Abstract
We investigated the possibility that manifestations of Lyme disease in certain hosts, such as arthritis and carditis, may be autoimmunity mediated due to molecular mimicry between the bacterium Borrelia burgdorferi and self-components. We first compared amino acid sequences of Streptococcus pyogenes M protein, a known inducer of antibodies that are cross-reactive with myosin, and B. burgdorferi and found significant homologies with OspA protein. We found that S. pyogenes M5-specific antibodies and sera from B. burgdorferi-infected mice reacted with both myosin and B. burgdorferi proteins by Western blots and enzyme-linked immunosorbent assay. To investigate the relationship between self-reactivity and the response to B. burgdorferi, NZB mice, models of autoimmunity, were infected. NZB mice infected with B. burgdorferi developed higher degrees of joint swelling and higher anti-B. burgdorferi immunoglobulin M cross-reactive responses than other strains with identical major histocompatibility complex (DBA/2 and BALB/c). These studies reveal immunological cross-reactivity and suggest that B. burgdorferi may share common epitopes which mimic self-proteins. These implications could be important for certain autoimmunity-susceptible individuals or animals who become infected with B. burgdorferi.
Lyme disease is caused by the spirochete Borrelia burgdorferi (4, 40). One isolate, B31, has been completely sequenced (15). The bacterium is complex, containing more plasmids than other bacteria, and over 90% of these plasmid genes have no similarity to genes outside Borrelia spp. (6, 15). Some of its plasmids are frequently lost during cultivation. Loss of some genes may block experimental in vivo infectivity from one host to another yet may permit pathogen survival even after antibiotic therapy (5). This may allow an inflammatory host response with certain clinical features that resemble autoimmune manifestations (41). Thus, it is plausible that individual clinical manifestations from an infection may vary depending on the combination of host response and pathogen properties. These factors prompted us to consider experiments to support or counter the possibility of an infection-triggered autoimmunity-like phenomenon in certain cases of Lyme disease, especially when the organism or its antigenic material persists beyond an acute period. We decided to pursue experimentation after our database search revealed common motifs between B. burgdorferi and human alpha myosin heavy chain.
There is precedent for infection-triggered autoimmunity or inflammation and a genetic predisposition. Molecular mimicry is one mechanism of autoimmunity following infection (16, 45) and may have relevance to the arthritis and carditis of Lyme disease. Arthritis and autoimmune carditis are common sequelae following infection with Streptococcus pyogenes (16, 45, 46). Autoimmune damage and arthritis can also result secondary to infection with Shigella, Salmonella, and Yersinia (24). Arthritis and arthralgia are common manifestations of Lyme disease (38) and are seen in autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis. It has been postulated that infectious agents may trigger and sustain chronic inflammatory arthritis due to the persistent release or presentation of immunogenic material in affected individuals (2, 3, 20, 32). Alternatively, arthritis may be due to the triggering of anti-self-reactivity from cross-reactive antibodies (Abs) which recognize both the infectious agent and self-components. Both may occur.
In this study, we used the murine NZB model of autoimmune disease for experimental B. burgdorferi infection to determine if it provokes demonstrable features such as joint swelling and anti-B. burgdorferi antibodies which bind to self-components. We also investigated whether antistreptococcal monoclonal Abs (MAbs) that react with streptococcal M proteins and self-components also react with B. burgdorferi-specific proteins.
MATERIALS AND METHODS
Mice.
All mice were obtained from Jackson Laboratories, Bar Harbor, Maine, and housed in the animal facilities at the University of Medicine and Dentistry of New Jersey. DBA/2, BALB/c, and NZB mice were employed in this study. Mice (five mice in a group) were infected with B. burgdorferi, and sera were obtained at various time points following infection. Joint swelling of tibiotarsal joints was assessed weekly. At the time of death, sera were obtained by retro-orbital sinus puncture, and tissues were saved for histopathological examination.
Sequence analysis of protein similarities between OspA of B. burgdorferi and M proteins of S. pyogenes.
GenInfo, a multiple-database information retrieval and analysis system at the National Center for Biotechnology Information, was used to reexamine previously noted sequence homologies between B. burgdorferi lipoproteins and S. pyogenes M proteins (30). Amino acid sequence comparisons between OspA (GenPept accession number AAC6620) and S. pyogenes M protein sequences in the Swiss-Prot, GenPept, and PIR databases were performed by using FASTA software homology comparisons (31). LFASTA was used to determine multiple similarities between B. burgdorferi OspA and S. pyogenes M5 sequences which had known cross-reactivity with self-proteins (8). Inclusion of conservative amino acid changes (amino acid substitutions that share the same properties) increased the overall similarities between these sequences. Peptides derived from the primary structure of the M5 protein were chemically synthesized for use as experimental controls with a DuPont RAMPS manual peptide synthesizer as previously described (19), and the high-performance liquid chromatography-purified synthetic peptide was confirmed by amino acid analysis. The peptide sequences were NT2 (KEALDKYELENHDLKTKN), NT3 (LKTKNEGLKTENEGLKTE), and NT4 (GLKTENEGLKTENEGLKTE).
Borrelia strain and infections.
The low-passage B. burgdorferi isolate 910255 used in these experiments has previously been described (23). Spirochetes were grown in vitro in Barbour-Stoenner-Kelly medium (1), harvested, and counted. Mice were injected subcutaneously with 5 × 105 B. burgdorferi spirochetes in a total volume of 0.3 ml.
Joint lesion assessment.
Arthritic joint lesion development in B. burgdorferi-infected mice was quantitated by measuring changes in the anterior-posterior tibiotarsal joint diameter over time. Before experimental B. burgdorferi infection, baseline joint diameters for all mice were measured and recorded. Measurement of joint swelling in B. burgdorferi-infected mice was performed at weekly intervals by using a spring-loaded microcaliper (Federal, Providence, R.I.). Data are reported as the difference in diameter from the uninfected time zero with each animal serving as its own control.
ELISA.
Enzyme-linked immunosorbent assays (ELISAs) were performed by using Immulon plates according to established protocol with minor modifications. Briefly, plates were coated with antigens at 10 μg/ml. Antigens employed were S. pyogenes M5 protein (obtained as described previously [12]), bovine myosin (Sigma-Aldrich, St. Louis, Mo.), and sonicated B. burgdorferi protein (strain 910255) and OspA (obtained from J. Dunn). Plates were coated with the various antigens for 2 h at room temperature, and 2% bovine serum albumin was used to block the plates. Antistreptococcal MAbs were hybridoma culture supernatants obtained from fusions of BALB/c spleens from mice immunized with purified M type 5 streptococcal membranes as described previously (12, 36). Sera (from mice immunized with B. burgdorferi) or MAbs were added to the blocked and washed triplicate wells and incubated for 1 h at 37°C. Sera from B. burgdorferi-infected animals were used at a 1:100 dilution. Antistreptococcal MAbs were tested as undiluted culture supernatants. Wells were then washed and incubated for 1 h at 37°C with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) (γ chain specific; Sigma-Aldrich) or conjugated goat anti-mouse IgM (μ chain specific; Sigma-Aldrich). After washing, the plates were developed with ABTS [2,2′azinobis(3-ethylbenzthiazolinesulfonic acid] or TMB peroxidase substrate (Kirkegaard & Perry, Gaithersburg, Md.). Specific Ab concentration was determined by incubation of sera on plates coated with either anti-IgM or anti-IgG, and optical density (OD) readings were compared to standards of known IgM or IgG concentrations. Competitive ELISAs to determine polyspecificity (9) were performed by preincubation of sera or monoclonal antibodies with specific antigens (10 μg/ml) at both 1 h at room temperature and overnight at 37°C prior to addition to the ELISA plates. Nonspecific competition was performed with mouse IgM. Percent inhibition was calculated by comparing the OD readings with values obtained with no preincubation as previously described (9).
Western blot analysis.
B. burgdorferi (B31) sonicates (kindly provided by Marc Golingtly, State University of New York, Stony Brook, N.Y.) or recombinant full-length OspA (expressed in Escherichia coli and purified by Triton X-114 and ion exchange chromatography as described previously [11]) was added to Laemmli gel sample reducing buffer consisting of 2% sodium dodecyl sulfate, 60 mM Tris-HCl (pH 6.8), 0.1 M dithiothreitol, 0.005% bromophenol blue, and 10% glycerol and boiled to load each well of the preparative gel with either 16 μg of whole B. burgdorferi sonicates or 1.6 μg of recombinant OspA protein. Molecular weight standards were run in tandem. The Western immunoblot was run and the blot was developed as previously described (35). Mouse sera were reacted with nitrocellulose strips at a 1:100 dilution. Antistreptococcal MAbs were concentrated 10-fold using a SpeedVac. Secondary Abs consisted of goat biotinylated anti-mouse IgM (μ chain specific) or biotinylated anti-mouse IgG (γ chain specific).
Microscopic examination and image acquisition.
High-quality computerized images were obtained from the hematoxylin- and eosin-stained sections by using a DM-RB compound microscope (Leica Microskopie und System GmbH) and the Image Pro Plus version 4.0 image processing and analysis system for Windows (Media Cybernetics, Silver Spring, Md.). A 10× PL Fluotar lens was used for this application. The images were acquired through a DEI-750 CE color camera (Optronics, Goleta, Calif.), and the digitized images were processed using Microsoft Windows NT Workstation, version 4.0, with a Trinitron Pentium III computer. Images were assessed for the number of nuclei present in a fixed area. The total area of the image was obtained as well, allowing for determination of the amount of nuclear area per total image area. While this method was unable to distinguish between cardiac cells and inflammatory cells, the actual nuclear area of cardiac cells for a given area should be roughly the same in both treated and untreated samples. Once the nuclear area and total area values were obtained, they were exported into Microsoft Excel for statistical analysis.
PCR amplification of B. burgdorferi DNA.
PCR amplification and detection of B. burgdorferi DNA followed previously reported procedures that have been shown to have high reliability in detection of B. burgdorferi DNA in mouse tissues (27). The OspA and OspB primers employed have been shown to have 92 to 96% reliability in detecting B. burgdorferi DNA in mouse tissues (27). Primers were as follows: primer set 15/272 (detects a 280-bp portion [positions 15 to 294] of the OspA gene), consisting of OspA6 (ATT GGG AAT AGG TCT AAT ATT AGC CT) and OspA7 (TGT GGT TTG ACC TAG ATC GTC AG); and primer set 1110/1428 (detects a 328-bp portion [positions 1110 to 1438] of the OspB gene), consisting of OspB-1110 (AAA CGC TAA ACA AGA CCT TCC TG) and OspB-1411 (AGC TTT GAG AGT TTC CTC TGT TAT TGA).
RESULTS
Overall, our results show that an autoimmunity-prone NZB mouse can develop a greater degree of clinicopathologic features than a non-autoimmunity-prone mouse. Furthermore, there is a greater antibody response to the inciting infectious pathogen, B. burgdorferi, which is cross-reactive with host tissue.
Joint swelling in mice infected with B. burgdorferi.
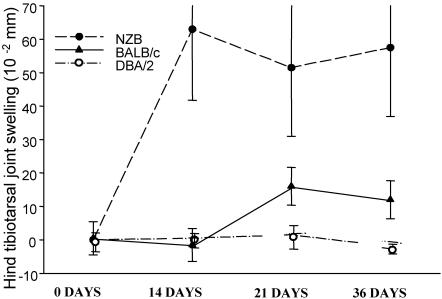
Autoimmune NZB and nonautoimmune mice were infected with B. burgdorferi, and tibiotarsal joint swelling was measured (Fig. 1). Joint swelling was moderate in the NZB mice and maximal at 2 weeks postinfection, while nonautoimmune H-2-matched control strains showed little joint inflammation. This was significant by the Student's t test (<0.05). NZB mice experienced increased joint swelling over the duration of the experiment.
FIG. 1.
Joint swelling in mice at various weeks after infection with B. burgdorferi. Mice were between 9 and 12 months of age at the time of initial infection. Data are means ± standard deviations (approximately five mice/group). Values are shown as the differences from uninfected time zero for each mouse. The NZB group was significantly different from the other groups on day 14 (P < 0.05; Student's t test).
Antibody response in mice infected with B. burgdorferi: anti-B. burgdorferi, anti-OspA, and anti-S. pyogenes M5.
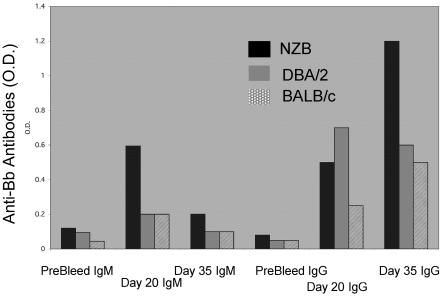
Analysis of the immune reactivity of the sera from all mice infected with B. burgdorferi indicated that the IgM anti-B. burgdorferi response was maximal on day 20 and that autoimmune NZB mice had the highest levels (Fig. 2). The IgM anti-B. burgdorferi response was temporally related to the peak joint swelling observed in these mice. The nonautoimmune strains of mice showed reduced IgM anti-B. burgdorferi responses and reduced joint swelling relative to that observed in the H-2-identical autoimmune NZB mice (Fig. 2). There was little difference between the NZB and the nonautoimmune mice in terms of IgG response at day 20, when joint swelling was observed in NZB mice, but at day 35, a significant increase in the IgG response in NZB mice was seen.
FIG. 2.
Levels of anti-B. burgdorferi antibodies in B. burgdorferi (Bb)-infected animals at various times pre- and postinfection using equivalent serum dilutions. Data in columns represent mean values for OD (O.D.) readings in an ELISA of pooled sera from each group. Sera (diluted 1:10) were obtained from animals studied at 9 to 12 months of age. Columns are IgM (left three groups) and IgG (right three groups) antibodies reactive with B. burgdorferi.
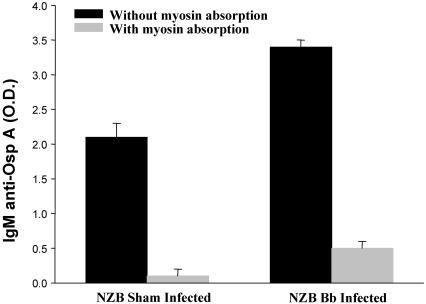
To further characterize the antibody response to B. burgdorferi, the response to a B. burgdorferi-specific protein, OspA, was studied. There was a significant increase in anti-OspA reactivity in NZB mice following infection. (Fig. 3). Restated, it appears that there is an inducible IgM-specific component of anti-OspA antibody when the animal is infected. Since only the NZB strain demonstrated the induction of anti-myosin cross-reactivity following infection with B. burgdorferi, competitive analysis was performed with this strain. No reactive changes to cardiolipin were observed.
FIG. 3.
Levels of IgM anti-OspA antibodies in control and B. burgdorferi-infected NZB mice infected between 5 and 6 months of age and bled on day 35 postinfection. Data in columns represent mean values for OD (O.D.) readings in an ELISA.
Anti-self-reactivity induced in B. burgdorferi-infected mice.
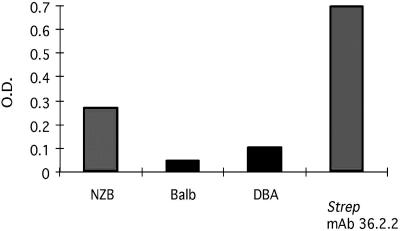
In Fig. 4, the induction of anti-myosin reactivity in mice infected with B. burgdorferi is shown. The NZB mice had more anti-myosin reactivity following B. burgdorferi infection than did the two nonautoimmune strains tested. In general, NZB strains are the most responsive strains (Fig. 5). Approximately 50% of the reactivity of S. pyogenes M MAb (MAb 36.2.2), which was cross-reactive to B. burgdorferi, was reduced (Fig. 6). The IgM level of NZB mice at day 20 was competitively reduced by approximately 90% (Fig. 6). The non-autoimmunity-prone strain, DBA, which had only a limited IgM response to B. burgdorferi after infection at day 20, had an insignificant reduction of that reactivity. Since anti-myosin reactivity is often generated following S. pyogenes infection, we looked for and found a sequence similarity in silico. This similarity was found between B. burgdorferi OspA and S. pyogenes M5 peptide already associated with cross-reactivity (the region of similarity between OspA and S. pyogenes M5 was in the previously identified NT2 region [amino acids 159 to 168]). The induction of anti-S. pyogenes M5 IgM antibodies following infection with B. burgdorferi was significant only in the autoimmunity-prone NZB mice and not with DBA or BALB/c mice on day 20 and day 35 postinfection (Fig. 5). As noted, in the competitive ELISA, only the NZB anti-B. burgdorferi antibodies were significantly decreased (90% reduction) by preincubation with S. pyogenes M5, and for comparison, an antistreptococcal MAb, 36.2.2, with known cross-reactivity with myosin is presented (Fig. 6). We found amino acid sequence similarity between B. burgdorferi OspA and streptococcal M protein, which suggested that B. burgdorferi and S. pyogenes share epitopes which cross-react with self-components. This reactivity was investigated further by Western analysis. Infected NZB and DBA/2 mice both demonstrated anti-B. burgdorferi IgM reactivity with a band at 31 kDa (marked by recombinant B. burgdorferi OspA) (Fig. 7). In addition, the antistreptococcal MAbs recognized this same 31-kDa OspA protein. We used NT3 and NT4 as negative controls. ELISA and Western blot results show that two anti-S. pyogenes M5 MAbs, 36.2.2 and 54.2.8, reacted with full S. pyogenes M5 but not NT3 or NT4 synthetic subpeptides (Fig. 7).
FIG. 4.
The mean ELISA OD (O.D.) readings of IgM anti-myosin reactivity in pooled sera of groups of mice infected with B. burgdorferi 35 days prior to readings. The anti-S. pyogenes (Strep) M5 MAb 36.2.2 was employed for comparison.
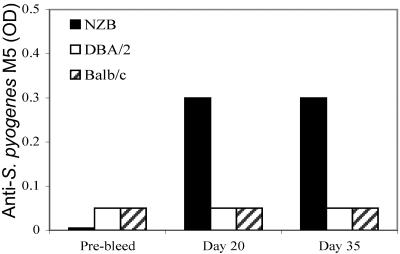
FIG. 5.
Levels of IgM anti-S. pyogenes M5 antibodies in B. burgdorferi-infected animals at various times pre- and postinfection. Data in columns represent mean values for OD readings in an ELISA. Pooled sera (diluted 1:10) were obtained from animals studied at 9 to 12 months of age (approximately five mice/group).
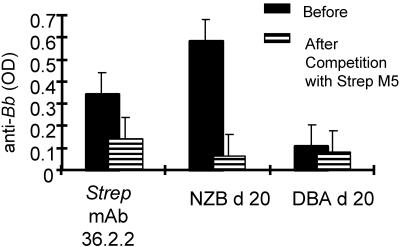
FIG. 6.
Competition of anti-B. burgdorferi (anti-Bb) reactivity with S. pyogenes M5 protein. Sera from 9- to 12-month-old individual NZB and DBA mice obtained on day 20 postinfection with B. burgdorferi or culture supernatants from anti-S. pyogenes (Strep) MAb 36.2.2 were preincubated with 10 μg of S. pyogenes M5 protein and then tested by ELISA for reactivity with B. burgdorferi lysates. Data represent mean OD values.
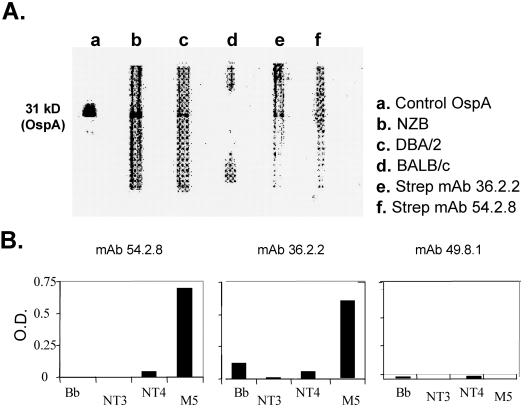
FIG. 7.
(A) Western blot immunoreactivity of serum or monoclonal antibodies to purified recombinant OspA lipoprotein. The control lane contains anti-OspA monoclonal antibody which detects the 31-kDa OspA protein. Sera were obtained from B. burgdorferi-infected NZB (lane b), DBA/2 (lane c), and BALB/c (lane d) mice 35 days following infection. Also shown are anti-S. pyogenes (Strep) M5 IgM MAbs 36.2.2 (lane e) and 54.2.8 (lane f). (Anti-OspA reactivity was detected with specific conjugated goat anti-mouse IgM in all lanes except the control lane, which was reacted with goat anti-mouse IgG.) (B) Anti-S. pyogenes monoclonal antibodies were screened for binding to ELISA plates coated with either B. burgdorferi sonicates, purified M5 protein, or control subpeptides NT3 and NT4. Data in columns represent mean values for OD readings in an ELISA.
Carditis following B. burgdorferi infection in NZB mice.
Heart tissue was studied for the presence of B. burgdorferi spirochetes and inflammation. Infected NZB mice remained positive for the presence of B. burgdorferi DNA throughout the duration of the experiment (Table 1). Controls were negative for the presence of spirochetes in the heart as well as inflammation. Microscopic examination of heart tissue showed that inflammation in the heart was readily observed in NZB mice following B. burgdorferi infection. This was quantified by enumerating cellular infiltrates (nuclear ratios). A statistically significant increase in cellular infiltration was observed in the B. burgdorferi-infected hearts. The computer analysis showed that there were 663 ± 36.9 cells in the control mice compared to 996.8 ± 72.4 cells in the infected mice.
TABLE 1.
Persistent presence of B. burgdorferi in the hearts of infected mice
| Mice | Time of B. burgdorferi infection | % PCR positive for OspA (no. positive/total)a |
|---|---|---|
| NZB | None (control) | 0 (0/3) |
| NZB | 6 weeks | 60 (3/5) |
| NZB | 8 months | 100 (4/4) |
Each group was tested for the OspA gene in the heart and spleen by semiquantitative PCR.
DISCUSSION
The present study investigated the possibility of epitope similarities between the infectious agent (B. burgdorferi) responsible for Lyme disease and host proteins. Our experiments took advantage of two well-characterized systems to study self-reactivity. The first system involved the relationship between the M5 protein of S. pyogenes and self-components. This relationship was analyzed further since we noted a sequence similarity between the B. burgdorferi protein OspA and the S. pyogenes M5 protein. The second system used the NZB strain of mice, which develops autoimmune disease characterized by IgM hypergammaglobulinemia due to a subset of B cells, B-1 cells, noted for the polyreactive nature of the IgM they produce. NZB mice were infected with B. burgdorferi to determine if cross-reactive antibodies were produced. The anti-B. burgdorferi response in NZB mice was compared to that of two nonautoimmune strains of mice, DBA/2 and BALB/c, as well as uninfected NZB mice. All three strains are myosin heavy chain identical, H-2d, to control for the fact that the development of Lyme arthritis in humans may be linked to the myosin heavy chain loci (39). We hypothesized that in autoimmunity-prone animals, much of the anti-B. burgdorferi response might also recognize the S. pyogenes M5 protein as well as myosin due to molecular mimicry. We found that the infected NZB strain developed higher levels of IgM anti-B. burgdorferi, which was temporally related to the higher levels of joint swelling observed in NZB mice compared to those of control strains. The IgM anti-B. burgdorferi response in NZB also cross-reacted with S. pyogenes M and myosin, both of which share sequence homology with B. burgdorferi OspA, suggesting a role for molecular mimicry in the generation of these Ab reactivities. These findings are consistent with prior evidence that immune responses to infectious agents may lead to deleterious autoimmune phenomena due to the immunological cross-reactivity of bacterial epitopes and self-components (8, 16).
In this report, sequence similarity between the NT2 peptide of the S. pyogenes M5 protein and B. burgdorferi OspA was noted. Based on the sequence similarity and secondary structure similarities between OspA, S. pyogenes M5 (NT2 peptide), and myosin, the immune response to B. burgdorferi which results in an anti-OspA response may have both beneficial and potentially harmful effects. OspA is a major outer surface protein of B. burgdorferi and is a basic lipoprotein with an approximate size of 31 kDa and demonstrates antigenic variability (29, 44). In the majority, but not all, of spirochetes involved at the onset of infection, OspA is downregulated, whereas OspC is upregulated (10). However, sufficient exposure occurs, since several studies have detected T- and B-cell responses to OspA in the acute phase (21, 25, 35). Antibodies specific for OspA occur in Lyme disease, and when anti-OspA antibodies are passively transferred, they protect (14, 37). Although antibodies play a role in resistance to B. burgdorferi (22), T cells and their associated cytokines are also involved in the type of immune response invoked during B. burgdorferi infections (22, 26, 28). OspA has been shown to cause polyclonal activation of B cells (43), which may result in increased production of polyreactive IgM antibodies which cross-react with self-components in B. burgdorferi-infected NZB mice. However, other mechanisms may be involved.
It is possible that cross-reactive antibodies initially triggered by B. burgdorferi lipoproteins such as OspA have initiated an autoimmune reaction. The IgM response to B. burgdorferi and the ability of S. pyogenes M5 to compete with this reactivity further suggest that cross-reactive antibodies induced by B. burgdorferi may be involved in mimicry to self-components and could play a role in certain cases of Lyme arthritis. Anti-B. burgdorferi antibodies, in particular anti-OspA antibodies, may be protective in Lyme disease; however, even healthy patients vaccinated with an OspA-containing formulation developed transient arthralgia (34). However, a reactive arthritis was not a universal finding in the OspA vaccine recipients (42). In some cases, the anti-B. burgdorferi response may have undesirable effects which may be more severe in individuals with a predisposition towards autoimmunity. A component of the anti-B. burgdorferi response is T-cell independent (13), and a subpopulation of T-cell-independent B. burgdorferi-responsive B cells may produce polyreactive antibodies which cross-react with self-components. In the present study, the fact that the anti-S. pyogenes M monoclonal antibodies (which recognized the NT2 peptide) also reacted with B. burgdorferi proteins suggests that the polyreactive nature of IgM antibodies elicited in response to both S. pyogenes and B. burgdorferi may play a role in autoimmunity and the development of arthritis. In addition to sequence similarity between B. burgdorferi proteins and S. pyogenes M proteins, other infectious agents such as coxsackie B3 virus, known to induce autoimmune damage, also have sequence similarity to S. pyogenes M5 (17, 19). These studies indicate that pathogenic immune response to cross-reactive self-epitopes may be responsible for cardiac injury following infection with a number of agents. In this report, we provide evidence that similar to the anti-S. pyogenes response, anti-B. burgdorferi antibodies may have the ability to cross-react with self-components and lead to autoimmune tissue reactivity or impairment of function.
Studies with non-autoimmunity-prone mice have shown that in the course of an immune response to foreign antigen, autoreactivity arises normally and is eliminated by apoptosis (33). Autoimmunity-prone individuals may have an increase in the production of polyspecific IgM antibodies which cross-react with self-components as well as defective apoptosis induction which would otherwise eliminate self-reactive B cells, including those which have acquired self-reactivity as a result of somatic mutation. This suggests that the basic mechanism leading toward the production of autoantibodies may lead to the induction of cross-reactive antibodies following some infections, as was observed in the present study. It is conceivable that cross-reactive Abs may remain at low circulating levels but may still be tissue bound.
Immunological response to B. burgdorferi may be responsible for many of the symptoms associated with Lyme disease, and Ab production may be crucial to clearance of this spirochete. It is unclear which isotype may be more effective in clearance, but Th1 or Th2 subsets determine the predominant immunoglobulin class involved. In patients who have treatment-resistant chronic Lyme disease, autoimmune mechanisms may play a role in persistent disease (18, 41).
We found that the majority of our infected animals were PCR positive for B. burgdorferi DNA. Thus, B. burgdorferi may be an active agent in causing cardiac damage or dysfunction (7), but the mechanism of the usual human transient conduction defections remains unexplained.
In summary, our data, albeit preliminary, suggest that the degrees of autoimmunity in Lyme disease warrant further investigation to distinguish pathogenetically related possibilities from epiphenomena.
Acknowledgments
We acknowledge A. Keane-Myers and J. Luan for technical contributions and M. E. McSweegan for his suggestions.
This work was supported in part by NIH grant CA71478-07 and the Lyme Disease Association.
REFERENCES
- 1.Barbour, A. G. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57:521-525. [PMC free article] [PubMed] [Google Scholar]
- 2.Barthold, S. W., K. D. Moody, G. A. Terwilliger, P. H. Duray, R. O. Jacoby, and A. C. Steere. 1988. Experimental Lyme arthritis in rats infected with Borrelia burgdorferi. J. Infect. Dis. 157:842-846. [DOI] [PubMed] [Google Scholar]
- 3.Barthold, S. W., K. D. Moody, G. A. Terwilliger, R. O. Jacoby, and A. C. Steere. 1988. An animal model for Lyme arthritis. Ann. N. Y. Acad. Sci. 539:264-273. [DOI] [PubMed] [Google Scholar]
- 4.Benach, J. L., E. M. Bosler, J. P. Hanrahan, J. L. Coleman, G. S. Habicht, T. F. Bast, D. J. Cameron, J. L. Ziegler, A. G. Barbour, W. Burgdorfer, R. Edelman, and R. A. Kaslow. 1983. Spirochetes isolated from the blood of two patients with Lyme disease. N. Engl. J. Med. 308:740-742. [DOI] [PubMed] [Google Scholar]
- 5.Bockenstedt, L. K., J. Mao, E. Hodzic, S. W. Barthold, and D. Fish. 2002. Detection of attenuated, noninfectious spirochetes in Borrelia burgdorferi-infected mice after antibiotic treatment. J. Infect. Dis. 186:1430-1437. [DOI] [PubMed] [Google Scholar]
- 6.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 7.Cuffe, M. S. 1999. Lyme disease: an active agent in dilated cardiomyopathy? Am. Heart J. 138:198-199. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham, M. W., S. M. Antone, J. M. Gulizia, B. M. McManus, V. A. Fischetti, and C. J. Gauntt. 1992. Cytotoxic and viral neutralizing antibodies crossreact with streptococcal M protein, enteroviruses, and human cardiac myosin. Proc. Natl. Acad. Sci. USA 89:1320-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham, M. W., and R. A. Swerlick. 1986. Polyspecificity of antistreptococcal murine monoclonal antibodies and their implications in autoimmunity. J. Exp. Med. 164:998-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Silva, A. M., S. R. Telford III, L. R. Brunet, S. W. Barthold, and E. Fikrig. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 183:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn, J. J., B. N. Lade, and A. G. Barbour. 1990. Outer surface protein A (OspA) from the Lyme disease spirochete, Borrelia burgdorferi: high level expression and purification of a soluble recombinant form of OspA. Protein Expr. Purif. 1:159-168. [DOI] [PubMed] [Google Scholar]
- 12.Fenderson, P. G., V. A. Fischetti, and M. W. Cunningham. 1989. Tropomyosin shares immunologic epitopes with group A streptococcal M proteins. J. Immunol. 142:2475-2481. [PubMed] [Google Scholar]
- 13.Fikrig, E., S. W. Barthold, M. Chen, I. S. Grewal, J. Craft, and R. A. Flavell. 1996. Protective antibodies in murine Lyme disease arise independently of CD40 ligand. J. Immunol. 157:1-3. [PubMed] [Google Scholar]
- 14.Fikrig, E., S. W. Barthold, F. S. Kantor, and R. A. Flavell. 1990. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science 250:553-556. [DOI] [PubMed] [Google Scholar]
- 15.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 16.Froude, J., A. Gibofsky, D. R. Buskirk, A. Khanna, and J. B. Zabriskie. 1989. Cross-reactivity between streptococcus and human tissue: a model of molecular mimicry and autoimmunity. Curr. Top. Microbiol. Immunol. 145:5-26. [DOI] [PubMed] [Google Scholar]
- 17.Gauntt, C. J., H. M. Arizpe, A. L. Higdon, H. J. Wood, D. F. Bowers, M. M. Rozek, and R. Crawley. 1995. Molecular mimicry, anti-coxsackievirus B3 neutralizing monoclonal antibodies, and myocarditis. J. Immunol. 154:2983-2995. [PubMed] [Google Scholar]
- 18.Hu, L. T., and M. S. Klempner. 1997. Host-pathogen interactions in the immunopathogenesis of Lyme disease. J. Clin. Immunol. 17:354-365. [DOI] [PubMed] [Google Scholar]
- 19.Huber, S. A., A. Moraska, and M. Cunningham. 1994. Alterations in major histocompatibility complex association of myocarditis induced by coxsackievirus B3 mutants selected with monoclonal antibodies to group A streptococci. Proc. Natl. Acad. Sci. USA 91:5543-5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston, Y. E., P. H. Duray, A. C. Steere, M. Kashgarian, J. Buza, S. E. Malawista, and P. W. Askenase. 1985. Lyme arthritis. Spirochetes found in synovial microangiopathic lesions. Am. J. Pathol. 118:26-34. [PMC free article] [PubMed] [Google Scholar]
- 21.Kalish, R. A., J. M. Leong, and A. C. Steere. 1995. Early and late antibody responses to full-length and truncated constructs of outer surface protein A of Borrelia burgdorferi in Lyme disease. Infect. Immun. 63:2228-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keane-Myers, A., C. R. Maliszewski, F. D. Finkelman, and S. P. Nickell. 1996. Recombinant IL-4 treatment augments resistance to Borrelia burgdorferi infections in both normal susceptible and antibody-deficient susceptible mice. J. Immunol. 156:2488-2494. [PubMed] [Google Scholar]
- 23.Keane-Myers, A., and S. P. Nickell. 1995. T cell subset-dependent modulation of immunity to Borrelia burgdorferi in mice. J. Immunol. 154:1770-1776. [PubMed] [Google Scholar]
- 24.Kingsley, G. H. 1993. Reactive arthritis: a paradigm for inflammatory arthritis. Clin. Exp. Rheumatol. 11(Suppl. 8):S29-S36. [PubMed] [Google Scholar]
- 25.Krause, A., G. R. Burmester, A. Rensing, C. Schoerner, U. E. Schaible, M. M. Simon, P. Herzer, M. D. Kramer, and R. Wallich. 1992. Cellular immune reactivity to recombinant OspA and flagellin from Borrelia burgdorferi in patients with Lyme borreliosis. Complexity of humoral and cellular immune responses. J. Clin. Investig. 90:1077-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim, L. C., D. M. England, N. J. Glowacki, B. K. DuChateau, and R. F. Schell. 1995. Involvement of CD4+ T lymphocytes in induction of severe destructive Lyme arthritis in inbred LSH hamsters. Infect. Immun. 63:4818-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malawista, S. E., S. W. Barthold, and D. H. Persing. 1994. Fate of Borrelia burgdorferi DNA in tissues of infected mice after antibiotic treatment. J. Infect. Dis. 170:1312-1316. [DOI] [PubMed] [Google Scholar]
- 28.Matyniak, J. E., and S. L. Reiner. 1995. T helper phenotype and genetic susceptibility in experimental Lyme disease. J. Exp. Med. 181:1251-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGrath, B. C., J. J. Dunn, G. Gorgone, D. Guttman, D. Dykhuizen, and B. J. Luft. 1995. Identification of an immunologically important hypervariable domain of major outer surface protein A of Borrelia burgdorferi. Infect. Immun. 63:1356-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norris, S. J., C. J. Carter, J. K. Howell, and A. G. Barbour. 1992. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect. Immun. 60:4662-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearson, W. R., and D. J. Lipman. 1988. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 85:2444-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Priem, S., G. R. Burmester, T. Kamradt, K. Wolbart, M. G. Rittig, and A. Krause. 1998. Detection of Borrelia burgdorferi by polymerase chain reaction in synovial membrane, but not in synovial fluid from patients with persisting Lyme arthritis after antibiotic therapy. Ann. Rheum. Dis. 57:118-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ray, S. K., C. Putterman, and B. Diamond. 1996. Pathogenic autoantibodies are routinely generated during the response to foreign antigen: a paradigm for autoimmune disease. Proc. Natl. Acad. Sci. USA 93:2019-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoen, R. T., F. Meurice, C. M. Brunet, S. Cretella, D. S. Krause, J. E. Craft, and E. Fikrig. 1995. Safety and immunogenicity of an outer surface protein A vaccine in subjects with previous Lyme disease. J. Infect. Dis. 172:1324-1329. [DOI] [PubMed] [Google Scholar]
- 35.Schutzer, S. E., P. K. Coyle, J. J. Dunn, B. J. Luft, and M. Brunner. 1994. Early and specific antibody response to OspA in Lyme Disease. J. Clin. Investig. 94:454-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shikhman, A. R., N. S. Greenspan, and M. W. Cunningham. 1993. A subset of mouse monoclonal antibodies cross-reactive with cytoskeletal proteins and group A streptococcal M proteins recognizes N-acetyl-beta-D-glucosamine. J. Immunol. 151:3902-3913. [PubMed] [Google Scholar]
- 37.Simon, M. M., U. E. Schaible, M. D. Kramer, C. Eckerskorn, C. Museteanu, H. K. Muller-Hermelink, and R. Wallich. 1991. Recombinant outer surface protein a from Borrelia burgdorferi induces antibodies protective against spirochetal infection in mice. J. Infect. Dis. 164:123-132. [DOI] [PubMed] [Google Scholar]
- 38.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 39.Steere, A. C., E. Dwyer, and R. Winchester. 1990. Association of chronic Lyme arthritis with HLA-DR4 and HLA-DR2 alleles. N. Engl. J. Med. 323:219-223. [DOI] [PubMed] [Google Scholar]
- 40.Steere, A. C., R. L. Grodzicki, A. N. Kornblatt, J. E. Craft, A. G. Barbour, W. Burgdorfer, G. P. Schmid, E. Johnson, and S. E. Malawista. 1983. The spirochetal etiology of Lyme disease. N. Engl. J. Med. 308:733-740. [DOI] [PubMed] [Google Scholar]
- 41.Steere, A. C., D. Gross, A. L. Meyer, and B. T. Huber. 2001. Autoimmune mechanisms in antibiotic treatment-resistant Lyme arthritis. J. Autoimmun. 16:263-268. [DOI] [PubMed] [Google Scholar]
- 42.Steere, A. C., V. K. Sikand, F. Meurice, D. L. Parenti, E. Fikrig, R. T. Schoen, J. Nowakowski, C. H. Schmid, S. Laukamp, C. Buscarino, D. S. Krause, et al. 1998. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. N. Engl. J. Med. 339:209-215. [DOI] [PubMed] [Google Scholar]
- 43.Tai, K. F., Y. Ma, and J. J. Weis. 1994. Normal human B lymphocytes and mononuclear cells respond to the mitogenic and cytokine-stimulatory activities of Borrelia burgdorferi and its lipoprotein OspA. Infect. Immun. 62:520-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilske, B., A. G. Barbour, S. Bergstrom, N. Burman, B. I. Restrepo, P. A. Rosa, T. Schwan, E. Soutschek, and R. Wallich. 1992. Antigenic variation and strain heterogeneity in Borrelia spp. Res. Microbiol. 143:583-596. [DOI] [PubMed] [Google Scholar]
- 45.Zabriskie, J. B. 1969. The relationship of streptococcal cross-reactive antigens to rheumatic fever. Transplant. Proc. 1:968-975. [PubMed] [Google Scholar]
- 46.Zabriskie, J. B. 1986. Rheumatic fever: a model for the pathological consequences of microbial-host mimicry. Clin. Exp. Rheumatol. 4:65-73. [PubMed] [Google Scholar]