devices that monitor and provide feedback on physical activity may not offer an advantage over standard behavioral weight loss approaches.
Clinical Trials Registration
INTRODUCTION
Overweight and obesity have high prevalence1 and are associated with numerous health conditions.2 Interventions emphasizing both diet and physical activity are effective for weight loss, resulting in 6 month weight loss of 8–10% of initial weight.3 However, challenges remain to sustaining weight loss long-term.3
There is wide availability of commercial technologies for physical activity and diet.4 These technologies include wearable devices to monitor physical activity, with many also including an interface to monitor diet. Short-term studies have shown these technologies to result in modest improvements in weight loss when added to a behavioral intervention.5,6 These technologies may provide a method to improve longer-term weight loss; however, there are limited data on the effectiveness of such technologies for modifying health behaviors long-term.4
This randomized trial examined whether adding wearable technology to a behavioral intervention would improve weight loss across 24 months among young adults 18 to 35 years of age. Additional outcomes included body composition, fitness, physical activity, and dietary intake.
METHODS
Design
IDEA (Innovative Approaches to Diet, Exercise and Activity) was a randomized clinical trial conducted at the University of Pittsburgh, and was one of the studies within the Early Adult Reduction of weight through LifestYle intervention (EARLY) Trials consortium, with each study implementing a unique intervention in young adults.7 Participants in IDEA were randomized to one of two groups. Both groups received a behavioral weight loss intervention for 6 months after which the two groups received different interventions until month 24. Randomization was to: 1) a behavioral weight loss intervention that, after the initial 6 months, added text messaging prompts and a study website containing study-related education materials and the ability to self-monitor diet and physical activity behaviors (SBWI), or 2) the same intervention described for SBWI but after the initial 6 months the study website was only used to access education materials and wearable technology was provided along with a web-based interface to monitor physical activity and diet (EWLI). Randomization was stratified by sex and race (white or non-white) using a computer program that applied randomly selected block sizes of 2 and 4 with the sequence of randomization kept confidential to the other investigators. The primary outcome was weight change at 24 months.
Participants
Recruitment occurred across 10 recruitment periods that took place between October 2010 and October 2012 at the University of Pittsburgh using direct mail, mass media advertisements, or referral from clinical research registries. Eligibility was assessed based on self-reported medical history, and clearance from the participant’s physician was also obtained. Procedures were approved by the University of Pittsburgh Institutional Review Board and all participants provided informed consent.
Eligibility criteria included age between 18 to 35 years, body mass index (BMI) of 25.0 to <40.0 kg/m2, access to a cellular telephone that could receive text messages, and a computer with internet access. Exclusion criteria have been previously published.8
Intervention
Intervention Contact
Both SBWI and EWLI received regular intervention contact. Group-based sessions were scheduled weekly for the initial 6 months and monthly between months 7 to 24. If a participant was unable to attend a scheduled group session, attempts were made to engage the participant in a make-up session. Theory-based strategies were used to promote adherence to weight loss behaviors.9–13 At each session participants were given feedback on weight change and were provided materials to complement the topic of the session. Beginning with month 7 these materials were posted on the study website along with a weekly behavioral tip.
During months 7 to 24 participants were also scheduled to receive a brief (≤10 minutes) individual telephone contact once per month and weekly text messages. The telephone contacts were conducted by intervention staff and followed a standard script. Text messages were provided once or twice per week, and were used to prompt engagement in weight loss behaviors or to remind participants of upcoming intervention sessions. Participants were compensated $5 per month to offset the cost of receiving text messages.
Dietary Intervention
Calorie intake in both SBWI and EWLI was prescribed based on baseline weight at 1200, 1500, and 1800 kcal/d for individuals who were <90.7 kg, 90.7 to <113.4 kg, and ≥113.4 kg, respectively. If weight loss exceeded 6% during each 4 week period or if BMI was ≤22 kg/m2, prescribed individual calorie intake was increased. Dietary fat was prescribed at 20 to 30% of total calorie intake and sample meal plans were provided to facilitate adoption of the prescribed dietary recommendations. During months 1 to 6 participants were instructed to self-monitor dietary intake in a diary that was returned to the interventionists at the conclusion of each week, and the intervention staff provided feedback prior to returning diaries to the participants. During months 7 to 24 SBWI self-reported their daily intake using a website designed for this study, and this information was available to the staff during the intervention telephone contacts. EWLI self-monitored their dietary patterns using the technology described below.
Physical Activity
Non-supervised moderate-to-vigorous physical activity (MVPA) in both SBWI and EWLI was initially prescribed at 100 minutes per week and increased at 4 week intervals until a prescription of 300 minutes per week was achieved. Participants were instructed to engage in structured forms of MVPA that were ≥10 minutes in duration. During months 1 to 6 participants were instructed to self-monitor their MVPA in a diary that was returned to the interventionists at the conclusion of each week. The intervention staff provided feedback on these diaries. During months 7 to 24 SBWI self-reported their daily MVPA using a website designed for this study, and this information was available to the staff during the intervention telephone contacts. EWLI self-monitored their MVPA using the technology described below.
Technology used within EWLI
EWLI was provided and encouraged to use a commercially available wearable technology that included a web-based interface (BodyMedia Fit, Pittsburgh, PA). This system included a multi-sensor device worn on the upper arm that provided feedback to the participant on energy expenditure and physical activity through a small display or through web-based software developed by the manufacturer. While the display provided information about total MVPA, the web-based software also provided feedback on MVPA performed in durations of ≥10 minutes. The web-based software also allowed for self-monitoring of dietary intake. Intervention staff had access to this information during the scheduled telephone contacts.
Outcome Assessments
Measures occurred at 0, 6, 12, 18, and 24 months. Participants received $100 for completing each of the 6, 12, 18, and 24 month assessments. Assessment staff were masked to prior data at each assessment to minimize potential bias.
Weight was assessed to the nearest 0.1 kg with the participant clothed in a hospital gown or lightweight clothing. Height was measured only at baseline to the nearest 0.1 cm with shoes removed. BMI was computed as kg/m2.
Body composition was assessed using dual-energy x-ray absorptiometry from a total body scan. Prior to this scan, females had a urine pregnancy test and a positive result excluded the participant from further study participation.
Cardiorespiratory fitness was assessed with a submaximal graded exercise test that was performed on a motorized treadmill.8 Oxygen consumption was assessed using a metabolic cart.
Physical activity was assessed using a portable device that was worn for one week14,15. Data were considered valid if the participant wore the device for ≥10 hours per day for ≥4 days during the observation period.16,17 Minute-by-minute data were used to identify minutes and MET-min/week of sedentary (awake time, <1.5 metabolic equivalents [METs]), light-intensity physical activity (LPA: 1.5 to <3.0 METs) and moderate-to-vigorous intensity physical activity (MVPA: ≥3.0 METs) physical activity. Percent sedentary time was calculated as sedentary time identified by the activity monitor divided by the monitor wear time.
Diet over the past month was assessed using the web-based version of the Diet History Questionnaire18,19 and DietCalc software (version 1.5.0).
Percent weight loss was included as a post-hoc outcome.
For safety, depressive symptoms were assessed using the 10-item Center for Epidemiology Studies questionnaire.20 Participants with a score of ≥13 were referred to their primary care physician and provided a list of community resources to assist in obtaining treatment. Resting blood pressure was assessed following a 5 minute seated resting period using an automated system, with systolic blood pressure of ≥140 mmHg or diastolic blood pressure of ≥90 mmHg referred to their primary care physician. Participants were queried regarding the occurrence of overnight hospitalizations and conditions to assess for adverse and serious adverse events.
Sex, race, ethnicity, education, income, employment status, smoking status, alcohol consumption, depressive symptoms20 were assessed by self-report using questionnaires.
Statistical Methods
Sample Size
The mean (standard deviation) weight loss (in kg) from baseline to month 24 in the SBWI group was projected to be approximately 3.4 kg at 24 months, with these estimates based on data from prior weight loss studies that included young adults.21–23 We specified 2.3 kg or more average weight loss for EWLI compared to SBWI so that the average weight loss in the EWLI group was expected to be 5.7 kg at the end of month 24. This would allow EWLI to maintain a clinically meaningful weight loss of at least a 5%.3 Using a standard deviation of 6.8 kg for both groups, a two-sided t-test at 5% level of significance had 90% power to detect a mean difference of 2.3 kg (effect size of .33) between EWLI and SBWI if 24 month data were available for at least 191 patients in each group. Based on an expected attrition rate of 20%, the recruitment goal was 238 participants per group.
Analysis Plan
Descriptive statistics were used to describe the participants in the two groups. Statistical significance of group differences in distributions was tested using Wilcoxon’s test for continuous and Pearson’s chi-square test or exact tests for categorical variables, as appropriate.
It was expected that the likelihood of missingness could be predicted by the observed data prior to missing so missing data were assumed to be at random and a likelihood-based analysis was used. Thus, the primary hypothesis of EWLI achieving different weight loss than SBWI was tested by fitting a linear mixed effect model via maximum likelihood with weight over time as the outcome, including race, sex, time (assessment, treated as discrete, baseline, 6, 12, 18, and 24 months), intervention (EWLI vs. SBWI) and intervention by time interaction as fixed effects, and participants and recruitment periods as random effects. Weights measured during or after pregnancy were excluded from the analyses. Significance of the difference in distributions of weight was tested with a likelihood ratio test of the null hypothesis H0: β = 0, with β as the coefficient of the intervention by 24 month visit interaction in the linear mixed effect model.
For all of the models, if the intervention by time interaction was statistically significant (p < 0.05), the equality of mean changes in the two intervention groups at each intermediate time point was tested. The mean change at each time point, estimated using the least-square means, are presented by intervention along with the corresponding 95% confidence intervals. P-values were adjusted by Holm’s method for multiplicity when the differences were tested at multiple time points.24 No adjustment for multiple comparisons were made for the primary outcome. P-values for all other secondary outcome analyses were adjusted for multiplicity using Holm’s method.
Multiple imputation was used for sensitivity analysis. Specifically, 10 Monte Carlo Markov Chain imputations based on the observed variables (intervention group, sex, race, ethnicity, education, income, employment status, waist circumference, smoking status, alcohol consumption, depression, and weights) at previous assessments were used to impute the missing weights for the sensitivity analysis. The estimates from the imputed datasets were averaged to see if they were similar to the likelihood-based estimates from the primary analysis. A similar approach was used for the secondary outcomes.
Fisher’s exact test conducted separately for each time interval was used for comparing adverse events and other alerts. All tests were two-sided and a level of less than 0.05 was used as the cut-off for statistical significance. All analyses were conducted using the SAS statistical software package (version 9.3, SAS Institute Inc., Cary, North Carolina).
RESULTS
This study randomized 471 participants with specific exclusion criteria by participant shown in Figure 1. However, prior to the start of the intervention, one participant was discovered to be ineligible and was removed from the study. Thus, 470 participants received the intervention and are included in the analysis. Descriptive characteristics for SBWI and EWLI are shown in Table 1. Weight data at 24 months was available on 74.5% of the sample (Figure 1, 72.5% in SBWI, 76.4% in EWLI). The 20 women in SBWI and 9 in TECH who became pregnant after randomization discontinued participation in the study for safety. When these women are excluded, 79.3% of those in SBWI and 79.4% in EWLI had weight measured at 24 months.
Figure 1.
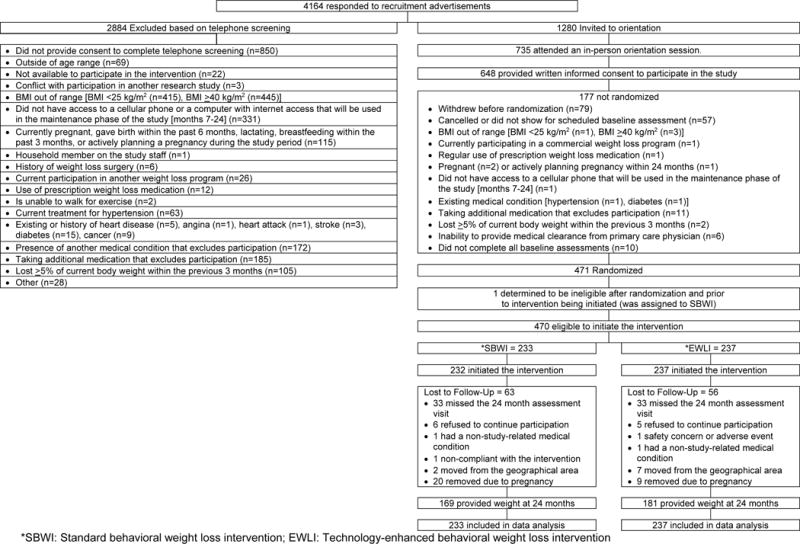
Consort Diagram
Table 1.
Baseline characteristics of participants by intervention condition.
| Total (N=470) |
SBWI Group (N=233) |
EWLI Group (N=237) |
|
|---|---|---|---|
| Age, years | |||
| Median (25th, 75th percentile) | 30.9 (27.8, 33.7) | 30.9 (28.0, 33.9) | 31.0 (27.4, 33.3) |
| Range | 18.4–35.9 | 18.4–35.9 | 19.3–35.9 |
| Gender | |||
| Male | 136 (28.9) | 67 (28.8) | 69 (29.1) |
| Female | 334 (71.1) | 166 (71.2) | 168 (70.9) |
| Race | |||
| White | 363 (77.2) | 180 (77.3) | 183 (77.2) |
| Non-white | 107 (22.8) | 53 (22.8) | 54 (22.8) |
| Hispanic/Latino | |||
| Yes | 20 (4.3) | 11 (4.7) | 9 (3.8) |
| No | 450 (95.7) | 222 (95.3) | 228 (96.2) |
| Weight, kg | |||
| Median (25th, 75th percentile) | 90.0 (79.6, 101.7) | 88.5 (79.2, 101.2) | 90.6 (80.8, 101.9) |
| Range | 60.1–146.1 | 62.8–129.6 | 60.1–146.1 |
| Body mass index, kg/m2 | |||
| Median (25th, 75th percentile) | 31.2 (28.4, 34.3) | 30.9 (28.7, 34.2) | 31.5 (28.2, 34.3) |
| Range* | 24.4–39.9 | 35.0–39.8 | 24.4–39.9 |
| Waist Circumference (iliac crest)**, cm | |||
| Median (25th, 75th percentile) | 101.5(93.5,109.9) | 100.7(92.6, 110.3) | 102.7(94.3, 109.6) |
| Range | 76.4–134.8 | 76.7–134.8 | 76.4–132.7 |
| Waist Circumference (umbilicus)***, cm | |||
| Median (25th, 75th percentile) | 104.8(96.8, 114.1) | 104.3(96.8, 114.4) | 105.7(97.0, 113.9) |
| Range | 78.4–136.3 | 79.2–136.3 | 78.4–132.2 |
| Education | |||
| High school graduate or Graduate Equivalency Degree (GED) | 117 (24.9) | 57 (24.5) | 60 (25.3) |
| College graduate or higher | 323 (75.1) | 176 (75.5) | 177 (74.7) |
| Relationship status | |||
| Married/like married**** | 233 (49.6) | 118 (50.6) | 115 (48.5) |
| Other | 237 (50.4) | 115 (49.4) | 122 (51.5) |
| Student status | |||
| Not student | 349 (74.3) | 169 (72.5) | 180 (76.0) |
| Currently a student (part-time or full-time) | 121 (25.7) | 64 (27.5) | 57 (24.1) |
| Employment Status | N=468 | N=232 | N=236 |
| Full-time for pay | 359 (76.4) | 174 (74.7) | 185 (78.1) |
| Part-time for pay | 65 (13.8) | 34 (14.6) | 31 (13.1) |
| Other | 44 (9.4) | 24 (10.3) | 20 (8.4) |
| Household income | N=466 | N=231 | N=235 |
| Less than $25,000 | 58 (12.4) | 27 (11.6) | 31 (13.1) |
| $25,000 and more | 408 (86.8) | 204 (87.6) | 204 (86.1) |
| Smoking Status | |||
| Current Smoker | 42(8.9) | 20(8.6) | 22(9.3) |
| Depressive Symptoms***** | |||
| Median (25th, 75th percentile) | 4.5(2,7) | 5(2,7) | 4(2,7) |
| Range | 0–22 | 0–19 | 0, 22 |
| Alcohol consumption | |||
| Had at least one alcoholic beverage in the last 30 days | 418(88.9) | 207(88.8) | 211(89.0) |
| Days with at least 1 drink | N=418 | N=207 | N=211 |
| Median (25th, 75th percentile) | 5(3,8) | 5(3,8) | 5(3,8) |
| Range | 1–29 | 1–28 | 1–29 |
| Average number of drinks | N=418 | N=207 | N=211 |
| Median (25th, 75th percentile) | 2(1,3) | 2(1,3) | 2(1,3) |
| Range | 1–15 | 1–12 | 1–15 |
| Times had 4(female) or 5 (male) drinks | N=418 | N=207 | N=211 |
| Median (25th, 75th percentile) | 1(0,2) | 1(0,2) | 1(0,2) |
| Range | 0–15 | 0–14 | 0–15 |
Abbreviations: SBWI, standard behavioral weight loss intervention; EWLI, technology-enhanced weight loss intervention.
Values are expressed as no. (%) unless otherwise indicated.
Eligibility based on screening with data based on baseline assessment.
measured horizontally at the level of the iliac crest
measured horizontally at the level of the umbilicus
self-identified as currently married or in a marriage-like relationship
Based on Center for Epidemiologic Studies Depression Score
There was significant change in weight over time (Time p < 0.0001), and the change differed significantly between EWLI and SBWI (Group × Time p=0.003), with less weight loss in EWLI (Table 2). Estimated mean weights for EWLI group were 96.3(95% CI: 94.2, 98.5) kg, 92.8 (95% CI: 90.6 to 95.0) kg and 3.5 kg (95% CI: 2.6, 4.5). Corresponding values for SBWI were 95.2 (95% CI: 93.0 to 97.3) kg at baseline and 89.3 (95% CI: 87.1, 91.5) kg at 24 months, resulting in a mean loss of 5.9 kg (95% CI: 5.0, 6.8). At 24 months weight loss was 2.4 kg (95% CI: −3.7, −1.0) lower in EWLI compared to SBWI (p=0.002). Results from the sensitivity analysis using multiple imputation were similar with weight loss at 24 months of 3.3 kg (95% CI: 2.5, 4.0) in EWLI and 5.3 kg (95% CI: 4.5, 6.2) in SBWI.
Table 2.
Change in weight, body mass index, body composition, and cardiorespiratory fitness by intervention condition.
| Least-Squares Mean (95% CI)* | ||||||||
|---|---|---|---|---|---|---|---|---|
| Change from baseline | P value** | |||||||
| Baseline | 6 months | 12 months | 18 months | 24 months | Group | Time | Group × Time | |
| Weight, kg**** | 0.07 | <0.0001 | 0.003 | |||||
| SBWI group | 95.2 (93, 97.3) | −8.6 (−9.5, −7.7) | −8.3 (−9.2, −7.4) | −7.3 (−8.3, −6.4) | −5.9 (−6.8, −5) | |||
| EBWI group | 96.3 (94.2, 98.5) | −8 (−8.8, −7.1) | −6.7 (−7.6, −5.8) | −5.4 (−6.3, −4.4) | −3.5 (−4.5, −2.6) | |||
| Difference | −0.7 (−1.9, 0.6) | −1.6 (−2.8, −0.3) | −2 (−3.3, −0.6) | −2.4 (−3.7, −1) | ||||
| P value*** | 0.29 | 0.032 | 0.011 | 0.002 | ||||
| Weight change from baseline, %***** | – | 0.014 | <0.0001 | <0.001 | ||||
| SBWI group | – | −9.4 (−10.2, −8.5) | −8.9 (−9.8, −8) | −7.9 (−8.9, −7) | −6.4 (−7.4, −5.5) | |||
| EBWI group | – | −8.4 (−9.3, −7.6) | −7 (−7.9, −6.1) | −5.6 (−6.5, −4.6) | −3.6 (−4.5, −2.7) | |||
| Difference | – | −0.9 (−2.2, 0.3) | −1.9 (−3.2, −0.6) | −2.4 (−3.7, −1) | −2.8 (−4.2, −1.5) | |||
| P value*** | – | 0.15 | 0.008 | 0.002 | <0.001 | |||
| Body mass index, kg/m2 | 0.41 | <.0001 | 0.63 | |||||
| SBWI group | 32.4 (31.5 to 33.3) | −2.9 (−3.7 to −2.2) | −2.8 (−3.5 to −2.0) | −2.5 (−3.3 to −1.7) | −1.8 (−2.6 to −1.0) | |||
| EBWI group | 32.3 (31.4 to 33.2) | −2.7 (−3.4 to −1.9) | −2.1 (−2.9 to −1.4) | −1.9 (−2.7 to −1.1) | −1.1 (−1.9 to −0.3) | |||
| Difference | −0.3 (−1.3 to 0.8) | −0.7 (−1.7 to 0.4) | −0.6 (−1.8 to 0.5) | −0.7 (−1.9 to 0.4) | ||||
| Fat mass, kg | >0.99 | <0.0001 | 0.52 | |||||
| SBWI group | 36.8 (35.4, 38.3) | −7 (−7.8, −6.3) | −7 (−7.7, −6.2) | −6 (−6.8, −5.2) | −5.1 (−6, −4.3) | |||
| EBWI group | 37.2 (35.7, 38.7) | −6.5 (−7.2, −5.8) | −5.7 (−6.5, −4.9) | −4.8 (−5.6, −4) | −3.4 (−4.3, −2.6) | |||
| Difference | −0.6 (−1.6, 0.5) | −1.3 (−2.4, −0.2) | −1.2 (−2.3, −0.1) | −1.7 (−2.9, −0.6) | ||||
| Lean mass, kg | >0.99 | <0.0001 | >0.99 | |||||
| SBWI group | 54.9 (53.9, 55.8) | −1.3 (−1.5, −1.1) | −1.3 (−1.5, −1.1) | −1.1 (−1.3, −0.8) | −0.9 (−1.1, −0.6) | |||
| EBWI group | 55.6 (54.7, 56.6) | −1.2 (−1.5, −1) | −1.1 (−1.4, −0.9) | −0.7 (−0.9, −0.4) | −0.6 (−0.8, −0.3) | |||
| Difference | 0 (−0.4, 0.3) | −0.1 (−0.5, 0.2) | −0.4 (−0.7, 0) | −0.3 (−0.7, 0) | ||||
| Percent body fat, % | >0.99 | <0.0001 | 0.52 | |||||
| SBWI group | 38.9 (38, 39.8) | −4.7 (−5.2, −4.2) | −4.6 (−5.2, −4.1) | −4 (−4.5, −3.5) | −3.5 (−4, −3) | |||
| EBWI group | 38.8 (37.8, 39.7) | −4.1 (−4.6, −3.6) | −3.7 (−4.2, −3.2) | −3.2 (−3.7, −2.7) | −2.4 (−3, −1.9) | |||
| Difference | −0.6 (−1.3, 0.1) | −1 (−1.7, −0.3) | −0.8 (−1.6, −0.1) | −1.1 (−1.9, −0.3) | ||||
| Tissue****** percent body fat, % | >0.99 | <0.0001 | 0.53 | |||||
| SBWI group | 40.2 (39.2, 41.1) | −4.7 (−5.2, −4.2) | −4.7 (−5.2, −4.2) | −4 (−4.6, −3.5) | −3.5 (−4.1, −3) | |||
| EBWI group | 40 (39, 40.9) | −4.1 (−4.6, −3.6) | −3.7 (−4.2, −3.2) | −3.2 (−3.8, −2.7) | −2.4 (−3, −1.9) | |||
| Difference | −0.6 (−1.3, 0.1) | −1 (−1.7, −0.3) | −0.8 (−1.6, −0.1) | −1.1 (−1.9, −0.3) | ||||
| Bone Mass, kg | >0.99 | <0.0001 | >0.99 | |||||
| SBWI group | 3008.5 (2957.5, 3059.6) | −13.4 (−19, −7.8) | −15.5 (−21.4, −9.6) | −17.4 (−23.5, −11.3) | −18.7 (−25, −12.4) | |||
| EBWI group | 3033.2 (2982.6, 3083.8) | −10 (−15.6, −4.4) | −8.7 (−14.6, −2.8) | −8 (−14.2, −1.9) | −9.2 (−15.6, −2.9) | |||
| Difference | −3.4 (−11.3, 4.6) | −6.8 (−15.1, 1.5) | −9.4 (−18, −0.7) | −9.5 (−18.4, −0.5) | ||||
| Total Body Bone Mineral Density, g/cm2 | >0.99 | <0.001 | 0.54 | |||||
| SBWI group | 1.3 (1.3, 1.4) | −0.005 (−0.007, −0.002) | −0.006 (−0.009, −0.003) | −0.007 (−0.010, −0.004) | −0.005 (−0.008, −0.002) | |||
| EBWI group | 1.3 (1.3, 1.4) | −0.001 (−0.003, 0.002) | −0.002 (−0.004, 0.001) | −0.002 (−0.005, 0.001) | 0.002 (−0.002, 0.005) | |||
| Difference | −0.004 (−0.008, −0.000) | −0.004 (−0.008, −0.000) | −0.005 (−0.009, −0.001) | −0.006 (−0.011, −0.002) | ||||
| Cardiorespiratory Fitness (ml/kg/min) | >0.99 | <0.0001 | >0.99 | |||||
| SBWI group | 27.3 (26.4, 28.1) | 3.8 (3.2, 4.4) | 3.1 (2.4, 3.7) | 3.1 (2.4, 3.7) | 2 (1.3, 2.6) | |||
| EBWI group | 27.3 (26.5, 28.2) | 3.9 (3.3, 4.5) | 2.3 (1.6, 2.9) | 2.6 (1.9, 3.3) | 1.7 (1, 2.4) | |||
| Difference | −0.1 (−0.9, 0.8) | 0.8 (−0.1, 1.7) | 0.5 (−0.4, 1.4) | 0.2 (−0.7, 1.2) | ||||
Model includes group (fixed), recruitment period (random), time (categorical, fixed), race (fixed), sex (fixed), time and group interaction (fixed), time and recruitment period (random).
P-values are adjusted for multiple outcomes using Holm’s approach, except for the definitive primary outcome of weight, and the exploratory outcome percent change in body weight.
P-values for comparison at specific time points are adjusted for multiple testing across time points using Holm’s method and only provided when there was a significant time and group interaction.
Primary outcome.
Post-hoc analysis of a non-pre-specified primary or secondary outcome.
Percent body fat excluding bone mass.
In post-hoc analysis, percent weight loss differed significantly between SBWI and EWLI (Group p<0.001) (Table 2). While there was no significant difference between groups at 6 months (estimated means: SBWI = 9.4% vs. EWLI = 8.4%, p=0.15), percent weight loss was significantly greater in SBWI compared to EWLI at 12 (estimated means: 8.9% vs. 7.0%, p=0.01), 18 (estimated means: 7.9% vs 5.6%, p=0.002) and 24 months (estimated means: 6.4% vs 3.6%, p<0.001).
SBWI and EWLI did not differ significantly for fat mass, lean mass, percent body fat, bone mineral content, bone mineral density, or cardiorespiratory fitness (Group p for all ≥0.05), though there were significant changes across time among all participants (Time p for all <0.01). Data are presented in Table 2.
Differences between intervention groups for physical activity and dietary intake were not significant (Table 3). Regardless of the intervention conditions, there was a significant change in % sedentary time, sedentary time, and LPA across time (Time p for all <0.001). Although total MVPA (min/week or MET-min/week) did not change significantly over time, MVPA performed in bouts of ≥10 minutes significantly changed across the intervention (p<0.0001 for min/week and MET-min/week). Approximately 95% of participants providing weight data also provided valid physical activity data across the assessment periods (see eTable 1 in the Supplement).
Table 3.
| Least-Squares Mean (95% CI)**** | ||||||||
|---|---|---|---|---|---|---|---|---|
| Change from baseline | P value***** | |||||||
| Baseline | 6 months | 12 months | 18 months | 24 months | Group | Time | Group × Time | |
| Sedentary (% of monitor wear time***) | >0.99 | <0.0001 | >0.99 | |||||
| SBWI group | 64.2 (61.9, 66.5) | −3.8 (−5.8, −1.8) | −0.3 (−2.4, 1.8) | −3.4 (−5.6, −1.2) | −2 (−4.3, 0.3) | |||
| EBWI group | 64.2 (62, 66.5) | −0.4 (−2.4, 1.6) | 2.9 (0.8, 5) | −0.9 (−3.1, 1.4) | −0.5 (−2.8, 1.8) | |||
| Difference | −3.4 (−6.3, −0.5) | −3.2 (−6.2, −0.3) | −2.5 (−5.7, 0.6) | −1.5 (−4.8, 1.7) | ||||
| Sedentary (hr/day) | >0.99 | <0.001 | >0.99 | |||||
| SBWI group | 8.9 (8.6, 9.2) | −0.5 (−0.8, −0.2) | −0.1 (−0.4, 0.2) | −0.5 (−0.9, −0.2) | −0.3 (−0.7, 0) | |||
| EBWI group | 8.9 (8.6, 9.2) | −0.1 (−0.4, 0.2) | 0.3 (0, 0.6) | −0.2 (−0.5, 0.2) | 0 (−0.4, 0.3) | |||
| Difference | −0.4 (−0.8, 0) | −0.4 (−0.9, 0) | −0.4 (−0.8, 0.1) | −0.3 (−0.8, 0.2) | ||||
| LPA (min/week) | >0.99 | <0.0001 | >0.99 | |||||
| SBWI group | 1587.4 (1488.4, 1686.4) | 179.6 (83.9, 275.3) | 14.3 (−84.6, 113.2) | 177.3 (72.7, 281.9) | 82.4 (−25.8, 190.6) | |||
| EBWI group | 1566.7 (1467.7, 1665.6) | 60.8 (−34.9, 156.6) | −76.7 (−175.9, 22.4) | 97 (−9.2, 203.2) | 49.5 (−58.3, 157.4) | |||
| Difference | 118.8 (−16.6, 254.2) | 91 (−49, 231.1) | 80.3 (−68.7, 229.4) | 32.8 (−120, 185.6) | ||||
| Total MVPA (min/week) | >0.99 | 0.10 | 0.89 | |||||
| SBWI group | 520.5 (461.2, 579.7) | 68.4 (16.4, 120.5) | 8.8 (−45, 62.5) | 31.6 (−25.3, 88.5) | 35.5 (−23.3, 94.3) | |||
| EBWI group | 527.6 (468.4, 586.8) | −30.2 (−82.2, 21.9) | −78.4 (−132.4, −24.5) | −24.1 (−81.9, 33.6) | 5.5 (−53.2, 64.1) | |||
| Difference | 98.6 (25, 172.2) | 87.2 (11, 163.3) | 55.7 (−25.3, 136.8) | 30 (−53.1, 113.1) | ||||
| Total MVPA (MET-min/wk) | >0.99 | 0.10 | >0.99 | |||||
| SBWI group | 1959.9 (1728.1, 2191.6) | 221.3 (15.1, 427.5) | 13.4 (−199.7, 226.4) | 106.3 (−119, 331.5) | 124 (−109.1, 357.1) | |||
| EBWI group | 1974.4 (1742.9, 2206) | −133 (−339.3, 73.3) | −306.3 (−520, −92.7) | −100.8 (−329.6, 128) | 14.5 (−217.9, 247) | |||
| Difference | 354.3 (62.7, 645.9) | 319.7 (18, 621.4) | 207.1 (−114, 528.2) | 109.5 (−219.7, 438.6) | ||||
| >10 minute sessions of MVPA (min/week) | >0.99 | <0.0001 | >0.99 | |||||
| SBWI group | 158.9 (117.8, 199.9) | 189.1 (149.9, 228.4) | 126.3 (85.8, 166.8) | 147.1 (104.3, 189.9) | 134.3 (90, 178.6) | |||
| EBWI group | 174.6 (133.6, 215.5) | 113.1 (73.9, 152.4) | 66.5 (25.9, 107.1) | 105.8 (62.4, 149.3) | 107.6 (63.4, 151.8) | |||
| Difference | 76 (20.5, 131.5) | 59.8 (2.5, 117.2) | 41.2 (−19.8, 102.3) | 26.7 (−35.9, 89.2) | ||||
| >10 minute sessions of MVPA (MET-min/week) | >0.99 | <0.0001 | >0.99 | |||||
| SBWI group | 724.4 (537.5, 911.4) | 790.7 (612.8, 968.6) | 547.3 (363.5, 731.1) | 629.7 (435.4, 824) | 572.8 (371.8, 773.8) | |||
| EBWI group | 778.6 (591.7, 965.4) | 494 (316, 671.9) | 280 (95.7, 464.2) | 448.5 (251.1, 645.8) | 460.9 (260.4, 661.3) | |||
| Difference | 296.7 (45.1, 548.3) | 267.4 (7.1, 527.6) | 181.2 (−95.7, 458.1) | 111.9 (−171.9, 395.8) | ||||
| Total calories (kcal/day) | >0.99 | <0.0001 | >0.99 | |||||
| SBWI group | 1987.1 (1872.6, 2101.6) | −450.6 (−551.8, −349.4) | −444.9 (−549.3, −340.5) | −330.6 (−439.6, −221.5) | −374.4 (−487.8, −260.9) | |||
| EBWI group | 2006.3 (1892.8, 2119.7) | −542.4 (−641.9, −443) | −484.6 (−589, −380.2) | −500 (−608.8, −391.1) | −543.7 (−656.7, −430.7) | |||
| Difference | 91.9 (−50, 233.7) | 39.7 (−107.9, 187.4) | 169.4 (15.4, 323.5) | 169.3 (9.2, 329.4) | ||||
| % calories carbohydrates | >0.99 | <0.0001 | >0.99 | |||||
| SBWI group | 47.8 (46.6, 49.1) | 2.8 (1.6, 3.9) | 2.5 (1.3, 3.6) | 0.9 (−0.3, 2.1) | 0.2 (−1.1, 1.5) | |||
| EBWI group | 47.9 (46.7, 49.1) | 2.5 (1.4, 3.6) | 1.7 (0.5, 2.9) | 0.4 (−0.9, 1.6) | −0.4 (−1.7, 0.8) | |||
| Difference | 0.3 (−1.3, 1.9) | 0.8 (−0.9, 2.4) | 0.5 (−1.2, 2.3) | 0.7 (−1.2, 2.5) | ||||
| % calories protein | >0.99 | <0.001 | >0.99 | |||||
| SBWI group | 15.5 (15, 16) | 0.7 (0.2, 1.2) | 0.4 (−0.1, 0.9) | 0.3 (−0.2, 0.9) | 0.5 (0, 1) | |||
| EBWI group | 15.5 (15, 16) | 0.8 (0.4, 1.3) | 0.5 (0, 1) | 0.6 (0.1, 1.1) | 0.9 (0.4, 1.5) | |||
| Difference | −0.1 (−0.8, 0.6) | 0 (−0.7, 0.7) | −0.3 (−1, 0.5) | −0.4 (−1.2, 0.3) | ||||
| % calories fat | >0.99 | <0.0001 | >0.99 | |||||
| SBWI group | 35.7 (34.6, 36.8) | −3.7 (−4.7, −2.7) | −2.7 (−3.7, −1.7) | −1.6 (−2.6, −0.6) | −0.2 (−1.3, 0.9) | |||
| EBWI group | 35.3 (34.2, 36.4) | −3.1 (−4, −2.1) | −1.6 (−2.6, −0.6) | −0.8 (−1.8, 0.2) | −0.4 (−1.4, 0.7) | |||
| Difference | −0.6 (−2, 0.7) | −1.1 (−2.5, 0.3) | −0.8 (−2.3, 0.6) | 0.2 (−1.4, 1.7) | ||||
MET: metabolic equivalent; LPA: light-intensity physical activity (1.5 to <3.0 METs); MVPA: moderate-to-vigorous physical activity (≥3.0 METs).
Based on data from objective assessment of physical activity for a 1 week period.
Based on data from the Diet History Questionnaire
Monitor wear time defined as the time that the device was worn per day.
Model includes group (fixed), recruitment period (random), time (categorical, fixed), race (fixed), sex (fixed), time and group interaction (fixed), time and recruitment period (random).
P-values are adjusted for multiple outcomes using Holm’s approach
Total calorie intake and the percent of energy intake consumed as dietary fat, carbohydrates, and protein changed significantly over time (Time p for all <0.001).
Of the 237 participants randomized to EWLI, 191 participants received the wearable device that was a component of the intervention starting after month 6 and wore the device for ≥1 day [median days worn = 170.0 (25th, 75th percentile: 68.0, 347)]. On days that the device was worn the median wear time was 241.1 (25th, 75th percentile: 99.3, 579.1) minutes per day. User experience with this technology is included in eTable 2 in the Supplement.
There were no significant differences between groups in the number of safety alerts, non-serious adverse events, and serious adverse events (Table 4).
Table 4.
Safety alerts, non-serious adverse events, and serious adverse events by intervention condition (n=470).
| From Signing Informed Consent through Baseline Assessment* | Following Baseline and Through 6 Month Assessment* | Following 6 Month and through 12 Month Assessment* | Following 12 Month and through 18 Month Assessment* | Following 18 Month and Through 24 Month Assessment* | |
|---|---|---|---|---|---|
| Resting Blood Pressure Alert** | |||||
| SBWI group | 4 | 3 | 2 | 2 | 0 |
| EBWI group | 5 | 3 | 3 | 4 | 3 |
| Depression Alert*** (CES-D score of 13 or higher) alert | |||||
| SBWI group | 11 | 8 | 4 | 3 | 4 |
| EBWI group | 7 | 10 | 7 | 7 | 8 |
| Rapid Weight Loss Alert**** | |||||
| SBWI group | – | 22 | 2 | 0 | 1 |
| EBWI group | – | 18 | 0 | 0 | 0 |
| Non-Serious Adverse Events | |||||
| SBWI group | 10 | 36 | 38 | 20 | 27 |
| EBWI group | 5 | 47 | 34 | 32 | 34 |
| Serious Adverse Events***** | |||||
| SBWI group | 0 | 1 | 2 | 2 | 1 |
| EBWI group | 0 | 1 | 3 | 5 | 2 |
Data presented as number of participants
Resting systolic blood pressure >140 mmHg or resting diastolic blood pressure >90 mmHg.
Score of >13 of the Center for Epidemiologic Studies Depression (CES-D) Questionnaire.
>6% weight loss during a 4 week period of the intervention, with the alert based on weight assessed during an intervention visit.
All serious adverse events were a result of an overnight hospitalization or surgery.
DISCUSSION
In this study, the addition of wearable technology to a behavioral intervention was less effective for 24-month weight loss. This may be a result of the technology not being as effective for changing diet or physical activity behaviors compared to what was achieved in SBWI; however, the study found no significant difference in these measures between SBWI and EWLI. Thus, the reason for this difference in weight loss between SBWI and EWLI warrants further investigation.
The few studies that have shown promise for adding wearable technology at the onset of a weight loss intervention have been short in duration and have included relatively small samples of participants.5,6 However, in one 9-month intervention, combining a group-based weight loss intervention with wearable technology improved weight loss compared to the group-based treatment alone.25 Furthermore, the group-based treatment resulted in a mean weight loss of approximately 2 kg, whereas our SBWI resulted in mean weight loss of approximately 8 kg at both 6 and 12 months. Thus, questions remain regarding the effectiveness of wearable technologies over and above a SBWI and how to best use them to modify physical activity and diet behaviors in adults seeking weight loss.
Although this study showed weight loss across the 24 month intervention in young adults, similar to trials of middle-aged and older-aged adults,22,23,26,27 the benefits achieved at 6 months were not fully sustained long-term. Thus, regardless of age, challenges remain to prevent or minimize weight regain following initial weight loss in adults. These findings are important because of the lack of data to support the effectiveness of approaches for weight loss in young adults, who have a high prevalence for overweight and obesity.1 The interventions used in this study resulted in substantially greater weight loss than what was recently reported for young adults in response to a 24-month low-intensity, technology-based intervention.28 Given that there was not a no treatment control condition in this study, the degree to which the observed change in weight is a direct result of the intervention versus other factors cannot be determined. However, the importance of examining effective weight loss strategies for young adults is supported by a recent report showing that this age demographic has a higher prevalence rate of obesity (32.3%) compared to youth 12–19 years of age (20.5%) but lower than the prevalence found in middle-aged adults (40.2%).29 This may suggest that young adulthood is an important transition period for weight gain and the development of obesity.29
There were limitations to this study. The study sample was restricted to young adults, so results cannot be generalized to other ages. The multi-sensor wearable device was worn on the upper arm, which may not reflect the effectiveness of more contemporary devices that are worn on the wrist. However, the accuracy of wrist-worn devices to monitor physical activity and energy expenditure compared to the arm worn device has been questioned,30 which may also limit their effectiveness and this may not be consequential. Moreover, the use of wearable technology was not initiated at the onset of the intervention, which may have influenced how the participants adopted and utilized the technology during their weight loss efforts. The device that was used was also commercially available, and therefore the investigators did not have control over any additional information that may have been provided through the website that was available for use with this device. Dietary intake was assessed using self-report, which may have affected the accuracy of this measure and therefore influenced the understanding of how the intervention influenced this aspect of energy balance. Additional investigation is also needed to examine for whom wearable devices and other technologies may be effective within the context of weight loss efforts, and how these technologies influence other components of weight loss, namely eating behavior and dietary intake.
Approximately 75% of the participants provided outcome data at the 24 month assessment. Of the 120 participants missing 24 month weight, approximately one-third (n=38) had missing weight due to either being terminated for pregnancy (n=29) or moving out of the area (n=9), which are unlikely to bias the results. Linear mixed models used all available data from participants with missing data (i.e., from earlier time points) to gain efficiency. Although multiple imputation was used to account for missing data in a sensitivity analysis, the loss of outcome data most likely resulted in reduced precision for the parameter estimates. Moreover, it is possible that the results could be biased in the event that the lost to follow-up was not missing at random. Assessment staff were also aware that individuals were engaged in a weight loss trial, which may have introduced additional bias.
CONCLUSIONS
Among young adults with a BMI between 25 to <40 kg/m2, the addition of a wearable technology device to a standard behavioral intervention resulted in less weight loss over 24 months. Devices that monitor and provide feedback on physical activity may not offer an advantage over standard behavioral weight loss approaches.
Supplementary Material
KEY POINTS.
Question
Is the addition of a wearable device to monitor and provide feedback on physical activity effective for improving weight loss within the context of a behavioral weight loss intervention?
Findings
In this randomized trial that included 470 young adults, weight loss was significantly less (by 2.4 kg) in response to a behavioral intervention when a wearable device that monitored and provided feedback on physical activity was included within the intervention.
Meaning
Devices that monitor and provide feedback on physical activity may not offer an advantage over standard behavioral weight loss approaches.
Acknowledgments
We recognize the contribution of the staff and graduate students at the Physical Activity and Weight Management Research Center and the Epidemiology Data Center at the University of Pittsburgh who received salary support for their effort on this project.
Dr. Jakicic had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr. Wahed (University of Pittsburgh) and Dr. King (University of Pittsburgh) conducted and take responsibility for the data analysis.
Funding Support:
This study was supported by grant U01 HL096770 from the National Institutes of Health and the National Heart, Lung, and Blood Institute.
Role of the Sponsor:
The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or in the preparation, review, approval, or decision to submit the manuscript for publication. However, because this grant was funded as a cooperative agreement (U-award) the sponsor provided input on outcome measurements prior to implementation, and the Program Officers of the sponsor (NHLBI) were invited to participate in meetings of the DSMB.
Footnotes
Financial Disclosures: Dr. Jakicic received an honorarium for serving on the Scientific Advisory Board for Weight Watchers International, was the Principal Investigator on a grant to examine the validity of activity monitors awarded to the University of Pittsburgh by Jawbone, Inc., a co-investigator on a grant award to the University of Pittsburgh by HumanScale, a co-investigator on a grant awarded to the University of Pittsburgh by Weight Watchers International, and a co-investigator on a grant awarded to the University of Pittsburgh by Ethicon/Covidien. Dr. Rogers was the Principal Investigator on a grant awarded to the University of Pittsburgh by Weight Watchers International. Dr. Marcus received an honorarium for serving on the Scientific Advisory Board for Weight Watchers International. None of the other authors had conflicts of interest to report.
Contributor Information
John M. Jakicic, University of Pittsburgh, Department of Health and Physical Activity, Physical Activity and Weight Management Research Center, Pittsburgh, PA.
Kelliann K. Davis, University of Pittsburgh, Department of Health and Physical Activity, Physical Activity and Weight Management Research Center, Pittsburgh, PA.
Renee J. Rogers, University of Pittsburgh, Department of Health and Physical Activity, Physical Activity and Weight Management Research Center, Pittsburgh, PA.
Wendy C. King, University of Pittsburgh, Department of Epidemiology, Pittsburgh, PA.
Marsha D. Marcus, University of Pittsburgh, Department of Psychiatry, Pittsburgh, PA.
Diane Helsel, Bastyr University, Department of Nutrition and Exercise Science, Kenmore, WA.
Amy D. Rickman, Slippery Rock University of Pennsylvania, Department of Exercise & Rehabilitative Sciences, Slippery Rock, PA.
Abdus S. Wahed, University of Pittsburgh, Department of Biostatistics, Pittsburgh, PA.
Steven H. Belle, University of Pittsburgh, Department of Epidemiology and Department of Biostatistics, Pittsburgh, PA.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institutes of Health National Heart Lung and Blood Institute. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults – The Evidence Report. Obes Res. 19986(suppl. 2) [PubMed] [Google Scholar]
- 3.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and The Obesity Society. Circulation. 2013;129(25 Suppl 2):S102–138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piwek L, Ellis DA, Andrews S, Joinson A. The rise of consumer health wearable: promises and barriers. PLoS Med. 2016;13(2):e1001953. doi: 10.1371/journal.pmed.1001953. doi: 1001910.1001371/journal.pmed.1001953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pellegrini CA, Verba SD, Otto AD, Helsel DL, Davis KK, Jakicic JM. The comparison of a technology-based system and an in-person behavioral weight loss intervention. Obesity. 2012;20(2):356–363. doi: 10.1038/oby.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polzien KM, Jakicic JM, Tate DF, Otto AD. The efficacy of a technology-based system in a short-term behavioral weight loss intervention. Obesity. 2007;15(4):825–830. doi: 10.1038/oby.2007.584. [DOI] [PubMed] [Google Scholar]
- 7.Lytle LA, Svetkey LP, Patrick K, et al. The EARLY Trials: A consortium of studies targeting weight control in young adults. Translational Behavioral Medicine. 2014;4(3):304–313. doi: 10.1007/s13142-014-0252-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jakicic JM, King WC, Marcus MD, et al. Short-term weight loss with diet and physical activity in young adults: the IDEA Study. Obesity. 2015;23(12):2385–2397. doi: 10.1002/oby.21241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- 10.Janz KF. Use of heart rate monitors to assess physical activity. In: Welk GJ, editor. Physical Activity Assessment for Health-Related Research. Champaign, IL: Human Kinetics; 2002. [Google Scholar]
- 11.Marlatt GA, Gordon JR. Relapse Prevention. New York: Guilford Press; 1985. [Google Scholar]
- 12.Perri MG, Nezu AM, McKelvey WF, Shermer RL, Renjilian DA, Viegener BJ. Relapse prevention training and problem solving therapy in the long-term management of obesity. J Consult Clin Psychol. 2001;69:722–726. [PubMed] [Google Scholar]
- 13.Rosenstock IM. Historical origins of the health belief model. Health Education Monograph. 1974;2:1–9. [Google Scholar]
- 14.Jakicic JM, Marcus MD, Gallagher KI, et al. Evaluation of the SenseWear Pro Armband ™ to assess energy expenditure during exercise. Med Sci Sports Exerc. 2004;36(5):897–904. doi: 10.1249/01.mss.0000126805.32659.43. [DOI] [PubMed] [Google Scholar]
- 15.St-Onge M, Mignault D, Allison DB, Rabasa-Lhoret R. Evaluation of a portable device to measure daily energy expenditure in free-living adults. Am J Clin Nutr. 2007;85:742–749. doi: 10.1093/ajcn/85.3.742. [DOI] [PubMed] [Google Scholar]
- 16.Masse LC, Fuemmeler BF, Anderson CB, et al. Accelerometer data reduction: a comparison of four reduction algorithms on select outcome variables. Med Sci Sports Exerc. 2005;37(11 Suppl):S544–S554. doi: 10.1249/01.mss.0000185674.09066.8a. [DOI] [PubMed] [Google Scholar]
- 17.Miller GD, Jakicic JM, Rejeski WJ, et al. Effect of varying accelerometry criteria on physical activity: the Look AHEAD Study. Obesity. 2013;21(1):32–44. doi: 10.1038/oby.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America’s Table Study. Am J Epidemiol. 2001;154(12):1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 19.Thompson FE, Subar AF, Brown CC, et al. Cognitive reseearch enhances accuracy of food frequency questionnaire reports: results of an experimental validation study. J Am Diet Assoc. 2002;102(2):212–225. doi: 10.1016/s0002-8223(02)90050-7. [DOI] [PubMed] [Google Scholar]
- 20.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychologial Measurement. 1977;1(3):385–401. [Google Scholar]
- 21.Jakicic JM, Marcus BH, Gallagher KI, Napolitano M, Lang W. Effect of exercise duration and intensity on weight loss in overweight, sedentary women. A randomized trial. JAMA. 2003;290:1323–1330. doi: 10.1001/jama.290.10.1323. [DOI] [PubMed] [Google Scholar]
- 22.Jakicic JM, Marcus BH, Lang W, Janney C. Effect of exercise on 24-month weight loss in overweight women. Arch Int Med. 2008;168(14):1550–1559. doi: 10.1001/archinte.168.14.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakicic JM, Winters C, Lang W, Wing RR. Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women: a randomized trial. Journal of the American Medical Association. 1999;282(16):1554–1560. doi: 10.1001/jama.282.16.1554. [DOI] [PubMed] [Google Scholar]
- 24.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65–70. [Google Scholar]
- 25.Shugar SL, Barry VW, Sui X, et al. Electronic feedback in a diet- and physical activity-based lifestyle intervention for weight loss: a randomized controlled trial. Int J Behav Nutr Phys Act. 2011;8:41–49. doi: 10.1186/1479-5868-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jakicic JM, Tate D, Davis KK, et al. Effect of a stepped-care intervention approach on weight loss in adults: The Step-Up Study Randomized Trial. JAMA. 2012;307(24):2617–2626. doi: 10.1001/jama.2012.6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Look AHEAD Research Group. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors with type 2 diabetes: four year results of the Look AHEAD Trial. Arch Int Med. 2010;170(17):1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Svetkey LP, Barch BC, Pao-Hwa L, et al. Cell phone intervention for you (CITY): A randomized, controlled trial of behavioral weight loss intervention for young adults using mobile technology. Obesity. 2015;23(11):2133–2141. doi: 10.1002/oby.21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. Prevalence of obesity among young adults and youth: United States, 2011–2014. NCHS Data Brief. 2015 Nov219 [Google Scholar]
- 30.Jung-Min L, Kim Y, Welk GJ. Validity of consumer-based physical activity monitors. Med Sci Sports Exerc. 2014;46(9):1840–1848. doi: 10.1249/MSS.0000000000000287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


