Abstract
Airway mucosal surfaces are potentially subjected to a variety of oxidant stresses. Airway submucosal glands secrete a variety of compounds that may protect the airways from injury. Cholinergically induced nasal submucosal gland secretion has recently been found to contain a low molecular weight nasal antioxidant. In this report, the isolation and identification of this nasal secretory antioxidant are described. Concentrated, cholinergically induced human nasal secretions were fractionated through a 10-kDa sieve and subjected to DEAE anion-exchange chromatography. Fractions containing antioxidant activity were subjected to gel filtration with Bio-Gel P-2 gel (resolution range, 200-2000 Da). The resultant antioxidant fractions were then desalted by gel filtration over the same column equilibrated in HPLC-grade water, yielding only a single peak with antioxidant activity. The absorption spectrum of the purified antioxidant revealed peaks at 238 and 292 nm at pH 7. These peaks shifted to 230 and 280 nm in 0.1 M HCl and 226 and 296 nm in 0.1 M NaOH. Sodium borohydride reduction of the antioxidant had no effect on the UV absorption, whereas platinum-catalyzed hydrogenation ablated all absorption peaks. Uric acid had identical absorption peaks and showed the same chromatographic behavior as the nasal antioxidant activity on both gel filtration and DEAE columns. Uricase (which degrades uric acid) metabolized both uric acid and the purified antioxidant. Uric acid was shown to have antioxidant activity at concentrations greater than 1.5 microM. These data indicate that nasal secretions contain uric acid that serves as an antioxidant.
Full text
PDF
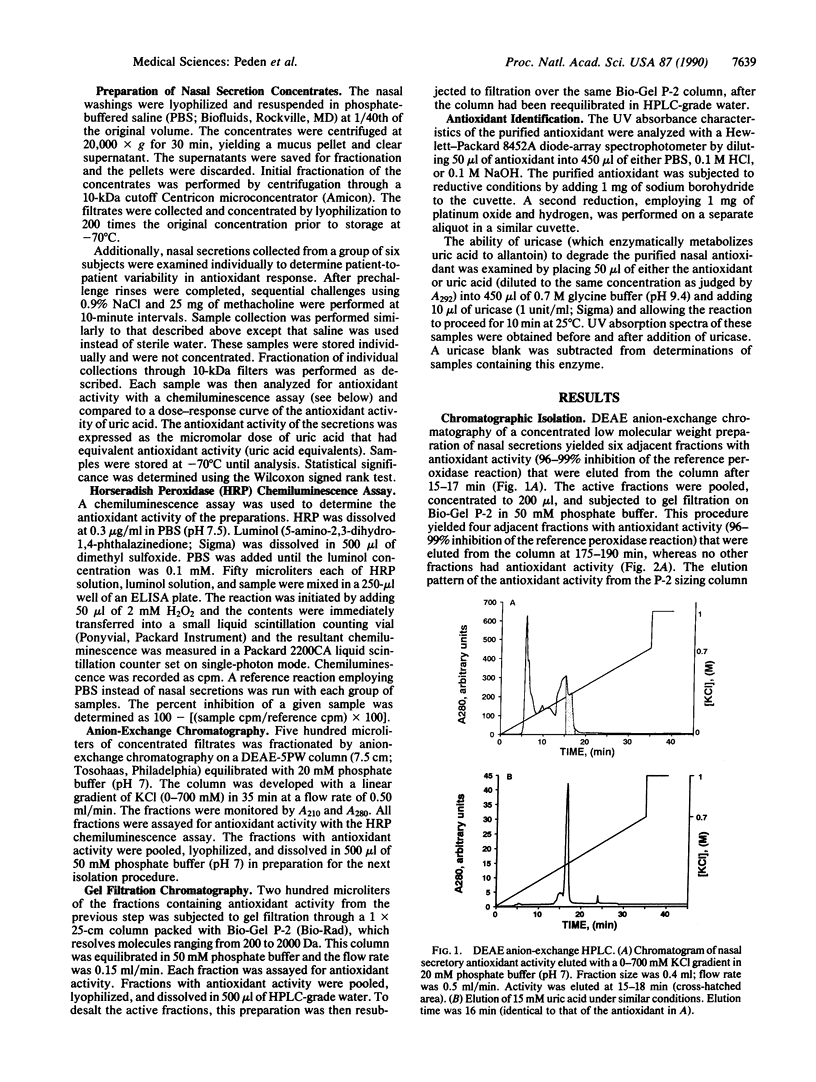
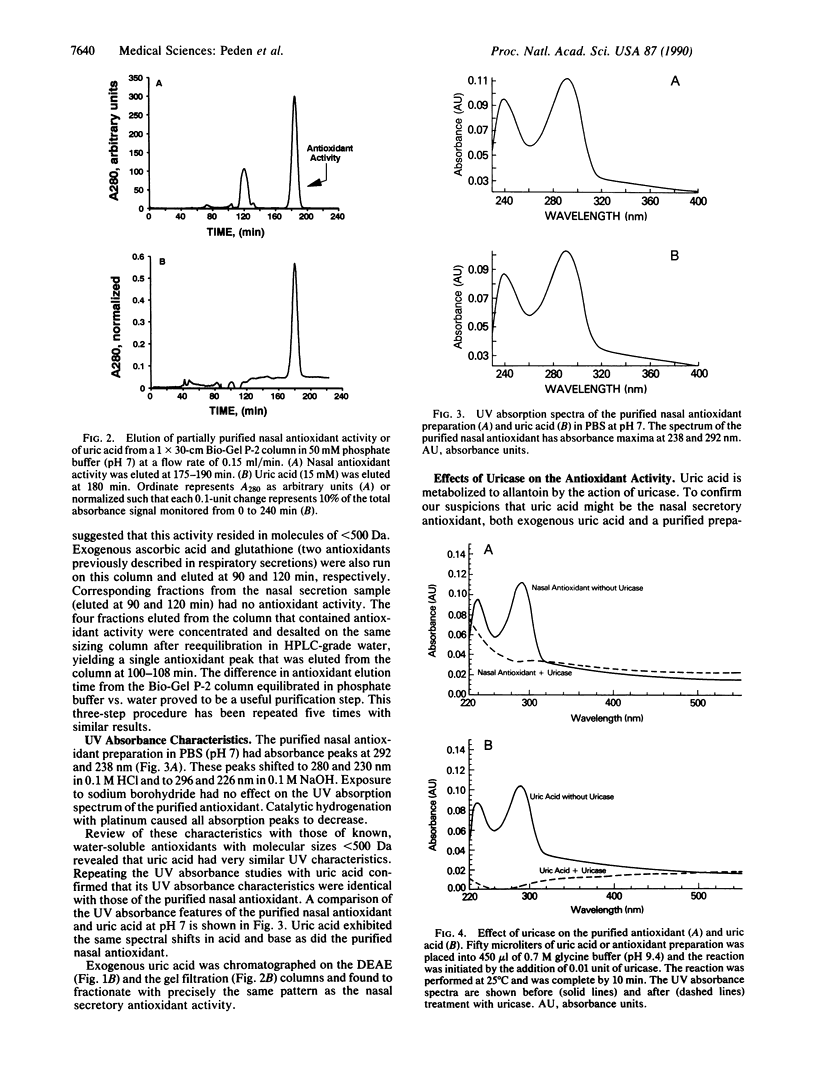
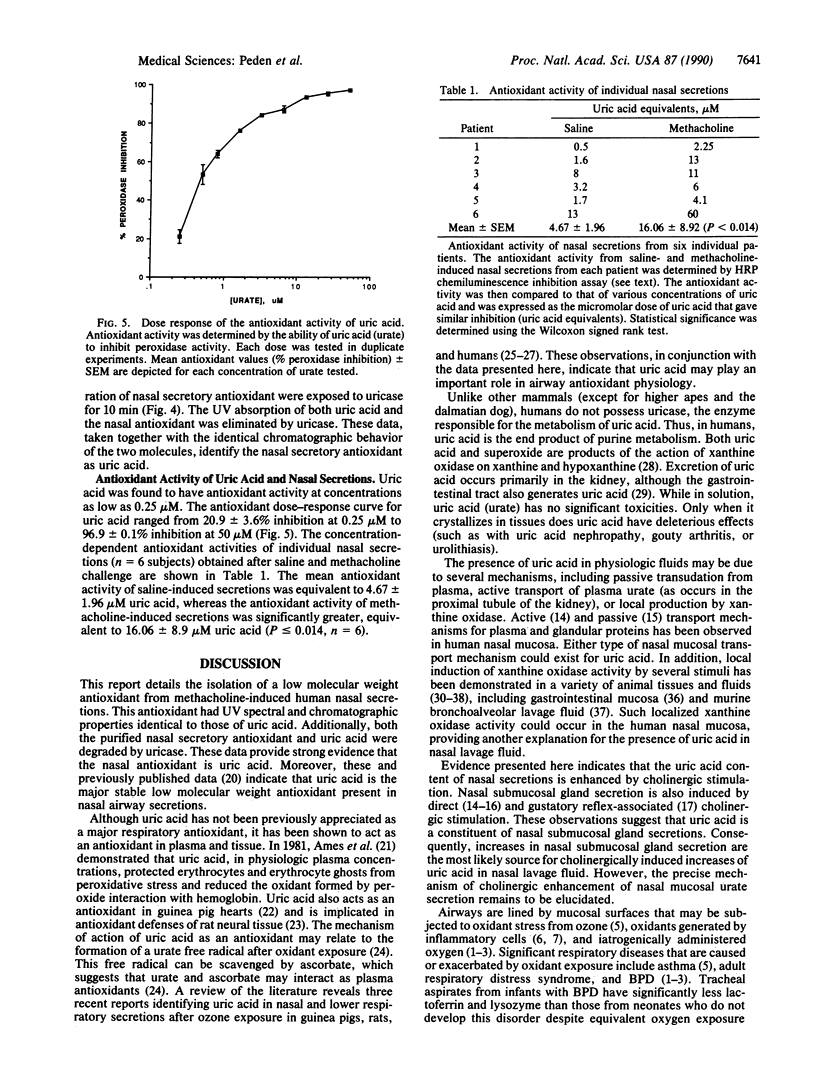

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike T., Ando M., Oda T., Doi T., Ijiri S., Araki S., Maeda H. Dependence on O2- generation by xanthine oxidase of pathogenesis of influenza virus infection in mice. J Clin Invest. 1990 Mar;85(3):739–745. doi: 10.1172/JCI114499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames B. N., Cathcart R., Schwiers E., Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin S. R., Simon R. H., Grum C. M., Ketai L. H., Boxer L. A., Devall L. J. Oxidant activity in expired breath of patients with adult respiratory distress syndrome. Lancet. 1986 Jan 4;1(8471):11–14. doi: 10.1016/s0140-6736(86)91895-7. [DOI] [PubMed] [Google Scholar]
- Baraniuk J. N., Lundgren J. D., Goff J., Peden D., Merida M., Shelhamer J., Kaliner M. Gastrin-releasing peptide in human nasal mucosa. J Clin Invest. 1990 Apr;85(4):998–1005. doi: 10.1172/JCI114577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker B. F., Reinholz N., Ozçelik T., Leipert B., Gerlach E. Uric acid as radical scavenger and antioxidant in the heart. Pflugers Arch. 1989 Nov;415(2):127–135. doi: 10.1007/BF00370582. [DOI] [PubMed] [Google Scholar]
- Cantin A. M., North S. L., Hubbard R. C., Crystal R. G. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol (1985) 1987 Jul;63(1):152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- Cassutto B. H., Misra H. P., Pfeiffer C. J. Intestinal post-ischemic reperfusion injury: studies with neonatal necrotizing enterocolitis. Acta Physiol Hung. 1989;73(2-3):363–369. [PubMed] [Google Scholar]
- Cochrane C. G., Spragg R., Revak S. D. Pathogenesis of the adult respiratory distress syndrome. Evidence of oxidant activity in bronchoalveolar lavage fluid. J Clin Invest. 1983 Mar;71(3):754–761. doi: 10.1172/JCI110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins R. A., Parsons K. R., Field T. R., Bramley A. J. Histochemical localization and possible antibacterial role of xanthine oxidase in the bovine mammary gland. J Dairy Res. 1988 Feb;55(1):25–32. doi: 10.1017/s0022029900025814. [DOI] [PubMed] [Google Scholar]
- Deitch E. A., Ma L., Ma W. J., Grisham M. B., Granger D. N., Specian R. D., Berg R. D. Inhibition of endotoxin-induced bacterial translocation in mice. J Clin Invest. 1989 Jul;84(1):36–42. doi: 10.1172/JCI114164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl H. P., Till G. O., Ryan U. S., Ward P. A. Mediator-induced activation of xanthine oxidase in endothelial cells. FASEB J. 1989 Nov;3(13):2512–2518. doi: 10.1096/fasebj.3.13.2806779. [DOI] [PubMed] [Google Scholar]
- Friedl H. P., Till G. O., Trentz O., Ward P. A. Roles of histamine, complement and xanthine oxidase in thermal injury of skin. Am J Pathol. 1989 Jul;135(1):203–217. [PMC free article] [PubMed] [Google Scholar]
- Heffner J. E., Repine J. E. Pulmonary strategies of antioxidant defense. Am Rev Respir Dis. 1989 Aug;140(2):531–554. doi: 10.1164/ajrccm/140.2.531. [DOI] [PubMed] [Google Scholar]
- Kennedy T. P., Rao N. V., Hopkins C., Pennington L., Tolley E., Hoidal J. R. Role of reactive oxygen species in reperfusion injury of the rabbit lung. J Clin Invest. 1989 Apr;83(4):1326–1335. doi: 10.1172/JCI114019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinuta Y., Kikuchi H., Ishikawa M., Kimura M., Itokawa Y. Lipid peroxidation in focal cerebral ischemia. J Neurosurg. 1989 Sep;71(3):421–429. doi: 10.3171/jns.1989.71.3.0421. [DOI] [PubMed] [Google Scholar]
- Koren H. S., Devlin R. B., Graham D. E., Mann R., McGee M. P., Horstman D. H., Kozumbo W. J., Becker S., House D. E., McDonnell W. F. Ozone-induced inflammation in the lower airways of human subjects. Am Rev Respir Dis. 1989 Feb;139(2):407–415. doi: 10.1164/ajrccm/139.2.407. [DOI] [PubMed] [Google Scholar]
- Koren H. S., Hatch G. E., Graham D. E. Nasal lavage as a tool in assessing acute inflammation in response to inhaled pollutants. Toxicology. 1990 Jan-Feb;60(1-2):15–25. doi: 10.1016/0300-483x(90)90159-e. [DOI] [PubMed] [Google Scholar]
- Kreit J. W., Gross K. B., Moore T. B., Lorenzen T. J., D'Arcy J., Eschenbacher W. L. Ozone-induced changes in pulmonary function and bronchial responsiveness in asthmatics. J Appl Physiol (1985) 1989 Jan;66(1):217–222. doi: 10.1152/jappl.1989.66.1.217. [DOI] [PubMed] [Google Scholar]
- Lynch M. J., Grum C. M., Gallagher K. P., Bolling S. F., Deeb G. M., Morganroth M. L. Xanthine oxidase inhibition attenuates ischemic-reperfusion lung injury. J Surg Res. 1988 May;44(5):538–544. doi: 10.1016/0022-4804(88)90159-x. [DOI] [PubMed] [Google Scholar]
- Malech H. L., Gallin J. I. Current concepts: immunology. Neutrophils in human diseases. N Engl J Med. 1987 Sep 10;317(11):687–694. doi: 10.1056/NEJM198709103171107. [DOI] [PubMed] [Google Scholar]
- Maples K. R., Mason R. P. Free radical metabolite of uric acid. J Biol Chem. 1988 Feb 5;263(4):1709–1712. [PubMed] [Google Scholar]
- O'Brodovich H. M., Mellins R. B. Bronchopulmonary dysplasia. Unresolved neonatal acute lung injury. Am Rev Respir Dis. 1985 Sep;132(3):694–709. doi: 10.1164/arrd.1985.132.3.694. [DOI] [PubMed] [Google Scholar]
- Pacht E. R., Davis W. B. Role of transferrin and ceruloplasmin in antioxidant activity of lung epithelial lining fluid. J Appl Physiol (1985) 1988 May;64(5):2092–2099. doi: 10.1152/jappl.1988.64.5.2092. [DOI] [PubMed] [Google Scholar]
- Pacht E. R., Kaseki H., Mohammed J. R., Cornwell D. G., Davis W. B. Deficiency of vitamin E in the alveolar fluid of cigarette smokers. Influence on alveolar macrophage cytotoxicity. J Clin Invest. 1986 Mar;77(3):789–796. doi: 10.1172/JCI112376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panus P. C., Shearer J., Freeman B. A. Pulmonary metabolism of reactive oxygen species. Exp Lung Res. 1988;14 (Suppl):959–976. doi: 10.3109/01902148809064186. [DOI] [PubMed] [Google Scholar]
- Petreccia D. C., Nauseef W. M., Clark R. A. Respiratory burst of normal human eosinophils. J Leukoc Biol. 1987 Apr;41(4):283–288. doi: 10.1002/jlb.41.4.283. [DOI] [PubMed] [Google Scholar]
- Raphael G. D., Davis J. L., Fox P. C., Malech H. L., Gallin J. I., Baraniuk J. N., Kaliner M. A. Glandular secretion of lactoferrin in a patient with neutrophil lactoferrin deficiency. J Allergy Clin Immunol. 1989 Dec;84(6 Pt 1):914–919. doi: 10.1016/0091-6749(89)90389-8. [DOI] [PubMed] [Google Scholar]
- Raphael G. D., Druce H. M., Baraniuk J. N., Kaliner M. A. Pathophysiology of rhinitis. 1. Assessment of the sources of protein in methacholine-induced nasal secretions. Am Rev Respir Dis. 1988 Aug;138(2):413–420. doi: 10.1164/ajrccm/138.2.413. [DOI] [PubMed] [Google Scholar]
- Raphael G. D., Jeney E. V., Baraniuk J. N., Kim I., Meredith S. D., Kaliner M. A. Pathophysiology of rhinitis. Lactoferrin and lysozyme in nasal secretions. J Clin Invest. 1989 Nov;84(5):1528–1535. doi: 10.1172/JCI114329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael G., Raphael M. H., Kaliner M. Gustatory rhinitis: a syndrome of food-induced rhinorrhea. J Allergy Clin Immunol. 1989 Jan;83(1):110–115. doi: 10.1016/0091-6749(89)90484-3. [DOI] [PubMed] [Google Scholar]
- Snyder A., Skoza L., Kikkawa Y. Comparative removal of ascorbic acid and other airway substances by sequential bronchoalveolar lavages. Lung. 1983;161(2):111–121. doi: 10.1007/BF02713849. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T., Ueda S., Naito Y., Takahashi S., Oyamada H., Morita Y., Yoneta T., Kondo M. Role of oxygen-derived free radicals in gastric mucosal injury induced by ischemia or ischemia-reperfusion in rats. Free Radic Res Commun. 1989;7(3-6):285–291. doi: 10.3109/10715768909087953. [DOI] [PubMed] [Google Scholar]