Abstract
Purpose
Fatigue is a prevalent and burdensome effect of breast cancer. Fatigue has been linked to chronic inflammation, and diets high in antioxidant nutrients have been associated with lesser prevalence and severity of fatigue. Studies are needed, however, to test if antioxidant-rich diets could improve fatigue.
Methods
Pilot, randomized, trial conducted between January 2014 and April 2015, to investigate if a 3-month diet rich in fruit, vegetables, whole grains, and omega-3 fatty acid-rich foods, named the fatigue reduction diet (FRD), improved fatigue and sleep compared to an attention control, named the general health curriculum (GHC). 30 stage 0 to III breast cancer survivors, who had completed cancer treatments, were randomized: 15 receiving the FRD and 15 the GHC. Primary outcome was change in fatigue, as measured by the brief fatigue Inventory, from baseline to 3 months analyzed using linear mixed models. Secondary analyses were changes in sleep quality, serum carotenoids, and fatty acids.
Results
From baseline to 3-month fatigue improved by 44 ± 39% in FRD compared to 8 ± 34% in GHC (p = 0.01); sleep quality improved by 2.5 ± 3.3 points in FRD, and diminished by 0.9 ± 2.3 in GHC (p = 0.03); serum total carotenoids (p < 0.01), β-cryptoxanthin (p = 0.02), lutein (p = 0.05), zeaxanthin (p = 0.01), lycopene (p = 0.05), omega-3 fatty acids (p < 0.01), and ratio of omega-3:omega-6 fatty acids (p = 0.02) were significantly increased, and percent saturated fatty acids were decreased (p = 0.04) in FRD; γ-tocopherol was significantly increased in GHC (p = 0.03), and there was a significant visit by group difference for α-carotene between the study groups (p = 0.05).
Conclusions
The FRD intervention improved fatigue and sleep in breast cancer survivors compared to the GHC. FRD diet could provide a non-toxic treatment strategy for persistent fatigue.
Keywords: Cancer-related fatigue; Sleep quality; Breast cancer survivor; Diet, omega-3 fatty acids; Fruits; Vegetables; Whole grains; Carotenoids
Background
Persistent fatigue is one of the most prevalent and burdensome late-term effects of breast cancer treatment [41, 44]. Even ten years after the completion of their breast cancer treatment, as many as one-third of breast cancer survivors experience moderate-to-severe persistent fatigue [10, 31, 38]. Persistent fatigue also often co-occurs with depression [26, 33, 45], poor sleep [3], and decreased quality of life [53], and some research suggest that more severe fatigue is independently associated with shorter times to breast cancer recurrence and shorter overall survival [29].
The etiology of persistent fatigue is currently unknown; however, research suggests that peripheral pro-inflammatory markers including cytokines and C-reactive protein (CRP) are consistently elevated in fatigued breast cancer survivors [8, 9, 20, 48]. This increased inflammation in the periphery can lead to a cascade of inflammatory response throughout the brain [22, 54] potentially causing CNS-induced sickness behavior in which fatigue plays a predominant role [35, 40]. It has been posited that diets high in antioxidants and other specific micronutrients could help to counter the elevated inflammatory state and thus help to alleviate fatigue. In several observational studies in breast cancer survivors, higher quality diets [27, 32]; diets high in fiber, lower in total fat [30], and high in fruits and vegetables [1]; and those high in omega-3 fatty acids relative to omega-6 fatty acids [2] were associated with a lower likelihood of having persistent fatigue in breast cancer survivors [2]. Likewise, we found that diets high in fatty fish, nuts and seeds, whole grains, and vegetables (in particular green leafy vegetables and tomatoes) were associated with both a lower chance of having fatigue and less severe fatigue in adult cancer survivors, and cancer survivors reporting no-fatigue had significantly higher intakes of certain antioxidant nutrients [55]. All these studies are, however, cross-sectional and thus cannot determine if diet is improving fatigue or if higher diet quality is simply associated with other healthy behaviors such as increased exercise or achieving and maintaining a normal body weight, both of which are known to improve persistent cancer-related fatigue.
To determine if diet quality, independent of weight loss, could improve persistent cancer-related fatigue, we tested the hypothesis that a three-month randomized pilot clinical trial of a fatigue reduction diet (FRD) high in fruits, vegetables, whole grains and omega-3 rich foods (fatty fish, nuts, and seeds) compared to an attention control named the general health curriculum (GHC), which was focused on education about general health topics, could decrease persistent cancer-related fatigue and improve sleep quality. We also examined the ability of the FRD compared to the attention control group to increase serum concentrations of carotenoids and omega-3 fatty acids, while decreasing serum concentrations of omega-6 fatty acids, and if these changes in serum nutrients were associated with changes in either fatigue or sleep quality.
Methods
Subjects and eligibility
The study was approved by the University of Michigan Institutional Review Board, and written informed consent was obtained from all participants. Participants were recruited between January 2014 and April 2015 from the University of Michigan Breast Cancer Clinics (Ann Arbor, Michigan), the surrounding community or from participants of a completed study in fatigued breast cancer survivors, who had indicated that they were willing to be contacted for future studies. Eligible participants were women 18 years of age and older with a body mass index (BMI) between 18.5 and 35 kg/m2, and with a diagnosis of local regional breast cancer (stage 0-IIIa), who had completed all cancer-related treatments, except for hormonal therapy and Herceptin, at least one year previously. Eligible women also had to report persistent fatigue starting on or after their cancer diagnosis and score ≥4 on the brief fatigue inventory (BFI) and have a low fruit and vegetable intake of less than 5.5 servings per day, not including potatoes and iceberg lettuce. Women who had a diagnosis of untreated anemia, hypo-, or hyper-thyroid; who were supplementing with omega-3 fatty acids; on medically prescribed diets; who were pregnant; wanting to become pregnant or lactating; and planning on starting or stopping any chronic supplements or medications within six weeks prior to or throughout the study were ineligible to participate. All women were asked to maintain their typical exercise program and were considered ineligible if they were planning on stopping or starting a new exercise routine during the course of the study.
Study design, blinding, randomization, allocation, and study visits
A 3-month randomized, parallel, pilot clinical trial comparing the FRD to a general health curriculum (GHC) was conducted. Because of the nature of the intervention, study staff was not able to be blinded; however, all data analyses were conducted in a blinded fashion. The randomization code was computer-generated in blocks of size 6 by the study biostatistician. Study personnel, who had no contact with participants or study data, placed the randomization assignments in sequentially numbered opaque envelopes. Upon randomization, the next number in the sequence was chosen, and the envelope opened indicating treatment assignment.
There were three in-person research visits: screening, baseline, and 3-month (end of treatment), and six telephone counseling visits: weeks 1, 2, 3, 4, 6, 8, 10, and 12. Seven-day diet records were asked to be completed the week before the baseline and 3-month visits. BFI, Pittsburgh sleep quality index (PSQI), weight, height, sociodemographic, clinical characteristics, concomitant medications, and fasting blood samples (obtained from the arm) were collected at baseline and at the 3-month visit. Three unannounced 24-h dietary recalls, one each on Tuesday, Thursday, and Sunday, were conducted during the 3 months of the study. Study visits were conducted at the Michigan Clinical Research Unit (MCRU) at the University of Michigan (Ann Arbor).
Interventions
Both the FRD and the GHC employed individualized counseling using the theoretical framework of Bandura’s social cognitive theory [5, 13], and with the exception of the baseline and 3-month visits, which were conducted inperson, were delivered using six brief 15-minute telephone counseling (once per week for the first 4 weeks and then once every other week) by a MCRU registered dietitian (for the FRD) or a study staff member (for the GHC). The FRD maintained a woman on a diet with her baseline caloric intake (isocaloric diet) and replaced some of her calories with the following foods on a daily basis:
(1) At least half of grain intake from whole grains; (2) five servings of vegetables (one leafy green, one tomato, and one yellow or orange); (3) two servings of fruit (one high in vitamin C); (4) one serving of fatty fish and (5) one serving of omega-3 fatty acid-rich nuts, seeds, or their associated oils (two servings of nuts or seeds for vegetarians). The counseling for this diet used participant daily self-monitoring checklists to help participants achieve goal intakes for the target foods. The checklists were mailed or faxed to the dietitian before the telephone counseling session and discussed.
For the control arm, we employed an attention control, which we named the GHC. The GHC sessions were matched in amount of time spent (~15 min), frequency (8 sessions), and method of delivery to those in the FRD arm. The 8 GHC topics were: (1) oral health, (2) healthy eyesight, (3) over-the-counter and prescription drug disposal, (4) healthy skin and hair, (5) cell phones and health, (6) hearing loss, (7) colorectal cancer screening, and (8) preventing colds and flu. None of the GHC topics contained dietary information.
Study outcome measures
We used the BFI [36] to assess severity and impact of fatigue in cancer patients. The BFI is validated in cancer patients, and it correlates highly with other fatigue measure [39]. It has a Cronbach alpha exceeding 0.95 [6]. The BFI has 9 items, each measuring a different aspect of fatigue, and is calculated from the mean of all completed items giving a 0-10 score. Clinically-relevant fatigue scores are defined as mean BFI scores of ≥4 [36].
The 19-item PSQI was used to assess sleep quality. The PSQI evaluates sleep interferences over the past month. The PSQI yields seven component scores and a global score, and the global PSQI score has an alpha coefficient of 0.81 [6]. In the general US population, a global PSQI score of ≥5 indicates poor sleep quality [15].
To assess dietary intakes at baseline and 3 months, 7-day food records and 24-h recalls were analyzed using the University of Minnesota Nutrition Data System Research software (Nutrition Coordinating Center, University of Minnesota, version 2014; http://www.ncc.umn.edu/).
Adherence to dietary goals was assessed using the daily food check lists and serum fatty acids and carotenoids. Fasting blood samples were stored at −70 °C until analysis. Analytical methods for both serum fatty acids and carotenoids have both been reported previously in detail. In brief, carotenoids were extracted with hexane and quantified using high-pressure liquid chromatography, and serum fatty acids were measured as fatty acid methyl ester by gas chromatography [24].
Statistical analysis
Descriptive statistics for baseline sociodemographic and clinical characteristics were analyzed between groups using an independent sample t test for continuous measures or Pearson’s Chi-square for categorical variables. To investigate the change through time both within and between groups for BFI, PSQI, changes in dietary measures, e.g., green leafy vegetable intake, BMI, calories, serum fatty acids, and serum carotenoids we conducted an intent-to-treat (ITT) analysis using linear mixed models (LMM). For each LMM, a random subject intercept was included to account for subject clustering, and fixed effects were visit, group, and the interaction term (visit by group). The models for BFI and PSQI were also controlled for the fixed effects of baseline age and BMI, while the serum fatty acids and carotenoids were further controlled for the fixed effects of smoking and analytical batch. Smoking status, BMI, and the analytical batch that the fatty acid and carotenoids were analyzed in were added to the models as these variables have been shown to impact the variability in these measures in other studies [18, 49].
Normality of the residuals derived from the LMM was assessed using the Shapiro—Wilk test and q–q plots. For non-normal results, a Box—Cox transformation was utilized [12]. A square root transformation was used for serum α-carotene, β-carotene, β-cryptoxanthin, lutein, total carotenoids, and PSQI. The other carotenoids, tochopherols, and fatty acids required no transformation. Associations between changes in concentrations of serum nutrients and changes in fatigue and sleep quality from baseline to 3 months were investigated using pairwise correlations for the FRD group (Spearman rank correlation coefficient for variables with non-normal distributions and Pearson correlation coefficients for all other variables).
In all analyses, a p value of ≤0.05 was considered statistically significant except for correlations between change in serum nutrients and change in fatigue and sleep quality between baseline and the 3-month visits where a p value of ≤0.10 was considered significant. As this was a pilot trial with a small sample size, we allowed for the larger p value of ≤0.10 when examining the serum correlations to allow for hypothesis generation. All tests were two-sided.
Our power analysis was based on detecting a time-by-group interaction term in the mixed effects regression model for BFI, our primary outcome measure. At baseline, both diet arms are assumed to have a mean BFI of 6. At the 3-month follow-up, the control arm was assumed to reduce the average BFI value to 5, whereas the FRD was assumed to experience a reduction of three points, bringing the mean BFI down to 3. The between-subject variance was assumed to be 4, and the intra-subject correlation is taken to be 0.3, a moderate value. These assumed values are estimates based on our pilot data [55]. For this configuration, the power for detecting a significant time-by-group interaction is 0.89 with a sample size of 15 per diet arm and a 5% level of significance.
Results
Screening, enrollment, and withdrawals
We screened 50 women of whom 30 were enrolled in the study with 15 randomized to the GHC and 15 to the FRD group. Twenty-nine of the 30 participants were evaluable for the primary outcome of change in BFI from baseline through month 3. All study visits were completed by 100% of women in the GHC group, and 93% in the FRD. Figure 1 documents exclusions and reasons for discontinuing the interventions.
Fig. 1.
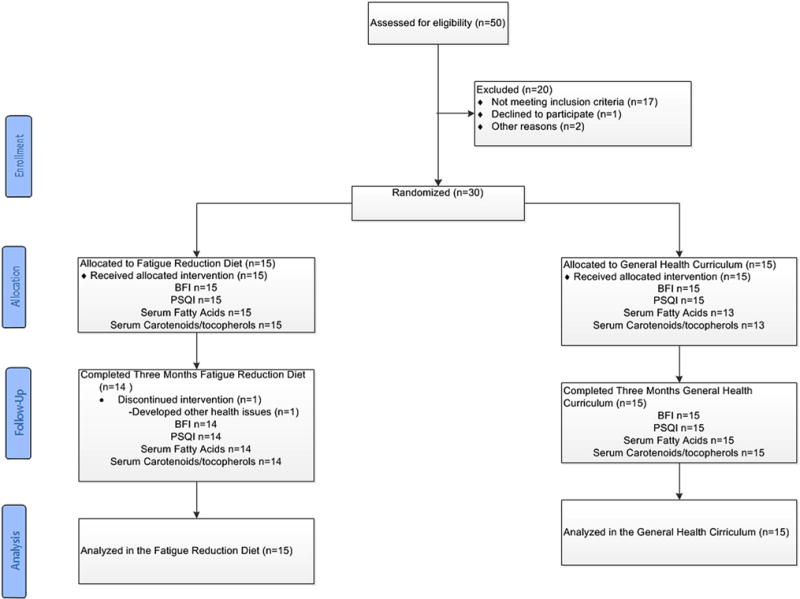
Consort flow diagram BCRT
Sociodemographic and clinical characteristics
There were no significant differences between study groups at baseline by any sociodemographic or clinical characteristics (Table 1). Despite no significant differences in baseline age or BMI between groups, the women in the GHC group were on average 4 years younger than the women in the FRD group and the participants in the GHC group had a 2-point lower BMI. Participants were mainly white (n = 28, 93%) and none self-reported as Hispanic. Mean baseline fatigue was 5.5 ± 1.2, baseline PSQI was 7.2 ± 3.6 and baseline BMI was 28.2 ± 3.8. The participants’ ages ranged from 47 to 81 years with the average age being 62.4 ± 9.7 years. Women had been diagnosed with breast cancer on average 7.4 ± 3.9 years before enrollment into the study; the majority were postmenopausal at the time of their breast cancer diagnosis (n = 16, 53%), and most women had estrogen receptor-positive breast cancer (n = 24, 80%).
Table 1.
Sociodemographic and clinical characteristics at baseline
| Fatigue reduction diet (n = 15) | General health curriculum (n = 15) | |
|---|---|---|
| Demographics | ||
| Age (mean years) ± SD | 64.4 ± 10.0 | 60.4 ± 9.35 |
| Race n (%) | ||
| White | 14 (93) | 14 (93) |
| Clinical characteristics | ||
| BFI (mean ± SD) | 5.4 ± 1.1 | 5.6 ± 1.3 |
| PSQI (mean ± SD) | 6.8 ± 3.9 | 7.5 ± 3.4 |
| BMI (mean ± SD) | 27.2 ± 3.8 | 29.2 ± 3.6 |
| Stage of cancer n (%) | ||
| Stage 0a | 1 (7) | 5 (33) |
| Stage 1 | 4 (27) | 3 (20) |
| Stage 2 | 7 (47) | 4 (27) |
| Stage 3 | 2 (13) | 2 (13) |
| Unknown | 1 (7) | 0 (0) |
| Estrogen receptor status | ||
| Yes | 12 (80) | 12 (80) |
| No | 3 (20) | 2 (13) |
| Unknown | 0 (0) | 1 (7) |
| Menopausal statusb | ||
| Premenopausal | 3 (21) | 7 (47) |
| Perimenopausal | 2 (14) | 1 (7) |
| Postmenopausal | 9 (64) | 7 (47) |
| Time since cancer diagnosis in years (mean ± SD)c | 7.4 ± 3.8 | 7.5 ± 4.1 |
| Treatments (were received) n (%)c | ||
| Surgery | 15 (100) | 15 (100) |
| Chemotherapy | 11 (73) | 8 (53) |
| Radiation | 12 (86) | 11 (73) |
| Hormone therapy | 12 (80) | 10 (67) |
Percentages may not add up to 100% because participants can receive multiple treatments or diagnoses BFI brief fatigue inventory, BMI body mass index, PSQI Pittsburgh sleep quality index
Stage 0 includes ductal carcinoma in situ (DCIS) and Lobular carcinoma in situ (LCIS)
Menopausal status at the time of initial breast cancer diagnosis
Time since “cancer diagnosis” was calculated from on-study date and date of diagnosis in years
Change in calories, BMI, and dietary intake
There was no significant change in BMI between the baseline and the final visit for either group (p = 0.70). Nor was there any significant change in calories in the FDR group (p = 0.50). There was a significant decrease in calories between the baseline and the 3-month visit in the GHC group (−188 calories, p < 0.01). All targeted food groups in the FRD, with the exception of non-citrus fruits, were significantly increased (all p ≤ 0.01) roughly twofold between the baseline and 3-month visit, while no foods had changed in the GHC group (as shown in Table 2).
Table 2.
Change in dietary measures
| Measures | Fatigue reduction diet N = 15 Mean ± SD |
General health curriculum N = 15 Mean ± SD |
||
|---|---|---|---|---|
| Baseline | 3 months | Baseline | 3 months | |
| Calories per day | 1608 ± 381 | 1665 ± 359 | 1788 ± 313 | 1600 ± 330a |
| BMI | 27.2 ± 3.8 | 27.2 ± 3.9 | 29.2 ± 3.9 | 29.1 ± 3.6 |
| Fruit excluding citrusd | 0.9 ± 0.5 | 1.3 ± 0.7 | 1.0 ± 0.9 | 0.7 ± 0.5 |
| Citrus | 0.2 ± 0.2 | 0.8 ± 0.6a | 0.4 ± 0.3 | 0.5 ± 0.6 |
| Vegetablesc | 2.8 ± 0.8 | 5.7 ± 1.9a | 1.9 ± 1.0 | 2.0 ± 0.9 |
| Leafy greenc | 0.5 ± 0.6 | 1.2 ± 0.7a | 0.3 ± 0.4 | 0.3 ± 0.4 |
| Yellow/orangec | 0.3 ± 0.4 | 0.6 ± 0.5a | 0.1 ± 0.1 | 0.1 ± 0.2 |
| Tomatoesc | 0.4 ± 0.3 | 1.2 ± 0.7a | 0.4 ± 0.3 | 0.4 ± 0.2 |
| Otherc | 1.0 ± 0.5 | 2.1 ± 1.5a | 1.0 ± 0.6 | 1.0 ± 0.5 |
| Fish servings per dayd | 0.6 ± 0.9 | 1.5 ± 1.5b | 0.8 ± 0.9 | 0.6 ± 0.7 |
| Nuts and Seeds servings per day | 0.7 ± 0.7 | 1.4 ± 1.0b | 0.9 ± 1.0 | 0.9 ± 1.2 |
| Whole Grains servings per dayc | 1.2 ± 0.8 | 2.3 ± 0.9a | 1.2 ± 0.7 | 1.3 ± 1.1 |
Significantly (p < 0.01) different from baseline to 3 months for that study arm from mixed linear regression models
Significantly (p < 0.05) different from baseline to 3 months for that study arm from mixed linear regression models
A significant (p ≤ 0.01) group-by-time interaction from mixed linear regression models
A significant (p ≤ 0.05) group-by-time interaction from mixed linear regression models
Change in fatigue and sleep quality
In our adjusted models, the BFI decreased from baseline by 2.4 ± 2.0 points between baseline and the 3-month visit in the FRD group compared to a decrease of 0.77 ± 1.8 in the GHC group (p < 0.01). This represents an average % decrease of 44 ± 39% in FRD group and an 8 ± 34% decrease in the GHC group (p = 0.01, Table 3). The global PSQI score decreased by 2.5 ± 3.3 points between baseline and the 3-month visit in FRD group, while it increased by 0.9 ± 2.3 in the GHC group (p = 0.03). At the 3-month visit, the global PSQI score had decreased by 3.9 ± 2.8 in the FRD and 0.8 ± 2.0 (p = 0.02) in those women with baseline global PSQI scores of ≥5 (N = 23, 77%) (Table 3).
Table 3.
Fatigue and sleep measures by treatment group and visit
| Measures | Fatigue reduction diet N = 15 Mean ± SD |
General health curriculum N = 15 Mean ± SD |
||
|---|---|---|---|---|
| Baseline | 3 months | Baseline | 3 months | |
| Brief fatigue inventorya | 5.4 ± 1.1 | 3.0 ± 2.2a | 5.6 ± 1.3 | 4.9 ± 1.6 |
| PSQId total score (all)2 | 6.8 ± 3.9 | 3.9 ± 2.1 | 7.5 ± 3.4 | 8.5 ± 3.4 |
| PSQId total scoreb | ||||
| Greater than or equal to 5 | 8.3 ± 3.3 | 3.9 ± 2.4c | 8.8 ± 2.6 | 9.5 ± 3.3 |
A significant (p < 0.01) group-by-time interaction from mixed linear regression models adjusted for; baseline age and BMI
A significant (p < 0.05) group-by-time interaction from mixed linear regression models adjusted for; baseline age and BMI
Significantly (p < 0.01) different from baseline to 3 month for that study arm from mixed linear regression models adjusted for baseline age and BMI
PSQI Pittsburgh sleep quality index; PSQI ≥ 5 is considered someone who has poor sleep quality
Serum nutrient concentrations
Table 4 shows serum nutrient levels by visit and study groups. In the adjusted analyses serum total carotenoids (p < 0.01), β-cryptoxanthin (p = 0.02), lutein (p = 0.05), zeaxanthin (p = 0.01), lycopene (p = 0.05), percent omega-3 fatty acids (p < 0.01), and the ratio of omega-3 to omega-6 fatty acids (p = 0.02) were significantly increased, and percent saturated fatty acids were decreased (p = 0.04) between baseline and the 3-month visit in the FRD group. The only nutrient change in the GHC group was γ-tocopherol, which was significantly increased (p = 0.03). There was also a significant visit by group difference for α-carotene between the study groups (p = 0.05), increasing in the FRD group, while decreasing or staying approximately the same in the GHC group.
Table 4.
Serum concentrations of fatty acids, carotenoids, and tocopherols by treatment group and study visit
| Nutrients | Fatigue reduction diet N = 15 Mean ± SD
|
General health curriculum N = 15 Mean ± SD
|
||
|---|---|---|---|---|
| Baseline | 3 months | Baseline | 3 months | |
| Serum carotenoids | ||||
| Total carotenoids | 465 ± 255 | 694 ± 454b | 439 ± 316 | 459 ± 384 |
| α-Carotene (ng/mL)a | 50 ± 53 | 83 ± 85 | 40 ± 26 | 41 ± 29 |
| β-Carotene (g/mL) | 99 ± 139 | 169 ± 179 | 97 ± 141 | 86 ± 100 |
| β-Cryptoxanthin (ng/mL) | 107 ± 79 | 154 ± 162b | 92 ± 76 | 134 ± 243 |
| Lutein (ng/mL) | 121 ± 66 | 155 ± 121b | 104 ± 75 | 96 ± 55 |
| Zeaxanthin (ng/mL) | 27 ± 17 | 34 ± 26c | 24 ± 16 | 25 ± 17 |
| Lycopene (ng/mL) | 61 ± 47 | 99 ± 86b | 83 ± 46 | 76 ± 49 |
| Serum tocopherols | ||||
| α-Tocopherol (lg/mL) | 8.8 ± 3.7 | 8.5 ± 4.2 | 7.6 ± 3.8 | 7.7 ± 3.5 |
| γ-Tocopherol (lg/mL) | 0.8 ± 0.4 | 0.7 ± 0.3 | 0.7 ± 0.3 | 0.8 ± 0.3b |
| Serum fatty acids | ||||
| SFA (%) | 36.2 ± 3.8 | 34.3 ± 2.5b | 36.5 ± 4.1 | 36.1 ± 4.9 |
| MUFA (%) | 24.2 ± 2.6 | 23.5 ± 2.7 | 22.8 ± 4.3 | 21.9 ± 3.8 |
| N6 PUFA (%) | 35.2 ± 3.6 | 36.3 ± 3.7 | 35.5 ± 5.8 | 36.1 ± 6.5 |
| N3 PUFA (%) | 4.5 ± 1.1 | 5.8 ± 1.5c | 5.3 ± 1.3 | 5.9 ± 1.3 |
| N3/N6 fatty acid ratio | 0.13 ± 0.04 | 0.16 ± 0.05b | 0.15 ± 0.04 | 0.16 ± 0.03 |
SFA saturated fatty acid, MUFA monounsaturated fatty acid, PUFA polyunsaturated fatty acid, N6 omega-6 fatty acid, N3 omega-3 fatty acid
A significant (p < 0.05) group-by-time interaction from mixed linear regression models adjusted for batch; baseline age, BMI, and smoking status
Significantly (p < 0.05) different from baseline to 3 months for that study arm from mixed linear regression models adjusted for batch; baseline age, BMI, and smoking status
Significantly (p < 0.01) different from baseline to 3 months for that study arm from mixed linear regression models adjusted for batch; baseline age, BMI, and smoking status
Correlations between changes in serum nutrients, fatigue, and sleep quality
Change in fatigue was significantly inversely associated with both changes in omega-3-to-omega-6 fatty acid ratio (Pearson r = −0.45, p = 0.09) and percent eicosapentaenoic acid (EPA, Pearson r = −0.49, p = 0.07). No changes in serum nutrients were significantly correlated with changes in sleep quality in the FRD group (Table 5). There was no significant correlation between either baseline fatigue and sleep quality (Pearson r = 0.20, p = 0.30) or change in fatigue and change in sleep quality between baseline and the 3-month visit in the FRD group (Pearson r = 0.16, p = 0.59).
Table 5.
Correlations in changes in serum nutrients with changes in fatigue and sleep quality
| Fatigue | Sleep quality | |
|---|---|---|
| Serum fatty acids | ||
| SFA (%) | −0.42 | −0.11 |
| MUFA (%) | −0.08 | −0.23 |
| N6 PUFA (%) | 0.43 | 0.18 |
| N3 PUFA (%) | −0.22 | 0.32 |
| N3/N6 fatty acid ratio | −0.45a | 0.25 |
| DHA (%) | −0.22 | 0.46 |
| EPA (%) | −0.49a | 0.36 |
| Serum carotenoids | ||
| Total carotenoids | −0.06 | 0.12 |
| α-Carotene (ng/mL) | −0.01 | 0.08 |
| β-Carotene (ng/mL) | −0.20 | 0.15 |
| β-Cryptoxanthin (ng/mL) | −0.04 | −0.27 |
| Lutein (ng/mL) | 0.18 | −0.25 |
| Zeaxanthin (ng/mL) | 0.22 | −0.18 |
| Lycopene (ng/mL) | −0.19 | 0.42 |
| Serum tocopherols | ||
| α-Tocopherol (lg/mL) | −0.05 | 0.05 |
| γ-Tocopherol (lg/mL) | −0.15 | −0.29 |
SFA saturated fatty acid, MUFA monounsaturated fatty acid, PUFA polyunsaturated fatty acid, N6 omega-6 fatty acid, N3 omega-3 fatty acid, DHA docosahexaenoic acid, EPA eicosapentaenoic acid
A significant (p < 0.10) from bivariate correlations; Pearson correlation coefficients for zeraxanthin, total lycopene, γ-tocopherol, α-tocopherol, DHA, EPA, SFA, MUFA, N6 PUFA, N3 PUFA, and N3/N6 PUFA; Spearman correlation coefficients for α-carotene, β-carotene, β-cryptoxanthin, lutein, and total carotenoids
Feasibility of fatigue reduction diet and general health curriculum
Based on the daily food check lists, participants meet their daily food goals for vitamin C-rich fruit 92 ± 12%, other fruit 94 ± 7%, green leafy vegetables 78 ± 19%, deep yellow or orange vegetables 84 ± 15%, tomatoes 92 ± 8%, other vegetables 73 ± 25%, whole grains 73 ± 22%, and omega-3 rich foods 79 ± 16%. Phone counseling sessions ranged from 6 to 30 min with the average phone counseling time being 14.5 ± 4.1 min.
Discussion
A 3-month whole foods dietary intervention compared to an attention control was able to significantly improve persistent cancer-related fatigue by over 44% compared to only 8% in the attention control group. In addition, women randomized to the dietary intervention also experienced a significant improvement of nearly 50% in sleep quality, while women in the control group experienced no change or a slight decrease in their sleep quality. These findings were achieved independent of weight change as neither group had a significant difference in weight during the study. Decreases in fatigue, between the baseline and 3-month visits, were also significantly inversely associated with increases in both serum EPA and the omega-3-to-omega-6 omega fatty acid ratio.
Our research builds on prior studies in a number of important ways. All other studies have only been able to investigate the association between diet and fatigue prevalence and severity. These cross-sectional studies were not able to explore if differences in diet quality or nutrient content were causal in nature or instead just associated with other healthy behaviors know to decrease persistent fatigue in cancer survivors such as exercising or maintaining a healthy weight, and these other behaviors could explain the association between fatigue and diet quality [23, 37]. We also assessed the impact of diet on sleep, which none of the previous studies had investigated despite strong associations between sleep disruptions and persistent fatigue in breast cancer survivors [42]. This study also adds to the literature by showing that improving diet quality outside of weight loss or calorie restriction could be a viable option to improve burdensome symptoms and thus improve quality of life in breast cancer survivors. While the goal of achieving and maintaining a healthy weight is the ideal, many breast cancer survivors find losing weight particularly challenging. Improving diet quality offers a possibly more achievable goal as demonstrated by the high level of adherence in our study.
Our findings are consistent with previous observational studies that found higher quality diets [27, 32], as well as diets high in fiber [30], fruits, and vegetables [1], omega-3 fatty acids relative to omega-6 fatty acids [2] were associated with a lower likelihood and less severe persistent fatigue in breast cancer survivors. In contrast to our study, which emphasized at least two servings per day of omega-3 fatty acid-rich foods, and thus encouraged targeted fat consumption, one study found that diets high in fat were associated with increased fatigue [30]. Fat consumption in this study, however, was investigated as one group and not broken down into different types of fat. As such, fats which could have a potential negative impact on fatigue such as saturated fats were grouped with fats such as omega-3 polyunsaturated fatty acids (omega-3 PUFAs), which are associated with lower likelihood of fatigue. This combing of types of fat could obscure the association between fatigue and various types of fat. This same study also did not find an association between fruit and vegetable consumption and fatigue [30]. Unlike our study, fruit and vegetable intake was combined, and mean intake was 2.7 servings per day, which is lower than the 3.5 servings per day in our participants at baseline.
Previous research has suggested supplementation of omega-3 fatty acids may be a strategy to reduce fatigue in breast cancer survivors [2]. Our results, however, suggest a dietary approach versus a supplemental approach may be a more successful strategy. For instance, our results found that the ratio of omega-3 to omega-6 fatty acids appeared as an important correlation of fatigue improvement, not just an increase of omega-3 fatty acids by themselves. To achieve meaningful changes in fatty acid ratios, it may be necessary to decrease the levels of omega-6 in the diet while simultaneously increasing the levels of omega-3 fatty acids. Also, changes in serum fatty acids were associated with only some of the decrease in fatigue and none of the improvement in sleep quality suggesting that other nutrients are involved in bringing about changes in these symptoms. Indeed, recent research investigating the relationship between sleep and diet found that numerous other nutrients including lower levels of calcium, vitamin C, and selenium were associated with disrupted sleep [28], and that in postmenopausal women, a lower diet quality, but not individual foods, was associated with shorter sleep duration [51].
The FRD method of using brief telephone counseling and written food exchange lists appears to be a feasible intervention for breast cancer survivors. The self-reported 7-day food records and daily food check lists both demonstrated significant increases in daily intake of target foods, and these differences were objectively confirmed by significant increases in serum levels of carotenoids and omega-3 fatty acids. We also found that all of the serum carotenoids categories increased indicating that we were able to impact the intake of a wide variety of vegetables and fruits. The increases in lycopene, β-cryptoxanthin, and zeaxanthin are particularly encouraging as other dietary intervention studies that focused on increasing fruit and vegetable intakes were unable to impact the levels of these carotenoids [7, 16, 24, 34]. As higher levels of β-cryptoxanthin, lycopene, and zeaxanthin are associated with lower breast cancer recurrence [25, 50, 52], the ability of the FRD to increase these nutrients has potential positive implications beyond control of symptoms in breast cancer survivors. Further, these dietary changes were achieved through a brief telephone delivered dietary counseling sessions. This approach could prove to be cost-effective.
Our work suggests several areas for additional research including exploration of the mechanisms through which this dietary intervention was associated with decreasing persistent cancer-related fatigue and improved sleep quality. Also a better understanding of how specific constituent parts (increased leafy green vegetables, whole grains, etc.) of the FRD intervention may have facilitated decreased fatigue and improved sleep quality. A likely mechanism through which the FRD is working is by decreasing chronic inflammation and modulating immune activation [11, 46], and in particular, decreasing circulating inflammatory biomarkers CRP, Interleukin-6 (IL-6), and interleukin 1 receptor antagonist (IL-1RA), which are associated with fatigue in cancer patients [21, 43, 47, 56]. We have previously reported that individuals who eat diets high in antioxidant micronutrients [19], or have high intake of whole foods including fish, fruits, and vegetables, similar to the FRD, have significantly lower concentrations of circulating inflammatory biomarkers [4, 14, 17].
Our study has several limitations. First, we had a small sample size, which could overestimate the effect size of the intervention. The intervention, however, was designed to focus on feasibility, adherence, and to establish a preliminary effect of the FRD. While we posited that the FRD would work through anti-inflammatory mechanisms, future studies will be needed to explore if this is true or if other mechanisms are important. Third, we were unable to measure other phytochemicals present in the FRD that could have been responsible for improving fatigue and sleep. Nonetheless, we did have serum measures, which were found in high levels in our targeted food groups, and that have been shown to have significant impacts on both fatigue and sleep. Also, we were unable to explore the impact of diet on symptoms such as hot flashes or arthralgias, which are prevalent in breast cancer survivors and can also cause poor sleep and fatigue.
This study also had several strengths including an attention control group that was matched for the frequency and duration of contacts in the dietary intervention. This is especially important for subjective patient reported outcomes such as fatigue where participating in a study independent of an intervention can lead to significant improvements in symptoms. Other strengths of this study include objective measures of dietary adherence in addition to patient reported intakes and a very high completion rate with only one participant withdrawing from the study.
Conclusions
A dietary intervention (the FRD rich in fruits, vegetables, whole grains, and omega-3 fatty acid-rich foods) delivered via brief phone counseling sessions significantly decreased fatigue and improved sleep quality over the course of 3 months compared to an attention control in persistently fatigued breast cancer survivors.
Acknowledgments
This study was supported by grants from the James Stuart and Barbara Padnos Research Funds for Cancer Research and the National Institutes of Health (NIH) CTSA Grant Number 2UL1TR000433-06. The funders had no role in the design of the study and collection, analysis, and interpretation of data, or the writing of the study. We would also like to thank Jianwei Ren for analyzing the blood samples.
Abbreviations
- FRD
Fatigue reduction diet
- GHC
General health curriculum
- RD
Registered dietitian
- HEI-2010
Healthy eating index
- BFI
Brief fatigue inventory
- PSQI
Pittsburgh sleep quality index
- BMI
Body mass index
- EPA
Eicosapentaenoic acid
- CRP
C-reactive protein
- LMM
Linear mixed models
- CNS
Central nervous system
- ITT
Intent-to-treat
- MCRU
Michigan Clinical Research Unit
- IL-6
Interleukin-6
- IL-1RA
Interleukin 1 receptor antagonist
Footnotes
Authors’ Contributions Dr. Zick made substantial contributions to conception and design, data analysis and interpretation of data, and acquisition of data; was involved in drafting the manuscript revising the manuscript critically for important intellectual content and gave final approval of the version to be published. Dr. Djuric made substantial contributions to conception and design, data analysis, and interpretation of data, was involved in revising the manuscript critically for important intellectual content and gave final approval of the version to be published. Dr. Colacino made substantial contributions to conception and design, data analysis and interpretation of data, and he was involved in revising the manuscript critically for important intellectual content and gave final approval of the version to be published. Ms. Brash, Surnow, Khabir, Cornellier, and Mr. Ren made substantial contributions to acquisition of data, and were involved in revising the manuscript critically for important intellectual content and gave final approval of the version to be published.
Conflicts of interest Drs. Zick, Djuric and Colacino and Ms. Cornellier, Surnow and Khabir declare that they have neither financial nor non-financial competing interest to disclose nor do they have any conflicts of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Alfano CM, Day JM, Katz ML, Herndon JE, 2nd, Bittoni MA, Oliveri JM, Donohue K, Paskett ED. Exercise and dietary change after diagnosis and cancer-related symptoms in long-term survivors of breast cancer: CALGB 79804. Psycho-oncology. 2009;18:128–133. doi: 10.1002/pon.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfano CM, Imayama I, Neuhouser ML, Kiecolt-Glaser JK, Smith AW, Meeske K, McTiernan A, Bernstein L, Baumgartner KB, Ulrich CM, Ballard-Barbash R. Fatigue, inflammation, and omega-3 and omega-6 fatty acid intake among breast cancer survivors. J Clin Oncol. 2012;30:1280–1287. doi: 10.1200/JCO.2011.36.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ancoli-Israel S, Liu L, Rissling M, Natarajan L, Neikrug AB, Palmer BW, Mills PJ, Parker BA, Sadler GR, Maglione J. Sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer before and after treatment: a 1-year longitudinal study. Support Care Cancer. 2014;22:2535–2545. doi: 10.1007/s00520-014-2204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arthur AE, Peterson KE, Shen J, Djuric Z, Taylor JMG, Hebert JR, Duffy SA, Peterson LA, Bellile EL, Whitfield JR, Chepeha DB, Schipper MJ, Wolf GT, Rozek LS. Diet and proinflammatory cytokine levels in head and neck squamous cell carcinoma. Cancer. 2014;120:2704–2712. doi: 10.1002/cncr.28778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31:143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 6.Beck SL, Schwartz AL, Towsley G, Dudley W, Barsevick A. Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. J Pain Symptom Manag. 2004;27:140–148. doi: 10.1016/j.jpainsymman.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Bowen PE, Garg V, Stacewicz-Sapuntzakis M, Yelton L, Schreiner RS. Variability of serum carotenoids in response to controlled diets containing six servings of fruits and vegetables per day. Ann N Y Acad Sci. 1993;691:241–243. doi: 10.1111/j.1749-6632.1993.tb26182.x. [DOI] [PubMed] [Google Scholar]
- 8.Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64:604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Bower JE, Ganz PA, Aziz N, Fahey JL, Cole SW. T-cell homeostasis in breast cancer survivors with persistent fatigue. J Natl Cancer Inst. 2003;95:1165–1168. doi: 10.1093/jnci/djg0019. [DOI] [PubMed] [Google Scholar]
- 10.Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106:751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 11.Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun. 2013;30(Suppl):S48–S57. doi: 10.1016/j.bbi.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Box G, Cox DR. An analysis of transformations. J R Stat Soc. 1964;26:211–252. [Google Scholar]
- 13.Brownell KD, Cohen LR. Adherence to dietary regimens. 2: components of effective interventions. Behav Med (Washington, DC) 1995;20:155–164. doi: 10.1080/08964289.1995.9933732. [DOI] [PubMed] [Google Scholar]
- 14.Bullo M, Casas-Agustench P, Amigo-Correig P, Aranceta J, Salas-Salvado J. Inflammation, obesity and comorbidities: the role of diet. Public Health Nutr. 2007;10:1164–1172. doi: 10.1017/S1368980007000663. [DOI] [PubMed] [Google Scholar]
- 15.Buysse D, Hall M, Strollo P, Kamarck T, Owens J, Lee L, Reis S, Matthews K. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med. 2008;4:563–571. [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell DR, Gross MD, Martini MC, Grandits GA, Slavin JL, Potter JD. Plasma carotenoids as biomarkers of vegetable and fruit intake. Cancer Epidemiol Biomark Prev. 1994;3:493–500. [PubMed] [Google Scholar]
- 17.Casas R, Sacanella E, Estruch R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr Metab Immune Disord Drug Targets. 2014;14:245–254. doi: 10.2174/1871530314666140922153350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chai W, Conroy SM, Maskarinec G, Franke AA, Pagano IS, Cooney RV. Associations between obesity and serum lipid-soluble micronutrients among premenopausal women. Nutr Res. 2010;30:227–232. doi: 10.1016/j.nutres.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colacino JA, Arthur AE, Ferguson KK, Rozek LS. Dietary antioxidant and anti-inflammatory intake modifies the effect of cadmium exposure on markers of systemic inflammation and oxidative stress. Environ Res. 2014;131:6–12. doi: 10.1016/j.envres.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12:2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- 21.Cruz F, Munhoz B, Alves B, Gehrke F, Fonseca F, Kuniyoshi R, Cubero D, Peppone L, del Giglio A. Biomarkers of fatigue related to adjuvant chemotherapy for breast cancer: evaluation of plasma and lymphocyte expression. Clin Trans Med. 2015;4:1–9. doi: 10.1186/s40169-015-0051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demark-Wahnefried W, Campbell KL, Hayes SC. Weight management and its role in breast cancer rehabilitation. Cancer. 2012;118:2277–2287. doi: 10.1002/cncr.27466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Djuric Z, Ren J, Blythe J, VanLoon G, Sen A. A Mediterranean dietary intervention in healthy American women changes plasma carotenoids and fatty acids in distinct clusters. Nutr Res. 2009;29:156–163. doi: 10.1016/j.nutres.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eliassen AH, Liao X, Rosner B, Tamimi RM, Tworoger SS, Hankinson SE. Plasma carotenoids and risk of breast cancer over 20 y of follow-up. Am J Clin Nutr. 2015;101:1197–1205. doi: 10.3945/ajcn.114.105080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galiano-Castillo N, Ariza-Garcia A, Cantarero-Villanueva I, Fernandez-Lao C, Diaz-Rodriguez L, Arroyo-Morales M. Depressed mood in breast cancer survivors: associations with physical activity, cancer-related fatigue, quality of life, and fitness level. Eur J Oncol Nurs. 2014;18:206–210. doi: 10.1016/j.ejon.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 27.George SM, Alfano CM, Neuhouser ML, Smith AW, Baumgartner RN, Baumgartner KB, Bernstein L, Ballard-Barbash R. Better postdiagnosis diet quality is associated with less cancer-related fatigue in breast cancer survivors. J Cancer Surviv. 2014;8:680–687. doi: 10.1007/s11764-014-0381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grandner MA, Jackson N, Gerstner JR, Knutson KL. Sleep symptoms associated with intake of specific dietary nutrients. J Sleep Res. 2014;23:22–34. doi: 10.1111/jsr.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groenvold M, Petersen MA, Idler E, Bjorner JB, Fayers PM, Mouridsen HT. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res Treat. 2007;105:209–219. doi: 10.1007/s10549-006-9447-x. [DOI] [PubMed] [Google Scholar]
- 30.Guest DD, Evans EM, Rogers LQ. Diet components associated with perceived fatigue in breast cancer survivors. Eur J Cancer Care. 2013;22:51–59. doi: 10.1111/j.1365-2354.2012.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrington CB, Hansen JA, Moskowitz M, Todd BL, Feuerstein M. It’s not over when it’s over: long-term symptoms in cancer survivors—a systematic review. Int J Psychiatry Med. 2010;40:163–181. doi: 10.2190/PM.40.2.c. [DOI] [PubMed] [Google Scholar]
- 32.Huang X, Zhang Q, Kang X, Song Y, Zhao W. Factors associated with cancer-related fatigue in breast cancer patients undergoing endocrine therapy in an urban setting: a cross-sectional study. BMC Cancer. 2010;10:453. doi: 10.1186/1471-2407-10-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SH, Son BH, Hwang SY, Han W, Yang JH, Lee S, Yun YH. Fatigue and depression in disease-free breast cancer survivors: prevalence, correlates, and association with quality of life. J Pain Symptom Manag. 2008;35:644–655. doi: 10.1016/j.jpainsymman.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Le Marchand L, Hankin JH, Carter FS, Essling C, Luffey D, Franke AA, Wilkens LR, Cooney RV, Kolonel LN. A pilot study on the use of plasma carotenoids and ascorbic acid as markers of compliance to a high fruit and vegetable dietary intervention. Cancer Epidemiol Biomark Prev. 1994;3:245–251. [PubMed] [Google Scholar]
- 35.Lee BN, Dantzer R, Langley KE, Bennett GJ, Dougherty PM, Dunn AJ, Meyers CA, Miller AH, Payne R, Reuben JM, Wang XS, Cleeland CS. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuro Immuno Modulation. 2004;11:279–292. doi: 10.1159/000079408. [DOI] [PubMed] [Google Scholar]
- 36.Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 37.Meneses-Echavez JF, Gonzalez-Jimenez E, Ramirez-Velez R. Effects of supervised exercise on cancer-related fatigue in breast cancer survivors: a systematic review and meta-analysis. BMC Cancer. 2015;15:77. doi: 10.1186/s12885-015-1069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minton O, Stone P. How common is fatigue in disease-free breast cancer survivors? A systematic review of the literature. Breast Cancer Res Treat. 2008;112:5–13. doi: 10.1007/s10549-007-9831-1. [DOI] [PubMed] [Google Scholar]
- 39.Minton O, Stone P. A systematic review of the scales used for the measurement of cancer-related fatigue (CRF) Ann Oncol. 2009;20:17–25. doi: 10.1093/annonc/mdn537. [DOI] [PubMed] [Google Scholar]
- 40.Myers JS. Proinflammatory cytokines and sickness behavior: implications for depression and cancer-related symptoms. Oncol Nurs Forum. 2008;35:802–807. doi: 10.1188/08.ONF.802-807. [DOI] [PubMed] [Google Scholar]
- 41.Noal S, Levy C, Hardouin A, Rieux C, Heutte N, Segura C, Collet F, Allouache D, Switsers O, Delcambre C, Delozier T, Henry-Amar M, Joly F. One-year longitudinal study of fatigue, cognitive functions, and quality of life after adjuvant radiotherapy for breast cancer. Int J Radiat Oncol Biol Phys. 2011;81:795–803. doi: 10.1016/j.ijrobp.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 42.Palesh O, Aldridge-Gerry A, Ulusakarya A, Ortiz-Tudela E, Capuron L, Innominato PF. Sleep disruption in breast cancer patients and survivors. J Natl Compr Cancer Netw. 2013;11:1523–1530. doi: 10.6004/jnccn.2013.0179. [DOI] [PubMed] [Google Scholar]
- 43.Pertl MM, Hevey D, Boyle NT, Hughes MM, Collier S, O’Dwyer AM, Harkin A, Kennedy MJ, Connor TJ. C-reactive protein predicts fatigue independently of depression in breast cancer patients prior to chemotherapy. Brain Behav Immun. 2013;34:108–119. doi: 10.1016/j.bbi.2013.07.177. [DOI] [PubMed] [Google Scholar]
- 44.Reinertsen KV, Cvancarova M, Loge JH, Edvardsen H, Wist E, Fossa SD. Predictors and course of chronic fatigue in long-term breast cancer survivors. J Cancer Surviv. 2010;4:405–414. doi: 10.1007/s11764-010-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romito F, Cormio C, Giotta F, Colucci G, Mattioli V. Quality of life, fatigue and depression in Italian long-term breast cancer survivors. Support Care Cancer. 2012;20:2941–2948. doi: 10.1007/s00520-012-1424-9. [DOI] [PubMed] [Google Scholar]
- 46.Saligan LN, Olson K, Filler K, Larkin D, Cramp F, Yennurajalingam S, Escalante CP, del Giglio A, Kober KM, Kamath J, Palesh O, Mustian K. The biology of cancer-related fatigue: a review of the literature. Support Care Cancer. 2015;23:2461–2478. doi: 10.1007/s00520-015-2763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sen A, Ren J, Ruffin MT, Turgeon DK, Brenner DE, Sidahmed E, Rapai ME, Cornellier ML, Djuric Z. Relationships between serum and colon concentrations of carotenoids and fatty acids in randomized dietary intervention trial. Cancer Prev Res (Philadelphia, PA) 2013;6:558–565. doi: 10.1158/1940-6207.capr-13-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sisti JS, Lindstrom S, Kraft P, Tamimi RM, Rosner BA, Wu T, Willett WC, Eliassen AH. Premenopausal plasma carotenoids, fluorescent oxidation products, and subsequent breast cancer risk in the nurses’ health studies. Breast Cancer Res Treat. 2015;151:415–425. doi: 10.1007/s10549-015-3391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stern JH, Grant AS, Thomson CA, Tinker L, Hale L, Brennan KM, Woods NF, Chen Z. Short sleep duration is associated with decreased serum leptin, increased energy intake and decreased diet quality in postmenopausal women. Obesity (Silver Spring, Md) 2014;22:E55–E61. doi: 10.1002/oby.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L, Li B, Pan MX, Mo XF, Chen YM, Zhang CX. Specific carotenoid intake is inversely associated with the risk of breast cancer among Chinese women. Br J Nutr. 2014;111:1686–1695. doi: 10.1017/s000711451300411x. [DOI] [PubMed] [Google Scholar]
- 53.Wu HS, Harden JK. Symptom burden and quality of life in survivorship: a review of the literature. Cancer Nurs. 2015;38:E29–E54. doi: 10.1097/NCC.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 54.Yarlagadda A, Alfson E, Clayton AH. The blood brain barrier and the role of cytokines in neuropsychiatry. Psychiatry (Edgmont) 2009;6:18–22. [PMC free article] [PubMed] [Google Scholar]
- 55.Zick SM, Sen A, Han-Markey TL, Harris RE. Examination of the association of diet and persistent cancer-related fatigue: a pilot study. Oncol Nurs Forum. 2013;40:E41–E49. doi: 10.1188/13.ONF.E41-E49. [DOI] [PubMed] [Google Scholar]
- 56.Zick SM, Zwickey H, Wood L, Foerster B, Khabir T, Wright B, Ichesco E, Sen A, Harris RE. Preliminary differences in peripheral immune markers and brain metabolites between fatigued and non-fatigued breast cancer survivors: a pilot study. Brain Imaging Behav. 2014;8:506–516. doi: 10.1007/s11682-013-9270-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


