Abstract
Objective
Arthritis and vitamin D insufficiency are prevalent in older adults and are risk factors for disability. The objective of this study was to examine the effect of co-ocurring arthritis and vitamin D defficiency on upper-lower extremity functional limitations and disability in older adults.
Methods
We examined 1,533 participants aged ≥50 years from a subsample of the Mexican Health and Aging Study. Measures included sociodemographics, body mass index, comorbid conditions, falls, physical activity, physical function tests, functional limitations, activities of daily living (ADL), and vitamin D. Participants were categorized into four groups according to arthritis and vitamin D status: no vitamin D insufficiency and no arthritis (58.80%), vitamin D insufficiency only (27.49%), arthritis only (8.47%), and arthritis and vitamin D insufficiency (5.24%).
Results
Fourteen percent reported arthritis and 31.2% had vitamin D insufficiency. The arthritis and vitamin D insufficiency group was associated with upper-lower extremity functional limitations (odds ratio [OR] =1.82, 95% Confidence Interval[CI]=1.06-3.15, and OR=1.90, 95%CI=1.00-3.62, respectively) and ADL disability (OR=3.00, 95%CI=1.63-5.51) when compared with the no vitamin D insufficiency and no arthritis group (reference group). The arthritis only group was three times more likely to report upper-lower extremity functional limitations and ADL disability. The vitamin D insufficiency only group was not significantly associated with functional limitations nor ADL disability.
Conclusion
Arthritis and vitamin D insufficiency increased the risk of ADL disability in this population. However, the effect of arthritis and vitamin D insufficiency on upper- lower extremity functional limitations was not higher than the effect of arthritis only, but higher than the effect on vitamin D insufficiency alone.
Keywords: self-reported arthritis, vitamin D, ADL disability, elderly
Introduction
Disability is a public health priority and musculoskeletal diseases are the most common cause of disability worldwide [1]. People with disabilities constitute the world's largest minority and approximately 80% of them live in developing countries [2]. Data from the Health, Well-Being, and Aging in Latin America and the Caribbean Study (SABE) studies, showed that 23.8% to 55.6% of adults aged 60 years and above had self-reported arthritis and was significantly associated with limitation in activities of daily living (ADL) in all studied populations [3]. Data from The Community Oriented Program for the Control of Rheumatic Diseases (COPCORD) questionnaire showed a 9.5% prevalence of osteoarthritis in Mexico, the primary form of arthritis to affect middle and older adults, increasing to 21.4% in those 65 years and over [4]. Based on the 2010-2012 National Health Interview Survey in the U.S., by 2040 the prevalence of arthritis in adults is expected to increase to 25.9%, and the number of adults with activity limitation due to arthritis will increase to 11.4% [5].
Arthritis and several other factors, including non-modifiable risk factors (such as age and gender) and modifiable risk factors (such as diseases, obesity, impairments, sedentary lifestyles, and environmental obstacles) are associated with physical disability [6]. Studies have shown an association between low vitamin D levels and higher risk of disability, muscle weakness, reduced physical performance, balance impairment, and falls [7–11]. Prevalence of vitamin D deficiency/insufficiency for all ages in Latin America ranges from 23.8% to 87% [12–14]. Low vitamin D levels have been found prevalent among persons with arthritis. Emergent evidence suggests that vitamin D plays an important role in immune regulation, and epidemiological data suggest that vitamin D deficiency may be a risk factor for development of autoimmune and chronic diseases [16–17]. Vitamin D has been found to play a role in multiple articular structures, including cartilage, subchondral bone and periarticular muscle, which are risk factors for the progression of knee osteoarthritis [18–19]. Studies have shown that lower serum levels of vitamin D associated with greater knee pain, higher prevalence of radiographic osteoarthritis (OA), incidence of knee pain, progression of radiographic OA, loss of joint space, and osteophyte growth [20–22].
In a report from The World Bank, Latin America and the Caribbean largely mirrored global tendencies in the principal causes of disability [23]. Mexico has experienced important epidemiologic and demographic changes, including declining fertility rate, decreasing mortality rate, increasing life expectancy, and increasing chronic disease burden [24]. As arthritis and vitamin D insufficiency are risk factors for disability, especially in older adult populations which are vulnerable to the disabling effects of arthritis, there is a critical need to reduce the incidence and progression of these conditions. The objective of this study was to examine the effect of co-occurring arthritis and vitamin D defficiency on upper-lower extremity functional limitations and ADL disability among Mexican older adults. We hypothesized that older adults with arthritis and vitamin D insufficiency would have higher upper-lower extremity functional limitations and ADL disability than those with arthritis only or vitamin D insufficiency only.
Patients and Methods
Study population
The sample for this study was drawn from the Mexican Health and Aging Study (MHAS), an ongoing nationally representative longitudinal study of adults in Mexico aged 50 years or older and their spouse or partner, beginning in 2001 [25–26]. Participants were followed in 2003 and 2012. In 2012, a new sample of 5,896 participants was added to the ongoing cohort interviewed in 2001 and 2003 who remained in the study for the third follow-up (n= 12,569), comprising a sample of 18,465 individuals [25]. Experienced professionals from the Instituto Nacional de Salud Pública de Mexico collected intravenous and capillary blood from a subsample of 2,086 participants in 2012, providing data on biomarkers including vitamin D. The details on the study methodology have been reported elsewhere [25]. The study received approval from the University's institutional review board.
Study subsample
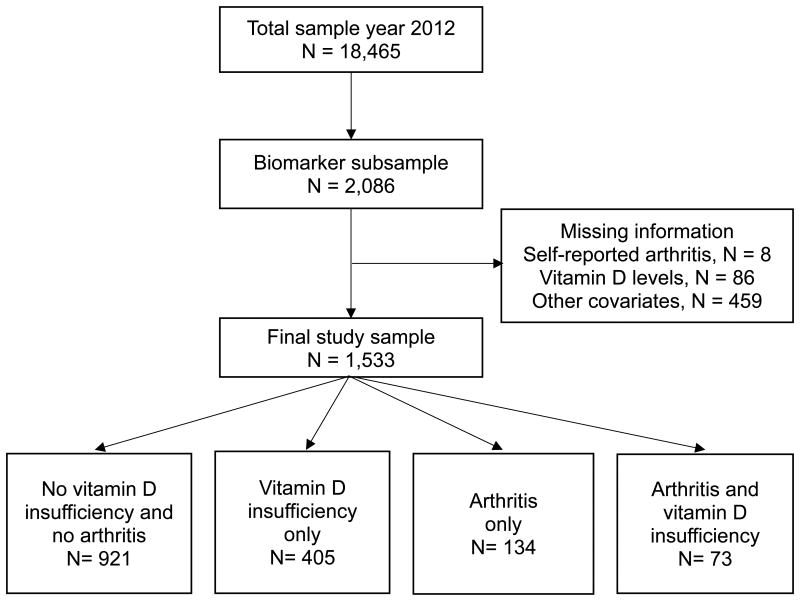
The target subsample included participants providing biomarker data and interviewed in 2012 in four Mexican states. These states included a highly rural state, a highly urban state, a high-U.S.-migration state (people who migrated to the United States and came back to Mexico), and a state with a high prevalence of diabetes [25]. Of 2,086 participants, 8 had missing information on self-reported arthritis, 86 on vitamin D levels, and 459 on any of the covariates (Figure 1). The excluded participants were older, unmarried, less educated, more likely to be male, report any ADL disability and lower extremity limitations, and less likely to live in an urban residence and engage in physical activity than those included for the analyses. No significant difference was found in the percent of self-reported arthritis nor vitamin D insufficiency between our final study sample (n= 1,533) and the biomarker subsample (n= 2,086), with 13.50% vs. 13.52%, and 31.18% vs. 32.20%, respectively [27–28].
Figure 1.
Flow chart of the whole sample.
Independent variables
Arthritis
Self-reported arthritis was established by the answer to the question “Has a doctor or medical personnel ever diagnosed you with arthritis or rheumatism?” Participants with arthritis were also asked about pain and treatment through the questions: “Do you feel pain, stiffness, or swelling in your joints?” and “Are you taking medication or are you receiving other treatment for your arthritis or rheumatism?” The arthritis case definition has been validated in previous studies for surveillance purposes [29–30]. Similar questions have been used in the National Health and Nutrition Examination Surveys [31].
Vitamin D
Serum 25-hydroxyvitamin D, 25(OH)D, was used to measure vitamin D status. This metabolite was estimated through ARCHITECT 25-OH Vitamin D assay, a chemiluminescent microparticle immunoassay (CMIA) for the quantitative serum and plasma determination of 25(OH)D [32]. Participants with serum 25(OH)D levels below 20 ng/dL were categorized as having vitamin D insufficiency [33].
Covariates
Sociodemographic variables included age (as continuous variable), gender, marital status (married vs. unmarried), years of formal education (categorized into four groups: none, 1–5, 6, and 7 years or more of education), residence (urban vs. rural), and residence in a high-U.S.-migration state (selected from the five Mexican states with the highest levels of migration to the U.S.). Self-reported comorbid conditions included hypertension, cardiovascular disease (heart attack and/or stroke), cancer, and fractures. Falls was assessed by asking participants how many times they had fallen and landed on the floor or ground during the past 12 months. Physical activity was assessed by asking participants whether they participated in vigorous physical activity or exercise three times a week or more on average over the last 2 years (yes or no). Body mass index (BMI) was computed as weight in kilograms divided by height in meters squared. Physical function tests included balance and 3-meter walk. The balance test was conducted by asking the participants to stand on any leg and then on the other one for ten seconds each; if they could not stand on any leg for 10 seconds, the test was considered unsuccessful. The walk test was performed twice by measuring the time (in seconds) the participants needed to walk a 3 meter distance. Handgrip strength was measured in both hands with a hand-held dynamometer (Baseline® Dynamometer, Smedley Spring, 220 lb Capacity) [28].
Outcomes
Upper-lower extremity functional limitations
Participants were asked to answer the question, “Please tell me if you have any difficulty with each of the activities that I mention. If you do not do any of the following activities, simply tell me. Do not include difficulties that you believe will last less than three months.” Respondents were asked to indicate ‘yes”, “no”, “cannot do” or “does not do” the activity. Upper extremity activities included reaching/extending their arms above shoulder level, pulling/pushing large objects like a living-room chair, lifting/carrying objects that weigh over 5 kg, and picking up a coin from the table. Lower extremity activities included walking one/several blocks, sitting for about two hours, getting up from a chair after sitting for long periods, climbing one/several flights of stairs without resting, and stooping, kneeling or crouching. Each activity was dichotomized into 1) respondents who answered “yes” or “cannot do” the activity, and 2) those who answered “no limitation” in the activity or “does not do” the activity. Any upper extremity functional limitation was dichotomized as having difficulty or not in performing one or more of the four activities. Any lower extremity functional limitation was dichotomized as having difficulty or not in performing one or more of the seven activities. These questions have been used in previous studies [34 – 35].
Disability included difficulty performing ADLs. Participants were asked to answer the question, “Please tell me if you have any difficulty doing each of the daily activities that I am going to read. Don't include difficulties that you believe will last less than three months.” Respondents were asked to indicate ‘yes”, “no”, “cannot do” or “does not do” the activity. These activities included walking, bathing, eating, going to bed, using the toilet and dressing. Each activity was dichotomized into 1) respondents who answered “yes”, “cannot do” or “does not do” but received help to perform the activity, and 2) those who answered “no” limitation” in the activity. Any disability was dichotomized as having difficulty or not in performing one or more of the six ADLs.
Statistical analysis
To examine the distribution of the unweighted variables by self-reported arthritis and vitamin D categories we used Chi-square test for categorical variables and analysis of variance with post hoc Tukey's test for continuous variables. Unweighted logistic regression analysis was first conducted separately by arthritis (Model 1) and vitamin D insufficiency (Model 2) to show the independent effect of each condition on upper and lower extremity functional limitation and ADL disability. Further analyses were performed by the 4 groups of arthritis and vitamin D insufficiency status (Model 3) to assess their co-occurring effect on upper and lower extremity functional limitation and ADL disability. All 3 models were adjusted for age, gender, marital status, education, residence (rural vs. urban), living in a high-U.S.-migration state, BMI, handgrip strength, 3-meter walk test, balance test, physical activity, comorbid conditions (hypertension, diabetes, cardiovascular disease, and fractures) and falls The interaction effect between arthritis and vitamin D insufficiency was not statistically significant for any of the outcomes. All analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
Results
The average age of the study participants was 61.15 years (Standard Deviation [SD]= 11.68), 58.51% were female, 70.84% married, 36.07% had more than 7 years of education, 60.80% lived in urban residence and 55.51% lived in a high-U.S.-migration state. Fourteen percent reported arthritis and 31.18% had vitamin D insufficiency. The most frequent comorbid conditions were hypertension (40.83%) and diabetes (20.35%). Almost forty percent of the participants reported falls. The mean vitamin D serum level was 23.45 ng/dL (SD= 7.19) in those with arthritis and 24.67 ng/dL (SD= 8.72) in those without arthritis (p= 0.0006).
Of 1,533 participants, 921 (60.08%) were identified as having no arthritis and no vitamin D insufficiency, 405 (26.42%) as having vitamin D insufficiency only, 134 (8.74%) as having arthritis only, and 73 (4.76%) as having arthritis and vitamin D insufficiency. Table 1 presents the descriptive characteristics by arthritis and vitamin D insufficiency grouping. Compared to those with no arthritis and no vitamin D insufficiency, participants with arthritis and vitamin D insufficiency were significantly more likely to be female, unmarried, have fewer years of education, live in urban residences, engage in less physical activity, and report more comorbid conditions. The age mean difference was significant between the arthritis and vitamin D insufficiency group with all groups. The BMI mean difference was significant between the no vitamin D insufficiency and no arthritis group and the vitamin D insufficiency only group. The vitamin D levels mean difference was significant between all groups except for the no vitamin D insufficiency and no arthritis group and the arthritis only group and the arthritis and vitamin D insufficiency group. For gait speed the mean difference was significant comparing all groups with the no vitamin D insufficiency and no arthritis group. For hand grip strength in males, the mean difference was significant between the no vitamin D insufficiency and no arthritis group and the arthritis only group, and between the vitamin D insufficiency only group and the arthritis only group. For hand grip strength in females, the difference was significant between all groups with the no vitamin D insufficiency and no arthritis group.
Table 1.
Descriptive characteristics of the sample by arthritis and vitamin D insufficiency status, n= 1,533.
| Variables | No Vitamin D insufficiency and No Arthritis N (%) |
Vitamin D insufficiency only N (%) |
Arthritis only N (%) |
Arthritis and vitamin D insufficiency N (%) |
P value |
|---|---|---|---|---|---|
| Total | 921 (60.08) | 405 (26.42) | 134 (8.74) | 73 (4.76) | |
| Age, mean (SD) | 59.93 (9.61) | 61.80 (10.58) | 62.81 (9.06) | 67.52 (9.54) | <0.0001 |
| Sex (female) | 482 (52.33) | 270 (66.67) | 87 (64.93) | 58 (79.45) | <0.0001 |
| Marital status (married) | 680 (73.83) | 272 (67.16) | 92 (68.66) | 42 (57.53) | 0.0042 |
| Years of education | |||||
| 0 years | 124 (13.46) | 47 (11.60) | 33 (24.63) | 14 (19.18) | 0.0001 |
| 1–5 years | 254 (27.58) | 92 (22.72) | 34 (25.37) | 24 (32.88) | |
| 6 years | 229 (24.86) | 86 (21.23) | 26 (19.40) | 17 (23.29) | |
| ≥ 7 years | 314 (34.09) | 180 (44.44) | 41 (30.60) | 18 (24.66) | |
| Residence (urban) | 524 (56.89) | 303 (74.81) | 60 (44.78) | 45 (61.64) | <0.0001 |
| High-U.S.-migration state | 561 (60.91) | 189 (46.67) | 66 (49.25) | 35 (47.95) | <0.0001 |
| BMI, mean (SD) | 28.67 (5.13) | 29.53 (5.29) | 29.36 (4.82) | 29.61 (6.20) | 0.0219 |
| Comorbid conditions | |||||
| Hypertension | 351 (38.11) | 171 (42.22) | 68 (50.75) | 36 (49.32) | 0.0130 |
| Diabetes | 153 (16.61) | 113 (27.90) | 25 (18.66) | 21 (28.77) | <0.0001 |
| Fractures | 42 (4.56) | 23 (5.68) | 6 (4.48) | 11 (15.07) | 0.0018 |
| CVD | 46 (4.99) | 17 (4.20) | 4 (2.99) | 5 (6.85) | 0.5640 |
| Cancer | 18 (1.95) | 10 (2.47) | 4 (2.99) | 4 (5.48) | 0.2607 |
| Falls | 349 (37.89) | 159 (39.26) | 59 (44.03) | 27 (36.99) | 0.5727 |
| Vitamin D, mean (SD) | 28.48 (7.60) | 15.99 (3.02) | 27.38 (5.64) | 16.24 (2.76) | <0.0001 |
| UEA limitation | 260 (28.23) | 136 (33.58) | 85 (63.43) | 42 (57.53) | <0.0001 |
| LEA limitation | 459 (49.84) | 212 (52.35) | 109 (81.34) | 57 (78.08) | <0.0001 |
| ADL disability | 92 (9.99) | 61 (15.06) | 30 (22.39) | 25 (34.25) | <0.0001 |
| Balance test ≤ 10 sec. | 483 (52.44) | 255 (62.96) | 79 (58.96) | 58 (79.45) | <0.0001 |
| Walk test, mean (SD) | 4.63 (2.06) | 5.34 (3.84) | 5.45 (2.30) | 5.92 (2.77) | <0.0001 |
| Handgrip, mean (SD) | |||||
| Male | 35.22 (11.18) | 35.94 (11.12) | 29.19 (8.88) | 32.27 (12.71) | 0.0020 |
| Female | 24.98 (9.26) | 22.72 (6.90) | 20.69 (7.99) | 20.17 (8.09) | <0.0001 |
| Physical activity | 466 (50.60) | 150 (37.04) | 62 (46.27) | 27 (36.99) | <0.0001 |
| Pain, stiffness, swelling | ──── | ──── | 126 (94.03) | 58 (79.45) | ──── |
| Treatment for arthritis | ──── | ──── | 72 (53.73) | 46 (63.01) | ──── |
Values are presented as mean (SD) or n (%).
Chi-square tests were used for categorical variables and t tests for continuous variables.
BMI = body mass index. CVD = cardiovascular disease. UEA = upper extremity activity. LEA = lower extremity activity. ADL = activities of daily living.
The majority of participants (94%) with arthritis only reported pain/stiffness/swelling; of these, about half (53.73%) were taking any treatment or medication for this condition. On the other hand, a lower proportion of participants with both arthritis and vitamin D insufficiency (79%) reported pain/stiffness/swelling; of these, 63.01% were taking any medication or treatment.
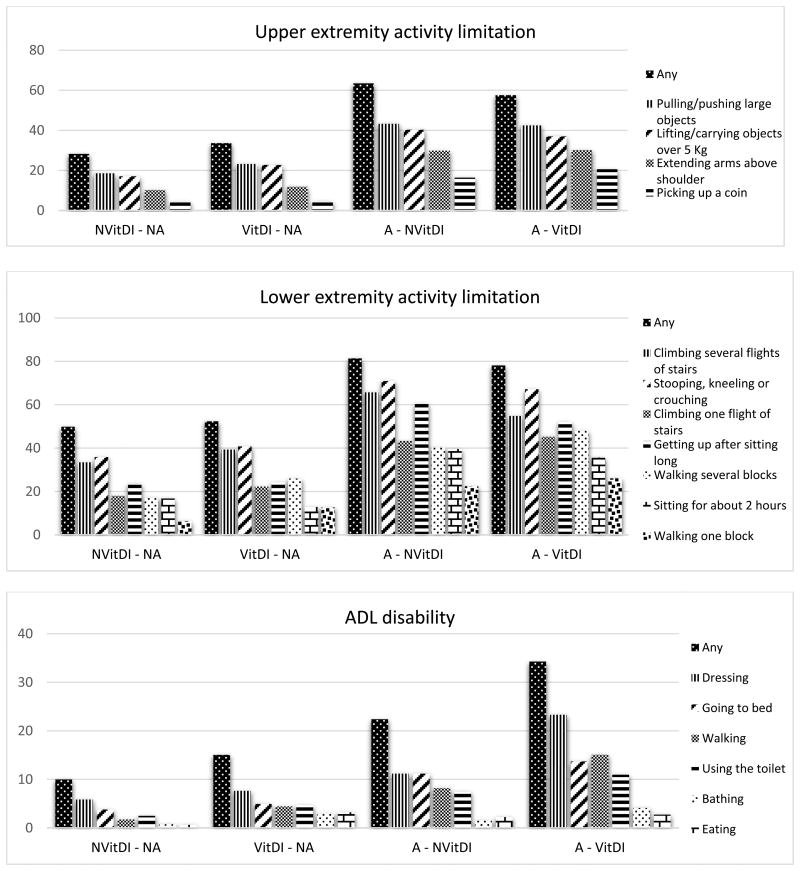
Figure 2 shows the prevalence of each upper-lower extremity functional activity limitations and ADL disability according to arthritis and vitamin D insuficiency status. The percent of participants who reported limitations in upper extremity activities ranged from 28.23% to 63.43%, being higher in the arthritis only group. Pulling/pushing large objects, and lifting/carrying objects over 5 Kg were the most reported limitations in all groups. Further, limitations in lower extremity activities ranged from 49.84% to 81.34%, being higher in the arthritis only group. Climbing one/several flights of stairs, and stooping, kneeling or crouching were the most reported limitations in all groups. ADL disability ranged from 9.99% to 34.25%, being higher in the arthritis and vitamin D insufficiency group. Dressing, going to bed, and walking were the most reported disabilities in all groups, being higher in the arthritis and vitamin D insufficiency group.
Figure 2.
Prevalence of functional limitations and disability by arthritis and vitamin D insufficiency status, n= 1,533.
NVitDI - NA= No Vitamin D Insufficiency - No Arthritis. VitDI - NA= Vitamin D Insufficiency only. A - NVitDI= Arthritis only. A - VitDI= Arthritis and Vitamin D Insufficiency. ADL = activities of daily living.
Table 2 shows the full adjusted logistic regression analysis for any upper-lower extremity functional limitations and for any ADL disability as a function of arthritis (Model 1), vitamin D insufficiency (Model 2) and co-occurring arthritis and vitamin D insufficiency status (Model 3). In Model 1, arthritis was significantly associated with any upper extremity functional limitation [odds ratio (OR)=2.88, 95% Confidence Interval (CI)=2.06-4.04), any lower extremity functional limitation (OR=3.03, 95% CI=2.04-4.49) and ADL disability (OR=2.06, 95% CI=1.39-3.07). In Model 2, vitamin D insufficiency was not associated with any upper-lower extremity functional limitations nor ADL disability. In Model 3, arthritis and vitamin D insufficiency were significantly associated with any upper extremity functional limitation (OR=1.82, 95% CI=1.06-3.15), any lower extremity functional limitation (OR=1.90, 95% CI=1.00-3.62) and ADL disability (OR=3.00, 95% CI=1.63-5.51) when compared with the group of no vitamin D insufficiency and no arthritis (reference group). The arthritis only group was significantly associated with any upper extremity functional limitation (OR=3.50, 95% CI=2.30-5.33), any lower extremity functional limitation (OR=3.43, 95% CI=2.10-5.61) and ADL disability (OR=2.99, 95% CI=1.63-5.51) when compared with the reference group. The group of vitamin D insufficiency only was not significantly associated with any upper-lower extremity functional limitations nor ADL disability when compared with the reference group.
Table 2.
Multivariate logistic regression analysis for functional limitation and disability as a function of arthritis and vitamin D insufficiency status, n= 1,533.
| Variables | Any upper extremity functional limitation OR (95% CI) |
Any lower extremity functional limitation OR (95% CI) |
ADL disability OR (95% CI) |
|---|---|---|---|
| Arthritis (Model 1) | |||
| No arthritis | Reference | Reference | Reference |
| Arthritis | 2.88 (2.06 – 4.04) | 3.03 (2.04 – 4.49) | 2.06 (1.39 – 3.07) |
|
| |||
| Vitamin D insufficiency (Model 2) | |||
| No vitamin D insufficiency | Reference | Reference | Reference |
| Vitamin D insufficiency | 0.84 (0.64 – 1.10) | 0.77 (0.59 – 1.00) | 1.36 (0.96 – 1.93) |
|
| |||
| Co-occurring arthritis and vitamin D insufficiency groups (Model 3) | |||
| No vitamin D insufficiency and no arthritis | Reference | Reference | Reference |
| Vitamin D insufficiency only | 0.90 (0.67 – 1.22) | 0.78 (0.59 – 1.03) | 1.30 (0.88 – 1.94) |
| Arthritis only | 3.50 (2.30 – 5.33) | 3.43 (2.10 – 5.61) | 1.92 (1.15 – 3.20) |
| Arthritis and vitamin D insufficiency | 1.82 (1.06 – 3.15) | 1.90 (1.00 – 3.62) | 2.99 (1.63 – 5.51) |
CI = confidence interval; OR = odds ratio; ADL = activities of daily living.
All models were controlled for age, gender, marital status, education, residence (rural vs. urban), living in a high-U.S.-migration state, BMI, handgrip strength, gait speed, balance, physical activity, comorbid conditions (hypertension, diabetes, cardiovascular disease, and fractures) and falls.
We repeated the analysis, redefining functional limitations and ADL disability using thresholds of at least 2 and 3 reported limitations in upper-lower extremities and ADLs to define disabled state. Patterns were similar to those given in Table 2.
Discussion
This study examined the effect of co-occurring arthritis and vitamin D defficiency on any upper-lower extremity functional limitations and ADL disability among Mexican older adults. Fourteen percent reported arthritis and 31.2% had vitamin D insufficiency. Arthritis and vitamin D insufficiency increased the risk of ADL disability in this population. However, the effect of arthritis and vitamin D insufficiency on any upper- lower extremity functional limitations was not higher than the effect of arthritis only, but higher than the effect of vitamin D insufficiency alone.
Our results are consistent with the previous literature that demonstrates the association between vitamin D and arthritis and disability in older adults. Studies have shown that low 25(OH)D increases osteoblastic activity and bone turnover [36]. These changes play an essential role in the onset and progression of cartilage lesions, by causing aberrant bone remodeling, sclerosis, and osteophyte formation, findings seen in osteoarthritis (OA) [37]. Additionally, in vitro studies have found vitamin D receptors (VDR) on several immune cells and that vitamin D metabolites modulate T cell proliferation and dendritic cell function, suggesting that vitamin D deficiency may be a risk factor for development of autoimmune and chronic diseases like rheumatoid arthritis (RA) [17].
Moreover, skeletal muscle cells also express VDR and its effects include increased calcium handling and muscle cell differentiation and proliferation [38]. Some studies have shown an association between vitamin D deficiency and chronic pain, skeletal muscle weakness, deterioration of cognitive function in the elderly, and consequently disability [39–40]. Low vitamin D levels are common in older adults because of reduced efficacy of previtamin D synthesis in the skin and reduced exposure to sunlight; in addition, the few natural food sources of vitamin D means that dietary intake of vitamin D is often inadequate [41]. Hence, vitamin D insufficiency adds an extra risk factor for disability, especially in older adult populations in whom arthritis is the most common disabling condition. In our study, participants with arthritis and vitamin D insufficiency were more likely to have poor performance in the walk test, balance test and handgrip strength. Therefore, functional limitations in the arthritis groups might also be related to deconditioned muscles in the elderly. Additionally, individuals with arthritis and vitamin D insufficiency were more likely to be older, have a higher BMI, and engage in less physical activity. The more inflammation and less mobility, the more likely older adults are to develop pain and hence difficulty upon performing physical activities.
Rossini et al. examining the association of arthritis and serum vitamin D levels with disability in patients with RA found that those not taking vitamin D supplements, lower 25(OH)D levels were correlated with more functional limitations (including activities such as reaching and getting down a 5 pound object from above head level, standing up from a straight chair, and climbing up five steps), higher disease activity, and ADL disability [17]. In another study of consecutive RA patients without vitamin D supplementation, vitamin D deficiency was detected in 76.3% of patients; and low 25(OH)D serum levels were correlated with higher disease activity, functional limitation and depression, while high 25(OH)D levels were correlated with higher physical activity and better quality of life [16].
In our study, participants with arthritis and vitamin D insufficiency were more likely to take medication/treatment and hence less likely to report pain and limitations, in comparison to those with arthritis and without vitamin D insufficiency. This may be because vitamin D has anti-inflammatory properties as well, and low 25(OH)D levels might enhance pain sensitization [42]. However, a previous literature review that sought high quality evidence from randomized controlled trials (RCT) on vitamin D to manage pain in chronic conditions found no consistent pattern that vitamin D treatment was better than placebo for any chronic painful condition [43].
Regarding arthritis, a meta-analysis found significant associations of low 25(OH)D levels with prevalent knee joint space narrowing and with progressive knee OA [44]. Similarly, it has been suggested that 25(OH)D deficiency leads to suboptimal anti-inflammatory immune responses [37]. Consistent with this hypothesis, epidemiological studies have reported an inverse association between serum 25(OH)D concentrations and RA disease activity and severity [45]. Although by far the strongest data suggest that low levels of serum 25(OH)D are associated with arthritis progression, inferring causality is difficult because disease severity can also restrict patient mobility, limit access to sunlight and hence diminish epidermal synthesis of vitamin D.
Inconsistencies between vitamin D supplementation RCT have been reported, some of which showed that vitamin D supplementation can significantly improve muscle function and physical performance among older adults [46–47]; other studies found no effect on physical function or falls [48–49]. These discrepancies may be the result of study population characteristics, inadequate vitamin D supplementation, low calcium intake, or vitamin D status. However, overall data from RCT and meta-analysis support a positive effect of vitamin D supplementation on muscle function, mainly in older individuals with vitamin D insufficiency, where a daily dose of 1,000 International Units (IU) seems to be appropriate to obtain significant improvements [50].
In a Mexican study, 43.6% of adults 14 years and older presented with vitamin D insufficiency [15]. Given the widespread high prevalence of vitamin D insufficiency, and that this disorder may be a key contributor to the progression and severity of arthritis and disability, it stands to reason that vitamin D supplementation could be a simple and relatively inexpensive therapy for people with these conditions, especially older people with vitamin D insufficiency.
Strengths and limitations
Our study has some limitations. First, we used cross-sectional data from 2012 which limits the interpretation of causality between arthritis, vitamin D insufficiency, and disability. It is possible that those participants with functional limitations were less active and had lower vitamin D levels for this reason. Second, we used a subsample from the larger sample interviewed in 2012, and our subsample of participants with arthritis and vitamin D insufficiency was relatively small (n= 73), which limits the generalizability of results to the national Mexican population [26]. Third, we used self-reported arthritis; a similar definition of doctor-diagnosed arthritis has been shown to be a valid measure for surveillance purposes in population-based studies [51]. Fourth, we did not have information on subtypes of arthritis or arthritis severity. Fifth, we had no access to medical records to obtain detailed information on health service use, which may influence the number of patients with arthritis and the treatment they were taking. Sixth, our study lacks information on the type of medication taken, or vitamin D supplementation, which may alter the number of patients with pain, limitations and vitamin D insufficiency. Finally, our measure of physical activity was self-reported, which introduces potential bias in estimating outcomes. The study strengths include a large sample of adults living in rural and urban regions in Mexico and careful collection of physical function measurements by trained interviewers.
Conclusions
Arthritis and vitamin D insufficiency are prevalent among Mexican older adults. Those participants with arthritis and vitamin D insufficiency reported more ADL disabilities when compared to those without arthritis nor vitamin D insufficiency. However, the effect of arthritis and vitamin D insufficiency on any upper-lower extremity functional limitations was not higher than the effect of arthritis only, but higher than the effect of vitamin D insufficiency alone. To prevent disability, early detection and treatment of arthritis and vitamin D insufficiency should be made for Mexican older adults.
Acknowledgments
The study sponsors had no role in the study design, analysis, or interpretation of the data. Study sponsors did not have any role in the writing of the article or the submission to a journal.
Funding: This study was supported by the National Institutes of Health (R01- AG018016, R.W. [PI]) and by the UTMB Sealy Center on Aging.
Footnotes
Compliance with ethical standards: Conflict of interest: The authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent: informed consent was obtained from all individual participants included in the study.
References
- 1.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81(9):646–56. [PMC free article] [PubMed] [Google Scholar]
- 2.United Nations. Convention on the Rights of Persons with Disabilities - Some Facts about Persons with Disabilities. [Accessed 28 June 2016];2006 http://www.un.org/disabilities/convention/facts.shtml.
- 3.Al Snih S, Ray L, Markides KS. Prevalence of self-reported arthritis among elders from Latin America and the Caribbean and among Mexican Americans from the southwestern United States. J Aging Health. 2006;18(2):207–23. doi: 10.1177/0898264305285661. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Amado J, Moreno-Montoya J, Alvarez-Nemegyei J, Goycochea-Robles MV, Sanin LH, Burgos-Vargas R, et al. The Social Gap Index and the prevalence of osteoarthritis in the community: a cross-sectional multilevel study in Mexico. Clin Rheumatol. 2016;35(1):175–82. doi: 10.1007/s10067-014-2776-y. [DOI] [PubMed] [Google Scholar]
- 5.Hootman J, Helmick C, Barbour K, Theis K, Boring M. Updated Projected Prevalence of Self-Reported Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation Among US Adults, 2015-2040. Arthritis Rheumatol. 2016;68(7):1582–7. doi: 10.1002/art.39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heikkinen E. What are the main risk factors for disability in old age and how can disability be prevented? Health Evidence Network report; WHO Regional Office for Europe: 2004. [Google Scholar]
- 7.Houston DK, Cesari M, Ferrucci L, Cherubini A, Maggio D, Bartali B, et al. Association between vitamin D status and physical performance: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2007;62(4):440–6. doi: 10.1093/gerona/62.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houston DK, Tooze JA, Hausman DB, Johnson MA, Nicklas BJ, Miller ME, et al. Change in 25-Hydroxyvitamin D and Physical Performance in Older Adults. J Gerontol A Biol Sci Med Sci. 2011;66A(4):430–6. doi: 10.1093/gerona/glq235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mowé M, Haug E, Bøhmer T. Low serum calcidiol concentration in older adults with reduced muscular function. J Am Geriatr Soc. 1999;47(2):220–6. doi: 10.1111/j.1532-5415.1999.tb04581.x. [DOI] [PubMed] [Google Scholar]
- 10.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Hu FB, Zhang Y, Karlson EW, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr. 2004;80(3):752–8. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 11.Zamboni M, Zoico E, Tosoni P, Zivelonghi A, Bortolani A, Maggi S, et al. Relation between vitamin D, physical performance, and disability in elderly persons. J Gerontol A Biol Sci Med Sci. 2002;57(1):M7–11. doi: 10.1093/gerona/57.1.m7. [DOI] [PubMed] [Google Scholar]
- 12.Oliveri B, Plantalech L, Bagur A, Wittich AC, Rovai G, Pusiol E, et al. High prevalence of vitamin D insufficiency in healthy elderly people living at home in Argentina. Eur J Clin Nutr. 2004;58(2):337–42. doi: 10.1038/sj.ejcn.1601786. [DOI] [PubMed] [Google Scholar]
- 13.Riera-Espinoza GS, Ramos J, Belzares E. Vitamin D deficiency in postmenopausal women with osteoporosis in Venezuela. Bone. 2009;44:S359. [Google Scholar]
- 14.Zanchetta J. Epidemiology, costs & burden of osteoporosis in 2012. International Osteoporosis Foundation; 2012. The Latin America Regional Audit. [Google Scholar]
- 15.Raczkiewicz A, Kisiel B, Kulig M, Tłustochowicz W. Vitamin D Status and Its Association With Quality of Life, Physical Activity, and Disease Activity in Rheumatoid Arthritis Patients. J Clin Rheumatol. 2015;21(3):126–30. doi: 10.1097/RHU.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 16.Rossini M, Maddali Bongi S, La Montagna G, Minisola G, Malavolta N, Bernini L, et al. Vitamin D deficiency in rheumatoid arthritis: prevalence, determinants and associations with disease activity and disability. Arthritis Res Ther. 2010;12(6):R216. doi: 10.1186/ar3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker-LePain JC, Lane NE. Role of bone architecture and anatomy in osteoarthritis. Bone. 2012;51(2):197–203. doi: 10.1016/j.bone.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinans H, Siebelt M, Agricola R, Botter SM, Piscaer TM, Waarsing JH. Pathophysiology of peri-articular bone changes in osteoarthritis. Bone. 2012;51(2):190–6. doi: 10.1016/j.bone.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Ding C, Cicuttini F, Parameswaran V, Burgess J, Quinn S, Jones G. Serum levels of vitamin D, sunlight exposure, and knee cartilage loss in older adults: the Tasmanian older adult cohort study. Arthritis Rheum. 2009;60(5):1381–9. doi: 10.1002/art.24486. [DOI] [PubMed] [Google Scholar]
- 20.Heidari B, Heidari P, Hajian-Tilaki K. Association between serum vitamin D deficiency and knee osteoarthritis. Int Orthop. 2011;35(11):1627–31 21. doi: 10.1007/s00264-010-1186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang FF, Driban JB, Lo GH, Price LL, Booth S, Eaton CB, et al. Vitamin D Deficiency Is Associated with Progression of Knee Osteoarthritis. J Nutr. 2014;144(12):2002–8. doi: 10.3945/jn.114.193227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Institute for Health Metrics and Evaluation, Human Development Network, The World Bank. The global burden of disease: generating evidence, guiding policy - Latin America and Caribbean regional edition. 2013:74. [Google Scholar]
- 23.Partida-Bush V. Demographic transition, demographic bonus and ageing in Mexico. Mexico City: United Nations; 2007. [Google Scholar]
- 24.Wong R, Michaels-Obregon A, Palloni A. Cohort profile: the Mexican Health and Aging Study (MHAS) Int J Epidemiol. 2015 doi: 10.1093/ije/dyu263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Instituto Nacional de Estadística y Geografía. Mexican Health and Aging Study. MHAS 2012-Sample design. [Accessed 28 June 2016];2013 http://mhasweb.org/Resources/DOCUMENTS/2012/Methodological_Document_2012%E2%80%93SEC.pdf.
- 26.Instituto Nacional de Estadística y Geografía. Mexican Health and Aging Study. Section C, Health. [Accessed 28 June 2016];2013 http://mhasweb.org/Resources/DOCUMENTS/2012/Codebook/Section_C_Health_2012.pdf.
- 27.Instituto Nacional de Salud Pública. Mexican Health and Aging Study. MHAS 2012 - Manual of Procedures Anthropometrics and Biological Sample. [Accessed 28 June 2016];2012 http://mhasweb.org/Resources/DOCUMENTS/2012/MHAS_Procedures_for_Biomarkers_2012.pdf.
- 28.Sacks JJ, Harrold LR, Helmick CG, Gurwitz JH, Emani S, Yood RA. Validation of a surveillance case definition for arthritis. J Rheumatol. 2005;32(2):340–7. [PubMed] [Google Scholar]
- 29.Bombard JM, Powell KE, Martin LM, Helmick CG, Wilson WH. Validity and reliability of selfreported arthritis: Georgia senior centers, 2000–2001. Am J Prev Med. 2005;28(3):251–8. doi: 10.1016/j.amepre.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Controls and Prevention, National Center for Health Statistics. 2015-2016 National Health and Nutrition Examination Survey (NHANES) [Accessed 28 June 2016];2016 https://www.cdc.gov/nchs/data/nhanes/nhanes_15_16/MCQ_I.pdf.
- 31.Abbott. Architect System - 25-OH Vitamin D. Abbott Laboratories; 2011. [Google Scholar]
- 32.Department of Health and Human Services; Center for Disease Control and Prevention. Fat-Soluble Vitamins & Micronutrients: Vitamin D. Centers for Disease Control and Prevention; 2008. National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population 1999-2002. [Google Scholar]
- 33.Fonda S, Regula A. Survey Research Center, University of Michigan; Ann Arbor, MI: 2014. [Accessed 24 October 2016]. Documentation of Physical Functioning Measured in the Health and Retirement Study and the Asset and Health Dynamics among the Oldest Old Study. http://hrsonline.isr.umich.edu/sitedocs/userg/dr-008.pdf. [Google Scholar]
- 34.Albala C, Lebrão ML, León-Díaz EM, Ham-Chandel R, Hennis AJ, Palloni A, et al. The Health, Well-Being, and Aging (“SABE”) survey: methodology applied and profile of the study population. Rev Panam Salud Publica. 2005;17(5-6):307–322. doi: 10.1590/s1020-49892005000500003. [DOI] [PubMed] [Google Scholar]
- 35.Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95(2):471–8. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mabey T, Honsawek S. Role of Vitamin D in Osteoarthritis: Molecular, Cellular, and Clinical Perspectives. Int J Endocrinol. 2015;2015:14. doi: 10.1155/2015/383918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ceglia L. Vitamin D and skeletal muscle tissue and function. Mol Aspects Med. 2008;29(6):407–14. doi: 10.1016/j.mam.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Anand S, Kaysen GA, Chertow GM, Johansen KL, Grimes B, Dalrymple LS, et al. Vitamin D deficiency, self-reported physical activity and health-related quality of life: the Comprehensive Dialysis Study. Nephrol Dial Transplant. 2011;26(11):3683–8. doi: 10.1093/ndt/gfr098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang W, Shah S, Long Q, Crankshaw AK, Tangpricha V. Improvement of pain, sleep, and quality of life in chronic pain patients with vitamin D supplementation. Clin J Pain. 2013;29(4):341–7. doi: 10.1097/AJP.0b013e318255655d. [DOI] [PubMed] [Google Scholar]
- 40.Nair R, Maseeh A. Vitamin D: The “sunshine” vitamin. J Pharmacol Pharmacother. 2012;3(2):118–26. doi: 10.4103/0976-500X.95506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glover TL, Horgas AL, Fillingim RB, Goodin BR. Vitamin D status and pain sensitization in knee osteoarthritis: a critical review of the literature. Pain Manag. 2015;5(6):447–53. doi: 10.2217/pmt.15.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Straube S, Derry S, Moore RA, McQuay HJ. Vitamin D for the treatment of chronic painful conditions in adults (Review) Cochrane Database Syst Rev. 2010;2010(1) doi: 10.1002/14651858.CD007771.pub2. CD007771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergink AP, Zillikens MC, Van Leeuwen JP, Hofman A, Uitterlinden AG, van Meurs JB. 25-Hydroxyvitamin D and osteoarthritis: A meta-analysis including new data. Semin Arthritis Rheum. 2016;45(5):539–46. doi: 10.1016/j.semarthrit.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 44.Hong Q, Xu J, Xu S, Lian L, Zhang M, Ding C. Associations between serum 25-hydroxyvitamin D and disease activity, inflammatory cytokines and bone loss in patients with rheumatoid arthritis. Rheumatology (Oxford) 2014;53(11):1994–2001. doi: 10.1093/rheumatology/keu173. [DOI] [PubMed] [Google Scholar]
- 45.Bischoff HA, Stähelin HB, Dick W, Akos R, Knecht M, Salis C, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18(2):343–51. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- 46.Dhesi JK, Jackson SH, Bearne LM, Moniz C, Hurley MV, Swift CG, et al. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing. 2004;33(6):589–95. doi: 10.1093/ageing/afh209. [DOI] [PubMed] [Google Scholar]
- 47.Latham NK, Anderson CS, Lee A, Bennett DA, Moseley A, Cameron ID, et al. A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: the Frailty Interventions Trial in Elderly Subjects (FITNESS) J Am Geriatr Soc. 2003;51(3):291–9. doi: 10.1046/j.1532-5415.2003.51101.x. [DOI] [PubMed] [Google Scholar]
- 48.Kenny AM, Biskup B, Robbins B, Marcella G, Burleson JA. Effects of vitamin D supplementation on strength, physical function, and health perception in older, community-dwelling men. J Am Geriatr Soc. 2003;51(12):1762–7. doi: 10.1046/j.1532-5415.2003.51561.x. [DOI] [PubMed] [Google Scholar]
- 49.Halfon M, Phan O, Teta D. Vitamin D: A Review on Its Effects on Muscle Strength, the Risk of Fall, and Frailty. Biomed Res Int. 2015;2015:11. doi: 10.1155/2015/953241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sacks JJ, Harrold LR, Helmick CG, Gurwitz JH, Emani S, Yood RA. Validation of a surveillance case definition for arthritis. J Rheumatol. 2005;32(2):340–7. [PubMed] [Google Scholar]