Abstract
In the present study, a variety of agro-industrial wastes have been utilized for meaningful purpose to produce valuable biocatalyst. All wastes used were low cost and easily accessible while, some available throughout the year. A number of bacterial strains isolated from rotten fruits and vegetables were screened for the production of industrially important polygalacturonase (PGase) using pectin present in these agro-industrial wastes. The strain producing maximum PGase was identified as Bacillus licheniformis KIBE-IB3 on the basis of taxonomic studies and 16S rDNA analysis. Among different agro-industrial wastes studied, high yield of PGase was achieved from fermentation broth having wheat bran (1.0%) as a substrate in to the medium supplemented with nitrogen sources in combination of NaNO3 and yeast extract while KH2PO4 was selected as suitable micronutrient. After optimizing fermentation parameters it was noticed that Bacillus licheniformis KIBE-IB3 was capable of producing maximum PGase at 37 °C, pH 7.0 and after 48 h of incubation time. From the current research, wheat bran was proven as a cheap and easily available source throughout the year for hyper production of pectinase. The utilization of the waste will also help to minimize the concerned environmental issues.
Keywords: Biotechnology, Environmental science
1. Introduction
In the modern era of biotechnology, there has been an increasing trend in the usage of microbial enzymes in different industries and currently, large number of enzymes are applicable for this purpose. Among them pectinase group of enzymes occupied a very important place. Pectinase group of enzymes include pectin methyl esterase, lyase and polygalacturonase. Pectin methyl esterase acts upon pectin to remove methoxyl groups from the C-6 carboxyl groups of galacturonate units by hydrolysis, pectin lyase cleaves (1->4)-alpha-D-galacturonan methyl ester to give oligosaccharides with 4-deoxy-6-O-methyl-alpha-D-galact-4-enuronosyl groups. While polygalacturonase hydrolyzes alpha-1,4 glycosidic bonds between galacturonic acid residues (Castilho et al., 1999a; Castilho et al., 2000). These enzymes are used for increasing juice extraction by decreasing the viscosity of concentrates in cellulose fiber preparation, coffee/tea fermentation, oligogalacturonides production and also for the clarification of juices and wines (Phutela et al., 2005; Gummadi and Panda, 2003; Croak and Corredig, 2006).
Polygalacturonase (PGase) is an important member of this group produced by many bacteria, fungi and some specific plant cells (Daas et al., 1999). Most of the pectinolytic enzymes are produce by fungi, however different species of genus Bacillus are also capable of producing these enzymes. Bacillus strains adapt changes in the growing conditions more easily and these factors contribute to better production of enzymes. A number of Bacillus species have been reported for the production of PGase enzymes such as B. subtilis, and B. licheniformis (Dharmik and Gomashe, 2013; Rehman et al., 2014). These bacteria are responsible for approximately 50% of the total enzymes production (Schallmey et al., 2004).
The demand of pectinase is increasing day by day but the application is limited due to high cost of medium that contribute 30–40% of production cost. Therefore, it is most important to explore alternative cost effective substrates for the production of pectinase (Palaniyappan et al., 2009). Several agro-industrial wastes incuding rice bran, wheat bran, wheat straw, sugarcane bagasse, and corncob are abundantly present in environment and could be used as low cost substrates for the production of industrially important enzymes (Bharathiraja et al., 2017). Utilization of these wastes for meaningful purpose is beneficial as increase amount of these waste creates health and environmental issues (Giuntini et al., 2006).
Currently, fermentation technique is considered as a powerful tool for utilization of these organic waste for the synthesis of valuable products by the help of microbes (Thomas et al., 2013). The technique also provide a solution for the disposal of these wastes (Mussatto et al., 2012). However, it has been reported that the process of fermentation is influenced by different physico-chemical parameters such as carbon and nitrogen sources, mineral salts, fermentation time, temperature and pH (Jacob and Prema, 2006; Palaniyappan et al., 2009).
In the light of aforementioned facts, current study was designed to utilize agro-industrial waste as a major source of substrate for production of pectinase that ultimately will decrease production cost of PGase on commercial scale. Among different isolates, Bacillus strain capable of producing PGase was selected and subjected to fermentation using these wastes.
2. Materials & methods
2.1. Qualitative screening for pectinase producer
Different strains of Bacillus licheniformis were isolated and tested for the production of extracellular polygalacturonase (PGase). These strains were cultured in pectin containing broth and incubated at 37 °C for 24 h. For qualitative screening assay, the strains were streaked on pectin containing agar plates and incubated at 37 °C for 24 h. After incubation, the plates were overlaid with potassium-iodide solution (Salomäo et al., 1996). A clear zone of hydrolysis around the colony was an indication of PGase production and on the basis of zone size Bacillus licheniformis KIBE-IB3 was selected for further studies.
2.2. Quantitative screening assay
Bacillus licheniformis KIBE-IB3 was further analyzed for PGase production using submerged fermentation technique. The medium containing citrus pectin (1%), yeast extract (0.3%), potassium dihyrogen phosphate (0.2%), dipotassium hydrogen phosphate (0.2%), potassium nitrate (0.2%), pH: 7.0 was used for extracellular production of enzyme (Rehman et al., 2012). After 24 h of incubation, fermented broth was centrifuged at 40,000 × g for 15 min at 4 °C. The cells were separated and the cell free filtrate (CFF) was assessed for pectinase production by performing enzyme assay.
2.3. Enzyme assay
The activity of PGase was determined by reacting enzyme with substrate (0.5% pectin in 0.025 M phosphate buffer, pH, 7.0) at 40 °C for 30 min. The reducing sugar (galacturonic acid) released by the degradation of pectin was measured by 3, 5, dinitrosalicylic acid method (Miller, 1959).
“One unit of enzyme activity is defined as the amount of polygalacturonase required to release 1.0 μmol of galacturonic acid per minute under standard assay condition”.
2.4. Determination of total protein
Total protein content of cell free filtrate was determined by Lowry’s method using bovine serum albumin as standard (Lowry et al., 1951).
2.5. Selection of agro industrial waste
Five agro-industrial wastes (apple peel, orange peel, lemon peel, potato peel and wheat bran) were selected for the production of PGase, because of their easy accessibility in abundant quantity during this season. For the comparison of pectinase production from these waste with that of commercial available substrate citrus pectin was used as control. All Agro-industrial wastes were dried and crumbled finely in electric grinder. The grinded wastes were then passed through sieve with opening of 9.5 mm, in order to have uniform size particles of the substrate. All the wastes and control (1%) were incorporated separately in selected medium. B. licheniformis KIBE-IB3 was inoculated in each medium and incubated at 37 °C for 24 h and after fermentation analyzed for PGase production.
2.6. Optimization of fermentation parameters
2.6.1. Time course for polygalacturonase (PGase) production
All the growth media having agro-industrial wastes were inoculated and incubated at 37 °C for different time intervals ranging from 24 to 120 h. Enzyme activity was measured after every 24 h from each fermented broth. Maximum enzyme production was observed in fermented broth supplemented with wheat bran after 48 h of incubation and therefore, wheat bran was selected as an appropriate agro waste for further study.
2.6.2. Optimization of wheat bran concentration
Due to increase PGase production from medium having wheat bran and also because of availability of this waste to the whole year, it was selected as a substrate for enzyme production and different concentrations of wheat bran ranging from 0.5% to 2.5% were optimized and incorporated separately in growth media. The media were subjected to fermentation at 37 °C for 48 h after microbial inoculation for pectinase production.
2.6.3. Optimization of fermentation temperature and pH
In order to attain high enzyme titer, the optimum fermentation temperature was determined by incubating broth media at different temperature ranging from 20 °C to 50 °C (pH 7.0). Similarly pH of the growth media was also adjusted from 5.0 to 9.0 to find pH suitable for fermentation and ultimately for maximum enzyme production. The inoculated broth were incubated for fermentation at 35 °C for 48 h.
2.6.4. Effect of organic and inorganic nitrogen sources on polygalacturonase production
To study the influence of organic and inorganic nitrogen sources on enzyme yield, different sources such as peptone and tryptone as organic while, urea and sodium nitrate as inorganic nitrogen sources at a concentration of 1% (w/v) were selected and incorporated in growth media separately. The media having supplemented nitrogen source along with wheat bran were then utilized for PGase production by fermentation.
2.6.5. Effect of salts on polygalacturonase production
Effect of potassium dihydrogen phosphate and dipotassium hydrogen phosphate on enzyme production was also studied by adding each salt (1.0%) separately as well as in combination of equal ratio into the cultivation medium.
3. Results and discussion
3.1. Screening of pectin degrading bacterial strain
Among several bacterial strains screened for polygalacturonase production, Bacillus licheniformis KIBGE-IB3 was selected as best pectinase producer on the basis of largest hydrolytic zone around the colony. Previously, other Bacillus species have also been identified as a pectinase producer by utilizing the same screening assay (Patil et al., 2012). The selected strain was already identified on the basis of phenotypic and genotypic characters. 16S rDNA sequence of Bacillus licheniformis KIBGE-IB3 submitted to GenBank and received accession number GU216260 (Ghani et al., 2013).
3.2. Selection of agro-industrial waste for PGase production
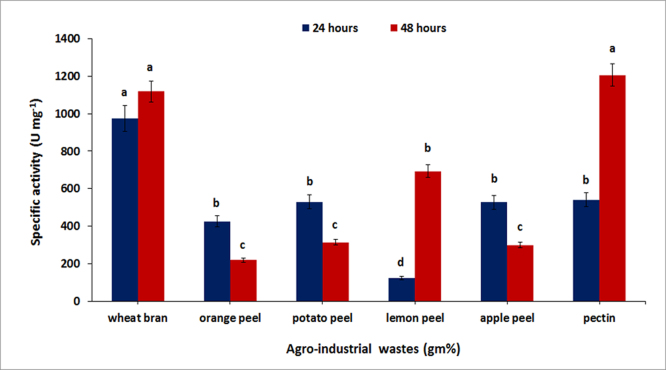
Production of polygalacturonase enzyme was assessed in each fermentation broth having specific agro-industrial waste. It was observed that the medium containing wheat bran at a concentration of 1% (w/v) resulted in high polygalacturonase production (974 U/mg) in contrast to medium having commercially available citrus pectin which showed low enzyme production (540 U/mg) after 24 h of incubation. It was also noticed that enzyme production was increased in both media up to 1118 U/mg and 1206 U/mg respectively after 48 h of fermentation (Fig. 1).
Fig. 1.
Effect of different agro industrial waste for the production of pectinase after 24 h and 48 h. Symbols (Means ± SE, n = 6) having similar letters are not significantly different from each other (Bonferroni test, P < 0.05).
The results revealed that wheat bran supported enhance enzyme production within 24 h as compared to other agro-wastes in the same concentration and the reason might be the presence of essential nutrients present in wheat bran that include pectin, vitamins, different proteins and other sugars contents (Abbasi et al., 2011). The enzyme production in media having other agro-wastes such as; potato peel, apple peel and lemon peel were 304 U/mg, 263U/mg, 493U/mg respectively within 48 h further increase in incubation decreased pectinase production in all the cases. It has been previously reported that the wheat bran act as a good substrate for production of polygalacturonase (Rehman et al., 2012; Abbasi et al., 2011).
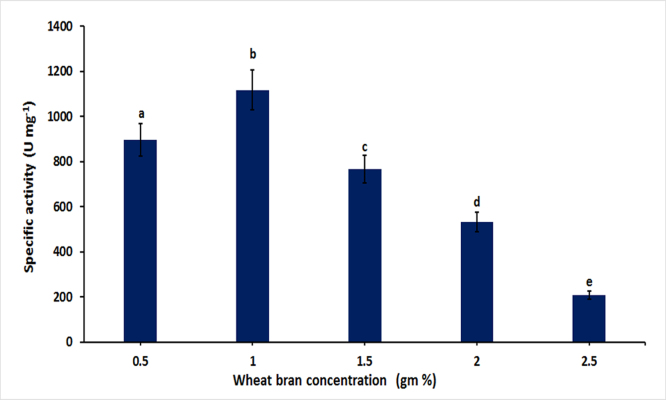
3.3. Optimization of wheat bran concentration
Various concentrations of wheat bran (0.5 gm% −2.5 gm%) were used and maximum enzymes production was observed in the medium containing 1% wheat bran (1119 U/mg). Further increase in concentration decline the PGase production and it was noticed that production was decreased from 52% to 32% when the cultivation media were supplemented with 1.5% and 2% (w/v) wheat bran respectively (Fig. 2). These results indicated that 1% wheat bran was the most suitable concentration for PGase production by Bacillus licheniformis KIBGE-IB3.
Fig. 2.
Effect of different concentration of wheat bran pectin on polygalacturonase production. Symbols (Means ± SE, n = 6) having similar letters are not significantly different from each other (Bonferroni test, P < 0.05).
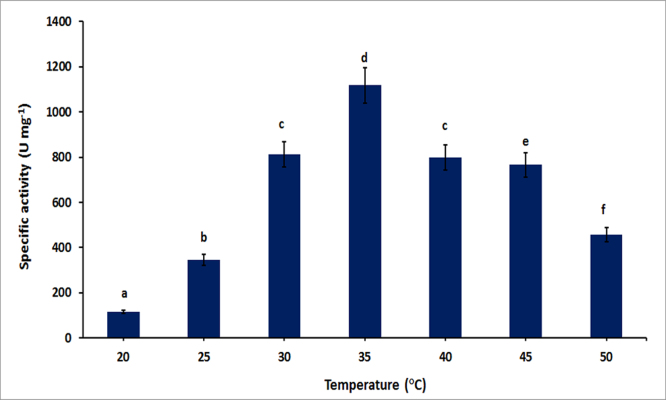
3.4. Effect of temperature on PGase production
The effect of temperature on polygalacturonase production was studied by growing the culture at different temperatures ranging from 20 °C to 50 °C. The results revealed that PGase production increases with the rise in temperature, reaching its maxima at 35 °C with a gradual decline afterward. It was noticed that the production was decreased to 29% and 32% when fermentation was carried out at 40 °C and 45 °C respectively (Fig. 3). A sharp declined in PGase production was observed at 50 °C and it could be due to decreased in bacterial growth as the strain utilized in the current study belongs mesophilic group. The maximum PGase production was also reported from other mesophilic bacteria in the temperature range from 32–37 °C (Soriano et al., 2005). Rehman et al., 2012 also reported 37 °C as the best temperature for PGase production by B. licheniformis (Rehman et al., 2012). However, the PGase production from Mucor circinelloides ITCC6025, Penicillium citrinum and Aspergillus fumigatus was previously mentioned at 42 °C, 40 °C and 60 °C respectively (Thakur et al., 2010; El-Batal et al., 2013; Sandri et al., 2015).
Fig. 3.
Effect of temperature on polygalacturonase production. Symbols (Means ± SE, n = 6) having similar letters are not significantly different from each other (Bonferroni test, P < 0.05).
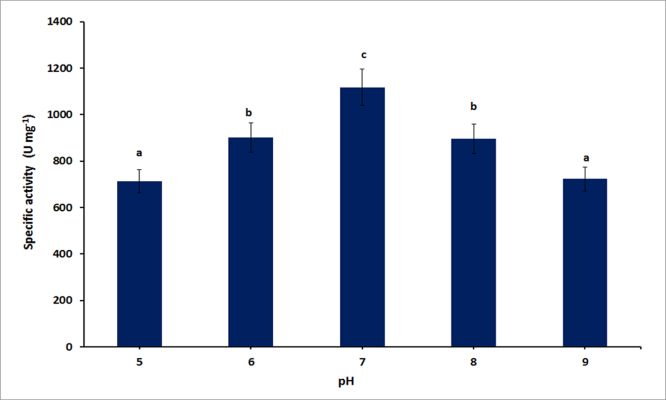
3.5. Effect of pH on PGase production
Effect of pH on PGase production was studied by growing culture overnight in the fermentation media having different pH values ranging from 5.0 to 9.0. It was observed that Bacillus licheniformis KIBGE-IB3 was able to produce pectinase in the pH range from 5.0 to 9.0 with a pH optima at 7.0. However, the enzyme titer reduced before and after the optimum pH value with a decline in 19.4% and 19.89% at pH 6.0 and 8.0 respectively (Fig. 4).
Fig. 4.
Effect of pH on polygalacturonase production. Symbols (Means ± SE, n = 6) having similar letters are not significantly different from each other (Bonferroni test, P < 0.05).
It has been previously reported by researchers that PGase could be produce from Bacillus species in the pH range from 7.0 to 10.0 (Kobayashi et al., 1999). While, in case of Mucor circinelloides ITCC 6025 and Penicillium citrinum maximum production was reported at pH 5.5 and 6.0 respectively (Thakur et al., 2010; El-Batal et al., 2013). Two other groups of scientist also reported 5.00 as suitable pH for the enzyme production from Aspergillus fumigatus (Sandri et al., 2015, Wang et al., 2015).
3.6. Effect of nitrogen sources on PGase production
To further enhance PGase production, different nitrogen sources and mineral salts were incorporated along with wheat bran into the fermentation medium. Among different organic nitrogen sources studied, high yield of PGase (1085.32 U/mg) was achieved in medium supplemented with yeast extract as it has been reported that yeast extract is the best inducer for exopolygalacturonse production from Aspergillus species due to the presence of vitamins, minerals and amino acids (Aguilar et al., 1991). The production was further enhanced (1118.12 U/mg), when from different inorganic nitrogen sources sodium nitrate in combination with yeast extract was added into the cultivation medium (Table 1). However, high production of PGase from B. licheniformis is also reported by using peptone, urea and KNO3 combination of nitrogen sources (Dharmik and Gomashe, 2013). Increase PGase production is already reported, when various nitrogen sources were supplemented along with wheat bran, (Kashyap et al., 2000).
Table 1.
Supplementation of medium with nitrogen sources and mineral salts for enhance PGase production.
| Nitrogen sources (1.0%) |
Specific activity (U mg−1) | Mineral salts (1.0%) | Specific activity (U mg−1) |
|---|---|---|---|
| Yeast extract (YE) | 1085.32 | K2HPO4 | 713.92 |
| Peptone | 585.36 | KH2PO4 | 1315.12 |
| Tryptone | 817.84 | K2HPO4 + KH2PO4 | 991.36 |
| YE + Sodium nitrate | 1118.12 | ||
| YE + Potassium nitrate | 892.37 | ||
| YE + Urea | 367.26 |
3.7. Effect of different salts on PGase production
Addition of different salts in growth medium affect the rate of enzyme production and these salts may cause a change in the osmotic pressure of the surrounding of the bacterial cells (Bazzolli et al., 2006). Therefore, enzyme production was studied by adding KH2PO4 + K2HPO4 separately and also in combination into the medium and it was found that increased PGase production was achieved in medium containing KH2PO4. The media containing both salts (KH2PO4 and K2HPO4) produced comparatively low enzyme (Table 1).
It was reported that KH2PO4 supported enzyme production when 0.05% was added in the medium (Bibi et al., 2014). On the other hand, K2HPO4 was also reported as an important factor to support cell growth and production of different microbial polysaccharides (Gao et al., 2010).
4. Conclusions
The finding explore in this study revealed that Bacillus licheniformis KIBGE IB-3 has potential to produce high amount of pectinase by utilizing different cheap substrates that can be easily accessible during their season. However, wheat bran was not only proven as a best source for hyper production of pectinase but rather, the accessibility of agro-wastes is not seasonal and it remains available in abundant quantity during the whole year. Therefore, the utilization of wheat bran as a substrate for the production of valuable polygalacturonase will reduce the production of enzyme to one side while on the other side helps to utilize agro industrial waste for beneficial purpose. The method used for the production of enzyme is a simple, optimized and eco-friendly.
Declarations
Author contribution statement
Nayyar Jahan, Faiza Shahid, Afsheen Aman, Talat Yasmeen Mujahid, Shah Ali Ul Qader: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abbasi H., Mortazavipour S.R., Setudeh M. Polygalacturonase (PG) production by fungal strains using agro-industrial bioproduct in solid state fermentation. Chem. Eng. Res. Bull. 2011;15(1):1–5. [Google Scholar]
- Aguilar G., Trejo B.A., Garcia J.M., Huitron C. Influence of pH on Endopectinase and Exopectinase Production by Aspergillus sp. Ch-Y-1043. Can. J. Microbiol. 1991;37:912–917. [Google Scholar]
- Bazzolli D.S., Ribon A.O., de Queiroz M.V., de Araújo E.F. Molecular characterization and expression profile of pectin-lyase-encoding genes from Penicillium griseoroseum. Can. J. Microbiol. 2006;52(11):1070–1077. doi: 10.1139/w06-070. [DOI] [PubMed] [Google Scholar]
- Bibi Z., Ansari A., Zohra R.R., Aman A., Ul Qader S.A. Production of xylan degrading endo-1, 4-β-xylanase from thermophilic Geobacillus stearothermophilus KIBGE-IB29. J. Radiat. Res. Appl. Sci. 2014;7:478–485. [Google Scholar]
- Bharathiraja S., Suriya J., Krishnan M., Manivasagan P., Kim S.K. Production of Enzymes From Agricultural Wastes and Their Potential Industrial Applications. Adv. Food Nutr. Res. 2017;80:125–148. doi: 10.1016/bs.afnr.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Castilho L.R., Alves T.L., Medronho R.A. Recovery of pectolytic enzymes produced by solid state culture of Aspergillus niger. Process Biochem. 1999;34(2):181–186. [Google Scholar]
- Castilho L.R., Medronho R.A., Alves T.L.M. Production and extraction of pectinases obtained by solid state fermentation of agroindustrial residues with Aspergillus niger. Bioresour. Technol. 2000;71:45–50. [Google Scholar]
- Croak S., Corredig M. The role of pectin in orange juice stabilization: effect of pectin methylesterase and pectinase activity on the size of cloud particles. Food Hydrocoll. 2006;20:961–965. [Google Scholar]
- Daas P.J., Meyer-Hansen K., Schols H.A., De Ruiter G.A., Voragen A.G. Investigation of the non-esterified galacturonic acid distribution in pectin with endopolygalacturonase. Carbohydr. Res. 1999;318:135–145. [Google Scholar]
- Dharmik P.G., Gomashe A.V. Bacterial polygalacturonase (PG) production from agro industrial waste by solid state fermentation. Ind. J. Appl. Res. 2013;3:439–442. [Google Scholar]
- El-Batal A.I., Osman A.M., Ibrahim A.M.S. Optimization and characterization of polygalacturonase enzyme by gamma irradiated Penicillium citrinum. J. Chem. Pharm. Res. 2013;5:336–337. [Google Scholar]
- Gao W., Kim Y.J., Chung C.H., Li J., Lee J.W. Optimization of mineral salts in medium for enhanced production of pullulan by Aureobasidium pullulans HP-2001 using an orthogonal array method. Biotechnol. Bioprocess Eng. 2010;15(5):837–845. [Google Scholar]
- Ghani M., Ansari A., Aman A., Zohra R.R., Siddiqui N.N., Qader S.A.U. Isolation and characterization of different strains of Bacillus licheniformis for the production of commercially significant enzymes. Pak. J. Pharm. Sci. 2013;26(4):691–697. [PubMed] [Google Scholar]
- Giuntini E., Bazzicalupo M., Castaldini M., Fabiani A., Miclaus N., Piccolo R., Mengoni A. Genetic diversity of dinitrogen-fixing bacterial communities in soil amended with olive husks. Ann. Microbiol. 2006;56(2):83–88. [Google Scholar]
- Gummadi S.N., Panda T. Purification and biochemical properties of microbial pectinases—a review. Process Biochem. 2003;38(7):987–996. [Google Scholar]
- Jacob N., Prema P. Influence of Mode of Fermentation on Production of Polygalacturonase by a Novel Strain of Streptomyces lydicus. Food Technol. Biotechnol. 2006;44(2) [Google Scholar]
- Kashyap D.R., Chandra S., Kaul A., Tewari R. Production, purification and characterization of pectinase from a Bacillus sp. DT7. World J. Microbiol. Biotechnol. 2000;16(3):277–282. [Google Scholar]
- Kobayashi T., Koike K., Yoshimatsu T., Higaki N., Suzumatsu A., Ozawa T., Ito S. Purification and properties of a low-molecular-weight, high-alkaline pectate lyase from an alkaliphilic strain of Bacillus. Biosci. Biotechnol. Biochem. 1999;63(1):65–72. doi: 10.1271/bbb.63.65. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31(3):426–428. [Google Scholar]
- Mussatto S.I., Teixeira J.A., Ballesteros L.F., Martins S. INTECH Open Access Publisher; 2012. Use of agro-industrial wastes in solid-state fermentation processes. [Google Scholar]
- Palaniyappan M., Vijayagopal V., Viswanathan R., Viruthagiri T. Screening of natural substrates and optimization of operating variables on the production of pectinase by submerged fermentation using Aspergillus niger MTCC 281. Afr. J. Biotechnol. 2009;8:682–686. [Google Scholar]
- Patil R.C., Murugkar T.P., Shaikh S.A. Extraction of pectinase from pectinolytic bacteria isolated from carrot waste. Int. J. Pharma Bio Sci. 2012;3:B261–B266. [Google Scholar]
- Phutela U., Dhuna V., Sandhu S., Chadha B.S. Pectinase and polygalacturonase production by a thermophilic Aspergillus fumigatus isolated from decomposting orange peels. Braz. J. Microbiol. 2005;36:63–69. [Google Scholar]
- Rehman H.U., Qader S.A.U., Aman A. Polygalacturonase: Production of pectin depolymerising enzyme from Bacillus licheniformis KIBGE IB-21. Carbohydr. Polym. 2012;90:387–391. doi: 10.1016/j.carbpol.2012.05.055. [DOI] [PubMed] [Google Scholar]
- Rehman H.U., Aman A., Zohra R.R., Qader S.A.U. Immobilization of pectin degrading enzyme from Bacillus licheniformis KIBGE IB-21 using agar-agar as a support. Carbohydr. Polym. 2014;102:622–626. doi: 10.1016/j.carbpol.2013.11.073. [DOI] [PubMed] [Google Scholar]
- Salomäo T.M.F., Amorim A.C.R., Alves V.M.C., Coelho J.L.C., Silva D.O., Araujo E.F.D. Isolation of pectinase hyperproducing mutants of Penicillium expansum. Rev. Microbiol. 1996;27(1):15–18. [Google Scholar]
- Sandri I.G., Fontana R.C., da Silveira M.M. Influence of pH and temperature on the production of polygalacturonases by Aspergillus fumigatus. LWT-Food Scie. Technol. 2015;61(2):430–436. [Google Scholar]
- Schallmey M., Singh A., Ward O.P. Developments in the use of Bacillus species for industrial production. Canadian J. Microbiol. 2004;50(1):1–17. doi: 10.1139/w03-076. [DOI] [PubMed] [Google Scholar]
- Soriano M., Diaz P., Pastor F.I.J. Pectinolytic systems of two aerobic sporogenous bacterial strains with high activity on pectin. Curr. Microbiol. 2005;50:114–118. doi: 10.1007/s00284-004-4382-8. [DOI] [PubMed] [Google Scholar]
- Thakur A., Pahwa R., Singh S., Gupta R. Production, purification, and characterization of polygalacturonase from Mucor circinelloides ITCC 6025. Enzyme Res. 2010 doi: 10.4061/2010/170549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L., Larroche C., Pandey A. Current developments in solid-state fermentation. Biochem. Eng. J. 2013;81:146–161. [Google Scholar]
- Wang S., Lian Z., Wang L., Yang X., Liu Y. Preliminary investigations on a polygalacturonase from Aspergillus fumigatus. Bioresour. Bioprocess. 2015;2(1):1–13. [Google Scholar]