Abstract
To investigate whether genetic variants of A. fumigatus are found among clinical isolates, four isolates that were originally identified as poorly sporulating strains of Aspergillus fumigatus were subjected to molecular analysis. DNA sequence analysis of the alkaline protease genes of these isolates showed that each is genetically distinct and each shows substantial variation (7 to 11%) from the A. fumigatus nucleotide sequence. Subsequent morphological examination suggested that all of the isolates could be classified as Aspergillus viridinutans. To clarify the taxonomic status of these four clinical isolates and of two previously identified as atypical A. fumigatus isolates, partial β-tubulin and 18S rRNA gene sequences were determined. Each of the six atypical strains had a unique β-tubulin sequence, whereas the sequences of three standard isolates of A. fumigatus, which were included as controls, were identical to the published A. fumigatus β-tubulin sequence. The very low level of DNA sequence variation detected in standard isolates of A. fumigatus compared with other isolates from members of Aspergillus section Fumigati suggests that it may be a relatively recently evolved species. The 18S rRNA gene of two of the atypical isolates differed from that of A. fumigatus at a single nucleotide position. Phylogenetic analyses do not support the classification of all of these isolates as A. viridinutans. Thus, some of these isolates represent new species which are potential opportunistic pathogens.
Aspergillus fumigatus is the species most commonly associated with aspergillosis in immunocompromised patients. A number of DNA-based methods have been developed to detect A. fumigatus for diagnostic purposes and to type strains to study the origin and transmission of nosocomial infections. Diagnostic methods using PCR rely on amplification of specific DNA sequences, which could vary between strains. Sequence analysis of protein coding genes such as the cytochrome b gene (21) has revealed little or no sequence variation between standard isolates of A. fumigatus. In contrast, seven isolates of Aspergillus viridinutans, another asexual species in the section Fumigati, showed considerable genetic variability in β-tubulin gene sequences (19).
Members of the Aspergillus section Fumigati are distinguished by the profiles of mycotoxins and secondary metabolites that they produce (2) and, in the case of species with known sexual states (genus Neosartorya), the morphology of the ascospores (17). The evolutionary relationship of A. fumigatus to other species from Aspergillus section Fumigati has been investigated using DNA sequence data obtained from the β-tubulin gene (3), the hydrophobin gene (3), and the mitochondrial cytochrome b gene (21). These analyses have shown that morphological variation in isolates of A. fumigatus is not necessarily accompanied by variation in chemical profiles or DNA sequences (2, 21).
In a previous study, DNA sequence analysis of the alkaline protease gene (Alp) was used to investigate two atypical isolates of A. fumigatus (NSW3 and FRR 1266) (8). The sequence obtained from an isolate with standard morphology (QLD1) showed greater than 99% identity with published sequences for three human isolates. However, the two atypical isolates differed by more than 6 and 10% of the nucleotides, respectively (8). Subsequent phylogenetic analysis of β-tubulin sequences by Varga et al. (20) indicated that FRR 1266 is closely related to A. viridinutans. In this study, we use DNA sequence analysis of the Alp, β-tubulin, and 18S rRNA genes to clarify the taxonomic status of these isolates and four additional clinical isolates with similar characteristics.
MATERIALS AND METHODS
Fungal strains.
The strains used in this study are listed in Table 1. Isolates MK245, MK246, MK284, and MK285 were obtained from the Mycology Reference Laboratory, Royal North Shore Hospital, Sydney, New South Wales, Australia, for identification. Strains NSW3 and QLD1 were obtained from ostriches suffering from aspergillosis (7). These six isolates were considered to be the causative agent of aspergillus infections. FRR 581, FRR 582, and FRR 1266 were provided by Ailsa Hocking and John Pitt of the Commonwealth Scientific and Industrial Research Organisation, Food Science Australia, North Ryde, New South Wales, Australia. The six atypical isolates described in this paper have been deposited in the Commonwealth Scientific and Industrial Research Organisation FRR Culture Collection, North Ryde, New South Wales, 1670 Australia.
TABLE 1.
Source of fungal strains
| Strain | Source | Country of origin | Morphologya | FRR no.b |
|---|---|---|---|---|
| MK245 | Human transplant tissue Recipient, lung | Australia | Atypical | 5678 |
| MK246 | Cat, thoracic mass | Australia | Atypical | 5679 |
| MK284 | Cat, retrobulbar abscess | Australia | Atypical | 5680 |
| MK285 | Cat, respiratory tract | Australia | Atypical | 5681 |
| NSW3 | Ostrich, air sac-lungs | Australia | Atypical | 5677 |
| FRR 1266 | Soil | Australia | Atypical | 1266 |
| QLD1 | Ostrich, air sac-lungs | Australia | Standard | |
| FRR 581 | City refuse | Indonesia | Standard | 581 |
| FRR 582 | Soil | Indonesia | Standard | 582 |
Strains exhibiting colony morphology typical of A. fumigatus are listed as standard.
FRR Culture Collection, Food Science Australia, P.O. Box 52, North Ryde, NSW 1670, Australia.
PCR amplification.
The PCR primers that were used in this study are listed in Table 2.
TABLE 2.
PCR primers used in this study
| Namea | Target gene | Sequence |
|---|---|---|
| rRNA1 | 18S rRNA | 5′-GTGAAACTGCGAATGGCTCA-3′ |
| rRNA2 | 18S rRNA | 5′-CCAACTTTCCGGCTCTGGGG-3′ |
| alp1 | Alkaline protease | 5′-AAACGCAATCTGGAGCGTCG-3′ |
| alp2 | Alkaline protease | 5′-CATTGCCATTGTAGGCAAGC-3′ |
| alp3 | Alkaline protease | 5′-ATTCCTGGCAAGTACATCGTGACCTTCAAG-3′ |
| alp4 | Alkaline protease | 5′-ATTGCCATTGTAGGCAAGCTTGTTGGGGCT-3′ |
| benA1 | β-Tubulin | 5′-AATAGGTGCCGCTTTCTGG-3′ |
| benA2 | β-Tubulin | 5′-AGTTGTCGGGACGGAAGAG-3′ |
Primer rRNA1 corresponds to nucleotides 43 to 62 and primer rRNA2 is complementary to nucleotides 1669 to 1688 in the 18S rRNA gene of A. fumigatus (11). Primer alp1 corresponds to nucleotides 480 to 499 and primer alp2 is complementary to nucleotides 1676 to 1695 in the Alp gene of A. fumigatus (6). Primer alp3 corresponds to nucleotides 393 in the Alp protease gene of A. fumigatus (6). Primers benA1 and benA2 are described in Geiser et al. (3).
DNA sequencing.
The DNA sequences of the PCR products were determined by a combination of direct sequencing of the PCR product and sequencing of the cloned PCR products and subclones derived thereof by standard molecular techniques (16). The sequences of both strands of the Alp gene PCR products were determined. The 18S rRNA gene PCR products were sequenced on one strand and compared to the sequence of the 18S rRNA gene of A. fumigatus. When nucleotide sequence differences were detected, they were confirmed by sequencing both strands. The β-tubulin PCR products were sequenced with the benA1 and benA2 primers as described in Geiser et al. (3).
Fungal morphology.
The morphology of strains MK245, MK246, MK284, MK285, NSW3, and FRR 1266 was examined by John Pitt and Ailsa Hocking at Food Science Australia, North Ryde, New South Wales, Australia.
RESULTS
Alkaline protease gene amplification.
Genetic variation in four clinical isolates (MK245, MK246, MK284, and MK285) was initially assessed by DNA sequence analysis of the Alp gene. Primers alp1 and alp2, which were used previously to amplify part of the Alp gene of A. fumigatus and genetic variants (7, 8), failed to yield amplification products from two of the isolates, MK246 and MK284. Longer primers (alp3 and alp4), based on regions of high similarity in the Alp genes of A. fumigatus, Aspergillus oryzae, Aspergillus nidulans, and Aspergillus flavus, were synthesized (Table 2) and were successfully used to amplify a 1.3-kb section of the Alp gene.
Phylogenetic analysis of alkaline protease gene sequences.
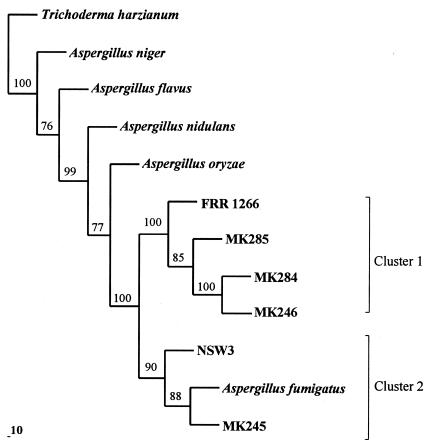
The sequences of the Alp PCR products from the four clinical isolates were compared to the sequences from two strains which we had analyzed previously (NSW3 and FRR 1266) and published sequences for A. fumigatus and other Aspergillus species (Table 3). The isolates fell into two classes. The Alp gene sequence of MK245 showed the highest similarity to NSW3, and the Alp gene of both of these isolates showed a similar degree of nucleotide similarity to the Alp of A. fumigatus (93%) (Table 4). MK246, MK284, and MK285 showed the highest similarity to FRR 1266, and the Alp genes from this group of strain showed 88 to 89% identity to the A. fumigatus Alp gene. The Alp gene sequences of the four clinical isolates were each unique, but the MK246 and MK284 sequences differed by only 2%. Phylogenetic analysis of the Alp sequences supports the division of the six isolates into two groups (Fig. 1). MK245 and NSW3 form a cluster with A. fumigatus (cluster 1), whereas MK246, MK284, MK285, and FRR 1266 form a separate cluster in this tree (cluster 2).
TABLE 3.
Sequences used in phylogenetic analyses
| Species | Strain | Gene | Accession no.a | Reference |
|---|---|---|---|---|
| Aspergillus sp. | MK245 | Alkaline protease | AY590134 (G) | This study |
| β-Tubulin | AY590128 (G) | This study | ||
| Aspergillus sp. | MK246 | Alkaline protease | AY590135 (G) | This study |
| β-Tubulin | AY590129 (G) | This study | ||
| Aspergillus sp. | MK284 | Alkaline protease | AY590136 (G) | This study |
| β-Tubulin | AY590130 (G) | This study | ||
| Aspergillus sp. | MK285 | Alkaline protease | AY590137 (G) | This study |
| β-Tubulin | AY590133 (G) | This study | ||
| Aspergillus sp. | NSW3 | Alkaline protease | Y15871 (E) | 8 |
| β-Tubulin | AY590132 (G) | This study | ||
| Aspergillus sp. | FRR 1266 | Alkaline protease | Y15873 (E) | 8 |
| β-Tubulin | AY590131 (G) | This study | ||
| A. brevipes | NRRL 2439 | β-Tubulin | AF057311 (G) | 3 |
| Aspergillus clavatus | H522 | β-Tubulin | AF057312 (G) | 3 |
| A. flavus | 28 | Alkaline protease | S67840 (E) | 14 |
| Aspergillus fumigatus var. ellipticus | NRRL 5109 | β-Tubulin | AF057314 (G) | 3 |
| A. fumigatus | HD133 | β-Tubulin | AF057315 (G) | 3 |
| CHUV-192-88 | Alkaline protease | Z11580 (Y) | 6 | |
| A. nidulans | MH2 | Alkaline protease | L31778 (G) | 9 |
| A. oryzae | ATCC 20386 | Alkaline protease | S79617 (G) | 10 |
| Aspergillus niger | N400 | Alkaline protease | L19059 (G) | 5 |
| A. viridinutans | IMI 062875 | β-Tubulin | AF134779 (G) | 19 |
| A. viridinutans | IMI 133982 | β-Tubulin | AF134775 (G) | 19 |
| A. viridinutans | IMI 182127 | β-Tubulin | AF134777 (G) | 19 |
| A. viridinutans | IMI 280490 | β-Tubulin | AF134780 (G) | 19 |
| A. viridinutans | NRRL 6106 | β-Tubulin | AF134778 (G) | 19 |
| N. aureola | NRRL 2244 | β-Tubulin | AF057319 (G) | 3 |
| N. fischeri | NRRL 181 | β-Tubulin | AF057322 (G) | 3 |
| N. pseudofischeri | NRRL 20748 | β-Tubulin | AF057325 (G) | 3 |
| N. spinosa | NRRL 5034 | β-Tubulin | AF057329 (G) | 3 |
| Trichoderma harzianum | IMI 206040 | Alkaline protease | M87518 (G) | 4 |
Sequences were obtained from GenBank (G) or EMBL (E) DNA databases through the Australian National Genome Information Service (ANGIS).
TABLE 4.
DNA sequence similarity in the alkaline protease genes of Aspergillusa
| Organismb | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 A. fumigatus | 100 | |||||||||||
| 2 NSW3 | 93 | 100 | ||||||||||
| 3 MK245 | 93 | 95 | 100 | |||||||||
| 4 FRR 1266 | 88 | 92 | 90 | 100 | ||||||||
| 5 MK285 | 89 | 91 | 90 | 94 | 100 | |||||||
| 6 MK246 | 89 | 91 | 91 | 94 | 94 | 100 | ||||||
| 7 MK284 | 89 | 91 | 91 | 94 | 95 | 98 | 100 | |||||
| 8 A. oryzae | 72 | 73 | 72 | 70 | 71 | 71 | 71 | 100 | ||||
| 9 A. nidulans | 68 | 69 | 69 | 69 | 69 | 69 | 69 | 65 | 100 | |||
| 10 A. flavus | 67 | 67 | 67 | 66 | 66 | 66 | 66 | 67 | 64 | 100 | ||
| 11 A. niger | 61 | 62 | 62 | 61 | 61 | 61 | 61 | 60 | 59 | 62 | 100 | |
| 12 T. harzianum | 47 | 48 | 48 | 48 | 47 | 47 | 47 | 47 | 47 | 49 | 45 | 100 |
FIG. 1.
Phylogenetic tree based on the DNA sequences corresponding to nucleotides 480 to 1664 in the alkaline protease gene of A. fumigatus (6). The sources of the DNA sequences used in the analysis are given in Table 3. The tree was constructed using the PILEUP, ESEQBOOT, EDNAPARS, and ECONSENSE programs in the PHYLIP computer package (1) through the Australian National Genome Information Service (ANGIS). Only bootstrap values of >60% are shown.
Morphology.
To determine whether the two groups of isolates were distinguished by morphological differences, the isolates were reexamined by John Pitt and Ailsa Hocking at Food Science Australia without prior knowledge of the results of the phylogenetic analysis. They concluded that all the isolates could be classified as A. viridinutans. Colonies of A. viridinutans resemble those of A. fumigatus, except that sporulation is reduced, so colonies are pale green blue rather than deep blue, and growth at 37°C is slower with a colony diameter of 50 mm after 7 days on Czapek yeast agar, rather than >70 mm. A. viridinutans is distinct microscopically in having stipes that are short, <50 μm long, and often bent just below the vesicle to give an asymmetrical appearance, termed nodding by Raper and Fennell (15). Stipes of A. fumigatus are straight and often 300 μm long. Vesicles are small and up to 15 μm wide, while those of A. fumigatus are up to 30 μm in diameter. A. viridinutans appears to be endemic to Australia (J. Pitt, personal communication).
Analysis of β-tubulin gene sequences.
Partial β-tubulin gene sequences were available for a number of A. viridinutans isolates (19) and other species in Aspergillus section Fumigati (3, 20). To allow comparison of the six atypical isolates with other members of Aspergillus section Fumigati, the same section of the β-tubulin gene used in previous analyses was amplified with the primers designed by Geiser et al. (3), and the DNA sequences of the PCR products were determined.
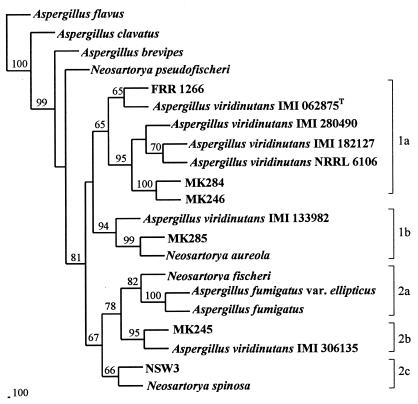
Phylogenetic analysis of the β-tubulin gene sequences from the six atypical isolates, six isolates of A. viridinutans (19), and other species from Aspergillus section Fumigati is shown in Fig. 2. The phylogenetic tree generated by the DNA sequence parsimony method is similar to that shown in Fig. 1 in that it places the cluster 1 and 2 isolates in separate lineages. However, the topology within cluster 1 differs with respect to the position of MK285 (cluster 1b). The cluster 1a isolates (MK246, MK284, and FRR 1266) are grouped with four of the six A. viridinutans isolates, including the type strain IMI 062875. The tree also indicates that MK285 is closely related to Neosartorya aureola (cluster 1b), NSW3 to Neosartorya spinosa (cluster 2c), and MK245 to A. viridinutans IMI 306135 (cluster 2b). The neighbor-joining method gave a tree with identical topology, except that the positions of Aspergillus brevipes and Neosartorya pseudofischeri were exchanged (data not shown).
FIG. 2.
Phylogenetic tree based on the DNA sequences corresponding to nucleotides 1 to 453 in the partial β-tubulin gene sequence of A. fumigatus (3). The sources of the DNA sequences used in the analysis are given in Table 3. The tree was constructed using the PILEUP, ESEQBOOT, EDNAPARS, and ECONSENSE programs in the PHYLIP computer package (1) available through ANGIS. Only bootstrap values of >60% are shown. The type strain (T) of A. viridinutans is indicated.
Partial β-tubulin gene sequences were obtained from three standard isolates of A. fumigatus (Table 1). All sequences were identical to the A. fumigatus sequence reported by Geiser et al. (3). In contrast, a maximum of 98% similarity was observed in the β-tubulin gene sequences of the other isolates that were analyzed (Table 5).
TABLE 5.
DNA sequence similarity in the β-tubulin genes of Aspergillusa
| Organismb | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 A. fumigatus | 100 | ||||||||||
| 2 A. viridinutans IMI 062875 | 90 | 100 | |||||||||
| 3 NSW3 | 92 | 96 | 100 | ||||||||
| 4 MK245 | 91 | 94 | 95 | 100 | |||||||
| 5 MK285 | 90 | 96 | 95 | 93 | 100 | ||||||
| 6 FRR 1266 | 90 | 96 | 96 | 93 | 95 | 100 | |||||
| 7 MK246 | 89 | 95 | 94 | 92 | 94 | 94 | 100 | ||||
| 8 MK284 | 89 | 94 | 95 | 92 | 93 | 94 | 98 | 100 | |||
| 9 N. fischeri | 94 | 93 | 95 | 94 | 93 | 93 | 92 | 91 | 100 | ||
| 10 N. aureola | 88 | 93 | 95 | 93 | 98 | 94 | 93 | 92 | 92 | 100 | |
| 11 N. spinosa | 92 | 97 | 98 | 95 | 96 | 96 | 95 | 94 | 96 | 95 | 100 |
Percent identical nucleotides are given for the sequences corresponding to nucleotides 1 to 453 in the partial β-tubulin gene sequence of A. fumigatus (3). For each pair of sequences, the shorter sequence was used in calculating percent identical nucleotides.
The source of the β-tubulin gene sequences is given in Table 3.
Sequence analysis of the 18S rRNA gene.
The DNA sequence of a 347-bp section of the 18S rRNA gene of NSW3 and FRR 1266 had previously been shown to be identical to the sequence of the 18S rRNA gene of A. fumigatus (8). To further investigate the taxonomic status of these two strains and the four atypical clinical isolates, 1.6 kb of the 18S rRNA gene from each isolate was determined. The DNA sequence of the PCR product generated with primers rRNA1 and rRNA2 (Table 2) from the cluster 1a isolates MK246 and MK285 contained a single nucleotide difference, T instead of C at position 483 of the A. fumigatus 18S rRNA gene sequence reported by Nikkuni et al. (11). The sequences of the remaining four atypical isolates in clusters 1 and 2 were identical to the A. fumigatus 18S rRNA gene.
DISCUSSION
Though morphological examination of the two isolates from cluster 2 (NSW3 and MK245) indicated that they could be classified as A. viridinutans, phylogenetic analysis did not. It has been shown that species recognition based on morphological differences often leads to the inclusion of two or more species, as defined by phylogenetic analyses or mating tests, in a single morphological species (18). For example, nine species were recognized within Fusarium graminearum, based on the phylogenetic analysis of 11 genes (12). Morphological characters, which could be used to distinguish some of these phylogenetically distinct species, were subsequently identified. It may be that further phenotypic examination of the isolates will reveal characteristics which distinguish the atypical isolates in cluster 1 from cluster 2.
The partial β-tubulin gene sequences of two of the atypical isolates show 98% identity with Neosartorya species, NSW3 with N. spinosa (cluster 2c) and MK285 with N. aureola (cluster 1b). These results suggest that these two isolates may represent asexual mutants derived from Neosartorya species or may be closely related asexual species.
MK245 (cluster 2b) grouped with A. viridinutans IMI 306135 (isolated from soil in western Australia) which Varga suggested may represent a new species or a highly unusual isolate of A. fumigatus based on toxin profiles, mitochondrial DNA, restriction fragment length polymorphism analyses, and partial β-tubulin sequences (19). Thus, cluster 2b represents an undescribed species of Aspergillus that is capable of infecting immunocompromised patients.
The partial β-tubulin gene sequences of all the atypical isolates and strains classified as A. viridinutans were unique. In contrast, the partial β-tubulin gene sequences of three standard A. fumigatus isolates from Australia and Indonesia are identical to the sequence obtained from A. fumigatus strain HD133 from the Institut Pasteur in Paris, France. The very low level of DNA sequence variation detected in A. fumigatus compared with other isolates from members of Aspergillus section Fumigati suggests that it may be a relatively recently evolved species.
DNA sequence analysis of two protein-encoding genes of the cluster 1a isolates revealed a considerable degree of genetic variation in this group of strains (from 2 to 8% in pairwise comparisons) (Table 5 and data not shown). The high level of DNA sequence variation and phylogenetic analyses indicate that this group may contain several cryptic species. However, the separate clades within cluster 1a are not all strongly supported. Furthermore, the phylogenetic tree of Alp sequences includes MK285 within this group of atypical isolates, whereas the tree of β-tubulin sequences does not. The phylogenetic species recognition approach proposed by Taylor et al. (18) is based on concordance of multiple gene genealogies. As only two polymorphic genes have been analyzed and the resulting two trees are not concordant, the asexual strains within cluster 1 could be considered a single species (A. viridinutans) by this method. Analysis of additional genes will be required to clarify the taxonomic status of this group.
Analysis of the 18S rRNA gene showed that four of the isolates had a sequence identical to A. fumigatus, and the others (MK246 and MK284) differed at only one position. Thus, some of the isolates in clusters 1 and 2 share an identical 18S rRNA gene sequence. The sequence of the Neosartorya fischeri 18S rRNA gene (GenBank accession no. NFU21299) contains a single-base-pair deletion corresponding to position 1327 of the A. fumigatus sequence (8). Based on our experience with sequencing the 18S rRNA gene, this difference is likely to be due to a sequencing error, as we have observed sequencing anomalies at exactly that position. The sequences of the A. viridinutans, N. aureola, and N. spinosa 18S rRNA genes have not been determined. Sequence analysis of the 28S rRNA genes of A. fumigatus, N. fischeri, N. aureola, and N. spinosa strains has revealed sequence differences between these four species (13). There is no accepted rule on the number of nucleotide differences in rRNA genes which define different species or genera.
Acknowledgments
We gratefully acknowledge the technical assistance of Ron Wicks and Karen Gray. We thank John Pitt and Ailsa Hocking for morphological examinations and David Backhouse for helpful discussions.
REFERENCES
- 1.Felsenstein, J. 1989. PHYLIP: phylogeny inference package (version 3.2). Cladistics 5:164-166. [Google Scholar]
- 2.Frisvad, J. C., and R. A. Samson. 1990. Chemotaxonomy and morphology of Aspergillus fumigatus and related taxa, p. 201-208. In R. A. Samson and J. I. Pitt (ed.), Modern concepts in Penicillium and Aspergillus classification, Plenum Press, New York, N.Y.
- 3.Geiser, D. M., J. C. Frisvad, and J. W. Taylor. 1998. Evolutionary relationships in Aspergillus section Fumigati inferred from partial β-tubulin and hydrophobin DNA sequences. Mycologia 90:831-845. [Google Scholar]
- 4.Geremia, R. A., G. H. Goldman, D. Jacobs, W. Ardiles, S. B. Vila, M. Van Montagu, and A. Herrera-Estrella. Molecular characterization of the proteinase-encoding gene, prb1, related to mycoparasitism by Trichoderma harzianum. Mol. Microbiol. 8:603-613. [DOI] [PubMed]
- 5.Jarai, G., D. Kirchherr, and F. P. Buxton. 1994. Cloning and characterization of the pepD gene of Aspergillus niger which codes for a subtilisin-like protease. Gene 139:51-57. [DOI] [PubMed] [Google Scholar]
- 6.Jaton-Ogay, K., M. Suter, R. Crameri, R. Falchetto, A. Fatih, and M. Monod. 1992. Nucleotide sequence of a genomic and a cDNA clone encoding an extracellular alkaline protease of Aspergillus fumigatus. FEMS Microbiol. Lett. 92:163-168. [DOI] [PubMed] [Google Scholar]
- 7.Katz, M. E., S. C. J. Love, H. S. Gill, and B. F. Cheetham. 1996. Development of a method for the identification, using the polymerase chain reaction, of Aspergillus fumigatus isolated from ostriches. Aust. Vet. J. 74:50-54. [DOI] [PubMed] [Google Scholar]
- 8.Katz, M. E., M. McLoon, S. Burrows, and B. F. Cheetham. 1998. Extreme DNA sequence variation in isolates of Aspergillus fumigatus. FEMS Immunol. Med. Microbiol. 20:283-288. [DOI] [PubMed] [Google Scholar]
- 9.Katz, M. E., R. N. Rice, and B. F. Cheetham. 1994. Isolation and chracterization of an Aspergillus nidulans gene encoding an alkaline protease. Gene 150:287-292. [DOI] [PubMed] [Google Scholar]
- 10.Murakami, K., Y. Ishida, A. Masaki, H. Tatsumi, S. Murakami, E. Nakano, H. Motai, H. Kawabe, and H. Arimura. 1991. Isolation and characterization of the alkaline protease gene of Aspergillus oryzae. Agric. Biol. Chem. 55:2807-2811. [PubMed] [Google Scholar]
- 11.Nikkuni, S., H. Nakajima, S. Hoshina, M. Ohno, C. Suzuki, Y. Kashiwagi, and K. Mori. 1998. Evolutionary relationships among Aspergillus oryzae and related species based on the sequences of 18S rRNA genes and internal transcribed spacers. J. Gen. Appl. Microbiol. 44:225-230. [DOI] [PubMed] [Google Scholar]
- 12.O'Donnell, K., T. J. Ward, D. M. Geiser, H. C. Kistler, and T. Aoki. 2004. Genealogical concordance between the mating type locus and seven other nuclear genes supports formal recognition of nine phylogenetically distinct species within the Fusarium graminearum clade. Fungal. Genet. Biol. 41:600-623. [DOI] [PubMed] [Google Scholar]
- 13.Peterson, S. W. 2000. Phylogenetic relationships in Aspergillus based on rDNA sequence analysis, p. 323-355. In R. A. Samson and J. I. Pitt (ed.), Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Harwood Academic Press, Amsterdam, The Netherlands.
- 14.Ramesh, M. V., T. Sirakova, and P. E. Kolattukudy. 1994. Isolation, characterization, and cloning of cDNA and the gene for an elastinolytic serine proteinase from Aspergillus flavus. Infect. Immun. 62:79-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raper, K. B., and D. F. Fennel. 1965. The genus Aspergillus. Williams and Wilkins, Baltimore, Md.
- 16.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 17.Samson, R. A., P. V. Nielsen, and J. C. Frisvad. 1990. The genus Neosartorya: differentiation by scanning electron microscopy and mycotoxin profiles, p. 455-467. In R. A. Samson and J. I. Pitt (ed.) Modern concepts in Penicillium and Aspergillus classification. Plenum Press, New York, N.Y.
- 18.Taylor, J. W., D. J. Jacobson, S. Kroken, T. Kasuga, D. M. Geiser, D. S. Hibbett, and M. C. Fisher. 2000. Phylogenetic species recognition and species concepts in fungi. Fungal Genet. Biol. 31:21-32. [DOI] [PubMed] [Google Scholar]
- 19.Varga, J., B. Tóth, K. Rigó, F. Debets, and Z. Dozakiewicz. 2000. Genetic variability within the Aspergillus viridinutans species. Folia Microbiol. 45:423-428. [DOI] [PubMed] [Google Scholar]
- 20.Varga, J., Z. Vida, B. Tóth, F. Debets, and Y. Horie. 2000. Phylogenetic analysis of newly described Neosartorya species. Antonie Leeuwenhoek 77:235-239. [DOI] [PubMed] [Google Scholar]
- 21.Wang, L., K. Yokoyama, M. Miyaji, and K. Nishimura. 2000. Mitochondrial cytochrome b gene analysis of Aspergillus fumigatus and related species. J. Clin. Microbiol. 38:1352-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]