Abstract
Introduction
Viral and non-viral vectors have been used as methods of delivery in gene therapy for many CNS diseases. Currently, viral vectors such as adeno-associated viruses (AAV), retroviruses, lentiviruses, adenoviruses and herpes simplex viruses (HHV) are being used as successful vectors in gene therapy at clinical trial levels. However, many disadvantages have risen from their usage. Non-viral vectors like cationic polymers, cationic lipids, engineered polymers, nanoparticles, and naked DNA offer a much safer option and can therefore be explored for therapeutic purposes.
Areas covered
This review discusses different types of viral and non-viral vectors for gene therapy and explores clinical trials for CNS diseases that have used these types of vectors for gene delivery. Highlights include non-viral gene delivery and its challenges, possible strategies to improve transfection, regulatory issues concerning vector usage, and future prospects for clinical applications.
Expert opinion
Transfection efficiency of cationic lipids and polymers can be improved through manipulation of molecules used. Efficacy of cationic lipids is dependent on cationic charge, saturation levels, and stability of linkers. Factors determining efficacy of cationic polymers are total charge density, molecular weights, and complexity of molecule. All of the above mentioned parameters must be taken care for efficient gene delivery.
Keywords: Gene delivery, central nervous system, viral/non-viral vector, brain delivery, neurological disorders, nanobiotechnology
1. Introduction
Diseases of the Central Nervous System (CNS) impact the lives of many individuals worldwide.[1–5] Many CNS disorders can be treated through gene therapy.[6] The usage of gene therapy extends to delivering DNA-vector hybrids encoding for genes, proteins, and other factors that can correct and aid in the treatment of neurological disorders.[7] For example, Parkinson’s disease (PD) is the degeneration of neurons in the substantia nigra, which generate dopamine subsequently providing information to the basal ganglia.[8] In order to treat this disorder, gene therapy has been used to induce dopamine production, protect neurons in the substantia nigra, and enhance GABA (gamma-aminobutyric acid) to inhibit the subthalamic nucleus.[9] Alzheimer’s disease, another neurological disease, is depicted by high correlations of extracellular amyloid- beta peptide deposition, intracellular neurofibrillary tangle formation, decreased synaptic integrity, and neuronal loss specifically in the basal forebrain cholinergic complex.[10,11] Gene therapy has been used to induce the nerve growth factor (NGF), neurotrophin, which reduces neuronal loss.[12] Furthermore, promising studies for the removal of neurotoxic amyloid beta (Aβ) peptides through various methods have been done, for example delivering vaccines against Aβ peptide epitopes, induced expressions of Aβ proteases, small inhibitory RNAs that suppress amyloid precursor proteins that give rise to Aβ peptides, and cholesterol degrading enzymes.[13] Amyotrophic lateral sclerosis is a disorder arising from mutations in the superoxide dismutase-1 (SOD-1) gene that results in loss of motor neurons in the CNS causing muscle weakness. These mutations are thought to produce toxic protein species. Gene therapy treatments have consisted of delivering IGF-1 (insulin-like growth factor-1), vascular endothelial growth factors, and RNA-interference-based silencing alleles of mutant SOD-1.[13]
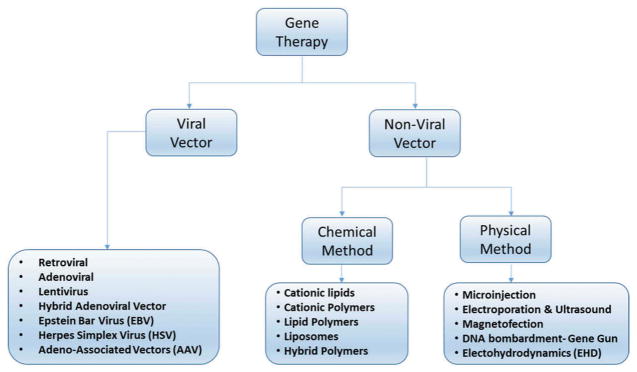
As previously mentioned, gene therapy has shown significant advancement in treating many diseases.[14] Nonetheless, due to the highly restrictive blood brain barrier (BBB),[15] effective treatments of CNS genetic disorders rely on the efficiency of the vectors used to deliver appropriate genes and other therapeutic compounds to the specific site in the brain.[16–20] Figure 1 summarizes the techniques and vectors used for both types of gene therapy (viral and nonviral) to deliver cargo to CNS.
Figure 1.
Types of gene delivery systems.
Viral vectors have been extensively investigated and remedied to promote transgene expression while reducing viral replication after successful transduction.[7,21–23] Currently, viruses such as adeno-associated virus (AAV), retroviruses, lentiviruses, adenoviruses, and herpes simplex virus (HHV) are being used as successful vectors in gene therapy.[23] While using viral vectors, gene transfection efficiency and long-term gene expression are high due to the exploitation of the viruses’ existing transfection mechanisms.[21] AAV vectors are the safest option among the viral vectors since they are considered nonpathogenic; however, they are costly, and difficult to target to a specific location, having limited transgene capacity and exhibiting high immunogenicity.[24,25] Viral vectors demonstrating increased gene transfection capabilities are lentiviruses and retroviruses, but are considered risky as a result of observed oncogenesis. HSV and adenovirus vectors showed better gene targeting, transgene efficacy, and larger transgene capacity. Unfortunately, these vectors are highly toxic and are significantly immunogenic.[9]
Article highlights.
Possible strategies to improve non-viral vector system
Non-viral vectors must have superior nuclear import ability, extended gene expression and persistence in nucleus.
Must have ability to proficiently interact with serum constituents without losing the therapeutic cargo.
Should have longer circulating time in the body and better biodistribution with better escaping ability from reticuloendothelial system or macrophage system.
Should have controlled intracellular trafficking ability (i.e. Endosomes release and escaping power from degradation by endonucleases).
Should have better cell targeting ability, ability to transcription and no cytotoxicity.
This box summarizes key points contained in the article.
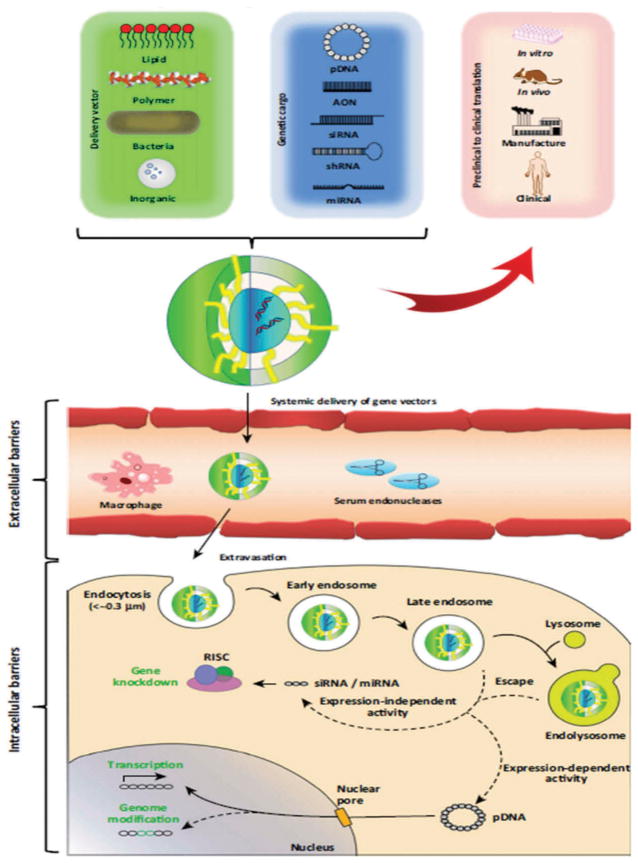
Cationic polymers, cationic lipids, engineered polymers, nanoparticles, and naked DNA have all been used to transfect genes nonvirally.[26] Cationic polymers allow the carried DNA to be condensed through electrostatic interactions and transport them by ligands, containing cell-targeting molecules, attached to the polymer’s functional group.[27–29] While their cell-targeting capabilities are a great improvement when compared to viral-vectors, their transfection efficiency can be hindered; nonetheless, poly(ethylenimine) (PEI)-based polyplexes have greater gene transfer success than HIV-derived vectors and share similar results to adenoviral vectors.[30] PEIs are attractive nonviral vectors because they show an increase in protection, cell binding, and uptake due to endosomal escape and exhibit the ability to deliver cargo (DNA, siRNA) efficiently.[31] Alternatively, cationic lipids have shown to be easy to use while being able to carry larger nucleic acids. Cationic lipids have been modified to PEGylated immunoliposomes (PILs), which have been observed to efficiently deliver nucleic acids by retaining colloidal stability in the blood when administered intravenously unlike previous cationic lipids.[30] Another type of nonviral vector is the use of polypeptides. Chimeric polypeptides have exhibited efficient transfection of neuronal cells due to their flexibility as they can be engineered to increase nucleic acid condensation, neuronal targeting, endosomal escape, and nuclear entry. Recently, nanoparticles have also demonstrated the capability to transfect plasmid DNA.[30] Figure 2 shows the general schematic representation of gene delivery mechanism using both viral and nonviral vectors at preclinical and clinical levels for gene delivery. This review will discuss the current market status of nonviral gene technologies, types of vectors or materials used for gene delivery, statuses of ongoing or finished clinical trials, the current challenges associated with their clinical translations, and finally considerations one must take into account when developing nonviral CNS gene therapy for clinical application will be reviewed.
Figure 2. Schematic representation of gene delivery mechanism.
Upon assembly of the selected nucleic acid cargo with the delivery vector construct, the composite particles (e.g. lipoplex or polyplex) must traverse various extracellular barrier (e.g., serum endonucleases) followed by cellular entry via endocytosis or by other biological means. Following uptake, gene particles modulate gene expression either in the cytosol (expression independent) or in the nucleus (expression dependent){Reproduce with permission [32]}.
2. Current status of clinical trials in gene delivery and development in nonviral systems
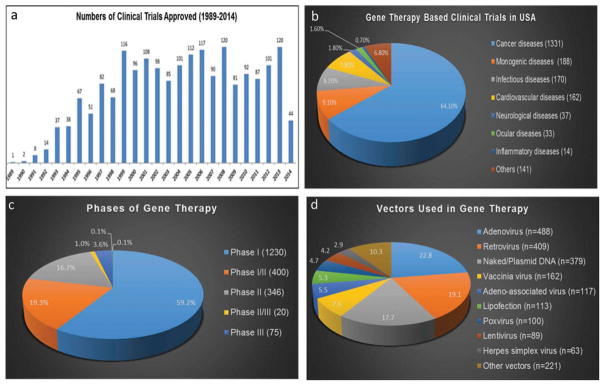
As per Markets and Markets (global market research analysis company), the global market for transfection products was estimated to be worth US $385 million in 2012 and is expected to reach US $601 million by 2017, growing at a compound annual growth rate of 9.32% from 2012 to 2017. [33] The cost of caring for those with neurodegenerative diseases was estimated to total $214 billion in 2014, and is projected to increase to $1.2 trillion (in today’s dollars) by 2050. According to data updated to June 2014 and presented by The Journal of Gene Medicine,[34] since the onset of the first gene therapy clinical trial in 1989, more than 2000 new clinical trials for gene therapy have been approved globally (Figure 3(a)). As shown in Figure 3(b), these trials address the most challenging diseases of today, which are cancer (64.1% of approved trials), monogenic diseases (9.1%) such as cystic fibrosis, infectious diseases (8.2%), and cardiovascular diseases (7.8%). Although at a lesser extent, neurological diseases (1.8%) are also subject to clinical trials with gene therapy. However, despite a growing number of successful phase-I CNS gene therapy clinical trials, clear clinical efficacy has been lacking and only few trials have reached phase-II. [35,36] At present, only 0.1% of all the gene therapy products approved for clinical trials have arrived to phase-IV (Figure 3(c)). In 2012, the European Medicine Agency approved for the first time a gene therapy product, Glybera, an adeno-associated viral vector engineered to express lipoprotein lipase in the muscle for the treatment of lipoprotein lipase deficiency.[37] One of the main reasons why gene therapy clinical trials are still few in number is the lack of suitable and safe approaches to deliver the genetic material to target cells.
Figure 3.
Indications addressed by gene therapy clinical trials (adapted from http://www.wiley.co.uk/genmed/clinical) [38] and data obtained from www.clinicaltrails.gov.
According to data updated in June 2014,[34] among the over 2000 clinical trials for gene therapy approved globally, 70% corresponds to trials using viral vectors (Figure 3(d)). As shown in Figure 3(d), there is a 17.7% of gene therapy clinical trials that use naked DNA, while 5.3% of trials use lipofection. [38] In short, we can say that the lack of measurable clinical efficacy in a number of clinical viral gene transfer trials in the CNS is below par. However, with the advent of novel expression systems, more refined and efficient nonviral vectors, and a greater understanding of the etiology of neurological diseases, the future of nonviral gene transfer is promising. The next section deals with a brief overview of the most commonly used nonviral vehicles and their preclinical and clinical development for the treatment of CNS genetic disorders.
3. Nonviral vectors for CNS delivery
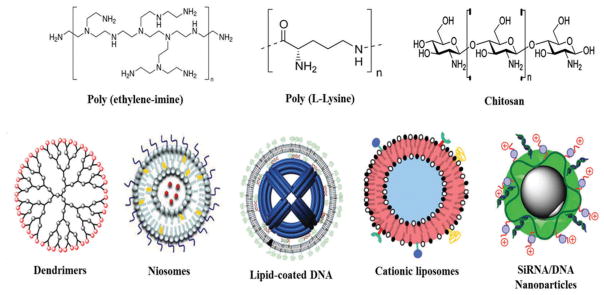
The success of gene therapy is mainly governed by the selection of appropriate transfection vectors, which must possess the following properties that is (1) it must safeguard nucleic acids against degradation by blood enzymes and endonucleases, (2) endorse internalization of the nucleic material into target cells, and (3) should release nucleic acids after reaching the target site within the cell. [38] Moreover, the ideal gene delivery system must be effective, specific, long-term, nontoxic (safe), easy to use, and inexpensive.[39] Lately, various types of nonviral materials (Figure 4) and approaches have been explored to overcome the issues associated with viral components. This section deals with a brief overview of all the preclinical and clinically used nonviral components used in CNS gene therapy.
Figure 4.
Schematic representation of different type of polymeric materials and nanocarriers used in the nonviral gene delivery systems.
3.1. Polymer based vectors
3.1.1. Polyethyleneimine
The most efficient cationic polymer in many cell types is PEI due to its ability to form homogenous nanoparticle (known as polyplexes) with its DNA.[40,41] Uniform polyplexes are formed because PEI has a high positive charge density, which interacts with the negatively charged DNA. The interaction between DNA and PEI causes the DNA to be tightly condensed into a spherical nanoparticle that can be taken up by the cell via endocytosis.[39,42] Additionally, PEI is quite versatile and can be designed to be different lengths, branched or linear, and can undergo functional group substitution and/or addition.[40,43] Functional group additions and substitutions allow for greater target specificity by increasing the binding of PEI–DNA to the cell membrane during receptor-mediated endocytosis. Furthermore, increasing the positive charge of the polyplex, prolonging incubation time, and raising the concentration of PEI–DNA enhances endocytosis. After entering the cell, the polyplex is subjected to the endolysosomal pathway. By having PEI attached to the DNA, the fusion1 of endosomes and lysosomes is prevented, thus DNA degradation is avoided. The inhibition of endosome and lysosome fusion is facilitated by the release of the PEI–DNA allowed by the proton sponge hypothesis. The proton sponge hypothesis states that the amine groups of the PEI absorb H+ into the endosomes inducing osmotic swelling, which results in the bursting of the endosome and discharge of the PEI–DNA. Furthermore, optimizing gene transfection of larger complexes is conveyed through the addition of positively charged protein transduction domains. These transduction domains enhance endocytosis and allows for the translocation of the complex toward the nucleus.[40,44]
3.1.2. Poly-(L-lysine)
Poly-(L-lysine) (PLL) is another successful cationic polymer that has been used for gene delivery due to their unique biodegradable and noncytotoxic nature.[40] Nonetheless, they possess limitations due to their inability to form stable complexes. Additionally, their transfection efficiency is not as high as a result of endosomolysis caused by a lack of amino groups; however, inserting histidine residues to the backbone of PLL can prevent this.[45]
3.1.3. Chitosan
Chitosan are advantageous vectors to transporting siRNA.[46] They are more biodegradable and biocompatible than PEI and are protected against DNAase degradation. Additionally, chitosans form stable and small complexes with DNA since they can induce electrostatic interactions with negatively charged molecules such as mucus and DNA.[47] Compared to other cationic polymers, chitosans have low gene transfection efficiency due to low water solubility and inability to efficiently release DNA. Gene unpacking can be improved via the introduction of hyaluronic acid (HA) or polyglutamic acid (PGA). The HA chain contains low-charge density, while PGA/chitosan/DNA complexes disintegrate into a number of even smaller subparticles after cellular internalization allowing for increased gene release efficiency in both cases.[48]
3.1.4. Dendrimers
Dendrimers are spherically, highly branched polymers that have been explored vastly in drug and gene delivery. The most common dendrimer explored in gene delivery is Poly (amidoamine) [PAMAM] due to its high transfection efficiency. PAMAM’s structure consists of a primary amine group on its surface that participates in DNA binding, compressing DNA, and promoting its uptake by the cell membrane; furthermore, a tertiary amine group is present inside the complex that acts as a proton sponge to enhance DNA release from endosome into the cytoplasm. However, dendrimers exhibit high toxicity due to its chemical structure and surface charge; this issue can be alleviated by polyethylene glycol (PEG) modification.[41,49,50]
3.2. Lipid polyplexes
3.2.1. Niosomes
Niosomes are synthetically self-assembled nonionic amphiphiles with similar structures to liposomes. Niosomes are considered to be advantageous due to their increased physicochemical stability, which increases their storage time to up to 84 months, when compared to phospholipid liposomes. Although they have been known to give positive results for gene delivery, they exhibit low target specificity as a result of their nonspecific interactions between their positive charges and plasma proteins causing complex dissociation. When treated with PEG, niosomes exhibit a relative reduction in these nonspecific interactions with said plasma proteins, increasing target specificity while still maintaining their storage stability and low costs.[48,51]
3.2.2. Lipid-coated DNA complexes and cationic liposomes
Cationic DNA/PEI complexes can be coated with 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and cholesteryl hemisuccinate by detergent removal producing lipid-coated DNA complexes (LCDCs) in order to protect the DNA–PEI center from extracellular polyanions interactions while still being able to fuse with membranes at acidic pH. DOPE facilitates the formation of a hexagonal arrangement under acidic pH at the endosomal level, which facilitates the destabilization of endosomal membranes aiding DNA release. DOPE also reduces the toxicity by achieving the lipid/DNA charge ratio required for optimal transfection.[2] Moreover, when compared to cationic polyplexes, LCDCs produced by condensing DNA with PEI first, then coating the polyplex with 1,2-dioleoyl-3-(trimethylammonium) propane and cholesterol at a lipid/DNA molar ratio of 17:1 showed higher transfection efficiency and lower toxicity.[48,52]
Cationic lipids are composed of a hydrophilic head group, a hydrophobic anchor and a linker. Currently, lipopolyamines, those with multiple charges at the head group, can condense DNA at lower mole ratios and provide unprotonated amine groups to evade lysosomal degradation.[52,53] Cationic liposomes are easy to use and are able to transfect larger nucleic acids efficiently. Lipofectamine 2000 (L2K) can transfect a relatively high percentage (20–25%) of primary neurons. Regrettably, these cationic liposomes aggregate in biological fluids; however, PILs avoid these limitations and maintain stability when administered intravenously. In addition, PILs can deliver genes to the CNS by binding targeting ligands such as transferrin or antibodies to the ends of the PEG strands.[30] While PEI can condense DNA and form more stable polymers than cationic lipid-based liposomes, liposomes have better biocompatibility leading to better gene delivery into the cytosol.[54] Conjoining PEI (Mw = 800) and cholesterol (PEI 800-Chol) has exploited these advantages creating polycation liposomes (PCLs) with lower cytotoxicity and higher transfection efficiency in HeLa cells. PCLs mixed with DOPE show an even higher transfection activity.[48]
3.3. Polypeptides/Proteins
Polypeptides can be engineered for optimal nucleic acid condensation, neuronal targeting, endosomal escape, and nuclear entry.[55] Tetanus toxin fragment C is being used as a foundation for recombinant fusion proteins that are targeted for the CNS.[56] These proteins have been enhanced with the translocation domain of diphtheria toxin for endosomal escape and the GAL4 transcription factor DNA-binding domain for DNA condensation. Likewise, NGF-derived targeting peptides with chimeric complexes consisting of either loop 4 of NGF or an NGF hairpin motif containing loops 1 and 2 fused to a DNA binding domain have successfully transfected neurons.[30]
3.4. Nanoparticles
The field of nanoparticles (NPs) for drug and gene therapy continues to grow greatly with the development of novel biomaterials/polymers.[57] Engineered gene nanocarriers have been produced to carry DNA into cells by coating DNA with synthetic NPs.[58] One example is coating PLL with iron oxide NPs; these carriers have successfully transfected into neurons and glia to mediate reporter gene expressions.[59] Another example is using amino-terminated organically modified silica (ORMOSIL) NPs. ORMOSIL coats plasmid DNA, allowing the transfection of neurons at similar success rates as HSV with lower toxicity and immunogenicity.[60]
3.5. Naked DNA/siRNA
Naked DNA and siRNA can be successfully transfected using multiple physical methods such as microinjection, needle injection, jet injection, gene gun, electroporation, sonoporation, and hydrodynamic gene transfer.[2] Observations of naked DNA transfecting cells in the brainstem have been reported when administered through intramuscular injection in the tongue or through intracisternal injections. Furthermore, low gene expression in neurons and astrocytes has been found when delivered to spinal cord injury sites. Nonetheless, naked nucleic acids tend to lack target specificity and subject to degradation, thus transfection efficiency is low.[1,30]
In conclusion, while the use of nonviral vectors has increased transfection efficiency, usage of naked DNA and siRNA has shown great promises. Over the years, scientists have developed and explored novel methods for viral and nonviral gene delivery. Table 1 summarizes and discusses the advantages and disadvantages of most commonly method for nonviral gene delivery.
Table 1.
Overview of different nonviral vector delivery methods.
| Delivery techniques | Mechanism of action | Target organ/Tissue | Advantages | Disadvantages |
|---|---|---|---|---|
| Naked/Plasmid DNA; direct delivery | Endocytosis | Muscle, skin, liver, cardiac muscle, and solid tumor | Safe; simple technique | Low transfection efficacy |
| Gene gun | High-pressure helium stream | Ovarian cancer | Flexible, low cytotoxicity, good efficiency | Low penetration |
| Electroporation | Enrichment of cell membrane permeability | Skin, muscle | Good efficiency, reproducible results | Tissue damage, ease of access of electrodes to internal organ are restricted |
| Ultrasound micro bubble | Enrichment of cell membrane permeability | Brain, cornea, kidney, peritoneal cavity, muscle, heart, vascular cells. | Safe, flexible | Low efficiency |
| Magnetofection | Pinocytosis/Endocytosis | Primary cells and cells difficult to transfect by other methods | Low cytotoxicity | Transient transfection |
| Lipoplexes, liposomes | Endocytosis, DNA condensation | Airway epithelial cells, endothelial cells, hepatocytes, muscle, brain cells | Safety low cytotoxicity targeting possible | Low-to-medium efficiency, very low immunogenicity |
| Polyplexes, dendrimers | Endocytosis, DNA condensation, proton sponge effect | Lung, oral cavity, brain | Low immunogenicity, fair efficiency targeting possible | Complement activation, low–moderate efficiency, cytotoxic |
4. State-of-the-art of clinical trials for gene delivery systems
Many clinical trials of gene and antisense therapy are currently underway. Of the entire nonviral gene delivery systems only two approaches: (1) naked plasmid-DNA delivery and (2) cationic liposomes have shown great promise.[1] At present, naked plasmid DNA delivery has reached the state of clinical trials. Meanwhile, cationic polymers have only been used in animal models and have not advanced into clinical trials due to various problems.[26] Table 2 shows the compilation of different types of vectors/carriers used in gene therapy and highlights the relative advantages and disadvantages of each strategy.
Table 2.
Types, advantage, and disadvantage of different types of gene delivery carriers.
| Carrier type | Advantages | Disadvantages | Material used |
|---|---|---|---|
| Viral vectors | High-to-moderate transfection efficiency; stable expression | Immunogenic, smaller size gene;<8 can be loaded | Retrovirus, adenovirus, adeno-associated virus, lentivirus |
| Polymers | Good biocompatibility, biodegradable, and non-immunogenic; can load larger size >10 kb gene/plasmid | Moderately toxic (PEI); transient expression; need surface modification for better targeting; low in-vivo efficiency | PEI (polyplexes), PLL, PLGA, PLA, chitosan, gelatin |
| Lipids | Non-immunogenic; high target affinity; can load larger size >10 kb gene/plasmid | Low in-vivo efficiency; transient expression; low-to-moderated inflammatory responses | Liposome, lipofectamine (lipolplexes), niosomes |
| Magnetic/Metal nanoparticles | Nontoxic; low side effects; target specificity; can load larger size >10 kb gene/plasmid | Limited loading; need surface modification; transient expression; low in-vivo efficiency; low-to-moderated inflammatory responses | Magnetic nanoparticle, gold nanoparticle |
PEI: Poly(ethylenimine); PLL: Poly-(L-lysine); PLGA: Poly(lactic-co-glycolic acid).
The first clinical trial of IFN-β gene therapy was started by patients with malignant glioma in April 2000.[61] Theyhaveused cationic multilamellar liposomes consisting of N-(α-trimethylammonioacetyl)-disodecyl-D-glutamate chloride, dilaurolylphosphatidylcholine and DOPE. In some patients, after treatment, brain tumor growth ceased with little change in size over 10 weeks. Various clinical trials are underway involving naked plasmid DNA especially in the gene therapy of CNS diseases. The following (Table 3) gives more information about various clinical trials in treating neurological CNS diseases through the usage of viral and nonviral vectors. Although some nonviral-gene-vector-based gene therapies have reached clinical trials, many have not. Clinical trials involving nonviral gene vectors have been sparse due to challenges presented below. The future of gene therapy relies on overcoming these challenges.
Table 3.
Current CNS diseases clinical trial status of gene therapy*.
| Vector | Disease | Virus/nanomaterials | Gene target | Trial phase | Trial number |
|---|---|---|---|---|---|
| Viral vectors | PD | AAV2-NTN | CERE-120 | Phase I/II | NCT00985517 |
| Lentivirus | AADC, TH, and GTP-CH1 (ProSavin) | Phase I/II | NCT00627588/NCT01856439 | ||
| AAV2 | GAD | Phase I | NCT00195143 | ||
| AAV2 | GAD 65 | Phase II | NCT00643890 | ||
| AAV2 | hAADC | Phase I | NCT01973543 | ||
| AAV2 | hAADC-2 | Phase I/II | NCT02418598 | ||
| AAV2 | GDNF | Phase I | NCT01621581 | ||
| Alzheimer’s disease | AAV2 | NGF/CERE-110 | Phase I/II |
NCT00876863 NCT00087789 |
|
| Lentivirus | BACE1-directed siRNA | Preclinical | [62] | ||
| Lentivirus | Becn1 | Preclinical | [62] | ||
| Multiple sclerosis | Retrovirus | Myelin basic protein | Phase I/II | US-0851 | |
| HIV-1 (neuroAIDS) | Lentivirus | shRNA for CCR5, HIV-1 fusion inhibitor, C46 | Phase I/II | NCT01734850 | |
| LSD | Phase I/II | NCT00001215 | |||
| Sanfilippo syndrome B III (LSD) | rAAV9 | hNAGLU | Preclinical | [63,64] | |
| Epilepsy | rAAV | Galanin gene | Preclinical | [65] | |
| rAAV2 | NPY | Preclinical | [65] | ||
| rAAV1/2 | NPY | Preclinical | [65] | ||
| rAAV1 | NPY | Preclinical | [65] | ||
| HSV | Glut-1 | Preclinical | [65] | ||
| HSV | Bcl-2 | Preclinical | [65] | ||
| Chronic peripheral nervous system pain | HSV | TRPV1-inhibitory genes | Preclinical | [66] | |
| Lentivirus | PKCγ antisense | Preclinical | [66] | ||
| AAV | antisense NMDA-R | Preclinical | [66] | ||
| HSV | Kv1.2 sense RNA fragment | Preclinical | [66] | ||
| antisense vectors to the GABA-R | Preclinical | [66] | |||
| HSV | antisense to CGRP | Preclinical | [66] | ||
| AAV | Antisense to GTP cyclohydrolase I | Preclinical | [66] | ||
| Non-viral vectors | PD | PEGylated Liposome attached to OX26 ab | TfR | Preclinical | [67] |
| Alzheimer’s disease | Ex-vivo | Human NGF | Phase-1 | NCT00017940 | |
| Painful diabetic neuropathy | Naked plasmid DNA (VM202) | Hepatocyte growth factor | Phase II Phase III |
NCT01475786 NCT02427464 |
|
| Cerebral ischemia | Lipoplexes decorated with transferrin | NGF gene | Preclinical | [67] | |
| Mucopolysaccharidosis | Liposomes associated to TfR ab | Beta-glucuronidase gene | Preclinical | [9,67] | |
| Antinociception | PLGA NP with a glycosylated hepta-peptide | Possible adsorption-mediated endocytosis | Preclinical | [67] |
Data obtained from http://clinicaltrials.gov/ and references cited in the table.
AAV2: Adeno-associated virus type 2; AADC: Aromatic l-amino acid decarboxylase (AADC); GAD: Glutamic acid decarboxylase; GDNF: Glial cell-derived neurotrophic factor; NGF: Nerve growth factor; AADC: aromatic L-amino acid decarboxylase; BACE-1: Beta-secretase 1; BECN1: Beclin 1; CCR5: C-C chemokine receptor type 5; NPY: neuropeptide Y; BCL2: B-cell lymphoma 2; TRPV1: Transient Receptor Potential Cation Channel, Subfamily V, Member 1; PKCγ: Protein Kinase Cγ; GABA: Gamma-aminobutyric acid; TfR: Transferrin receptor; PLGA: Poly(lactic-co-glycolic acid); HIV-1: human immunodeficiency virus, type-1; PD: Parkinson’s disease.
4.1. Challenges of nonviral gene therapy and future prospects
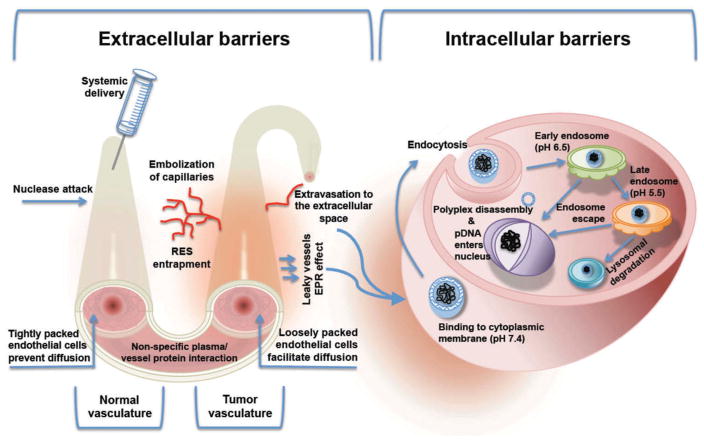
Nonviral gene therapy has emerged as a promising therapeutic approach for gene delivery. Although clinical practice of this field is still far into the future, much progress has been made in the last few years regarding both the optimization of the nonviral vector formulations and the exploration of alternative routes of administration. For transgene expression to occur, optimal nonviral vectors should not elicit an immune response and should be able, among other aspects, to protect the DNA cargo from degradation in circulation, enable extravasation from the blood-stream, traverse cellular membranes, enhance endosomal escape, and facilitate DNA transport to the nucleus.[38] Figure 5 shows the extracellular and intracellular barriers that vector/DNA complexes have to overcome in order to achieve an efficient transfection. Comprehensive understanding of these barriers has allowed the development of several strategies to surpass them. Most approaches are based on formulation modifications of the complexes and on the use of local routes of administration. Regarding this latter aspect, considerable evidence suggests that the optimization of noninvasive routes (e.g. intranasal delivery) of administration may provide safer and more effective gene delivery platforms in the future; therefore, it may be relevant to guide some efforts in this direction.[16] The major limitation of nonviral gene delivery, as mentioned repeatedly, is low transfection efficiency. Several strategies discussed increase transfection efficiency by providing NPs the ability to overcome extra- and intracellular barriers. However, there are two other aspects that are essential for developing optimal gene delivery platforms: targeting and long-term expression of the transgene. Both issues are crucial to applying nonviral gene delivery systems for clinical purposes, since they provide specificity and sustained effect of the treatment, respectively.
Figure 5.
Barriers faced by non-viral gene therapies following systematic delivery.
{Reproduce with permission from [68]}
Many efforts have been made in overcoming barriers (extracellular or intracellular) for delivering the therapeutic gene to the target location; however, further research is still required to achieve its clinical efficacy. In addition, other aspects such as the toxicity of the NPs and issues pertaining to manufacturing and regulations have to be carefully considered. Table 4 describes the possible approaches one can adapt to have better cell targeting and long-term expression of the transgene.
Table 4.
Strategies to improve gene transfer efficacy of nonviral vectors.
| Barriers | Functional moieties | Approaches | Materials/molecules used |
|---|---|---|---|
| Extracellular stability | Carrier molecules, hydrophobic moiety | DNA condensation protects from nucleases steric stability achieved by surface charge shielding | Protamine, lipids, gelation, PEGylation |
| Internalization | Targeting ligands | Receptor-mediated endocytosis | Transferrin, EGF, antibodies, RGD |
| Intracellular trafficking | Endosomal disruptive agent | Escape from endosomes and unpacking by proton sponge effect | PEI, DOPE |
| Nuclear entry | Nuclear localization signal | Nuclear entry | Tat, ReV |
DNA: Deoxyribonucleic acid; EGF: epidermal growth factor; RGD: arginyl-glycylaspartic acid; DOPE: 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, Tat: HIV-1-transactivating protein; ReV: HIV-1 Rev protein; PEI: polyethyleneimine.
4.2. Regulatory issues
Gene therapy is among the most highly regulated areas of human research worldwide.[69] In the United States, any new gene therapy study (as with any other type of new drug or device) must be approved and regulated by the US Food and Drug Administration (US FDA). In addition, prior to FDA approval, the Recombinant DNA Advisory Committee, an advisory committee of the National Institutes of Health (NIH) Office of Biotechnology Activities, must first review any study involving introduction of recombinant genetic material (DNA or RNA) into humans for any purpose.[38] This must be completed even if NIH funds the protocol, although as with any federally funded clinical trials, additional oversight is usually required by the relevant institute funding the trial. At the institutional level, gene therapy protocols must be approved by both the local Institutional Review Board and by the Institutional Biosafety Committee, a separate group regulated by NIH that is responsible for the oversight of biological agents.[70] Even for the seasoned investigator, navigating the regulatory process to obtain approval and satisfy regulation for human gene therapy can be daunting. Therefore, a review of the current regulatory process is provided as a guide for both new investigators who may wish to initiate a study as well as those who may wish to gain greater knowledge prior to participating in a multicenter study. Most of the regulatory information contained in this section is based upon guidelines published on federal government websites, which will be enumerated, and/or on personal experience of the author.
4.3. Targeting
Targeting is an essential requirement in gene delivery systems. Specific cell or tissue targeting can be achieved by modifying either the vehicle (the nonviral vector) or the cargo (the plasmid DNA). The most employed strategy is the attachment of specific ligands (such as transferrin for targeting the BBB) to nonviral vectors, which recognize particular receptors present in brain cells. Another possibility is to introduce modifications in the DNA cargo (instead of the vector) to achieve targeted expression of the transgene, this approach is known as “transcriptional targeting.” In this strategy the DNA would, in theory, be delivered to all tissues, but the expression of the transgene would only occur in the cell population where the particular transcription factors are present, that is, in the targeted cell population. The success of this method requires prior knowledge of differences in transcription factor expression between the targeted and normal tissue.[38,71] A common approach used to enhance cell or tissue targeting is outlined in Table 4. Beneficial aspects of targeted gene delivery include: increased bioavailability of the therapeutic product in the diseased tissue, reduced accumulation in healthy tissues (thus, reduced side effects), and enhanced patient compliance (due to reduction of drug dosage and reduced dosing frequency). All of these aspects help to increase the therapeutic efficacy and permit reduction in treatment costs.
4.4. Duration of gene expression
Long-term or sustained expression of transgene delivery constitutes a real challenge in nonviral gene therapy. It is a considerable limiting factor, since transient expression requires repeated dosing and makes the therapeutic effect unsustainable. Transgene expression can decrease in time due to several factors, including destruction by nucleases and gene loss through recombination, distribution to non-nuclear compartments and/or recognition and subsequent silencing of foreign DNA.[71] In dividing cells the percentage of transfected cells decreases at each division because while cells replicate, plasmids do not.
Strategies to increase duration of transgene expression have focused on plasmid DNA modifications rather than on vector modifications. Some of those strategies are aimed at integrating the transgenes into the host genome by using viral integrases, site-specific recombinases, and transposases, which are enzymes with the capacity to insert foreign DNA into the host genome.[71] However, this approach cannot be clinically applicable in humans because of its associated risks, such as the induction of insertional mutagenesis in the host cells. A different strategy to achieve sustained transgene expression is the use of autonomously replicating plasmids or episomes. This approach does not require integration in the host genome and, hence, avoids the risk of insertional mutagenesis.[71] In addition, episomally replicating plasmids usually yield high levels of transgene expression. These strategies incorporate genes that encode necessary cofactors for transcription of the plasmid to the therapeutic plasmid DNA making the transgene expression less dependent on host factors. An efficient approach is the incorporation of viral DNA, which allows the plasmid to replicate extrachromosomally. However, induction of the immune response and the risk of transformation are associated with replication inducing viral DNA elements. Alternatively, mammalian scaffold/matrix attachment regions have been discovered to incorporate into plasmid DNA instead of the aforementioned viral sequences. Further modifications can be also applied to the therapeutic plasmid DNA in order to increase the strength or the specificity of the therapeutic transgene expression. For instance, positive feedback loops can be integrated. Technologies incorporating positive feedback loops are estimated to increase the strength of weak, but highly specific regulatory elements.[71]
4.5. Toxicity and manufacturing issues
Besides increasing transfection efficiency through targeting and other strategies, careful attention to toxicity, and issues pertaining to manufacturing and regulation of nonviral delivery systems is mandatory. Scalability and long-term storage requirements are essential factors to be taken into serious consideration when developing nonviral formulations for potential commercial application and introduction in clinical practice.[38] A generally accepted advantage of nonviral vectors is their ease for large-scale manufacturing. However, this can become challenging as formulations increase in complexity when incorporating stabilizing components and bioactive targeting ligands. Characteristics such as size, charge, surface functionalization, shape, and architecture may contribute to the toxicity profile of lipoplexes/polyplexes/NPs. Nonviral vectors are thought to cause toxicity through different mechanisms, including membrane destabilization and lysis, due to induced oxidative stress, initiation of inflammatory response, and induction of global changes in gene expression profiles.[71] Additionally, the properties of the biomaterials used can influence toxicity, depending on the rate of degradation and persistence in organs. Continuous accumulations of biomaterials are more likely to induce an inflammatory response while products of the degradation of biomaterials/NPs can potentially cause toxicity. However, knowledge of how nonviral vectors are disassembled and metabolically processed is bare and further research is necessary. To conclude, the concept of a unique universal nonviral vector is nowadays abandoned, and it is increasingly accepted that future nonviral gene delivery platforms will be based on multifunctional vectors specifically tailored for different applications. However, there are some generally assumed features that all nonviral vectors should accomplish for efficient gene delivery. In short, three main factors should be taken into consideration when developing nonviral gene delivery platforms: (1) formulation components (of both the vector and the DNA) – the biomaterials/NP should be able to protect DNA over extra- and intracellular barriers and deliver the cargo into the nucleus of target cells; once inside the nucleus, the plasmids can be addressed to the nuclear matrix for episomal replication and sustained expression; (2) manufacturing issues – nonviral vector formulations should have potential for scale-up; and (3) safety issues – formulations should be nontoxic, non-immunogenic and should have suitable storage conditions. Although nonviral vectors are still far from clinical practice, they represent a safer alternative to conventional viral vectors. Several formulations and strategies are under investigation with the aim of overcoming extra- and intracellular barriers, enhancing targeted transfection, and in general, increasing transfection efficiency for the cure of CNS diseases. In addition, those formulations would ideally be suitable for administration through noninvasive routes, such as the intranasal administration to target the brain, topical ocular administration for the retina and aerosols for pulmonary diseases. Finally, the inclusion of novel functional modules within both the carrier and the DNA molecule will produce a range of nonviral vectors tailored for specific applications, including the safe and long-term expression of therapeutic genes in humans.[38,72] Therefore, reasonable hope suggests that next-generation gene delivery systems may be based on nonviral vector systems tailored for specific applications and suitable for noninvasive administration routes, representing an ideal platform to effectively shuttle the genetic material to target cells in a safe and controlled way.
5. Conclusion
The success of gene therapy is highly dependent on the delivery vector. Viral vectors have dominated clinical trials in gene therapy for its relatively high delivery efficiency. However, the improvement of efficacy of nonviral vectors has led to an increased number of products entering into clinical trials. A better understanding of the mechanisms governing the efficiency of transfection, from the formation of the complexes to their intracellular delivery, will lead to the design of better adapted nonviral vectors for gene therapy applications. A number of potentially rate-limiting steps in the processes of nonviral-mediated gene delivery have been identified. These include the efficiency of cell surface association, internalization, release of gene from intracellular compartments such as endosomes, transfer via the cytosol, translocation into the nucleus, and transcription efficacy. Insight into molecular features of each of these steps is essential in order to determine their effectiveness as a barrier and to identify means of overcoming these hurdles. Although nonviral vectors may work reasonably well in-vitro, clinical success is still far from ideal. Considering the number of research groups that focus their investigations on the development of new vectors for gene therapy, together with the advances in the development of new technologies to better understand their in vitro and in vivo behavior, the present limitations of nonviral vectors will be resolved rationally.
6. Expert opinion
Gene therapy, as a promising therapeutics to treat genetic or acquired diseases, has accomplished exhilarating growth in recent years. Fitting gene vectors can be vital for gene transfer. Cationic lipids and polymers are the most important class of nonviral vectors and have been explored for their advantages over viral vectors, for example less immunogenic, easy escape of various extra- and intracellular environments, ease of production, and not cytotoxic. Due to their unique properties of loading larger size gene/plasmid and target release, they hold the potential to supplant viral vectors in the future. While nonviral vectors products have many advantages over their viral counterparts, only a few have reached clinical stage thus lot of improvement is needed.
As a result, the need for safer vector substitutes has led to the advancement of novel drug delivery systems for example liposomes, cationic polyplexes, and nanoparticles (organic and inorganic). Even though these alternative vectors have shown good potential, only biodegradable NPs are the nonviral vectors that can provide a targeted intracellular delivery with controlled release properties, reduced toxicity and the escaping of accumulation within the target tissue after repeated administration. Further to increase the transfection efficiency and reduced cytotoxicity scientist have identified many factors that influences overall success of nonviral vectors. For example, in case of cationic lipid, excess cationic charge may result in lower gene delivery due to exceedingly tight nucleic acid binding or related increased cytotoxicity or degree of unsaturation in lipid carriers may play important role in achieving higher transfection efficiency. Also, we need to develop the lipid nanoformulation which can easily cleave and facilitate timely endosomal release of plasmid for better and long-term expression. In case of cationic polymer, various factors that is charge density of polymer, N/P ratios, polymer molecular weight, and degree of complexation plays an important role for achieving higher transfection efficiency and reduced cytotoxicity.
Also, a number of possibly rate-limiting steps in the processes of nonviral mediated gene delivery have been recognized, which include the efficiency of cell surface association, internalization, release of gene from intracellular compartments, for example endosomes, transfer via the cytosol, translocation into the nucleus, and transcription efficacy. Understanding into molecular mechanism of each of these steps is vital in order to determine their efficiency as a barrier and to identify means of overpowering these hurdles. As we know, nonviral vectors have worked reasonably well in vitro systems, but clinical success is still far from the expectation. Bearing in mind the number of research groups that focus their research on the advancement of new nonviral vectors for gene therapy, together with the advances in the development of new technologies to better understand their in vitro and in vivo performance, the present limitations of nonviral vectors will be resolved judiciously. Good understanding of internal trafficking and cell architecture will help us to recognize the likely hurdles and assist scientists to design more proficient nonviral vectors. Combinatorial Synthesis and High-Throughput Screening of polymer libraries may offer a better idea in developing high transfection efficient biodegradable polymer in future. Thus, in summary, we can say the strategies that amalgamate nonviral and viral free biological vectors might be advantageous to attain anticipated long lasting, competent, and nontoxic gene delivery system.
Acknowledgments
Author’s acknowledge Institute of NeuroImmune Pharmacology (INIP) and Center for Personalized NanoMedicine (CPNM) for the research support and facility.
Footnotes
The merger between lysosomes and endosomes begin the process of degradation and thus this escape from lysosomes increases the chances for a successful DNA transfection
Declaration of interest
This work was supported by the National Institute on Drug Abuse [RO1DA027049, RO1DA037838, RO1DA040537, RO1DA042706A]. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1••.Al-Dosari MS, Gao X. Nonviral gene delivery: principle, limitations, and recent progress. Aaps J. 2009;11(4):671–681. doi: 10.1208/s12248-009-9143-y. This paper highlights current non-viral methods and recent efforts toward refinement of non-viral approaches. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang W, Li W, Ma N, et al. Non-viral gene delivery methods. Curr Pharm Biotechnol. 2013;14(1):46–60. [PubMed] [Google Scholar]
- 3•.Nair M, Jayant RD, Kaushik A, et al. Getting into the brain: potential of nanotechnology in the management of neuroaids. Adv Drug Deliv Rev. 2016 doi: 10.1016/j.addr.2016.02.008. This paper highlights the issue of CNS delivery and approaches used to overcome BBB drug delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jayant RD, Atluri VS, Agudelo M, et al. Sustained-release nanoart formulation for the treatment of neuroAIDS. Int J Nanomedicine. 2015;10:1077–1093. doi: 10.2147/IJN.S76517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaushik A, Jayant RD, Nikkhah-Moshaie R, et al. Magnetically guided central nervous system delivery and toxicity evaluation of magneto-electric nanocarriers. Sci Rep. 2016:6. doi: 10.1038/srep25309. Article id: 25309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6•.Taylor RM. Gene therapy for inherited neurological disease. ILAR J. 1994;36(3–4):56–63. This paper highlights the CNS neurological diseases and rationale for gene therapy of neurological diseases. [Google Scholar]
- 7.Davidson BL, Breakefield XO. Viral vectors for gene delivery to the nervous system. Nat Rev Neurosci. 2003;4(5):353–364. doi: 10.1038/nrn1104. [DOI] [PubMed] [Google Scholar]
- 8.Alexander GE. Biology of parkinson’s disease: pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues Clin Neurosci. 2004;6:259–280. doi: 10.31887/DCNS.2004.6.3/galexander. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Simonato M, Bennett J, Boulis NM, et al. Progress in gene therapy for neurological disorders. Nat Rev Neurol. 2013;9(5):277–291. doi: 10.1038/nrneurol.2013.56. This review article describe the promising gene therapy strategies for neurological diseases and discuss prospects for future development of gene therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serrano-Pozo A, Frosch MP, Masliah E, et al. Neuropathological alterations in alzheimer disease. Cold Spring Harb Perspect Med. 2011;1(1):a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaushik A, Jayant RD, Tiwari S, et al. Nano-biosensors to detect beta-amyloid for Alzheimer’s disease management. Biosens Bioelectron. 2016;80:273–287. doi: 10.1016/j.bios.2016.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12••.Griesenbach U. Progress and prospects: gene therapy clinical trials (part 2) Gene Ther. 2007;14(22):1555–1563. doi: 10.1038/sj.gt.3303033. This article highlights the status of clinical trials for CNS disease treatment using gene therapy. [DOI] [PubMed] [Google Scholar]
- 13.Bowers WJ, Breakefield XO, Sena-Esteves M. Genetic therapy for the nervous system. Hum Mol Genet. 2011;20:R28–R41. doi: 10.1093/hmg/ddr110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho DY, Sapolsky RM. Gene therapy for the nervous system. Sci Am. 1997;276(6):116–120. doi: 10.1038/scientificamerican0697-116. [DOI] [PubMed] [Google Scholar]
- 15.Atluri VSR, Hidalgo M, Samikkannu T, et al. Effect of human immunodeficiency virus on blood-brain barrier integrity and function: an update. Front Cell Neurosci. 2015;9:212. doi: 10.3389/fncel.2015.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Upadhyay RK. Drug delivery systems, CNS protection, and the blood brain barrier. Biomed Res Int. 2014;2014:1–37. doi: 10.1155/2014/869269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Misra A, Ganesh S, Shahiwala A, et al. Drug delivery to the central nervous system: a review. J Pharm Pharm Sci. 2003;6(2):252–273. [PubMed] [Google Scholar]
- 18.Timbie KF, Mead BP, Price RJ. Drug and gene delivery across the blood–brain barrier with focused ultrasound. J Control Release. 2015;219:61–75. doi: 10.1016/j.jconrel.2015.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Bourdenx M, Dutheil N, Bezard E, et al. Systemic gene delivery to the central nervous system using adeno-associated virus. Front Mol Neurosci. 2014;7:50. doi: 10.3389/fnmol.2014.00050. This review highlights the recent results achieved in the last decade using systemic AAV-mediated delivery and propose their brief assessment on therapeutic value. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayant RD, Nair M. Nanotechnology for the treatment of neuroaids. J Nanomedicine Res. 2016;3(1):0047. [Google Scholar]
- 21.Bouard D, Alazardedici D, Cosset F-L. Viral vectors: from virology to transgene expression. Br J Pharmacol. 2009;157(2):153–165. doi: 10.1038/bjp.2008.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4(5):346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 23•.Gray SJ, Woodard KT, Samulski RJ. Viral vectors and delivery strategies for cns gene therapy. Ther Deliv. 2010;1(4):517–534. doi: 10.4155/tde.10.50. This review aims to provide a broad overview of the targets, challenges and potential for gene therapy in the CNS, citing specific examples. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samulski RJ, Muzyczka N. Aav-mediated gene therapy for research and therapeutic purposes. Annu Rev Virol. 2014;1:427–451. doi: 10.1146/annurev-virology-031413-085355. [DOI] [PubMed] [Google Scholar]
- 25.Daya S, Berns KI. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 2008;21(4):583–593. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26••.Ramamoorth M, Narvekar A. Non viral vectors in gene therapy-an overview. J Clin Diagn Res. 2015;9(1):GE01–GE06. doi: 10.7860/JCDR/2015/10443.5394. This review provides the details about various types of non-viral vectors and their recent advancement in translating them from “Bench to bedside”. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shmueli RB, Anderson DG, Green JJ. Electrostatic surface modifications to improve gene delivery. Expert Opin Drug Deliv. 2010;7(4):535–550. doi: 10.1517/17425241003603653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Q, Wang C-H, Pack DW. Polymeric carriers for gene delivery: chitosan and poly (amidoamine) dendrimers. Curr Pharm Des. 2010;16(21):2350–2368. doi: 10.2174/138161210791920469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barua S, Ramos J, Potta T, et al. Discovery of cationic polymers for non-viral gene delivery using combinatorial approaches. Comb Chem High Throughput Screen. 2011;14(10):908–924. doi: 10.2174/138620711797537076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Bergen JM, Park I-K, Horner PJ, et al. Nonviral approaches for neuronal delivery of nucleic acids. Pharm Res. 2008;25(5):983–998. doi: 10.1007/s11095-007-9439-5. This review article describe the challenges preventing successful nonviral delivery of nucleic acids to neurons and review strategies aimed at overcoming these challenges. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin H, Kanasty RL, Eltoukhy AA, et al. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15(8):541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 32•.Hill AB, Chen M, Chen C-K, et al. Overcoming gene-delivery hurdles: physiological considerations for nonviral vectors. Trends Biotechnol. 2015;34(2):91–105. doi: 10.1016/j.tibtech.2015.11.004. This article highlight the current status of viral and non viral US gene therapy clinical trials and also describes the rational of selecting the appropriate material for building the vector. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rohan N. Global transfection technologies market worth $601 million by 2017. Markets and Markets. 2016 Available from: http://www.marketsandmarkets.com/PressReleases/transfection-technologies.asp.
- 34.Ginn SL, Alexander IE, Edelstein ML, et al. Gene therapy clinical trials worldwide to 2012–an update. J Gene Med. 2013;15(2):65–77. doi: 10.1002/jgm.2698. [DOI] [PubMed] [Google Scholar]
- 35.Lim ST, Airavaara M, Harvey BK. Viral vectors for neurotrophic factor delivery: A gene therapy approach for neurodegenerative diseases of the CNS. Pharmacological Res. 2010;61(1):14–26. doi: 10.1016/j.phrs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manfredsson FP, Mandel RJ. Development of gene therapy for neurological disorders. Discov Med. 2010;9(46):204–211. [PubMed] [Google Scholar]
- 37.Ylä-Herttuala S. Endgame: glybera finally recommended for approval as the first gene therapy drug in the european union. Mol Ther. 2012;20(10):1831–1832. doi: 10.1038/mt.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38•.Villate-Beitia I, Puras G, Zarate J, et al. First insights into noninvasive administration routes for non-viral gene therapy. In: Hashad D, editor. Gene therapy-principles and challenges. InTech; 2015. pp. 145–177. Available from: http://www.intechopen.com/books/gene-therapy-principles-and-challenges/first-insights-into-non-invasive-administration-routes-for-non-viral-gene-therapy This book chapter highlights the possible future strategies to improve DNA transfection efficiency using non-viral vectors and focusing on the non-invasive routes of administration. [DOI] [Google Scholar]
- 39.Gascón AR, Pozo-Rodríguez AD, Solinís M. Non-viral delivery systems in gene therapy. Gene Ther–Tools Potential Appl. 2013 doi: 10.5772/52704. [DOI] [Google Scholar]
- 40.Morille M, Passirani C, Vonarbourg A, et al. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials. 2008;29(24):3477–3496. doi: 10.1016/j.biomaterials.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 41.Thomas M, Klibanov A. Non-viral gene therapy: polycation-mediated DNA delivery. Appl Microbiol Biotechnol. 2003;62(1):27–34. doi: 10.1007/s00253-003-1321-8. [DOI] [PubMed] [Google Scholar]
- 42••.Lungwitz U, Breunig M, Blunk T, et al. Polyethylenimine-based nonviral gene delivery systems. Eur J Pharmaceutics Biopharmaceutics. 2005;60(2):247–266. doi: 10.1016/j.ejpb.2004.11.011. This article provides the insight into strategies developed for PEI-based non-viral vectors to overcome intracellular obstacles, methods for polyplex preparation and the incorporation of endosomolytic agents or nuclear localization signals. [DOI] [PubMed] [Google Scholar]
- 43.Niidome T, Huang L. Gene therapy progress and prospects: nonviral vectors. Gene Ther. 2002;9(24):1647–1652. doi: 10.1038/sj.gt.3301923. [DOI] [PubMed] [Google Scholar]
- 44•.Akinc A, Thomas M, Klibanov AM, et al. Exploring polyethylenimine- mediated DNA transfection and the proton sponge hypothesis. J Gene Med. 2005;7(5):657–663. doi: 10.1002/jgm.696. This paper provides the PEI mechanism of action for gene therapy. [DOI] [PubMed] [Google Scholar]
- 45.Pack DW, Hoffman AS, Pun S, et al. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4(7):581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 46.Rudzinski WE, Aminabhavi TM. Chitosan as a carrier for targeted delivery of small interfering rna. Int J Pharm. 2010;399(1–2):1–11. doi: 10.1016/j.ijpharm.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 47.Mansouri S, Lavigne P, Corsi K, et al. Chitosan-DNA nanoparticles as non-viral vectors in gene therapy: strategies to improve transfection efficacy. Eur J Pharmaceutics Biopharmaceutics. 2004;57(1):1–8. doi: 10.1016/s0939-6411(03)00155-3. [DOI] [PubMed] [Google Scholar]
- 48.He C-X, Tabata Y, Gao J-Q. Non-viral gene delivery carrier and its three-dimensional transfection system. Int J Pharm. 2010;386(1–2):232–242. doi: 10.1016/j.ijpharm.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Dufès C, Uchegbu IF, Schätzlein AG. Dendrimers in gene delivery. Adv Drug Deliv Rev. 2005;15(57):2177–02. doi: 10.1016/j.addr.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 50•.Huang RQ, Qu YH, Ke WL, et al. Efficient gene delivery targeted to the brain using a transferrin-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. FASEB J. 2007;21(4):1117–1125. doi: 10.1096/fj.06-7380com. This article provides the insights into polymer based non vectors modification to achieve higher transfection rates. [DOI] [PubMed] [Google Scholar]
- 51.Huang Y, Rao Y, Chen J, et al. Polysorbate cationic synthetic vesicle for gene delivery. J Biomed Mater Research-Part. 2011;96(3):513–519. doi: 10.1002/jbm.a.32999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.De Ilarduya CT, Sun Y, Düzgüneş N. Gene delivery by lipoplexes and polyplexes. Eur J Pharm Sci. 2010;40(3):159–170. doi: 10.1016/j.ejps.2010.03.019. This article provide a framework for the future design and synthesis of optimal non-viral vectors for gene therapy. [DOI] [PubMed] [Google Scholar]
- 53•.Zuhorn IS, Engberts JB, Hoekstra D. Gene delivery by cationic lipid vectors: overcoming cellular barriers. Eur Biophys J. 2007;36(4–5):349–362. doi: 10.1007/s00249-006-0092-4. This article provides the mechanism by which cationic lipids can surpass the intra & extra cellular barrier to achieve higher transfection efficiency. [DOI] [PubMed] [Google Scholar]
- 54.Li W, Szoka FC., Jr Lipid-based nanoparticles for nucleic acid delivery. Pharm Res. 2007;24(3):438–449. doi: 10.1007/s11095-006-9180-5. [DOI] [PubMed] [Google Scholar]
- 55.Uherek C, Wels W. DNA-carrier proteins for targeted gene delivery. Adv Drug Deliv Rev. 2000;44(2–3):153–166. doi: 10.1016/s0169-409x(00)00092-2. [DOI] [PubMed] [Google Scholar]
- 56.Pellizzari R, Rossetto O, Schiavo G, et al. Tetanus and botulinum neurotoxins: mechanism of action and therapeutic uses. Philosophical Trans Royal Soc B: Biol Sci. 1999;354(1381):259–268. doi: 10.1098/rstb.1999.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaushik A, Jayant RD, Sagar V, et al. The potential of magneto-electric nanocarriers for drug delivery. Expert Opin Drug Deliv. 2014;11(10):1635–1646. doi: 10.1517/17425247.2014.933803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58•.Dobson J. Gene therapy progress and prospects: magnetic nanoparticle- based gene delivery. Gene Ther. 2006;13(4):283–287. doi: 10.1038/sj.gt.3302720. This article highlights the use of nanoparticle (magnetic) for the gene delivery as a non viral vector and their potential future application for CNS diseases. [DOI] [PubMed] [Google Scholar]
- 59.Mok H, Zhang M. Superparamagnetic iron oxide nanoparticle-based delivery systems for biotherapeutics. Expert Opin Drug Deliv. 2013;10(1):73–87. doi: 10.1517/17425247.2013.747507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xia T, Kovochich M, Liong M, et al. Polyethyleneimine coating enhances the cellular uptake of mesoporous silica nanoparticles and allows safe delivery of sirna and DNA constructs. ACS Nano. 2009;3(10):3273–3286. doi: 10.1021/nn900918w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshida J, Mizuno M, Wakabayashi T. Lnterferon-β gene therapy for cancer: basic research to clinical application. Cancer Sci. 2004;95(11):858–865. doi: 10.1111/j.1349-7006.2004.tb02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Santiago-Lopez AJ, Hovell CM, Lee H, et al. Neuroregeneration: disease modeling and therapeutic strategies for alzheimer’s and parkinson’s diseases. In: Jo H, Jun H-W, Shin J, et al., editors. Biomedical engineering: frontier research and converging technologies. Cham: Springer; 2016. pp. 293–325. [Google Scholar]
- 63.McBride KL, Truxal K, McNally K, et al. Design of a phase i/ii gene transfer clinical trial of raav9. Cmv. Hnaglu for mucopolysaccharidosis type iiib. Mol Genet Metab. 2016;117(2):S78–S79. [Google Scholar]
- 64•.Kalburgi SN, Khan NN, Gray SJ. Recent gene therapy advancements for neurological diseases. Discov Med. 2013;15(81):111–119. This article highlights the recent advancement in CNS gene transfer technology and future CNS-directed clinical trials. [PMC free article] [PubMed] [Google Scholar]
- 65.Vezzani A. Gene therapy in epilepsy. Epilepsy Currents. 2004;4(3):87–90. doi: 10.1111/j.1535-7597.2004.43001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guedon J-MG, Wu S, Zheng X, et al. Current gene therapy using viral vectors for chronic pain. Mol Pain. 2015;11(27) doi: 10.1186/s12990-015-0018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67••.Peluffo H, Unzueta U, Negro-Demontel ML, et al. BBB-targeting, protein-based nanomedicines for drug and nucleic acid delivery to the cns. Biotechnol Adv. 2015;33(2):277–287. doi: 10.1016/j.biotechadv.2015.02.004. This article highlights the engineering of functional proteins offers drug delivery tools for specific CNS diseases and how proteins can be engineered into ‘artificial viruses’ as to affordable therapeutics. [DOI] [PubMed] [Google Scholar]
- 68.Miyata K, Nishiyama N, Kataoka K. Rational design of smart supramolecular assemblies for gene delivery: chemical challenges in the creation of artificial viruses. Chem Soc Rev. 2012;41(7):2562–2574. doi: 10.1039/c1cs15258k. [DOI] [PubMed] [Google Scholar]
- 69.Koehler RN, Altevogt BM, Gostin LO. Oversight and review of clinical gene transfer protocols. Washington (DC): National Academies Press; 2014. [DOI] [PubMed] [Google Scholar]
- 70••.National Institutes of Health. Health UNIo: NIH guidelines for research involving recombinant or synthetic nucleic acid molecules. [cited 2016 Apr]. Available from: http://osp.od.nih.gov/sites/default/files/NIH_Guidelines.html This NIH report highlight the regulatory aspects of using recombinant technology for therapeutic applications.
- 71.Van Gaal EV, Hennink WE, Crommelin DJ, et al. Plasmid engineering for controlled and sustained gene expression for nonviral gene therapy. Pharm Res. 2006;23(6):1053–1074. doi: 10.1007/s11095-006-0164-2. [DOI] [PubMed] [Google Scholar]
- 72.Glover DJ, Glouchkova L, Lipps HJ, et al. Overcoming barriers to achieve safe, sustained and efficient non-viral gene therapy. Adv Gene Mol Ther. 2007;1(2):126–140. [Google Scholar]