Abstract
Purpose of Review
To present, summarize and interpret most recent advances in the study and understanding of the lamina cribrosa (LC) in glaucoma, in the context of previous work.
Recent Findings
The lamina is an active living structure that responds to strain and changes morphology at the micro- and macro-scales in glaucoma. Changes in LC morphology in glaucoma include posteriorization of the laminar insertion into the sclera, increased cupping or depth of the LC, and the development of focal LC defects. These LC changes are associated with disk hemorrhages and visual field damage, and are detectable with clinical imaging techniques such as optical coherence tomography (OCT). Glaucomatous changes in the LC are driven by cellular processes mediated by focal cyclical mechanical strain. Strain is eye specific and mediated by IOP, cerebrospinal fluid pressure (CSFP), and scleral and LC morphology and structural stiffness; deleterious LC strains can occur at all levels of mean IOP.
Summary
Laminar morphology is ever changing in health and disease, and recent studies have identified several promising morphological changes that are indicative of glaucoma susceptibility, onset and progression.
Keywords: glaucoma, optic nerve head, lamina cribrosa
Introduction
Glaucoma is an optic neuropathy resulting primarily from the damage to retinal ganglion cell (RGC) axons as they exit the eye at the optic nerve head (ONH). While there may be other primary or secondary factors in the retina and brain that contribute to RGC axonal damage and loss, the preponderance of evidence suggests that the laminar region of the ONH is a principal site of insult. The lamina cribrosa (LC) is a fenestrated, three-dimensional network of load-bearing trabeculae, many of which contain capillaries, that provides structural and nutrient support to the RGC axons as they leave the eye on their path to the brain. From a biomechanical perspective, the LC is a weak spot in an otherwise robust pressure vessel, the corneoscleral envelope. This arises from the fact that the LC is only approximately one-third the thickness of the sclera at the scleral canal, and its load-bearing connective tissue components only comprise about 40% of the tissue volume in the laminar region of the ONH. Thus, the LC is tasked with the conflicting tasks of providing structural support to the ONH by withstanding IOP-related mechanical strain, or local deformation, while also allowing the axons an open pathway to leave the eye. The three-dimensional LC trabecular structure also contains the vascular capillaries that nourish the axons and cells in the laminar region, so resisting high mechanical strains that may reduce vessel lumen size and blood flow is paramount as well (Figure 1).
Figure 1.
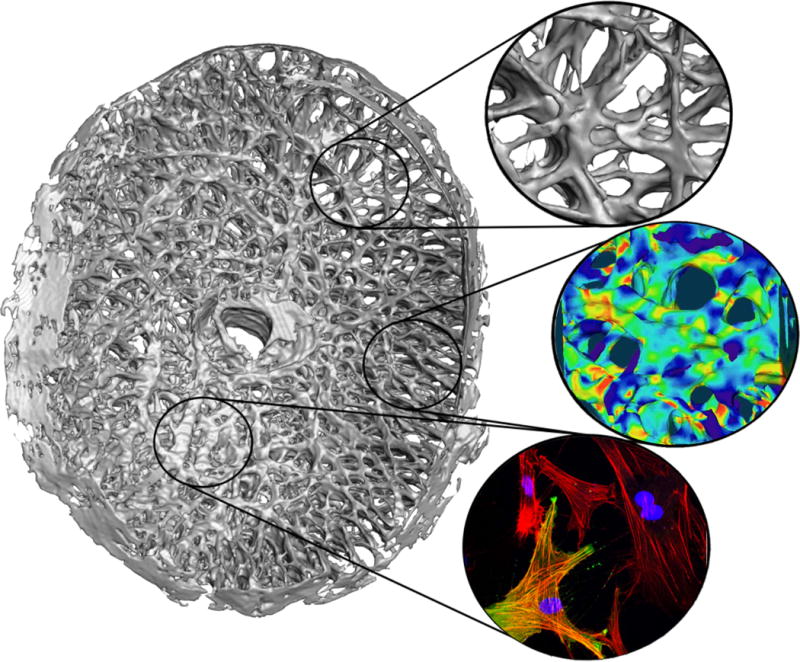
A high-resolution three-dimensional (3D) reconstruction of the lamina cribrosa from a human donor eye. The fluorescent histologic reconstruction was created using a custom, automated, microtome-based episcopic fluorescent image capture device that images the embedded ONH tissue block face after each thin section is cut away, thereby capturing the LC structure in an image volume at 1.5×1.5×1.5 micrometer voxel resolution. Regional differences in laminar density and beam orientation are evident. Callouts show the laminar microstructure (top), the typical mechanical strain in the laminar microstructure in response to an IOP elevation from 10 to 45 mmHg as estimated by a computational model (middle), and the F-actin (red) and αSMA (green) in activated fibroblastic cells that engender LC remodeling after cyclic strain is applied (bottom).
The LC as a Biomechanical Structure
The LC is protected somewhat from mechanical strain by the large circumpapillary ring of collagen and elastin fibers in the immediate peripapillary sclera, which serves as a reinforcement against excessive IOP-induced scleral canal expansion and the resulting LC stretch [1, 2]. It is within this paradigm that recent studies have confirmed that the net displacement of the LC in response to IOP elevation can be either inward or outward [3], depending largely on the initial position of the LC and the structural stiffness of the surrounding peripapillary sclera. However, while the overall inward or outward displacement of the LC may be minimal, the overall mechanical strain in the LC can be quite substantial, as even small scleral canal expansion will pull the LC radially and impart significant tensile strain [4]. The cells (astrocytes and LC cells) can sense strain through integrin receptors that tie their cytoskeletons directly to the adjacent fibrillar extracellular matrix (ECM) [5], and are therefore exquisitely sensitive to changes in the local biomechanical strain environment.
Laminar and ONH Remodeling in Health and Disease
ONH biomechanics change and adapt constantly with natural physiologic processes such as aging, wherein age-related glycation crosslinking of the fibrillar ECM increases the structural stiffness of both the sclera [2] and LC [6]. Also, recent studies suggest that racial differences in the magnitude and extent of age-related scleral stiffening in the posterior pole further complicate these relationships [7]. Finally, emerging evidence suggests that chronic exposure to elevated IOP may also spur remodeling-based stiffening of the sclera [8] and LC [9], presumably driven by cellular responses to elevated strain [10]. Connective tissue remodeling cascades in scleral fibroblasts and LC cells/astrocytes are preferentially activated in response to cyclic strain [11–14], analogous to IOP transients, which have been measured in both humans and animals. In summary, the ONH reacts to IOP and its transients as a structural system, and the resulting strains are wholly dependent on the structural characteristics of the individual eye. The structural stiffness of the ONH is always changing however, with age and IOP-related strain history, both of which are confounded with racial background and other ocular coat stiffness-related co-morbidities such as diabetes and myopia.
Pressures Effecting the ONH and LC
While intraocular pressure (IOP) is the primary driver of ocular biomechanics, the retrolaminar tissue pressure exerted by the cerebrospinal fluid pressure (CSFP) surrounding the optic nerve serves to counterbalance IOP at the LC to some degree [15]. Hence, the mechanical stress, or force distribution, in the LC is a combination of direct IOP-related forces acting on its inner surface, counteracted by CSFP-related retrolaminar tissue pressure acting on its outer surface, and the forces imparted by the sclera at the LC insertion. LC strains are therefore very complex, arising from the interactions of multiple forces, all mediated by the 3D geometry and local directional stiffness of the LC trabeculae themselves [16–18].
IOP Fluctuations
In terms of IOP, emerging evidence suggests that IOP is extremely variable [19, 20], resulting from transient increases due to blink, saccade, and ocular pulse amplitude (cyclical increases in IOP due to vascular filling). Studies of IOP “fluctuation” in glaucoma have focused solely on changes of mean IOP, whether analyzed over short time periods (hourly for 24 hours at most frequent), or longer periods such as the differences between IOPs measured during clinic visits months apart. These studies have yielded equivocal results, with some suggesting that mean IOP fluctuation is correlated with glaucoma progression [21, 22], and others not finding a relationship [23–25]. All of these previous studies have relied on snapshot measurements of mean IOP however, which totally ignore the transient fluctuations in IOP that occur on shorter timescales of milliseconds to minutes. While it might seem counterintuitive that these transient IOP fluctuations could cause damage since they occur constantly in all eyes, the cells in the ONH are much more tuned to react to these short-term transients than to slow changes in mean IOP. Given that the biomechanical strain in the LC is a complex derivative of eye-specific morphology and tissue stiffness, it is reasonable that transient IOP-related strains will be high in some eyes, even at normal levels of mean IOP. Hence, IOP transient reduction or damping may prove to be a new therapeutic pathway for glaucoma treatment.
Cellular Basis of Ocular Connective Tissue Remodeling
Studies of cells from the LC and sclera indicate that both larger strains and/or higher frequency strain cycling engender cellular changes that are consistent with ECM remodeling [14, 26, 27]. These same effects are seen in other collagenous soft tissues[28], and support the notion that IOP transients play an important role in the connective tissue remodeling signaling cascade. In addition, changes in ocular coat stiffness affect the magnitude of IOP transients[29], insofar as the eye is an elastic pressure vessel that can expand and contract to absorb some of the energy associated with transient events such as blink, saccade, and vascular pulse. Stiffer ocular coats can absorb less energy, so stiffer ocular coats engender larger IOP transients. IOP transients should therefore be larger in the stiffer eyes of the elderly [30], persons of African heritage [7], and those eyes that have been chronically exposed to elevated IOP [31, 32], and hence the ONH and LC in their eyes will be subjected to greater cyclical biomechanical insult. Larger IOP transients in these at-risk populations would occur at all IOP levels, and persist after IOP lowering in glaucoma patients, which may play a role in glaucoma risk. Clinical studies have not been done to link IOP transients with glaucoma pathogenesis or progression because continuous IOP measurement is not yet possible in humans. Ongoing studies in nonhuman primates (NHPs) instrumented with implantable wireless telemetry devices that measure IOP continuously will test these hypotheses.
Glaucomatous Remodeling of the ONH
Previous studies in both humans and animal models with an analogous collagenous LC microstructure have demonstrated that glaucoma, in stark contrast to other optic neuropathies [33, 34], generally involves progressive cupping and excavation of both the prelaminar neural tissues and the underlying LC [4, 35, 36]. Hence, even though individual eyes exhibit a wide range of susceptibility to IOP, biomechanics-driven LC remodeling is a central feature of the disease. Until recently, studies of this remodeling process were limited to postmortem histology in human eyes, as there were no methods available to assess progressive glaucomatous changes in the LC macro- or micro-architecture in vivo. Work in nonhuman primates (NHPs) has been the mainstay of this effort, wherein unilateral experimental glaucoma can be induced and then monitored frequently with surface topography imaging approaches until endpoint, with immediate postmortem histology [37]. In addition, glaucoma induced by elevated IOP in NHPs progresses much more quickly (3–18 months post IOP elevation) than human disease (5+ years), so longitudinal studies beginning with a normal eye and progressing through severe glaucoma can be performed in less time.
In Vivo Assessment of LC Remodeling in Glaucoma
A rapidly progressing inducible animal model of glaucoma with ONH and LC structures similar to humans has become increasingly important with the rapid advances in optical coherence tomography (OCT), adaptive optics, hyperspectral imaging, and other noninvasive approaches that translate to the clinic. Many of these technologies allow deep imaging of the LC structure that provide an anterior-to-posterior optical section through the ONH, which is not possible with more mature techniques such as optic disc photography or surface. Significant improvements in these new technologies come to market every year or two, which effectively limits longitudinal studies to timeframes much shorter than the 5–10 year follow up period typical in human studies of progressive glaucoma. Hence, NHP studies are critical to evaluate these evolving technologies in a timely manner to identify the most promising glaucoma biomarkers and speed clinical study and adoption.
OCT has matured somewhat and the instrument upgrade cycle is lengthening, so it is now being used in cross-sectional human glaucoma studies, and shorter duration longitudinal studies have reached endpoint and subsequent publication. These studies fall in several general categories: studies showing which ONH morphological features are associated with glaucoma and/or functional progression, studies showing that focal LC changes or defects are associated with focal glaucoma visual field loss, and studies showing which features of the LC microarchitecture are associated with glaucoma and which changes in LC microarchitecture one observes with glaucoma progression. Finally, there have been several recent studies that have evaluated the ONH mechanical response, essentially observations of ONH and/or LC biomechanical behavior, in response to acute or short-term IOP changes.
Glaucomatous Changes in LC Morphology
Recent OCT-based studies of ONH and morphology have generally focused on the changes in prelaminar neural tissue thickness and LC position, shape and thickness in glaucoma eyes versus normal controls, with a few exceptions. One of the more interesting studies involved asymmetric glaucoma patients in which one eye had more severe glaucoma with associated visual field defects, and the fellow eye showed no visual field loss[38]. Under the assumption that both ONHs within an individual patient begin as normal and ONH change in glaucoma is progressive, this study showed that prelaminar tissue is thinner and the LC is more posteriorly located in the more severely affected eye, and versus normal eyes in a healthy cohort as well [38]. This result matched several other studies showing that LC depth was more posterior in glaucoma eyes in general compared to normal eyes [39], and differentially more posterior in glaucoma eyes that presented with elevated IOP than those that presented with IOP in the normal range [40]. Not only was the average or maximum LC depth more posterior in glaucoma eyes, but the peripheral LC and LC insertion was more posterior in glaucoma eyes as well, and the magnitude of peripheral LC depth was associated with visual field loss [41]. This result indicates that the LC migrates posteriorly in glaucoma as is seen in NHPs [42], or that LCs that are more posterior in the scleral canal are predisposed to glaucoma. These studies are generally cross sectional in nature, and so don’t address the glaucoma related changes in the ONH or LC over time, but instead focus on the morphological features in glaucoma eyes versus controls. A recent longitudinal study in NHPs investigated the effect of age on LC morphology change in unilateral experimental glaucoma, and found that eyes from older animals exhibited less LC depth change than eyes from younger animals with similar levels of elevated IOP and axonal loss [9]; this study suggests that age and potentially other factors such as racial heritage that affect LC stiffness play a role in how the LC remodels in glaucoma. Characterization of progressive change in human LC morphology with glaucoma and how aging and racial heritage interact with those changes await additional longitudinal investigation.
There are several recent studies that investigated the change in ONH morphology after surgical IOP lowering or in response to acute IOP elevation. These studies assess the reversal of LC cupping, and/or assess the mechanical compliance of the ONH in vivo. In one recent longitudinal study of 34 Korean glaucoma patients, LC depth was assessed before surgical IOP reduction from a preoperative average of 24 mmHg to 11 mmHg, and 6 months and 2.5 years post-surgery. LC depth was reduced significantly at both follow up visits, which indicates that LC cupping was reversed after IOP lowering, and that reversal was largely sustained over time. The rate of postoperative RNFL thinning was significantly slower in those patients in whom IOP did not rise and LC depth retained its more anterior post-surgical position at the 2.5 year follow up visit [43]. Another longitudinal study showed that the rate of RNFL thinning was significantly associated with greater LC depth measured at presentation in a cohort of 110 Korean glaucoma patients [44]. Interestingly, these studies do not elucidate the morphological changes in the LC in progressive glaucoma, but do indicate that glaucomatous axon loss is associated with posterior LC position in the scleral canal at native IOP. Tun and colleagues studied the mechanical response of the ONH to acute IOP elevation of ~20 mmHg via ophthalmodynamometry, and found that LC depth was not significantly increased in normal, glaucoma, or ocular hypertensive eyes upon IOP elevation; the three-dimensional shape of the LC did change significantly however, which indicates that the LC’s mechanical response is very complex and full three-dimensional analysis may be necessary to fully capture ONH morphological change with IOP change and/or disease [45]. To this end, Girard and colleagues used an OCT volumetric image mapping approach [46] to calculate the mechanical strain relief in the visible portions of the prelaminar and laminar tissues following surgical IOP reduction. Not surprisingly, tissue strain relief after IOP-lowering was significant but very variable between patients, suggesting eye-specific responses, but was not associated with the magnitude of IOP lowering; the eyes with the highest strain relief, i.e., the largest local morphological change after IOP-lowering, exhibited the highest visual field loss [47]. This is supported by recent evidence in NHPs with induced experimental glaucoma has shown that the sclera and LC can become more compliant at the onset of ONH surface topography change [48], although no further analysis was done to associate increased compliance with axon or visual field loss in these animals.
Glaucomatous Changes in LC Microarchitecture
In vivo OCT imaging has also revealed that the LC is not a uniform fenestrated structure, but can exhibit focal defects including holes, pits, scleral disinsertions, and other features [49]. This is important from a structural and biomechanical standpoint, as local laminar strain is correlated to local laminar density [50]. Focal laminar defects have recently been associated with disk hemorrhages [51–53] and RNFL defects [54] in glaucoma patients, which suggests that focal damage or remodeling of the LC trabeculae may underlie many of the focal RNFL defects and resulting visual field scotomas in glaucoma. Global changes in the laminar microarchitecture have also been reported in recent NHP studies, including LC pore enlargement and increased laminar region volume and laminar connective tissue volume in glaucoma eyes compared to their fellow control eyes [55]. Hence, while focal defects are detectable clinically in human glaucoma, postmortem studies of NHP glaucoma eyes using high resolution 3D reconstructions of the LC reveal widespread remodeling of the laminar trabeculae as well.
Conclusions
It’s clear from recent studies that LC morphology and structural stiffness changes markedly in glaucoma beginning early in the disease process, and those changes are confounded with age and potentially racial heritage. Further, the IOP-related mechanical response of the LC is extremely complex, and is dependent on eye-specific LC morphology and stiffness [50], scleral stiffness [56], and the level of cerebrospinal fluid pressure [57]. Further studies are needed to fully elucidate the best biomarkers of laminar biomechanics, morphological change, blood flow, and cellular activity and their relationship to glaucoma pathogenesis and progression.
Key Points.
The lamina cribrosa is a complex three-dimensional structure that is tasked with the conflicting functions of providing structural support to the ONH by withstanding IOP-related mechanical strain, while also providing an open pathway for the retinal ganglion cell axons to leave the eye.
The lamina cribrosa is subjected to a constant barrage of IOP and cerebrospinal fluid pressure transients, which drive cyclical strain in the ONH through a complex interplay between laminar and scleral morphology and structural stiffness.
The lamina cribrosa remodels in response to both physiologic and pathophysiologic stimuli, which can manifest as both focal laminar defects and more generalized alterations in laminar beam thickness and pore morphology.
Changes in laminar morphology and focal laminar defects have been definitely linked to focal retinal nerve fiber layer defects and visual field loss, but there is less spatial correspondence with disk hemorrhages.
Clinical imaging technology can be used to assess laminar morphology at the macro and micro-scales, and detection of both focal and global changes laminar structure show great promise as eye-specific biomarkers for glaucoma susceptibility, onset and progression.
Acknowledgments
N/A
Financial support and sponsorship
This work was supported by the Department of Ophthalmology, UAB School of Medicine, Birmingham, Alabama, USA
Footnotes
Conflicts of interest
The authors have no relevant conflicts of interest
BIBLIOGRAPHY AND REFERENCES CITED
- 1.Sigal IA, et al. IOP-induced lamina cribrosa deformation and scleral canal expansion: independent or related? Invest Ophthalmol Vis Sci. 2011;52(12):9023–32. doi: 10.1167/iovs.11-8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grytz R, et al. Age- and race-related differences in human scleral material properties. Invest Ophthalmol Vis Sci. 2014;55(12):8163–72. doi: 10.1167/iovs.14-14029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agoumi Y, et al. Laminar and prelaminar tissue displacement during intraocular pressure elevation in glaucoma patients and healthy controls. Ophthalmology. 2011;118(1):52–9. doi: 10.1016/j.ophtha.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Downs JC. Optic nerve head biomechanics in aging and disease. Exp Eye Res. 2015;133:19–29. doi: 10.1016/j.exer.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrison JC. Integrins in the optic nerve head: potential roles in glaucomatous optic neuropathy (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:453–77. [PMC free article] [PubMed] [Google Scholar]
- 6.Albon J, et al. Age related compliance of the lamina cribrosa in human eyes. Br J Ophthalmol. 2000;84(3):318–23. doi: 10.1136/bjo.84.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fazio MA, et al. Human scleral structural stiffness increases more rapidly with age in donors of African descent compared to donors of European descent. Invest Ophthalmol Vis Sci. 2014;55(11):7189–98. doi: 10.1167/iovs.14-14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girard MJ, et al. Scleral biomechanics in the aging monkey eye. Invest Ophthalmol Vis Sci. 2009;50(11):5226–37. doi: 10.1167/iovs.08-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang H, et al. Age-related differences in longitudinal structural change by spectral-domain optical coherence tomography in early experimental glaucoma. Invest Ophthalmol Vis Sci. 2014;55(10):6409–20. doi: 10.1167/iovs.14-14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison JC, et al. Optic nerve head extracellular matrix in primary optic atrophy and experimental glaucoma. Arch Ophthalmol. 1990;108(7):1020–4. doi: 10.1001/archopht.1990.01070090122053. [DOI] [PubMed] [Google Scholar]
- 11.Kirwan RP, et al. Effect of cyclical mechanical stretch and exogenous transforming growth factor-beta1 on matrix metalloproteinase-2 activity in lamina cribrosa cells from the human optic nerve head. J Glaucoma. 2004;13(4):327–34. doi: 10.1097/00061198-200408000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Wallace DM, et al. The role of matricellular proteins in glaucoma. Matrix Biol. 2014;37:174–82. doi: 10.1016/j.matbio.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Murphy-Ullrich JE, Downs JC. The Thrombospondin1-TGF-beta Pathway and Glaucoma. J Ocul Pharmacol Ther. 2015;31(7):371–5. doi: 10.1089/jop.2015.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Qu J, et al. High-Magnitude and/or High-Frequency Mechanical Strain Promotes Peripapillary Scleral Myofibroblast Differentiation. Invest Ophthalmol Vis Sci. 2015;56(13):7821–30. doi: 10.1167/iovs.15-17848. This paper was the first to show that both increased strain levels AND increased frequency of strain fluctuations cause myofibroblast differentiation and increased cytoskeletal changes in scleral fibroblasts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Jonas JB, Ritch R, Panda-Jonas S. Cerebrospinal fluid pressure in the pathogenesis of glaucoma. Prog Brain Res. 2015;221:33–47. doi: 10.1016/bs.pbr.2015.06.002. This is an excellent review of the latest evidence that CSFP and the translaminar pressure gradient play a role in glaucoma. [DOI] [PubMed] [Google Scholar]
- 16.Downs JC, et al. Multiscale finite element modeling of the lamina cribrosa microarchitecture in the eye. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:4277–80. doi: 10.1109/IEMBS.2009.5332755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sigal IA, et al. Eye-specific IOP-induced displacements and deformations of human lamina cribrosa. Invest Ophthalmol Vis Sci. 2014;55(1):1–15. doi: 10.1167/iovs.13-12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18**.Wang B, et al. Decreased Lamina Cribrosa Beam Thickness and Pore Diameter Relative to Distance From the Central Retinal Vessel Trunk. Invest Ophthalmol Vis Sci. 2016;57(7):3088–92. doi: 10.1167/iovs.15-19010. This study demonstrated that LC microstructure can be quantified using AO-OCT, and that glaucoma eyes exhibit a larger decrease in LC beam thickness and pore size than healthy controls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coleman DJ, Trokel S. Direct-recorded intraocular pressure variations in a human subject. Arch Ophthalmol. 1969;82(5):637–40. doi: 10.1001/archopht.1969.00990020633011. [DOI] [PubMed] [Google Scholar]
- 20.Downs JC. IOP telemetry in the nonhuman primate. Exp Eye Res. 2015;141:91–8. doi: 10.1016/j.exer.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caprioli J, Coleman AL. Intraocular pressure fluctuation a risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology. 2008;115(7):1123–1129 e3. doi: 10.1016/j.ophtha.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 22.Nouri-Mahdavi K, et al. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology. 2004;111(9):1627–35. doi: 10.1016/j.ophtha.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Bengtsson B, Heijl A. Diurnal IOP fluctuation: not an independent risk factor for glaucomatous visual field loss in high-risk ocular hypertension. Graefes Arch Clin Exp Ophthalmol. 2005;243(6):513–8. doi: 10.1007/s00417-004-1103-8. [DOI] [PubMed] [Google Scholar]
- 24.Bengtsson B, et al. Fluctuation of intraocular pressure and glaucoma progression in the early manifest glaucoma trial. Ophthalmology. 2007;114(2):205–9. doi: 10.1016/j.ophtha.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 25.Medeiros FA, et al. Long-term intraocular pressure fluctuations and risk of conversion from ocular hypertension to glaucoma. Ophthalmology. 2008;115(6):934–40. doi: 10.1016/j.ophtha.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider M, Fuchshofer R. The role of astrocytes in optic nerve head fibrosis in glaucoma. Exp Eye Res. 2016;142:49–55. doi: 10.1016/j.exer.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Wallace DM, O’Brien CJ. The role of lamina cribrosa cells in optic nerve head fibrosis in glaucoma. Exp Eye Res. 2016;142:102–9. doi: 10.1016/j.exer.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 28**.Suki B, et al. Regulatory Roles of Fluctuation-Driven Mechanotransduction in Cell Function. Physiology (Bethesda) 2016;31(5):346–58. doi: 10.1152/physiol.00051.2015. This study provides a framework for understanding how strian fluctuations drive cellular activity and connective tissue remodeling cascades in both healthy cells and disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris HJ, et al. Correlation between biomechanical responses of posterior sclera and IOP elevations during micro intraocular volume change. Invest Ophthalmol Vis Sci. 2013;54(12):7215–22. doi: 10.1167/iovs.13-12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fazio MA, et al. Age-related changes in human peripapillary scleral strain. Biomech Model Mechanobiol. 2014;13(3):551–63. doi: 10.1007/s10237-013-0517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girard MJ, et al. Biomechanical changes in the sclera of monkey eyes exposed to chronic IOP elevations. Invest Ophthalmol Vis Sci. 2011;52(8):5656–69. doi: 10.1167/iovs.10-6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coudrillier B, et al. Biomechanics of the human posterior sclera: age- and glaucoma-related changes measured using inflation testing. Invest Ophthalmol Vis Sci. 2012;53(4):1714–28. doi: 10.1167/iovs.11-8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Fard MA, et al. Optic Nerve Head Morphology in Nonarteritic Anterior Ischemic Optic Neuropathy Compared to Open-Angle Glaucoma. Invest Ophthalmol Vis Sci. 2016;57(11):4632–40. doi: 10.1167/iovs.16-19442. This study showed the difference between glaucomatous optic neuropathy and the related LC cupping, as opposed to other optic neuropathies such as NAION. [DOI] [PubMed] [Google Scholar]
- 34.Lee EJ, et al. Comparison of the Deep Optic Nerve Structures in Superior Segmental Optic Nerve Hypoplasia and Primary Open-Angle Glaucoma. J Glaucoma. 2016;25(8):648–56. doi: 10.1097/IJG.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 35.Burgoyne CF, et al. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005;24(1):39–73. doi: 10.1016/j.preteyeres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Downs JC, Roberts MD, Sigal IA. Glaucomatous cupping of the lamina cribrosa: a review of the evidence for active progressive remodeling as a mechanism. Exp Eye Res. 2011;93(2):133–40. doi: 10.1016/j.exer.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burgoyne CF. The non-human primate experimental glaucoma model. Exp Eye Res. 2015;141:57–73. doi: 10.1016/j.exer.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38**.Kim DW, et al. Prelamina and Lamina Cribrosa in Glaucoma Patients With Unilateral Visual Field Loss. Invest Ophthalmol Vis Sci. 2016;57(4):1662–70. doi: 10.1167/iovs.15-18453. This study showed that the LC is more posterior in the scleral canal in the damaged glaacoma eye as compared to the preperimetric fellow eye in patients with assymmetric glaucoma. [DOI] [PubMed] [Google Scholar]
- 39*.Kim YW, et al. Clinical Assessment of Lamina Cribrosa Curvature in Eyes with Primary Open-Angle Glaucoma. PLoS One. 2016;11(3):e0150260. doi: 10.1371/journal.pone.0150260. This paper showed that the LC is more curved in glaucoma eye compared to normal controls, which demostrated the structural underpinnings of glaucomatous LC cupping. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40**.Li L, et al. Posterior displacement of the lamina cribrosa in normal-tension and high-tension glaucoma. Acta Ophthalmol. 2016;94(6):e492–500. doi: 10.1111/aos.13012. This study showed that the LC is more posteriorly displaced in high tension as compared to low tension glaucoma eyes, which suggests a link between IOP-related mechanical strain and LC remodeling in glaucoma. [DOI] [PubMed] [Google Scholar]
- 41**.Kim YW, et al. Peripheral lamina cribrosa depth in primary open-angle glaucoma: a swept-source optical coherence tomography study of lamina cribrosa. Eye (Lond) 2015;29(10):1368–74. doi: 10.1038/eye.2015.162. This paper showed that not only the central LC remodels posteriorly in glaucoma, but that the peripheral LC and its insertion into the scleral canal wall migrates posteriorly as well; this is important confirmation of a previous study showing this behavior in NHPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang H, et al. Posterior (outward) migration of the lamina cribrosa and early cupping in monkey experimental glaucoma. Invest Ophthalmol Vis Sci. 2011;52(10):7109–21. doi: 10.1167/iovs.11-7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Lee EJ, Kim TW. Lamina Cribrosa Reversal after Trabeculectomy and the Rate of Progressive Retinal Nerve Fiber Layer Thinning. Ophthalmology. 2015;122(11):2234–42. doi: 10.1016/j.ophtha.2015.07.020. This paper showed that the LC moves anteriorly in glaucoma eyes after surgical IOP lowering, and that rates of progressive RNFL thinning are lower in eyes that maintain that LC anteriorization over time. [DOI] [PubMed] [Google Scholar]
- 44*.Lee EJ, et al. Influence of lamina cribrosa thickness and depth on the rate of progressive retinal nerve fiber layer thinning. Ophthalmology. 2015;122(4):721–9. doi: 10.1016/j.ophtha.2014.10.007. This study showed that greater LC depth is associated with faster rates of RNFL thinning in gluacoma eyes. [DOI] [PubMed] [Google Scholar]
- 45*.Tun TA, et al. Shape Changes of the Anterior Lamina Cribrosa in Normal, Ocular Hypertensive, and Glaucomatous Eyes Following Acute Intraocular Pressure Elevation. Invest Ophthalmol Vis Sci. 2016;57(11):4869–4877. doi: 10.1167/iovs.16-19753. This study showed that LC deformation following acute IOP elevation is subtle, three-dimensional, and manifests as changes in LC surface shape rather than position. [DOI] [PubMed] [Google Scholar]
- 46.Girard MJ, et al. In vivo optic nerve head biomechanics: performance testing of a three-dimensional tracking algorithm. J R Soc Interface. 2013;10(87):20130459. doi: 10.1098/rsif.2013.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47**.Girard MJ, et al. In Vivo 3-Dimensional Strain Mapping of the Optic Nerve Head Following Intraocular Pressure Lowering by Trabeculectomy. Ophthalmology. 2016;123(6):1190–200. doi: 10.1016/j.ophtha.2016.02.008. This study was the first to demonstrate that eye-specific changes in IOP-related ONH mechanical strain can be measured and calculated using in vivo OCT volume scans of the ONH; this is an important advance, as strain could prove to be an important biomarker for glaucoma susceptibility. [DOI] [PubMed] [Google Scholar]
- 48**.Ivers KM, et al. In Vivo Detection of Laminar and Peripapillary Scleral Hypercompliance in Early Monkey Experimental Glaucoma. Invest Ophthalmol Vis Sci. 2016;57(9):Oct388–403. doi: 10.1167/iovs.15-18666. This is the first study to show that the acute, IOP-related biomechanical response of the LC and sclera change early in the glaucoma, which could prove to be a clinical biomarker for glaucoma onset in patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park SC, et al. Factors associated with focal lamina cribrosa defects in glaucoma. Invest Ophthalmol Vis Sci. 2013;54(13):8401–7. doi: 10.1167/iovs.13-13014. [DOI] [PubMed] [Google Scholar]
- 50.Roberts MD, et al. Correlation between local stress and strain and lamina cribrosa connective tissue volume fraction in normal monkey eyes. Invest Ophthalmol Vis Sci. 2010;51(1):295–307. doi: 10.1167/iovs.09-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee EJ, et al. Recent structural alteration of the peripheral lamina cribrosa near the location of disc hemorrhage in glaucoma. Invest Ophthalmol Vis Sci. 2014;55(4):2805–15. doi: 10.1167/iovs.13-12742. [DOI] [PubMed] [Google Scholar]
- 52*.Kim YK, Park KH. Lamina cribrosa defects in eyes with glaucomatous disc haemorrhage. Acta Ophthalmol. 2016;94(6):e468–73. doi: 10.1111/aos.12903. This sudy showed that focal LC defects were much more common in eyes with disk hemorrhages and the LC defects were highly spatially correlated with RNFL defects but less so with disk hemorrhages. [DOI] [PubMed] [Google Scholar]
- 53*.Sharpe GP, et al. Optic Disc Hemorrhages and Laminar Disinsertions in Glaucoma. Ophthalmology. 2016;123(9):1949–56. doi: 10.1016/j.ophtha.2016.06.001. This sudy showed that focal LC disinsertions were much more common in eyes with disk hemorrhages but those disinsertions were poorly spatially correlated with disk hemorrhages. [DOI] [PubMed] [Google Scholar]
- 54.Kim YK, Jeoung JW, Park KH. Effect of Focal Lamina Cribrosa Defect on Disc Hemorrhage Area in Glaucoma. Invest Ophthalmol Vis Sci. 2016;57(3):899–907. doi: 10.1167/iovs.15-18389. [DOI] [PubMed] [Google Scholar]
- 55*.Reynaud J, et al. Lamina Cribrosa Microarchitecture in Monkey Early Experimental Glaucoma: Global Change. Invest Ophthalmol Vis Sci. 2016;57(7):3451–69. doi: 10.1167/iovs.16-19474. This study showed that widespread remodeling of the LC occurs early in glaucoma, which consists of LC beam thickening, and LC connective tissue volume increase; this is important as it shows that many details of the remodeling process are likely to be invisible to current imaging modalities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56**.Coudrillier B, et al. Effects of Peripapillary Scleral Stiffening on the Deformation of the Lamina Cribrosa. Invest Ophthalmol Vis Sci. 2016;57(6):2666–77. doi: 10.1167/iovs.15-18193. This study showed that stiffening the peripapillary sclera can reduce strain in the LC, which is important as it is the forst study to demonstrate that scleral stiffness madulation can potentially be used as a therapeutic method to change LC strains. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57*.Feola AJ, et al. Finite Element Modeling of Factors Influencing Optic Nerve Head Deformation Due to Intracranial Pressure. Invest Ophthalmol Vis Sci. 2016;57(4):1901–11. doi: 10.1167/iovs.15-17573. This computational modeling study suggests that strains in the LC can be altered by changes in CSFP. [DOI] [PubMed] [Google Scholar]


