Abstract
Caproic acid is an emerging platform chemical with diverse applications. Recently, a novel biorefinery process, that is, chain elongation, was developed to convert mixed organic waste and ethanol into renewable caproic acids. In the coming years, this process may become commercialized, and continuing to improve on the basis of numerous ongoing technological and microbiological studies. This study aims to analyze the environmental performance of caproic acid production from mixed organic waste via chain elongation at this current, early stage of technological development. To this end, a life cycle assessment (LCA) was performed to evaluate the environmental impact of producing 1 kg caproic acid from organic waste via chain elongation, in both a lab-scale and a pilot-scale system. Two mixed organic waste were used as substrates: the organic fraction of municipal solid waste (OFMSW) and supermarket food waste (SFW). Ethanol use was found to be the dominant cause of environmental impact over the life cycle. Extraction solvent recovery was found to be a crucial uncertainty that may have a substantial influence on the life-cycle impacts. We recommend that future research and industrial producers focus on the reduction of ethanol use in chain elongation and improve the recovery efficiency of the extraction solvent.
1. Introduction
The amount of organic waste produced by society is increasing, alongside a growing demand for fuels and chemicals. Currently, fuels and chemicals are mainly produced from fossil resources, and production of such from food crops such as corn, sugar cane, and palm are expanding.1 The vast consumption of fossil resources contributes greatly to global warming and air pollution. Increasing use of food crops for fuel and chemical production may, on the other hand, compete with human food production.2 An alternative and more sustainable feedstock is needed to support our fuel and chemical consumption.
Mixed organic waste is a promising feedstock for fuel and chemical production.1,3,4 It refers to an organic waste stream with a highly heterogeneous composition, for example, food supply chain waste, industrial process food waste, and organic fraction of municipal solid waste (OFMSW).1,5 Mixed organic waste is generated in large quantities worldwide. It usually contains large amounts of readily biodegradable organic matter and various nutrients that are essential for biotechnological applications. The challenge, however, is to produce high value end-products from mixed organic waste to make the process more economically attractive than the current practices, for example, anaerobic digestion producing biogas and composting into soil amendment.6,7 There is a growing interest in producing bulk chemicals from mixed organic waste. It is argued that using organic waste as substrate for bulk chemicals yields higher value products than using it for heat, electricity and fuel.3,4,8 Bulk chemical production from organic waste is even more attractive when targeting an emerging platform chemical with a relatively small and specific niche market. This is because market potential is already guaranteed, and the economic competition from an established chemical process for the market is less threatening.6 Although the relatively small market potential, to certain extent, may limit short-term application of these waste-to-chemical bioprocesses, the increased availability of other applications9 may continue the impact of chain elongation.
Caproic acid is an emerging platform chemical that can be produced from low-grade mixed organic waste, as recently demonstrated in both lab-10−14 and pilot-scale systems, at high rates and specificities.15 Here we refer to caproic acid in its undissociated form, and to caproate in its dissasociated form. Caproic acid has a wide range of applications. It can be used directly as feed additives,16 antimicrobials17 and plant growth promoters.18 It can also be used as a precursor to various commodities including lubricants, fragrances, paint additives and pharmaceuticals.8,15,19 Currently, caproic acid is produced from food crops like palm and coconut, with oils containing less than one percent of caproic acid. Although the caproic acid produced from food crops is commercially available, the low caproic acid content in these crop oils leads to a high price and a limited market. Recently, an industrially applicable caproic acid production process using mixed organic waste as a feedstock was developed and implemented, based on a microbial fermentation process, that is, chain elongation via reversed β-oxidation pathway.20 In chain elongation, short-chain fatty acids (SCFAs; saturated fatty acids containing less than six carbons) and ethanol are converted by microorganisms into medium-chain fatty acids (MCFAs; saturated fatty acids containing six to 12 carbons). It was found that chain elongation can be performed under a nonsterile condition and in a continuous production mode.10,14 Moreover, the use of SCFAs, like acetate and butyrate, in chain elongation yields caproate as the most dominant end-product with a high production rate and specificity.21,22 Both acetate and butyrate are the main intermediates from anaerobic degradation of mixed organic waste like OFMSW. Ethanol addition during the anaerobic degradation of OFMSW has been shown to stimulate chain elongation of these SCFAs and the added ethanol to caproate as the main end-product.12,13 The highest caproate production rate via this process was 26 g/L/day with a concentration up to 12.6 g/L, which approximates the solubility of caproic acid in water and is advantageous to the downstream processes.13 A caproic acid production process using mixed organic waste and ethanol was thus developed. Four factors, namely the high caproate concentration, the high caproate production rate, the use of a mixed organic waste and the possibility to operate under a nonsterile condition make this caproic acid production process attractive and industrially applicable. Thus, a spin-off company from Wageningen University, ChainCraft B.V. (Amsterdam), has developed this proven technology into a pilot-scale system that continuously converts food processing waste and ethanol into economically viable caproic acid.
Continuous caproate production via chain elongation was demonstrated for the first time in 2011.14 Since then, several studies were completed to promote MCFA production from low-grade waste via chain elongation. Most of these studies addressed the substrate range,12,13,23−28 the bioprocessing,10,11,21,22,29 the microbiology23,30 and the downstream processes.31−33 Recent review articles on chain elongation also focus mostly on these aspects.15,34 Surprisingly, the environmental performance of this “sustainable” bioprocess has not been addressed in any of these studies. Analyzing the environmental sustainability of an emerging technology during its early stages is beneficial not only for orienting the future development toward an improved environmental performance, but also for supporting decision-making during the implementation, process design and commercialization stages.35 Life Cycle Assessment (LCA) has been widely applied to assessing the fuel and chemical production from biomass;36−38 the importance of applying LCA for improving the emerging biobased production processes was also addressed in literature.1
This study, therefore, aims to quantify the life-cycle environmental impacts of caproic acid production from mixed organic waste via chain elongation, and use the outcome to propose key factors for improving the environmental sustainability of this process. To this end, an early stage, gate-to-gate and attributional LCA was performed to quantify the environmental impact associated with the caproic acid production based on the existing chain elongation business case, that is, caproic acid production from mixed organic waste and ethanol. The result may help to identify environmental impact “hot-spots” within the life cycle of caproic acid production from organic waste or provide a benchmark for comparison with other existing processes.35 The potential outreach of the LCA outcome may provide environmental sustainability as an additional perspective for orienting future research on chain elongation as well as providing a basis for strategic improvement advices, which could prove vital to industrial producers of caproic acid.
2. Materials and Methods
2.1. Life Cycle Assessment (LCA)
The goal of this LCA is to quantify the environmental impact associated with caproic acid production from mixed organic waste via chain elongation. An attributional LCA was selected based on existing guidances.39,40 The functional unit (f.u.) is 1 kg of caproic acid (Purity >99%) produced from mixed organic waste and ethanol via chain elongation. A gate-to-gate life cycle of the caproic acid production is assessed. The gate-to-gate life cycle starts from the organic waste arriving at the caproic acid production site and ends with the product leaving the caproic acid production site. Environmental impacts associated with all waste treatments during the defined gate-to-gate life cycle are included. The emissions and environmental impacts associated with the generation of mixed organic waste, which is used as feedstock, are not considered. The reason for such exclusion is further elaborated in the Supporting Information (SI).
Data for the life cycle inventory (LCI) were collected from, in order of preference, internal data that were published,13,41 existing literature,19,20 internal data that were not published and personal communications with industrial producers and experts (see SI Table S1). The information required to carry out the life cycle impact assessment (LCIA) was sourced from literature and the Ecoinvent 3.1 database.42 The characterization method used was CML-IA baseline V3.02/EU25. Global warming potential (GWP; CO2-equivalent/f.u.), Eutrophication potential (EP; PO43–-equivalent/f.u.), and acidification potential (AP; SO2-equivalent/f.u.) were the selected impact categories based on the existing guidance43 and data availability. An overview of the data used in the LCIA is available in SI Table S2.
2.2. Production System and Cases
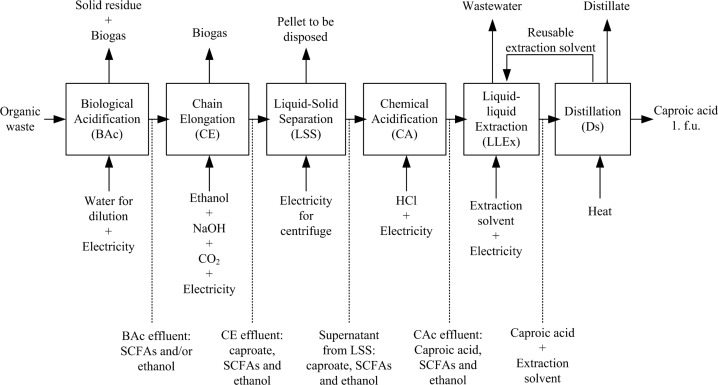
The assessed system consists of six main processes (Figure 1), which starts with the mixed organic waste and ends at the production of caproic acid. Two types of mixed organic waste were used for caproic acid production via chain elongation,15 including the organic fraction of municipal solid waste (OFMSW)12,33,41 and supermarket food waste (SFW) from the food residue processing industry (unpublished data). The SFW has been applied in a pilot-scale system (ChainCraft B.V., Amsterdam); the OFMSW was only applied in lab-scale bioreactor systems.15 In this study, OFMSW and SFW are selected because we aim to use a low-grade, mixed and geographically widespread waste stream as the feedstock. Moreover, a large quantity of internal data using OFMSW and SFW for caproic acid production via chain elongation is available in our institute. The use of yeast-fermentation beer for producing caproic acid via chain elongation was demonstrated10 but not included in this study, mainly because this raw, undistilled ethanol with a high ethanol titer (up to 152 g ethanol/L; ∼15%)10 is not produced from a mixed organic waste stream. Based on the process data of OFMSW and SFW we possess, three cases were developed (see SI Table S3 for more details). They are the lab-scale system using OFMSW (Case LO), the lab-scale system using SFW (Case LS) and the pilot-scale system using SFW (Case PS). Part of the Case PS was simulated using the data from Case LS considering the available data from the pilot plant. A detailed description for the entire life-cycle for producing caproic acid via chain elongation is available in the SI.
Figure 1.
Gate-to-product life cycle of caproic acid production assessed in this study. The life cycle starts at a mixed organic waste arriving at the caproic acid production site and ends at the caproic acid produced at the production site.
2.3. Sensitivity Analysis and Comparison to Other Studies
A sensitivity analysis was performed to evaluate the sensitivity of the calculated life-cycle environmental impacts of the alternative materials/data and several proposed improvement strategies (see SI Table S4 for details). The three study cases, that is, LO, LS, and PS, were used as baselines for the sensitivity analysis. In addition, the results of the LCA are compared to other studies published in the literature that address the life-cycle impact of treating organic waste with mixed culture biotechnologies.
3. Results and Discussion
3.1. The Life-Cycle Impacts
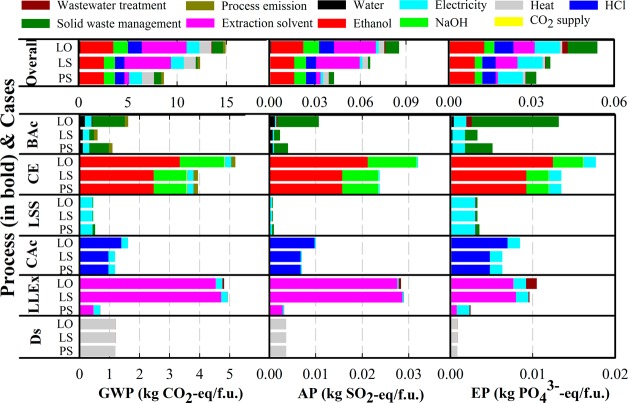
Figure 2 shows the sum as well as the breakdown of the overall life-cycle impacts of caproic acid production from mixed organic waste via chain elongation. The ethanol use in chain elongation (CE) is the dominant cause of environmental impact throughout all cases and impact categories assessed. The use of NaOH and HCl for neutralizing pH also contributed considerably to all three impact categories throughout the three cases. In Case LO (OFMSW in lab-scale system) and LS (SFW in lab-scale system), the extraction solvent used in the liquid–liquid extraction (LLEx) process was the dominant environmental impact source. In Case LO, the solid waste management had a considerably higher contribution to the life-cycle impact than Case LS and PS (SFW in pilot-scale system) due to the type of organic waste and biological acidification (BAc) used.
Figure 2.
Gate-to-product life cycle impact of 1 kg of caproic acid produced through chain elongation. Results are shown for global warning potential (GWP), acidification potential (AP), and eutrophication potential (EP) for each process used (in bold; see Figure 1) in each case (Case LO, LS, and PS; see section 2.2 and SI Table S3).
A large quantity of ethanol (1.8 kg/f.u. in Case LO and 1.5 kg/f.u. in Case LS and PS) was added during CE as an essential substrate for a high-rate caproic acid production from organic waste via chain elongation. It is both a carbon source and an electron donor, which provides energy for the chain-elongating microorganisms.15 The environmental impacts of the added ethanol account for at least 20% of the total life-cycle impacts for all impact categories in all cases. More than half of the impacts of the added ethanol originate from the feedstock production, that is, production of corn grains.44 The use of 1 kg of corn-based bioethanol gives 1.6 kg CO2-eq GWP, of which 0.9 kg CO2-eq GWP (56%) resulted from the production and transportation of the corn grain. For AP and EP, 75% and 78% of the overall impact of 1 kg of corn-based bioethanol are associated with the production of corn grains, respectively. The high AP and EP are related to nitrogen and phosphorus emissions from the soil due to fertilizers application during cultivation.45
There are three potential strategies for reducing the environmental impacts of ethanol addition. As the ethanol production system is beyond the system boundary of the present study, we therefore only discuss improvement strategies that can be implemented within our system boundaries. The first strategy is to stimulate the in situ ethanol formation within the organic waste during BAc. The ethanol consumption (1.8 kg/f.u.) and the ethanol concentration (19.3 g/L) required for caproic acid production in all cases were assumed to be the same. However, the amount of ethanol addition in Cases LS and PS is lower than that in Case LO, because there is in situ ethanol formation (up to 5.3 g/L; around 0.5 kg/f.u.) during the BAc of SFW. In contrast, during the BAc of OFMSW, there is hardly any in situ ethanol production.13 The higher ethanol addition in CE in Case LO consequently increased the life-cycle impacts. Substrate composition,46 pH47 and headspace hydrogen partial pressure46 are parameters that can affect the in situ ethanol production during BAc.48,49 In SFW, there were likely more carbohydrates that are easily fermented into ethanol compared with OFMSW.50 During the BAc of OFMSW and SFW, the pH was similar (between 5 and 5.5), and the hydrogen partial pressure in the headspace were not reported. Potential strategies that can stimulate in situ ethanol production during BAc may be further investigated.
The second strategy for reducing the ethanol addition is to improve the accuracy of the ethanol dose. Based on the currently known stoichiometry of the microbial chain elongation reaction,10,20 2.4 mol of ethanol and 1 mol of acetate is used to produce 1 mol of caproate. This means that the production of 1 f.u. requires 0.9 kg of ethanol (pure) in the most ideal condition, which is half of the totally available ethanol (i.e., the sum of the added ethanol and in situ ethanol formation) in all study cases. Thus, about half of the ethanol supply in CE in this study does not end in the final product. The ethanol that is not used for chain elongation to caproic acid either remains in the CE effluent, which is wasted, or it is consumed by other reactions during CE, for example, excessive ethanol oxidation to acetate and hydrogen that is not associated with chain elongation. This means that there is still room for reducing the ethanol addition via precisely controlling the ethanol dose during CE. However, the decreased ethanol dose may change the ethanol-acetate molar ratio in the medium, which was reported to be an influential parameter for caproate production in chain elongation.51 In Grootscholten et al. (2014), 420 mM ethanol and 60 mM acetate (ratio 7:1) was used in the medium,13 while in Liu et al. (2016) the highest used ratio was 3:1 (112.5 mM ethanol and 37.5 mM acetate).51 The caproate concentration achieved in the former was much higher than that for the latter (12.6 g/L versus 3.3 g/L). The effect of reducing ethanol dose, therefore, should be carefully assessed.
The third strategy is to employ a substitute for ethanol in CE. Hydrogen,14 renewable electricity,52 methanol,9,53 lactate25,26 and ethanol from more renewable sources, for example, syngas fermentation broth24 have been used to substitute the current ethanol use in CE,24 though they are still in an early stage of development. Moreover, though not included in this study, undistilled ethanol with a high ethanol titers may be used as an alternative ethanol source for upgrading mixed organic waste into caproic acid,10 provided that such stream is available on-site.
The use of NaOH in CE and HCl in CAc for adjusting pH contributed considerably to all three impact categories throughout the three cases. This is mainly due to the electricity used during the NaOH and HCl production process, i.e. the electrolysis of brine or so-called chloralkali process. The use of NaOH during the anaerobic fermentation of organic waste for propionic acid production was reported to contribute 11% of the life-cycle GWP,54 which is similar to the result of this study. The use of this base cannot be omitted if a high-rate caproic acid production is targeted, as the pH drop induced by caproic acid accumulation inhibits the microbial activities. Continuous removal of caproic acid from the fermentation broth inside the CE bioreactor, for example, via an in-line liquid–liquid membrane extraction, may help reduce the use of NaOH.10 A combination of the in-line extraction system and a membrane electrolysis for the caproic acid recovery can even further avoid the external supply of HCl in the chemical acidification (CAc) process, as the protons required to extract the caproic acid are produced in the electrochemical system.55 However, such a system would require several membranes, which are manufactured via energy-intensive processes56 and have to be replaced regularly. The trade-off between the additional impacts due to the use of membranes and the impact reduction due to the avoided use of NaOH and HCl should be carefully evaluated.
Case PS has a much lower life-cycle impact compared with Case LO and LS, mainly due to the recovery of a large portion of the extraction solvent (99% in Case PS versus. 90% in Case LO and LS). In the lab-scale system, the extraction solvent loss during LLEx and distillation (Ds) processes was assumed to be 5% of the total added solvent, respectively. A 90% solvent recovery was, therefore, assumed. This estimation was made to give the worst-case scenario. Based on this assumption, the extraction solvent consumption was the largest contribution to the life-cycle impacts in Cases LO and LS. This was due to both the high demand for solvent replenishment (1.7 kg/f.u.) and the high life-cycle impacts of the extraction solvent.42 According to ChainCraft B.V., a solvent recovery efficiency up to 99% is feasible in the pilot-scale system. A similar solvent recovery efficiency, that is, 98.5%, was also assumed to be feasible in a previous study using a similar extraction system for recovering propionic acid from the fermentation broth.54 If the solvent recovery efficiency can be up to 99%, the life-cycle impacts as well as the impact generated by the solvent consumption can be reduced significantly, as shown in the impact of LLEx in Case PS (Figure 2). However, the actual environmental impact that arose from the solvent consumption is quite uncertain and requires further investigation. This is because the distillation has not been performed in the lab-scale system, and the LLEx performed in the lab-scale system is not yet well developed.
In addition to the quantity of the solvent consumed, the data quality of the solvent is another uncertainty. In the current study, the life-cycle impact of ethyl caproate was simulated by using the life-cycle impact data of ethyl acetate derived from Ecoinvent 3.1. In Ecoinvent 3.1, the acetate required for manufacturing ethyl acetate is mainly produced from syngas, which is derived from the partial combustion of heavy fuel oil or coal, which are fossil-based. This combustion process contributed a large portion of the life-cycle GWP and AP of ethyl acetate. However, more environmentally sustainable acetate manufacturing processes using CO2 or organic waste as substrates are under development, the use of which may reduce the life-cycle impact of ethyl acetate considerably.57 Alternatively, the environmental impact of ethyl caproate may also be estimated by using the result of this LCA. More specifically, part of caproic acid produced from organic waste via chain elongation can be used for manufacturing ethyl caproate to extract the caproic acid itself. Based on this study, the life-cycle impacts of producing 1 kg caproic acid is still higher than that of producing 1 kg ethyl acetate (2.6 kg CO2-eq for GWP, 0.02 kg SO2-eq for AP and 0.004 kg PO43– for EP). In the future, when the caproic acid production via chain elongation is further improved, the potential benefits of using caproic acid produced via chain elongation for making the extraction solvent may be of interest to be further studied. Besides ethyl esters, several extraction solvents, for example, biodiesel derived from residual kitchen oil,33 or extraction process, for example, an in-line liquid–liquid membrane extraction,10,11 a membrane electrolysis31 and an electrodialysis with a bipolar membrane46 have been applied to recover caproic acid. The environmental sustainability of these alternatives should be further investigated, although these separation techniques have only been demonstrated in lab-scale systems.
The overall life-cycle impact of using OFMSW as a feedstock (i.e., Case LO) is higher than using SFW as the feedstock (i.e., Case LS and PS) throughout the three impact categories assessed. This is mainly due to the higher ethanol addition in CE in Case LO, and partially due to the large quantity of solid waste that remains after the BAc process. A large quantity of solid waste (33.3 kg/f.u.) remained after the dry anaerobic digestion of OFMSW, due to the high lignocellulosic content in the OFMSW (90% volume-to-volume garden waste) that was difficult to degrade biologically. The lignocellulosic fraction of the OFMSW may have to be pretreated to be effectively degraded, which was not employed in the previous chain elongation study (from which we obtained the data). However, the application and selection of the pretreatment methods have to be carefully evaluated as the application of pretreatments before anaerobic digestion (BAc in this study) can increase the life-cycle impact especially the eutrophication potential as well as the life-cycle cost.58
3.2. Sensitivity Analysis (SA)
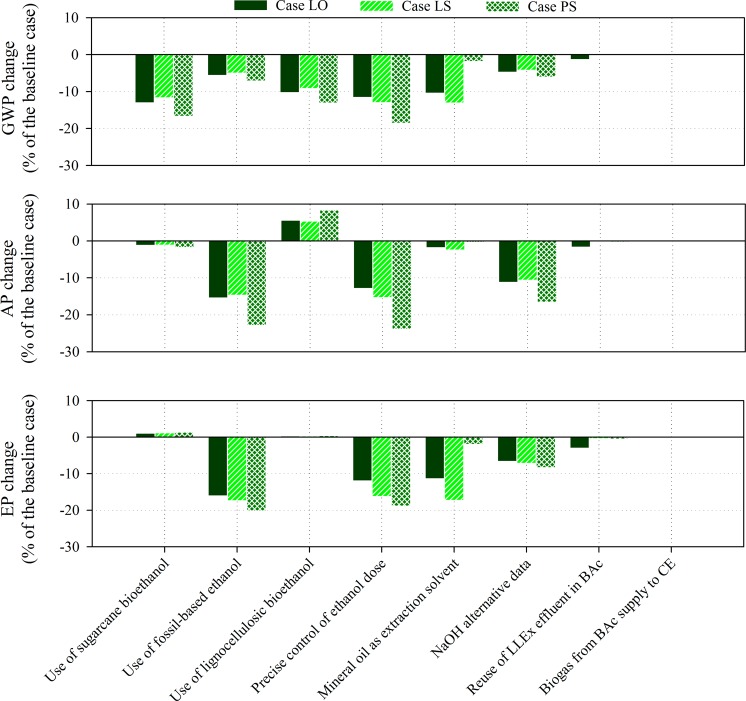
Corn-based bioethanol was reported to have a poorer environmental sustainability, especially in GWP and nutrient use efficiency compared with other crops like sugar cane.59 Sugar cane bioethanol is a potential alternative to corn ethanol as it is already commercially available at a large production scale.60 In the SA (Figure 3), the use of sugar cane bioethanol in CE reduces the life-cycle GWP considerably. Sugar cane bioethanol was reported to have a considerably lower GHG emission compared with other crop-based bioethanol from different feedstocks.59,61 This is likely due to the climate conditions where it grows and the crop properties. It is reported that, in general, temperate annual crops (like corn) have a lower productivity and net energy production compared with perennial crops growing in tropical zones (e.g., sugar cane in Brazil).59,62 Given the same amount of plantation area, sugar cane in the tropical zone could yield up to two times more bioethanol than corn in the temperate region.62 After replacing corn ethanol with sugar cane ethanol, the life-cycle GWP of all cases decreased significantly (>12%), whereas the life-cycle AP and EP remained similar.
Figure 3.
Overview of the sensitivity analysis results for three impact categories (GWP, AP, and EP) and the three study cases (LS, LO, and PS), in terms of relative changes in the life-cycle impact compared with the baseline cases (see Figure 2). Negative values indicate a reduction in the overall life-cycle impact, whereas positive values indicate an increase in the overall life-cycle impact. We refer to SI for a detailed description of the strategies considered in the sensitivity analysis (SI Table S4) and the results presented in terms of absolute value of the life-cycle impacts (SI Table S5).
When using a fossil-based ethanol (produced via hydration of ethylene) instead of a crop-based bioethanol, the environmental impact is lower, especially for the AP and EP (Figure 3). The reduction in AP and EP by using fossil-based ethanol was anticipated, as this has been reported in literature, mainly due to nutrient leaching and fertilizer application.60,63 On the other hand, GWP of fossil ethanol is lower than that of corn ethanol. This corresponds to the outcome of previous studies59,60 but is intuitively contradictory. A key reason for this may be the exclusion of the end-of-life of ethanol. When the end-of-life of ethanol is not included, fossil ethanol has a similar or, in some cases, lower life-cycle GWP compared to other crop bioethanol. However, when the end-of-life is included, bioethanol could have a lower GWP than fossil ethanol, because part of the carbon emission could be counted as biogenic carbon emission.60 For fossil ethanol, all carbon emissions in the use phase and the end-of-life phase are accounted as nonbiogenic carbon that contributes to GWP, regardless of the purpose of the ethanol use. However, for crop bioethanol, the purpose of the ethanol use has a substantial influence on the GWP associated with the end-of-life of bioethanol. If bioethanol is used as a fuel and combusted, most of the carbon emitted is in the form of biogenic CO2 that does not have any GWP. In the case that bioethanol is not combusted but used as an additive or precursor to chemicals, for example, caproic acid in the present study, part of the bioethanol will end up in the water phase and eventually form methane via biological degradation (e.g., anaerobic digestion), which cannot be counted as a biogenic emission and thus contributes to GWP.64 In the present study, the end-of-life of the ethanol is not yet included as the end-of-life of caproic acid is not within the system boundary (due to the various potential applications of caproic acid). In the future, when the life cycle of caproic acid production via chain elongation is assessed for a specified application, the feedstock as well as the end-of-life of the ethanol should be carefully addressed. For example, if the caproic acid is used as a feed additive and the caproic acid leaks into the environment due to the impropriate manure treatment, part of the caproic acid may end up as CH4 in the nature environment and contribute to GWP regardless of the feedstock for ethanol used for producing caproic acid. In contrast, if the caproic acid is used for making drop-in fuels,15,19 caproic acid will end up as CO2 after the fuel combustion. In this case, GWP of this end-of-life CO2 is accounted for only if the caproic acid production process employs fossil-based ethanol rather than bioethanol.
Lignocellulosic bioethanol is an alternative ethanol source that is becoming increasingly available in Europe.65 The use of lignocellulosic bioethanol produced from grass via saccharification and simultaneous fermentation (SSF) has a clear reduction on the life-cycle GWP, but the use of it increases the life-cycle AP (Figure 3). SSF is currently considered to be the most mature production process for lignocellulosic ethanol, which was therefore used in the Ecoinvent 3.1 database.44 The higher AP could be attributed to the steam (i.e., heat) used to pretreat the grass to yield higher ethanol production. The energy or chemical, for example, sulfuric acid, used for pretreating lignocellulosic biomass could also be one of the main causes for the high life-cycle AP.66 Overall, based on the present study, the use of lignocellulosic bioethanol produced from grass via SSF does reduce the life-cycle GWP but not the life-cycle AP and EP.
In addition to SSF, there are several emerging lignocellulosic ethanol production processes including the mixed culture syngas fermentation67,68 and the consolidated bioprocessing (CBP).69 The effluent of these emerging lignocellulosic ethanol production processes usually have a lower ethanol titer.70 The lower ethanol titer makes them difficult to be used for the caproic acid production system in this study, considering the substrate dilution (e.g., SCFAs and ethanol) after the mixing of the BAc effluent with the dilute-ethanol solutions. Nevertheless, use of these dilute ethanol as a sole substrate for caproic acid production can be a potential alternative caproic acid production strategy. Caproic acid production from a dilute-ethanol solution from a syngas fermentation (around 11 g ethanol/L)24 via chain elongation was demonstrated in lab-scale systems.24 Even though a mixed organic waste was not used in such system, it is still of interest to study the life-cycle environmental impact of such system due to the use of a waste stream, that is, lignocellulosic waste71 instead of crop-based ethanol for caproic acid production. We recommend that the future study also look into these systems where a dilute lignocellulosic ethanol solution is used as the feedstock for caproic acid production via chain elongation.
Reducing ethanol addition is another potential improvement strategy, as discussed in section 3.1. Based on the stoichiometry of the chain elongation reaction, the maximum possible ethanol reduction (in the form of a 95% ethanol solution) is about 1 kg/f.u. for all cases. This reduction in ethanol dose leads to a substantial reduction of all life-cycle impacts throughout all cases, especially in Case PS where the ethanol use dominates the life-cycle impacts. The ethanol use efficiency, as well as the possible reduction on ethanol addition in CE, have not yet been specifically addressed in previous studies on chain elongation, to authors’ best knowledge. Regarding the potentially substantial reduction of the life-cycle impacts, it is advised to study the maximal feasible reduction on ethanol addition.
The reuse of LLEx effluent in BAc is a potential improvement strategy, which reduces the water use for diluting the organic waste during BAc. The use of biogas produced during BAc as a CO2 supply to CE is another potential improvement strategy. In SA, both improvement strategies are evaluated (details in SI Table S4). The result suggested a limited reduction on the life-cycle impacts in all cases and impact categories (Figure 3 and SI Table S5). This is mainly due to the limited environmental impacts arose by the water and CO2 use during the entire life cycle. This confirms that future studies should first dedicate to the improvement strategies for the impact hot-spots such as the ethanol and extraction solvent use. In this discussion, we focused mainly on the SA for the ethanol use and the alternative process design; the discussion for other SA results is provided in the SI.
3.3. Comparison with Other Studies
Comparing the LCA outcome of this study with other competing technologies is of use to benchmark the technology assessed in this study. There are three ways to compare the outcome of this LCA with other studies (see also SI Table S6 for the studies used for the comparison in this section). The first is comparing it to other LCAs for caproic acid production processes using other feedstocks than organic waste, for example, palm oil. Both process data and LCAs for caproic acid production from palm oil are not available in literature, to authors’ current knowledge. However, based on the currently known parameters, we can make a best-case estimation for the life-cycle impact of caproic acid produced from palm oil. Crude palm oil (CPO) does not contain any caproic acid, but the byproduct of CPO, that is, palm kernel oil (PKO), contains about 0.5 wt % caproic acid.72,73 The life-cycle GWP of producing 1 tonne CPO is estimated to be up to 2305 kg CO2-eq (from plantations and mills, excluding land use change),74 and around 100 kg PKO is produced as byproduct.75 If we assume all caproic acid in PKO can be completely extracted and use a mass-based allocation, the GWP of caproic acid produced from palm oil is around 0.23 kg CO2-eq per kg caproic acid, excluding the impact arose from downstream processes (see SI Table S6 for the detailed estimation for the life-cycle AP and EP). Comparing with Case PS in this study, this estimation for the life-cycle impact of caproic acid from palm oil is considerably lower. However, there are many uncertainties in this estimation, including the effects of land use change,76 the unknown impacts of the downstream processes, the actual extraction efficiency of caproic acid from PKO and a proper allocation of the life-cycle impact of CPO to the produced biodiesel and caproic acid. Further studies on the actual life-cycle impacts of caproic acid production from crude palm oil is needed to make a sound comparison. Nevertheless, based on this best-case estimation, substantial improvements for the life-cycle impact of caproic acid production from mixed organic waste via chain elongation are still needed.
The second is to compare it to LCAs of other novel products from organic waste via emerging biotechnologies, for example, polyhydroxyalkanoates (PHA). Gurieff and Lant (2007) reported an LCA study for PHA that can be used to compare with the present study.77 The other LCA studies for PHA mostly used either a more homogeneous waste stream or a pure-culture bioprocess.78 In Gurieff and Lant (2007), a 20 g-COD/L mixed wastewater stream from the food industry was used to produce PHAs using a mixed culture under a nonsterile condition. They report that the life-cycle GWP of PHA from a mixed wastewater stream is about 20.4 kg CO2-eq/kg PHA or 3.92 kg CO2-eq/kg-CODfeed (excluding the environmental benefits of displacing fossil-based polymers). In Case PS of this study, the life-cycle GWP of caproic acid is about 8.7 kg CO2-eq/kg caproic acid or 2.3 kg CO2-eq/kg-CODfeed (based on the COD in the SFW), which is lower than that of PHA reported by Gurieff and Lant (2007). The main reason to exclude the environmental benefits of PHA is due to the uncertain application of caproic acid. When the environmental impact of displacing an existing fossil-based polymer is accounted for, the PHA production from the mixed wastewater has a negative environmental impact, that is, it gains environmental benefits while treating the wastewater. It is, therefore, recommended to identify a specific application of caproic acid in future LCA studies to effectively evaluate the environmental credentials by caproic acid. An example can be the use of caproic acid as a feed additive, which improves the feed conversion efficiency and the health of livestock.16 In this case, caproic acid can obtain environmental credentials through saving animal feed due to the higher feed conversion efficiency. Another interesting fact of this comparison (caproic acid versus PHAs) is the respective environmental impact hot-spots of both systems; namely the ethanol use in the caproic acid production and the electricity use in the PHA system for the downstream processes.77,78 As the environmental impacts from electricity use can be significantly reduced by introducing renewable electricity, the life-cycle impact of PHA production via a mixed culture bioprocess can be significantly reduced by employing a cleaner electricity.78 In contrast, ethanol use in caproic acid production is currently inevitable, though the ethanol dose may be significantly reduced. The development of alternative electron donors for chain elongation is of importance to improve the life-cycle environmental performance of the caproic acid production via chain elongation.
The third way is comparing this study to LCAs for other current ways of organic waste treatment, for example, anaerobic digestion (AD). AD has been implemented for treating organic waste, which yields biogas as a byproduct. According to a recent LCA of a large-scale AD on OFMSW, treating 1 kg OFMSW via AD generates 0.056 kg CO2-eq/kg OFMSW (recalculated from literature79), excluding the avoided environmental burden of the produced biogas. Assuming that the OFMSW used in this AD study has a similar COD concentration as the OFMSW used in our study (0.55 kgCOD/kg OFMSW), the environmental impact of this large-scale AD treating OFMSW is about 0.1 kg CO2-eq/kg CODOFMSW, considerably lower than that in Case PS. A similar result was reported by Gurieff and Lant (2007) when they compared the PHA production and AD for treating a mixed food industrial wastewater without crediting the end-products.77 The system scales may be a factor contributing to the significant difference in the environmental performances of these bioprocesses. It is known that lab-scale and pilot-scale systems, for example, this study and the PHA production,77 may have lower yields than commercial-scale systems such as large-scale AD.35,79 On the other hand, after giving environmental credentials to the product, the PHA production appears to be a more environmentally sustainable option for managing organic waste than AD.77 We anticipate the same outcome after the caproic acid is credited for the environmental impacts it avoided by using chain elongation production process. Future LCA studies should therefore identify an application for the produced caproic acid, which is useful for giving caproic acid an environmental credential and for further evaluating its environmental performance.
Acknowledgments
We thank Wageningen IPOP Research Programme Biorefinery and ChainCraft B.V. for funding this research. We acknowledge ChainCraft B.V. for providing their useful input and expert opinions. We also thank Kirsten Steinbusch and Mark Roghair for providing the internal experimental data.
Glossary
Abbreviations
- AP
acidification potential
- BAc
biological acidification
- CAc
chemical acidification
- Case LO
Lab-scale system employing OFMSW as the feedstock
- Case LS
Lab-scale system employing SFW as the feedstock
- Case PS
Pilot-scale system employing SFW as the feedstock
- CE
chain elongation
- CH4
methane
- CO2
carbon dioxide
- CO2-eq
carbon dioxide equivalent
- CPO
crude palm oil
- Ds
distillation
- EP
eutrophication potential
- f.u
functional unit
- GHG
greenhouse gases
- GWP
global warming potential
- HCl
hydrochloric acid
- kg
kilogram
- LCA
life cycle assessment
- LCI
life cycle inventory
- LCIA
life cycle impact assessment
- LLEx
liquid–liquid extraction
- LSS
liquid–solid separation
- MCFA
medium-chain fatty acids
- NaOH
sodium hydroxide
- OFMSW
organic fraction of municipal solid waste
- PKO
palm kernel oil
- PO43–-eq
phosphate equivalent
- SCFA
short-chain fatty acids
- SFW
supermarket food waste
- SO2-eq
sulfur dioxide equivalent
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.6b06220.
Explanation for excluding the feedstock emissions and environmental impacts. Detailed description for the caproic acid production system based on chain elongation. Sensitivity analysis for the extraction solvent and the base. Table S1, overview of parameters used for life cycle inventory; Table S2, overview of parameters used for life cycle impact assessment; Table S3, the setup of three baseline case studies; Table S4, overview of the parameters used for sensitivity analysis; Table S5, the overview of the results of the sensitivity analysis, in terms of the absolute value of the life-cycle impacts. Table S6, overview of data used for the comparison section in the discussion (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Cherubini F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manage. 2010, 51 (7), 1412–1421. 10.1016/j.enconman.2010.01.015. [DOI] [Google Scholar]
- Rathmann R.; Szklo A.; Schaeffer R. Land use competition for production of food and liquid biofuels: An analysis of the arguments in the current debate. Renewable Energy 2010, 35 (1), 14–22. 10.1016/j.renene.2009.02.025. [DOI] [Google Scholar]
- Tuck C. O.; Pérez E.; Horváth I. T.; Sheldon R. A.; Poliakoff M. Valorization of biomass: Deriving more value from waste. Science 2012, 337 (6095), 695–699. 10.1126/science.1218930. [DOI] [PubMed] [Google Scholar]
- Sanders J.; Scott E.; Weusthuis R.; Mooibroek H. Bio-refinery as the bio-inspired process to bulk chemicals. Macromol. Biosci. 2007, 7 (2), 105–117. 10.1002/mabi.200600223. [DOI] [PubMed] [Google Scholar]
- Lin C. S. K.; Pfaltzgraff L. A.; Herrero-Davila L.; Mubofu E. B.; Abderrahim S.; Clark J. H.; Koutinas A. A.; Kopsahelis N.; Stamatelatou K.; Dickson F.; Thankappan S.; Mohamed Z.; Brocklesby R.; Luque R. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 424–464. 10.1039/c2ee23440h. [DOI] [Google Scholar]
- Kleerebezem R.; Joosse B.; Rozendal R.; Van Loosdrecht M. C. M. Anaerobic digestion without biogas?. Rev. Environ. Sci. Bio/Technol. 2015, 14 (4), 787–801. 10.1007/s11157-015-9374-6. [DOI] [Google Scholar]
- Renkow M.; Rubin A. R. Does municipal solid waste composting make economic sense?. J. Environ. Manage. 1998, 53 (4), 339–347. 10.1006/jema.1998.0214. [DOI] [Google Scholar]
- Levy P. F.; Sanderson J. E.; Kispert R. G.; Wise D. L. Biorefining of biomass to liquid fuels and organic chemicals. Enzyme Microb. Technol. 1981, 3 (3), 207–215. 10.1016/0141-0229(81)90087-9. [DOI] [Google Scholar]
- Chen W.-S.; Huang S.; Strik D. P. B. T. B.; Buisman C. J. N. Isobutyrate biosynthesis via methanol chain elongation: Converting organic wastes to platform chemicals. J. Chem. Technol. Biotechnol. 2017, 92, 1370. 10.1002/jctb.5132. [DOI] [Google Scholar]
- Agler M. T.; Spirito C. M.; Usack J. G.; Werner J. J.; Angenent L. T. Chain elongation with reactor microbiomes: Upgrading dilute ethanol to medium-chain carboxylates. Energy Environ. Sci. 2012, 5 (8), 8189–8192. 10.1039/c2ee22101b. [DOI] [Google Scholar]
- Ge S.; Usack J. G.; Spirito C. M.; Angenent L. T. Long-term n-caproic acid production from yeast-fermentation beer in an anaerobic bioreactor with continuous product extraction. Environ. Sci. Technol. 2015, 49 (13), 8012–8021. 10.1021/acs.est.5b00238. [DOI] [PubMed] [Google Scholar]
- Grootscholten T. I. M.; Kinsky dal Borgo F.; Hamelers H. V. M.; Buisman C. J. N. Promoting chain elongation in mixed culture acidification reactors by addition of ethanol. Biomass Bioenergy 2013, 48, 10–16. 10.1016/j.biombioe.2012.11.019. [DOI] [Google Scholar]
- Grootscholten T. I. M.; Strik D. P. B. T. B.; Steinbusch K. J. J.; Buisman C. J. N.; Hamelers H. V. M Two-stage medium chain fatty acid (MCFA) production from municipal solid waste and ethanol. Appl. Energy 2014, 116, 223–229. 10.1016/j.apenergy.2013.11.061. [DOI] [Google Scholar]
- Steinbusch K. J. J.; Hamelers H. V. M; Plugge C. M.; Buisman C. J. N. Biological formation of caproate and caprylate from acetate: Fuel and chemical production from low grade biomass. Energy Environ. Sci. 2011, 4 (1), 216–224. 10.1039/C0EE00282H. [DOI] [Google Scholar]
- Angenent L. T.; Richter H.; Buckel W.; Spirito C. M.; Steinbusch K. J. J.; Plugge C. M.; Strik D. P. B. T. B.; Grootscholten T. I. M.; Buisman C. J. N.; Hamelers H. V. M Chain elongation with reactor microbiomes: Open-culture biotechnology to produce biochemicals. Environ. Sci. Technol. 2016, 50 (6), 2796–2810. 10.1021/acs.est.5b04847. [DOI] [PubMed] [Google Scholar]
- Zentek J.; Buchheit-Renko S.; Ferrara F.; Vahjen W.; Van Kessel A. G.; Pieper R. Nutritional and physiological role of medium-chain triglycerides and medium-chain fatty acids in piglets. Anim. Health Res. Rev. 2011, 12 (1), 83–93. 10.1017/S1466252311000089. [DOI] [PubMed] [Google Scholar]
- Woolford M. K. Microbiological screening of the straight chain fatty acids (C1-C12) as potential silage additives. J. Sci. Food Agric. 1975, 26 (2), 219–228. 10.1002/jsfa.2740260213. [DOI] [PubMed] [Google Scholar]
- Scalschi L.; Vicedo B.; Camañes G.; Fernandez-Crespo E.; Lapeña L.; González-Bosch C.; García-Agustín P. Hexanoic acid is a resistance inducer that protects tomato plants against Pseudomonas syringae by priming the jasmonic acid and salicylic acid pathways. Mol. Plant Pathol. 2013, 14 (4), 342–355. 10.1111/mpp.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agler M. T.; Wrenn B. A.; Zinder S. H.; Angenent L. T. Waste to bioproduct conversion with undefined mixed cultures: the carboxylate platform. Trends Biotechnol. 2011, 29 (2), 70–78. 10.1016/j.tibtech.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Seedorf H.; Fricke W. F.; Veith B.; Brüggemann H.; Liesegang H.; Strittmatter A.; Miethke M.; Buckel W.; Hinderberger J.; Li F.; Hagemeier C.; Thauer R. K.; Gottschalk G. The genome of Clostridium kluyveri, a strict anaerobe with unique metabolic features. Proc. Natl. Acad. Sci. U. S. A. 2008, 105 (6), 2128–2133. 10.1073/pnas.0711093105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootscholten T. I. M.; Steinbusch K. J. J.; Hamelers H. V. M; Buisman C. J. N. Improving medium chain fatty acid productivity using chain elongation by reducing the hydraulic retention time in an upflow anaerobic filter. Bioresour. Technol. 2013, 136, 735–738. 10.1016/j.biortech.2013.02.114. [DOI] [PubMed] [Google Scholar]
- Grootscholten T. I. M.; Steinbusch K. J. J.; Hamelers H. V. M; Buisman C. J. N. Chain elongation of acetate and ethanol in an upflow anaerobic filter for high rate MCFA production. Bioresour. Technol. 2013, 135 (0), 440–445. 10.1016/j.biortech.2012.10.165. [DOI] [PubMed] [Google Scholar]
- Coma M.; Vilchez-Vargas R.; Roume H.; Jauregui R.; Pieper D. H.; Rabaey K. Product diversity linked to substrate usage in chain elongation by mixed-culture fermentation. Environ. Sci. Technol. 2016, 50 (12), 6467–6476. 10.1021/acs.est.5b06021. [DOI] [PubMed] [Google Scholar]
- Vasudevan D.; Richter H.; Angenent L. T. Upgrading dilute ethanol from syngas fermentation to n-caproate with reactor microbiomes. Bioresour. Technol. 2014, 151, 378–382. 10.1016/j.biortech.2013.09.105. [DOI] [PubMed] [Google Scholar]
- Zhu X.; Tao Y.; Liang C.; Li X.; Wei N.; Zhang W.; Zhou Y.; Yang Y.; Bo T. The synthesis of n-caproate from lactate: a new efficient process for medium-chain carboxylates production. Sci. Rep. 2015, 5, 14360. 10.1038/srep14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucek L. A.; Nguyen M.; Angenent L. T. Conversion of l-lactate into n-caproate by a continuously fed reactor microbiome. Water Res. 2016, 93, 163–171. 10.1016/j.watres.2016.02.018. [DOI] [PubMed] [Google Scholar]
- Grootscholten T. I. M.; Steinbusch K. J. J.; Hamelers H. V. M; Buisman C. J. N. High rate heptanoate production from propionate and ethanol using chain elongation. Bioresour. Technol. 2013, 136, 715–718. 10.1016/j.biortech.2013.02.085. [DOI] [PubMed] [Google Scholar]
- Liang S.; Wan C. Carboxylic acid production from brewer’s spent grain via mixed culture fermentation. Bioresour. Technol. 2015, 182, 179–183. 10.1016/j.biortech.2015.01.082. [DOI] [PubMed] [Google Scholar]
- Roghair M.; Strik D. P. B. T. B.; Steinbusch K. J. J.; Weusthuis R. A.; Bruins M. E.; Buisman C. J. N. Granular sludge formation and characterization in a chain elongation process. Process Biochem. 2016, 51 (10), 1594–1598. 10.1016/j.procbio.2016.06.012. [DOI] [Google Scholar]
- Weimer P. J.; Nerdahl M.; Brandl D. J. Production of medium-chain volatile fatty acids by mixed ruminal microorganisms is enhanced by ethanol in co-culture with Clostridium kluyveri. Bioresour. Technol. 2015, 175, 97–101. 10.1016/j.biortech.2014.10.054. [DOI] [PubMed] [Google Scholar]
- Xu J.; Guzman J. J. L.; Andersen S. J.; Rabaey K.; Angenent L. T. In-line and selective phase separation of medium-chain carboxylic acids using membrane electrolysis. Chem. Commun. 2015, 51 (31), 6847–6850. 10.1039/C5CC01897H. [DOI] [PubMed] [Google Scholar]
- Xiong B.; Richard T. L.; Kumar M. Integrated acidogenic digestion and carboxylic acid separation by nanofiltration membranes for the lignocellulosic carboxylate platform. J. Membr. Sci. 2015, 489, 275–283. 10.1016/j.memsci.2015.04.022. [DOI] [Google Scholar]
- Kannengiesser J.; Sakaguchi-Söder K.; Mrukwia T.; Jager J.; Schebek L. Extraction of medium chain fatty acids from organic municipal waste and subsequent production of bio-based fuels. Waste Manage. 2016, 47 (Part A), 78–83. 10.1016/j.wasman.2015.05.030. [DOI] [PubMed] [Google Scholar]
- Spirito C. M.; Richter H.; Rabaey K.; Stams A. J. M.; Angenent L. T. Chain elongation in anaerobic reactor microbiomes to recover resources from waste. Curr. Opin. Biotechnol. 2014, 27 (0), 115–122. 10.1016/j.copbio.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Hetherington A. C.; Borrion A. L.; Griffiths O. G.; McManus M. C. Use of LCA as a development tool within early research: challenges and issues across different sectors. Int. J. Life Cycle Assess. 2014, 19 (1), 130–143. 10.1007/s11367-013-0627-8. [DOI] [Google Scholar]
- Pant D.; Singh A.; Van Bogaert G.; Gallego Y. A.; Diels L.; Vanbroekhoven K. An introduction to the life cycle assessment (LCA) of bioelectrochemical systems (BES) for sustainable energy and product generation: Relevance and key aspects. Renewable Sustainable Energy Rev. 2011, 15 (2), 1305–1313. 10.1016/j.rser.2010.10.005. [DOI] [Google Scholar]
- Ekman A.; Börjesson P. Environmental assessment of propionic acid produced in an agricultural biomass-based biorefinery system. J. Cleaner Prod. 2011, 19 (11), 1257–1265. 10.1016/j.jclepro.2011.03.008. [DOI] [Google Scholar]
- Cherubini F.; Strømman A. H. Life cycle assessment of bioenergy systems: State of the art and future challenges. Bioresour. Technol. 2011, 102 (2), 437–451. 10.1016/j.biortech.2010.08.010. [DOI] [PubMed] [Google Scholar]
- IES-JRC-EU, International Reference Life Cycle Data System (ILCD) Handbook - General Guide for Life Cycle Assessment - Detailed Guidance, 1st ed.; Publications Office of the European Union: Luxemburg, 2010. [Google Scholar]
- Ahlgren S.; Björklund A.; Ekman A.; Karlsson H.; Berlin J.; Börjesson P.; Ekvall T.; Finnveden G.; Janssen M.; Strid I. Review of methodological choices in LCA of biorefinery systems - key issues and recommendations. Biofuels, Bioprod. Biorefin. 2015, 9 (5), 606–619. 10.1002/bbb.1563. [DOI] [Google Scholar]
- Grootscholten T. I. M.Development of a mixed culture chain elongation process based on municipal solid waste and ethanol. Ph.D. Dissertation, Wageningen University, Wageningen, 2013. [Google Scholar]
- Weidema B. P. B., Ch; Hischier R.; Mutel Ch.; Nemecek T.; Reinhard J.; Vadenbo C. O.; Wernet G.. The Ecoinvent Database: Overview and Methodology, Data quality guideline for the ecoinvent database version 3; Ecoinvent: Zurich, Switzerland, 2013. [Google Scholar]
- Tufvesson L. M.; Tufvesson P.; Woodley J. M.; Börjesson P. Life cycle assessment in green chemistry: overview of key parameters and methodological concerns. Int. J. Life Cycle Assess. 2013, 18 (2), 431–444. 10.1007/s11367-012-0500-1. [DOI] [Google Scholar]
- Jungbluth N.; Chudacoff M.; Dauriat A.; Dinkel F.; Doka G.; Faist Emmenegger M.; Gnansounou E.; Kljun N.; Schleiss K.; Spielmann M.. Life Cycle Inventories of Bioenergy; Swiss Centre for Life Cycle Inventories: Dübendorf, 2007. [Google Scholar]
- Kim S.; Dale B. E. Life cycle assessment of fuel ethanol derived from corn grain via dry milling. Bioresour. Technol. 2008, 99 (12), 5250–5260. 10.1016/j.biortech.2007.09.034. [DOI] [PubMed] [Google Scholar]
- Arslan D.Selective short chain carboxylates production by mixed culture fermentation. Ph.D. Dissertation, Wageningen University, Wageningen, 2014. [Google Scholar]
- Ren N.; Wang B.; Huang J.-C. Ethanol-type fermentation from carbohydrate in high rate acidogenic reactor. Biotechnol. Bioeng. 1997, 54 (5), 428–433. . [DOI] [PubMed] [Google Scholar]
- Arslan D.; Steinbusch K. J. J.; Diels L.; De Wever H.; Buisman C. J. N.; Hamelers H. V. M Effect of hydrogen and carbon dioxide on carboxylic acids patterns in mixed culture fermentation. Bioresour. Technol. 2012, 118 (0), 227–234. 10.1016/j.biortech.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Steinbusch K. J. J.; Hamelers H. V. M; Buisman C. J. N. Alcohol production through volatile fatty acids reduction with hydrogen as electron donor by mixed cultures. Water Res. 2008, 42 (15), 4059–4066. 10.1016/j.watres.2008.05.032. [DOI] [PubMed] [Google Scholar]
- Arslan D.; Steinbusch K. J. J.; Diels L.; Hamelers H. V. M; Strik D. P. B. T. B.; Buisman C. J. N.; De Wever H. Selective short-chain carboxylates production: A review of control mechanisms to direct mixed culture fermentations. Crit. Rev. Environ. Sci. Technol. 2016, 46 (6), 592–634. 10.1080/10643389.2016.1145959. [DOI] [Google Scholar]
- Liu Y.; Lü F.; Shao L.; He P. Alcohol-to-acid ratio and substrate concentration affect product structure in chain elongation reactions initiated by unacclimatized inoculum. Bioresour. Technol. 2016, 218, 1140–1150. 10.1016/j.biortech.2016.07.067. [DOI] [PubMed] [Google Scholar]
- Van Eerten-Jansen M. C. A. A.; Ter Heijne A.; Grootscholten T. I. M.; Steinbusch K. J. J.; Sleutels T. H. J. A.; Hamelers H. V. M; Buisman C. J. N. Bioelectrochemical production of caproate and caprylate from acetate by mixed Cultures. ACS Sustainable Chem. Eng. 2013, 1 (5), 513–518. 10.1021/sc300168z. [DOI] [Google Scholar]
- Chen W. S.; Ye Y.; Steinbusch K. J. J.; Strik D. P. B. T. B.; Buisman C. J. N. Methanol as an alternative electron donor in chain elongation for butyrate and caproate formation. Biomass Bioenergy 2016, 93, 201–208. 10.1016/j.biombioe.2016.07.008. [DOI] [Google Scholar]
- Tufvesson P.; Ekman A.; Sardari R. R. R.; Engdahl K.; Tufvesson L. Economic and environmental assessment of propionic acid production by fermentation using different renewable raw materials. Bioresour. Technol. 2013, 149 (0), 556–564. 10.1016/j.biortech.2013.09.049. [DOI] [PubMed] [Google Scholar]
- Andersen S. J.; Candry P.; Basadre T.; Khor W. C.; Roume H.; Hernandez-Sanabria E.; Coma M.; Rabaey K. Electrolytic extraction drives volatile fatty acid chain elongation through lactic acid and replaces chemical pH control in thin stillage fermentation. Biotechnol. Biofuels 2015, 8 (1), 1–14. 10.1186/s13068-015-0396-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J. M.; Rozendal R. A.; Hertle C. K.; Lant P. A.; Rabaey K. Life Cycle Assessment of High-Rate Anaerobic Treatment, Microbial Fuel Cells, and Microbial Electrolysis Cells. Environ. Sci. Technol. 2010, 44 (9), 3629–3637. 10.1021/es100125h. [DOI] [PubMed] [Google Scholar]
- Jourdin L.; Freguia S.; Flexer V.; Keller J. Bringing high-Rate, CO2-based microbial electrosynthesis closer to practical implementation through improved electrode design and operating conditions. Environ. Sci. Technol. 2016, 50 (4), 1982–1989. 10.1021/acs.est.5b04431. [DOI] [PubMed] [Google Scholar]
- Carballa M.; Duran C.; Hospido A. Should we pretreat solid waste prior to anaerobic digestion? an assessment of its environmental cost. Environ. Sci. Technol. 2011, 45 (24), 10306–10314. 10.1021/es201866u. [DOI] [PubMed] [Google Scholar]
- de Vries S. C.; van de Ven G. W. J.; van Ittersum M. K.; Giller K. E. Resource use efficiency and environmental performance of nine major biofuel crops, processed by first-generation conversion techniques. Biomass Bioenergy 2010, 34 (5), 588–601. 10.1016/j.biombioe.2010.01.001. [DOI] [Google Scholar]
- Muñoz I.; Flury K.; Jungbluth N.; Rigarlsford G.; Canals L. M.; King H. Life cycle assessment of bio-based ethanol produced from different agricultural feedstocks. Int. J. Life Cycle Assess. 2014, 19 (1), 109–119. 10.1007/s11367-013-0613-1. [DOI] [Google Scholar]
- von Blottnitz H.; Curran M. A. A review of assessments conducted on bio-ethanol as a transportation fuel from a net energy, greenhouse gas, and environmental life cycle perspective. J. Cleaner Prod. 2007, 15 (7), 607–619. 10.1016/j.jclepro.2006.03.002. [DOI] [Google Scholar]
- Chum H. L.; Warner E.; Seabra J. E. A.; Macedo I. C. A comparison of commercial ethanol production systems from Brazilian sugarcane and US corn. Biofuels, Bioprod. Biorefin. 2014, 8 (2), 205–223. 10.1002/bbb.1448. [DOI] [Google Scholar]
- Yang Y.; Bae J.; Kim J.; Suh S. Replacing Gasoline with Corn Ethanol Results in Significant Environmental Problem-Shifting. Environ. Sci. Technol. 2012, 46 (7), 3671–3678. 10.1021/es203641p. [DOI] [PubMed] [Google Scholar]
- Muñoz I.; Rigarlsford G.; i Canals L. M.; King H. Accounting for greenhouse gas emissions from the degradation of chemicals in the environment. Int. J. Life Cycle Assess. 2013, 18 (1), 252–262. 10.1007/s11367-012-0453-4. [DOI] [Google Scholar]
- Balan V.; Chiaramonti D.; Kumar S. Review of US and EU initiatives toward development, demonstration, and commercialization of lignocellulosic biofuels. Biofuels, Bioprod. Biorefin. 2013, 7 (6), 732–759. 10.1002/bbb.1436. [DOI] [Google Scholar]
- Schmitt E.; Bura R.; Gustafson R.; Cooper J.; Vajzovic A. Converting lignocellulosic solid waste into ethanol for the State of Washington: An investigation of treatment technologies and environmental impacts. Bioresour. Technol. 2012, 104, 400–409. 10.1016/j.biortech.2011.10.094. [DOI] [PubMed] [Google Scholar]
- Liu K.; Atiyeh H. K.; Stevenson B. S.; Tanner R. S.; Wilkins M. R.; Huhnke R. L. Continuous syngas fermentation for the production of ethanol, n-propanol and n-butanol. Bioresour. Technol. 2014, 151, 69–77. 10.1016/j.biortech.2013.10.059. [DOI] [PubMed] [Google Scholar]
- Liu K.; Atiyeh H. K.; Stevenson B. S.; Tanner R. S.; Wilkins M. R.; Huhnke R. L. Mixed culture syngas fermentation and conversion of carboxylic acids into alcohols. Bioresour. Technol. 2014, 152, 337–346. 10.1016/j.biortech.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Brethauer S.; Studer M. H. Consolidated bioprocessing of lignocellulose by a microbial consortium. Energy Environ. Sci. 2014, 7 (4), 1446–1453. 10.1039/c3ee41753k. [DOI] [Google Scholar]
- Paulova L.; Patakova P.; Branska B.; Rychtera M.; Melzoch K. Lignocellulosic ethanol: Technology design and its impact on process efficiency. Biotechnol. Adv. 2015, 33 (6), 1091–1107. 10.1016/j.biotechadv.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Tilman D.; Socolow R.; Foley J. A.; Hill J.; Larson E.; Lynd L.; Pacala S.; Reilly J.; Searchinger T.; Somerville C.; Williams R. Beneficial biofuels- The food, energy, and environment trilemma. Science 2009, 325 (5938), 270–271. 10.1126/science.1177970. [DOI] [PubMed] [Google Scholar]
- Bagby M. O.; Johnson R. W.; Daniels R. W.; Contrell R. R.; Sauer E. T.; Keenan M. J.; Krevalis M. A., Carboxylic Acids. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, 2000. [Google Scholar]
- Young F. V. K. Palm Kernel and coconut oils: Analytical characteristics, process technology and uses. J. Am. Oil Chem. Soc. 1983, 60 (2), 374–379. 10.1007/BF02543521. [DOI] [Google Scholar]
- Stichnothe H.; Schuchardt F. Life cycle assessment of two palm oil production systems. Biomass Bioenergy 2011, 35 (9), 3976–3984. 10.1016/j.biombioe.2011.06.001. [DOI] [Google Scholar]
- Basiron Y. Palm oil production through sustainable plantations. Eur. J. Lipid Sci. Technol. 2007, 109 (4), 289–295. 10.1002/ejlt.200600223. [DOI] [Google Scholar]
- Fargione J.; Hill J.; Tilman D.; Polasky S.; Hawthorne P. Land Clearing and the Biofuel Carbon Debt. Science 2008, 319 (5867), 1235–1238. 10.1126/science.1152747. [DOI] [PubMed] [Google Scholar]
- Gurieff N.; Lant P. Comparative life cycle assessment and financial analysis of mixed culture polyhydroxyalkanoate production. Bioresour. Technol. 2007, 98 (17), 3393–3403. 10.1016/j.biortech.2006.10.046. [DOI] [PubMed] [Google Scholar]
- Heimersson S.; Morgan-Sagastume F.; Peters G. M.; Werker A.; Svanström M. Methodological issues in life cycle assessment of mixed-culture polyhydroxyalkanoate production utilising waste as feedstock. New Biotechnol. 2014, 31 (4), 383–393. 10.1016/j.nbt.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Evangelisti S.; Lettieri P.; Borello D.; Clift R. Life cycle assessment of energy from waste via anaerobic digestion: A UK case study. Waste Manage. 2014, 34 (1), 226–237. 10.1016/j.wasman.2013.09.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.