Abstract
Objective
The magnitude of acute tolerance is a strong predictor of the development of longer term, chronic tolerance and plays a decisive role in risky decisions (e.g., driving after drinking). Therefore, it is important to identify factors that increase the magnitude of this adaptive process. The present study explored whether acute tolerance magnitude varied as a function of the overall rate of increase in breath alcohol concentration (BrAC).
Methods
Twenty-nine young adult social drinkers (M age = 22.55, SD = 3.10; 62.1% female) consumed a moderate dose of alcohol (men: 0.86 g/kg, women: 0.75 g/kg) in a controlled laboratory setting. Subjective intoxication was assessed at matched BrACs (~0.060 g/dL) on each limb of the BrAC curve.
Results
Hierarchical regression results indicated that faster overall increases in BrAC on the ascending limb were associated with greater acute tolerance for subjective intoxication ratings (p < .01, R2 = .29).
Conclusions
These results present some of the first evidence that faster increases in BrAC may be associated with greater acute tolerance, as indicated by greater reduction in subjective intoxication across the limbs of the BrAC curve. This greater reduction may, in turn, promote heavier drinking and/or engagement in behaviors for which one is unfit (e.g., driving after drinking).
Keywords: Acute Tolerance, Alcohol Administration, Subjective Intoxication, Blood Alcohol Concentration
Acute alcohol tolerance has commonly been defined as the reduction in alcohol effects over time within a single exposure, independent of blood alcohol concentration (Martin & Moss, 1993). Researchers have reported acute tolerance in both animals and humans for a number of alcohol effects, using a wide range of physiological, behavioral, cognitive, and subjective measures (e.g., LeBlanc, Kalant, & Gibbins, 1975; Martin & Moss, 1993; Radlow & Hurst, 1985; Weafer & Fillmore, 2012). Moreover, studies have established that reduction over time in alcohol responses (i.e., acute tolerance) cannot be fully accounted for by practice effects (Cromer, Cromer, Maruff, & Snyder, 2010) or differences in the direction of change in BrACs (rising versus falling; Hendershot et al., 2015; O’Connor, Morzorati, Christian, & Li, 1998; Morzorati, Ramchandani, Flury, Li, & O’Connor, 2002; Ramchandani et al., 2002). Individual differences in the magnitude of acute tolerance have also been observed. This variability has been shown to predict subsequent levels of long term tolerance, and thus may increase risk of alcohol use disorder (Beirness & Vogel-Sprott, 1984). Differences in acute tolerance may also play a role in alcohol-related risk behaviors, such as driving after drinking (Amlung, Morris, & McCarthy., 2014; Marczinski & Fillmore, 2009, Morris, Treloar, Niculete, & McCarthy, 2013). Therefore, better understanding the factors that affect the magnitude of acute tolerance may have implications for alcohol use disorder risk and engagement in related behaviors.
Several factors have been suggested to affect the magnitude of acute tolerance to alcohol effects. Radlow (1994, 2006) postulated that the magnitude of acute tolerance increases as a linear function of exposure time. That is, the longer an organism’s system is exposed to alcohol the greater the reduction observed in alcohol effects. This notion has been supported in both animal and human experiments (Kaplan, Sellers, Hamilton, Naranjo, & Dorian, 1985; Lê & Kalant, 1992; Morzorati et al., 2002), with a few exceptions (see Martin & Moss, 1993). Others have suggested that acute tolerance varies as a function of prior drinking patterns (Evans & Levin, 2004; Fillmore & Weafer, 2012; Hiltunen, 1997; Portans, White, & Staiger, 1989). For instance, Marczinski and Fillmore (2009) reported that binge drinkers display greater acute tolerance, with larger reductions in subjective intoxication across the blood alcohol curve compared to non-binge drinkers.
A number of theoretical models have suggested that the degree of acute tolerance observed is influenced by the magnitude of initial drug effects (e.g., Koob & Le Moal, 1997; Poulos & Cappell, 1991; Ramsay & Woods, 1997; Solomon & Corbit, 1973). According to these models, the initial effects of a drug disrupt the internal homeostatic state of an organism, which in turn leads to the activation of adaptive processes to counteract the disturbance. The number of adaptive processes that are activated is dependent on the magnitude of the disturbance, such that greater disturbances activate more adaptive processes and greater acute tolerance (Poulos & Cappell, 1991; Ramsay & Woods, 1997).
Based on these theoretical models, factors that influence the strength of initial alcohol effects should also influence the magnitude of acute tolerance. One factor associated with the initial effects of alcohol is the overall rate at which brain alcohol concentration (approximated by breath alcohol concentration; BrAC) increases. Martin and Earleywine (1990) examined the association between the overall rate of increase in BrAC and subjective intoxication by experimentally manipulating participants’ duration of consumption. In this study, overall rate of increase in BrAC was calculated for each participant by dividing peak BrAC by the amount of time (min) it took for BrAC to peak. Results indicated that faster increases in BrAC were associated with greater feelings of intoxication. Similarly, Fillmore and Vogel-Sprott (1998) found that faster overall increases in BrAC (calculated by dividing BrAC at time of assessment by the amount of time elapsed since consumption) were associated with greater psychomotor impairment on a pursuit rotor task in a sample of young adult males. In fact, the rate of increase in BrAC was shown to be a better predictor of motor impairments than individual BrAC levels at any given moment (Fillmore & Vogel-Sprott, 1998). While the overall rate of increase in BrAC is associated with greater impairment, it is unclear whether this effect extends across both limbs of the BrAC curve. Based on the notion of acute tolerance as homeostatic adaptation, a faster increase in BrAC should lead to greater impairment on the ascending limb, but greater recovery on the descending limb due to increased activation of metabolic or pharmacodynamic adaptive responses (see Kalant, 2010; Nestler, 2001; Tabakoff & Rothstein, 1983). These adaptive responses may include alterations in cell membrane receptors or ion channels or intracellular changes in energy metabolism, among others (Kalant, 2010; Nestler, 2001).
The aim of the present study was to explore this possibility—the overall rate of increase in BrAC is associated with the magnitude of acute tolerance. Acute tolerance was quantified in the present study by comparing subjective intoxication ratings at matched BrACs (~0.060 g/dL) on the ascending and descending limbs of the breath alcohol curve. This method of quantifying acute tolerance was first described by Mellanby (1919) and has been widely utilized in the literature (e.g., Beirness & Vogel-Sprott, 1984; Marczinski & Fillmore, 2009; Weafer & Fillmore, 2012; Wetherill et al., 2012). Other methods of calculating acute tolerance have also been proposed (see Martin & Moss, 1993). Subjective intoxication was used to evaluate alcohol effects over other measures (e.g., motor and cognitive functions) for several reasons. Most importantly, subjective intoxication has been used extensively in prior human alcohol administration studies and has consistently been reported to exhibit acute tolerance (e.g., Martin & Moss, 1993; Portans et al., 1989; Radlow & Hurst, 1985; Weafer & Fillmore, 2012). Thus, there is greater certainty of observing the development of acute tolerance with this measure compared to other behavioral measures for which results have been less consistent (see Schweizer & Vogel-Sprott, 2008). Furthermore, subjective effects of alcohol are believed to play a decisive role in the regulation of drinking (Morean & Corbin, 2010). Thus, better understanding the factors that influence the magnitude of acute tolerance of subjective intoxication as assessed by the Mellanby method may have important clinical and theoretical implications.
Based on past findings (e.g., Martin & Earleywine, 1990; Fillmore & Vogel-Sprott, 1998), we anticipated that participants would display a greater response to alcohol on the ascending limb than they would on the descending limb at the same BrAC, reflecting acute tolerance in feelings of intoxication. More importantly, we expected that the overall rate of change in rising BrACs would be associated with the magnitude of acute tolerance to subjective intoxication, such that faster increases in BrAC would correspond with greater decreases in intoxication ratings across assessment points. Given the association of length of alcohol exposure and binge drinking with acute tolerance magnitude (e.g., Marczinski & Fillmore, 2009; Morzorati et al., 2002), we included both these variables in our analyses to better account for their effects.
Methods
Participants
Data for this study were drawn from a larger alcohol challenge experiment designed to test the effects of acute tolerance on judgments about driving after drinking (Amlung et al., 2014)1. Participants were young adults recruited from a large, Midwestern university and its surrounding community via fliers and university informational emails. Eligibility was determined based on a telephone interview that assessed typical drinking behaviors, along with physical and mental health. To reduce the likelihood of experiencing adverse effects in the laboratory, eligible respondents had to report consuming approximately 5 or more drinks on at least one occasion in the past 6 months. Additionally, they had to report no current or lifetime psychiatric disorder, substance use disorder, or head trauma. Respondents were excluded if they reported having any contraindications with alcohol, such as certain medical conditions (e.g., hepatitis, epilepsy) or taking certain medications (e.g., benzodiazepines, sedatives). Women who were pregnant or nursing were also excluded from participation.
Data from the 31 participants who consumed alcohol and finished the full study were used in the present analyses. Two of these participants were excluded from analyses: one due to extreme breath alcohol concentration readings (>3 SD away from the group mean) and the other due to sickness following alcohol consumption. The remaining 29 participants (62.1% female) ranged in age from 21 to 33 years old (M = 22.55, SD = 3.10). The majority of the sample was Caucasian (n = 20), with three African Americans, one Native American, three who identified as multi-racial, and one Other response (one participants did not indicate race).
Measures
Demographics
Demographic information such as age, sex, and race was assessed using a self-report questionnaire.
Alcohol Use
Past month alcohol use was assessed using three open-ended questions modified from the Monitoring the Future project (Johnston, O’Malley, Bachman, & Schulenberg, 2011). Participants were asked to report, based on the past 30 days, the number of occasions they consumed alcohol, the number of drinks they had per occasion, and the number of occasions they consumed 5 (men) or 4 (women) drinks or more in a single episode (i.e., heavy episodic drinking).
Subjective Alcohol Effects
Participants were asked to rate how intoxicated they felt at the moment using an integer scale ranging from 1 (“Not drunk at all”) to 10 (“More drunk than I’ve ever been”) (Giancola, 2004, 2006; Peterson, Rothfleisch, Zelazo, & Pihl, 1990). Subjective intoxication ratings were assessed on both the ascending and descending limbs at comparable breath alcohol concentrations. Participants also completed the Biphasic Alcohol Effects Scale (BAES; Martin, Earleywine, Musty, Perrine, & Swift, 1993) assessing subjective stimulation and sedation. However, data from the BAES were not examined in the current analyses.
Breath Alcohol Concentration
Breath alcohol concentration was measured using a FST Alco-Sensor (Intoximeters, Inc., St. Louis). Breath alcohol concentration has been shown to be a good approximation of actual brain alcohol concentration (Fein & Meyerhoff, 2000). To facilitate matching BrAC across limbs, breath samples were taken every 5 min during the ascending limb of the blood alcohol curve and every 10 min during the descending limb.
Alcohol Exposure
According to Radlow (1994, 2006), the magnitude of acute tolerance increases the longer alcohol is in an organism’s system. For this study, exposure time to alcohol was quantified as the duration of time (min) that elapsed from the onset of drinking to the descending limb assessment.
Overall Rate of Change in Ascending BrAC
An estimate of the overall rate of change in ascending BrAC (i.e., change in BrAC over change in time) was computed for each participant. The estimate was calculated by dividing participants’ BrAC at the time of the ascending assessment by the amount of time (min.) it took to reach this BrAC following the end of consumption. This method of quantifying the overall rate of change in BrAC is similar to that used in previous studies (Fillmore & Vogel-Sprott, 1998). However, it is worth noting that there are alternative methods to quantifying the rate of change in ascending BrAC (e.g., moment-to-moment change).
Procedure
All study procedures were approved by the Institutional Review Board at the University of Missouri. Sessions began at 11:00 AM and were conducted in a neutral laboratory setting. Participants were tested individually by two trained research assistants, one of which was blind to condition and interacted with the participant. Participants were instructed to abstain from drugs and alcohol for 24 hours prior to the session. Upon arrival to the laboratory, participants provided informed consent and confirmed their compliance to pre-session drug and alcohol restrictions (e.g., BrAC of 0.000 g/dL). Female participants were required to take a hormonal pregnancy test before continuing in the study. After verifying eligibility for the study, participants completed questionnaires on a desktop computer that assessed demographic and drinking information, as well as other individual difference variables not pertinent to the hypotheses of the current study.
Beverage Administration
Participants included in the present study expected to receive alcohol and consumed 190-proof pure grain alcohol mixed with orange juice in a 1:3 ratio. The alcohol dose was calculated based on estimated total body water (TBW) and time for consumption to achieve a peak BrAC of 0.100 g/dL an hour after the onset of drinking (see Curtin & Fairchild, 2003). Total body water was estimated using age, sex, height, and weight. Beverages were divided equally into two glasses. On average, the calculated alcohol doses for men and women were 0.86 (SD = .03) and 0.75 (SD = .05) g/dL TBW, respectively. Following procedures used in prior acute tolerance studies (Fillmore, Dixon, & Schweizer, 2000; Fillmore and Vogel-Sprott, 1998; Marczinski and Fillmore, 2009), each glass was consumed in one minute, with a five minute break between glasses. This dosing procedure was chosen because it allowed for more control over the rate at which participants consumed each beverage, which is important as variability in consumption rate can alter the rate of increase in BrAC (O’Neill, Williams, & Dubowski, 1983). The larger study utilized a between-subject design involving additional placebo and control groups. Given the focus on the overall change in rising BrAC and acute tolerance of subjective perceptions of intoxication, these groups were not included in the present study as no BrAC ratings were detected in either group.
Post-Consumption
Following consumption, BrAC was assessed every five minutes until a BrAC of approximately 0.060 g/dL was achieved. At such time, participants’ rated their subjective intoxication. Following the completion of these measures, BrAC assessments resumed at five minute intervals until a comparable BrAC was achieved on the descending limb. At such time, participants’ rated their subjective level of intoxication once again. Participants also completed a computerized version of the Wisconsin Card Sorting following assessment of intoxication ratings. Data from this task were not included in the present study because this task is prone to practice effects (Bartels, Wegrzyn, Wiedl, Ackerman, & Hannelore, 2010; Calamia, Markon, & Tranel, 2012), which prevent inferences about acute tolerance. After the study, participants were given a light meal and remained in the laboratory until their BrAC descended to 0.020 g/dL (NIAAA, 2005). Participants were transported home via a prepaid taxi or a friend and were paid $12 an hour for their participation. The study typically lasted approximately 6.5 hours.
Results
Descriptive Statistics
Participants reported drinking approximately ten times in the past month and consumed around four drinks per occasion. Additionally, one third of drinking occasions reportedly involved heavy drinking (five or more drinks). Independent sample t-tests indicated that, compared to females, males drank more frequently in the past 30 days, (Mmales = 13.26 days [SD = 4.24] vs. Mfemales = 7.44 days [SD = 5.62]; t(27) = 2.95, p < .01, d = 1.14), consumed more alcohol per occasion, (Mmales = 5.14 drinks [SD = 3.08] vs. Mfemales = 2.53 drinks [SD = 0.95]; t(27) = 3.37, p < .01, d = 1.30), and had more heavy drinking episodes in the past 30 days, (Mmales = 6.75 days [SD = 6.19] vs. Mfemales = 1.28 days [SD = 1.41]; t(27) = 3.65, p < .01, d = 1.40). Past drinking behaviors were not associated with intoxication ratings on either limb of the BrAC curve (rs = -.18 and -.20, ps > .30).
Breath Alcohol Concentration
Overall, participants’ mean BrAC peaked at 0.089 g/dL (SD = 0.016 g/dL) approximately 60 minutes (M = 58 min, SD = 17) after the onset of drinking. Results also confirms that ascending and descending assessments of subjective intoxication were administered at comparable BrACs (M = .067 g/dL, SD = .010 g/dL for both limbs), with a paired-sample t-test suggesting only trivial differences (within-person mean difference = .001 g/dL, range: -0.002 to 0.017; t(28) = 1.87, p = .07). Therefore, any differences observed between intoxication ratings on the ascending and descending limbs are likely a reflection of acute tolerance of the subjective rating of intoxication to alcohol. Figure 1 depicts the change in BrAC across the test session for upper, middle, and lower tertiles for overall change in BrAC.
Figure.1.
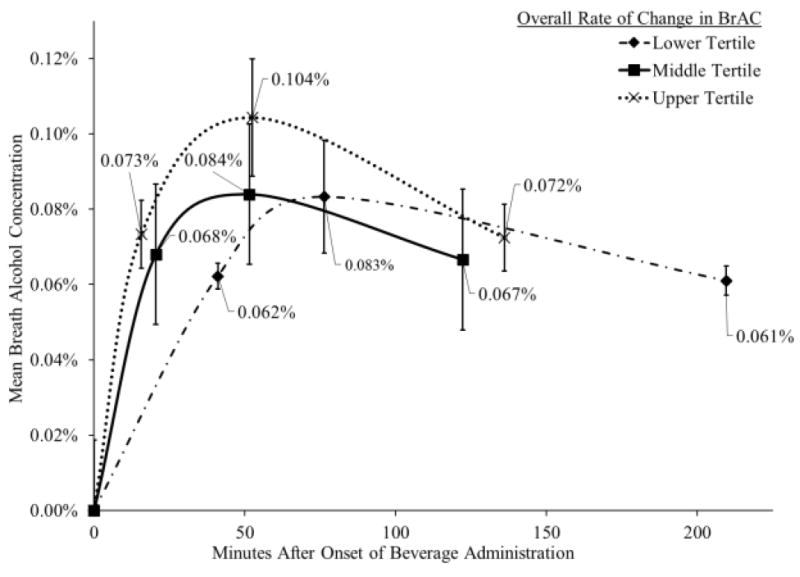
Separate mean breath alcohol concentrations (BrACs) trajectories for upper, middle, and lower tertiles for overall change in BrAC. Dotted line with × markers represents change in BrAC over time for participants in the upper tertile (i.e., faster) for overall change in BrAC. Solid line with filled square markers represents change in BrAC over time for participants in the middle tertile for overall change in BrAC. Dashed line with filled diamond markers represents change in BrAC over time for participants in the lower tertile (i.e., slower) for overall change in BrAC. Numerical values and capped vertical lines reflect the mean and standard deviation, respectively, at each time point.
Pharmacokinetic Factors and Initial Intoxication
Following alcohol consumption, participants’ BrACs increased at a mean rate of 0.0038 g/dL/min (SD = 0.002 g/dL/min). No sex differences were observed for the rate at which BrAC ascended (p = .23). Means for the amount of time that participants were exposed to alcohol before assessment on the ascending and descending limbs were 24 min (SD = 16) and 146 min (SD = 56), respectively. The latter was used in subsequent analyses as an indicator of alcohol exposure time consistent that proposed by Radlow (1994, 2006). Independent sample t-test indicated that males were exposed to alcohol for a longer duration than females (males: M = 173 min, SD = 56; females: M = 130 min, SD = 44; t(27) = 2.37, p < .05, d = 0.81). Therefore, sex was included as a covariate in subsequent analyses. The rate at which participants’ BrACs increased was not significantly related to the amount of time exposed to alcohol (r = .04, p = .83). Contrary to prior findings (Martin & Earleywine, 1990), the overall rate of increase in BrAC was not significantly associated with initial intoxication ratings on the ascending limb (p = .46). Likewise, the amount of time participants were exposed to alcohol was not significantly related to their initial intoxication rating (p = .28).
Acute Tolerance of Subjective Intoxication Ratings
Mean intoxication ratings for the ascending and descending limbs, respectively, were 3.93 (SD = 1.51) and 2.62 (SD = 2.09). A paired-sample t-test revealed a significant reduction in ratings from the ascending to descending limb, Mdifference = 1.31 (SD = 1.34), t(28) = 5.27, p < .001, dpaired = 0.98. This marked decrease in ratings suggests the presence of acute tolerance for subjective intoxication. Acute tolerance magnitude was calculated by subtracting intoxication ratings on the descending limb from those on the ascending limb. Greater positive values indicated greater acute tolerance. Mean magnitude of acute tolerance of subjective intoxication was 1.31 (SD = 1.34). No gender difference was observed for mean magnitude of acute tolerance of subjective intoxication (p > .31).
Association of Overall BrAC Ascent Rate with Acute Tolerance Magnitude
Multiple linear regression analyses were conducted to evaluate the association of the overall rate of change in ascending BrAC with the magnitude of Mellanby acute tolerance for subjective intoxication near 0.067 g/dL. Predictor variables were entered into the overall model in a hierarchical manner. Gender, alcohol exposure time, and binge drinking were entered into the model first (Step 1). Ascent rate was entered next (Step 2) to evaluate its association with the magnitude of acute tolerance for subjective intoxication beyond the effects of the other covariates. All variables were standardized prior to entry into the models.
Results revealed that the inclusion of gender, exposure time, and binge drinking (Step 1) did not significantly aide in the prediction of acute tolerance magnitude, R2 = .05, F(3, 25) = 0.43, p = .91. However, including overall ascent rate into the model (Step 2) significantly increased the predictive value of the model, ΔR2 = .28, R2 = .29, F(1, 24) = 10.03, p < .01. Moreover, results indicated that overall rate of increase in BrAC was the only variable uniquely associated with the magnitude of reduction in intoxication ratings, β = .56, p < .01, 95% confidence interval [.20, .93]. Figure 2 depicts this association. As the overall rate of change in ascending BrAC increased so did the magnitude of reduction in intoxication ratings from the ascending to descending limb of the breath alcohol curve.
Figure 2.
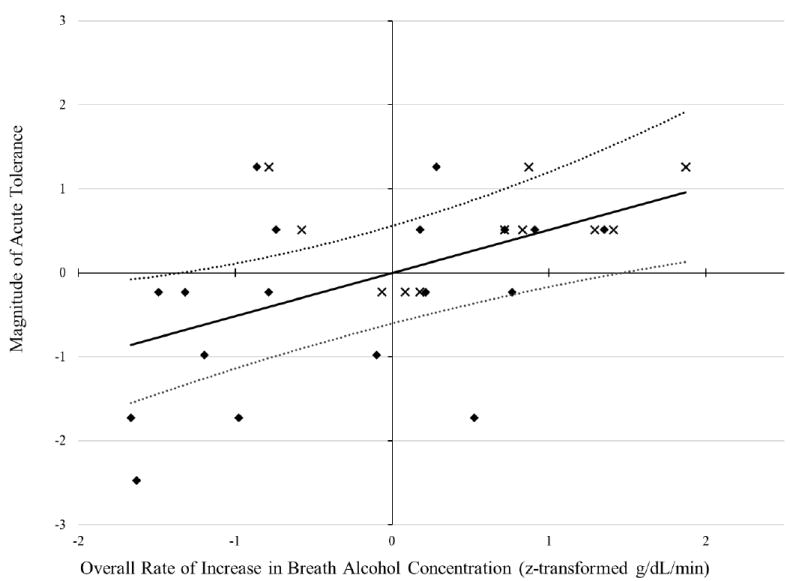
Plot of standardized regression equation predicting acute tolerance magnitude from the overall rate of increase in breath alcohol concentration (BrAC). The solid and dotted lines, respectively, depict the multiple linear regression and 95% confidence interval predictions from the full model with covariates. The diamond symbols represent the standardized raw data for women, while the × symbols represent the standardized raw data for men. The x- and y-axes have a mean and standard deviation of 0 and 1, respectively.
Supplementary Analyses
Despite both methodological (e.g., standardized consumption rate) and statistical (e.g., covaried binge drinking and alcohol exposure time) efforts to control for potential confounds, there are a number of factors that may have accounted for the association between the overall increase in BrAC and acute tolerance in subjective intoxication. For example, the overall rate of increase BrAC could have been related to the amount of time between assessments or participants’ BrACs at assessments, which could potentially account for the observed results. We therefore conducted supplementary analyses in order to rule out a number of potential third-variable explanations.
Results indicated that person-level variables had little association with the overall rate at which participants’ BrACs increased (height, age, weight, gender ps > .23). Faster overall increase in BrAC was associated with reaching peak BrAC earlier (r = -.71, p < 0.001). However, this likely does not explain study findings, as the overall rate at which BrAC increased was unrelated to the amount of time elapsed between assessments (p > .15). Faster overall increases in BrACs were significantly related to higher BrACs at the peak (r = .59, p < .001) but not with BrACs at the time of assessment on either limb (ascending: r = .21, p > .26; descending: r = .29,p < .13). Nevertheless, higher BrACs for those with sharper overall increases in BrAC could possibly correspond with greater intoxication ratings for both ascending and descending limb assessments. However, this possibility was not the case—faster increases in BrAC were unrelated to intoxications ratings on the ascending limb (r = -.14, p = .46) and were negatively related with ratings on the descending limb (r = -.43, p = .05).
Discussion
Prior research suggests that stronger initial levels of intoxication may be associated with more substantial reductions in intoxication on the descending limb of the breath alcohol curve (e.g., Koob and Le Moal, 1997; Poulos and Cappell, 1991; Ramsay and Woods, 1997; Solomon and Corbit, 1973). The aim of the present study was to test whether faster overall increases in ascending BrAC, which has been associated with greater intoxication and alcohol-induced impairment (Fillmore and Vogel-Sprott, 1998; Martin and Earleywine, 1990), related to greater acute tolerance to subjective intoxication. We found that while faster overall increase in BrAC was not associated with initial ratings of intoxication it was associated with greater development of acute tolerance to feelings of intoxication. This association persisted even after controlling for other factors (e.g., alcohol exposure time, binge drinking) that have been shown to contribute to differences in the magnitude of acute tolerance.
Contrary to prior findings (Fillmore and Vogel-Sprott, 1998; Martin and Earleywine, 1990), the overall rate of change in rising BrAC was not associated with the intensity of alcohol’s initial subjective effects on the ascending limb. This inconsistency in results may be due to methodological and idiosyncratic differences between studies. For instance, only males participated in those studies conducted by Fillmore & Vogel-Sprott (1998) and Martin & Earleywine (1990), whereas both males and females participated in the present study. Research has documented sex differences in the pharmacokinetics of alcohol (Baraona et al., 2001), suggesting that sex may moderate the association between overall changes in increasing BrAC and acute tolerance magnitude to subjective intoxication. The present study’s relatively small sample size prevents examination of the interaction between sex and overall rate of change in BrAC on acute tolerance magnitude to subjective intoxication. There are also differences in the dosing procedures utilized in the present study compared to those used in prior studies (Fillmore & Vogel-Sprott, 1998; Martin & Earleywine, 1990). The present study utilized a larger dose of alcohol compared to other studies (Fillmore & Vogel-Sprott, 1998) and involved faster consumption relative to other research (Martin & Earleywine, 1990).
The lack of association between the overall change in increasing BrAC and initial intoxication ratings suggests that the greater acute tolerance in subjective intoxication associated with faster overall increases in BrAC was not attributable to greater initial intoxication level. This lack of association is inconsistent with the belief that greater initial drug effects elicit a stronger adaptive physiological response, which in turn leads to greater acute tolerance (Koob and Le Moal, 1997; Poulos and Cappell, 1991; Ramsay and Woods, 1997). Instead, our data align with the alternative view that faster increases in the overall rate of change in ascending BrAC might lead to earlier activation of the body’s underlying homeostatic adaptive processes. This earlier activation may in turn elicit earlier recovery from intoxication, which would correspond with greater reduction across assessments. Prior research has demonstrated that the maximal effects of alcohol occur prior to peak BrAC (Portans et al., 1989; Radlow and Hurst, 1985). In the present study, faster increases in the overall change of rising BrAC were associated with earlier peak BrACs, which suggests that faster increases may also be associated with earlier maximal alcohol effects. However, since subjective intoxication was only assessed at a single time point on the ascending limb, it remains possible that the maximal effect of alcohol was not adequately captured by our assessment protocol. To circumvent this limitation, both BrAC and feelings of intoxication should be tracked at multiple time points in future studies.
Supplementary analyses further suggested that the association between the overall change in rising BrAC and acute tolerance in subjective intoxication was not attributable to person-level variables or characteristics of the assessment protocol. In the latter case, the present findings are not merely an artifact of the timing of assessments, as the rate of increase in BrACs was unrelated to the amount of time between assessments. Moreover, results indicated that the overall increasing change was not associated with BrACs at the time of assessments. Although generally faster increases were associated with higher BrACs at both assessments, they also tended to be associated with reduced feelings of intoxication on the descending limb.
This study provides some of the first data on the association between the overall rate of change in increasing BrAC and the magnitude of acute tolerance in subjective intoxication. Additional research is necessary to replicate and extend these results. Although the present study relied on the known inter-individual variability in rising BrAC (e.g., Ramchandi et al., 1999), future studies may utilize other approaches that directly manipulate this pharmacokinetic variable to clarify this association. Recent methodological advancements in IV administration have granted more precise control over the overall rate of change in rising BrACs (e.g., Plawecki et al., 2012; Wetherill et al., 2012). For example, physiologically-based pharmacokinetic modeling has been used to manipulate the infusion rate of alcohol, resulting in different rates of change in rising BrACs within individuals (Plawecki et al., 2012). Thus, IV administration has the unique advantage of more precise control of individual differences in alcohol pharmacokinetics. This may permit examination of how moment-to-moment changes in increasing BrAC relate to acute tolerance and other alcohol functions.
The present study utilized the Mellanby (1919) method to quantify acute tolerance magnitude. While this method is widely used in the literature (e.g., Beirness & Vogel-Sprott, 1984; Marczinski & Fillmore, 2009; Weafer & Fillmore, 2012; Wetherill et al., 2012), two other methods have been proposed for quantifying acute tolerance (see Martin & Moss, 1993). As explained by Martin and Moss (1993), the first of these methods is conceptually similar to that of Mellanby’s method and computes the area under the curve for measures of BrAC and alcohol response to quantify acute tolerance magnitude. The other method utilizes a slope function approach and computes the difference between the rate of change in BrAC and alcohol response from their respective maxima (Radlow, 1994). Greater acute tolerance is indicated by greater change in alcohol response compared to BrAC. The use of these other methods for calculating acute tolerance may be helpful in determining the replicability of the present findings.
Another important next step is to determine whether inter-individual variability in the overall rate of change in ascending BrAC is reflective of stable individual differences or merely the result of situation-specific differences that effect absorption kinetics (e.g., stomach content [Fraser, Rosalki, Gamble, & Pounder, 1995], rate of gastric emptying [Horowitz et al., 1989]). Research on this issue has proven challenging as it is difficult to control for all of the various transient environmental factors that alter changes in BrAC (Nagoshi & Wilson, 1989). Nevertheless, better understanding the reliability of individual differences in the overall change of BrAC may be particularly important, as it may serve as an indicator for the risk of greater acute tolerance, especially in regards to feelings of intoxication. This may have substantial theoretical and practical implications, as greater acute tolerance is associated with greater chronic tolerance (Beirness & Vogel-Sprott, 1984) and is believed to play a decisive role is risky decisions, such as driving after drinking (Amlung et al., 2014; Marczinski & Fillmore, 2009). As such, individuals who generally exhibit faster increase in BrAC may be at greater risk of alcohol-related problems and alcohol use disorders.
The present study focused on only one measure of alcohol impairment, subjective intoxication. We focused on this indicator of impairment because it consistently displays acute tolerance across studies (e.g., Martin & Moss, 1993; Portans et al., 1989; Radlow & Hurst, 1985; Weafer & Fillmore, 2012) and is thought to play an important role in the regulation of drinking and engagement in risky behaviors (Morean and Corbin, 2010; Quinn and Fromme, 2012; Quinn et al., 2013). Furthermore, feelings of intoxication are less likely to be susceptible to practice effects that commonly accompany task-based measures (Basso, Bornstein, & Lang, 1999; Collie, Maruff, Darby, & McStephen, 2003). However, it is unclear whether or not the association between ascent rate and acute tolerance holds for task-based measures of alcohol effects, especially given the inconsistencies in previous findings based on task measures (see Schweizer & Vogel-Sprott, 2008). Another important priority is to examine the association between rate of change in BrAC and other subjective indicators of alcohol effects, such as subjective stimulation and sedation. Thus, interpretation of the present results should be limited to subjective intoxication and may not extend to other alcohol effects. Future research is needed to test whether the present findings extend to other measures of alcohol effects.
Other limitations of the present study may have influenced our results. Overall change in increasing BrAC was calculated in the present study based on participants’ peak BrAC and the time it took them to achieve their peak BrAC. While this method of quantifying overall rate of increase in BrAC has been utilized in previous work (Fillmore & Vogel-Sprott, 1998; Martin & Earleywine, 1991), it assumes a steady linear increase in BrAC and may not fully capture the variability in the rate of change in BrAC common with oral alcohol administration. Furthermore, participants consumed a relatively large concentration of alcohol in a relatively short period of time. While this administration protocol was chosen to reduce variability in the duration of consumption, it may not reflect participants’ customary style of drinking outside of the laboratory. Likewise, the laboratory environment in which the study was conducted is unnatural compared to typical drinking environments. The relatively small sample size and recruitment from a single geographic location resulted in a somewhat homogenous sample, restricting the generalizability of the findings. The small sample limits statistical power, which might potentially account for the lack of significant associations between alcohol exposure time, binge drinking, and acute tolerance magnitude. Participants also had to report consuming five or more drinks to participate in the study. While this criterion was included to reduce the risk of adverse reaction to the alcohol administration, it may also limit the generalizability of the results to those who consume smaller amounts.
In summary, the present study demonstrates that faster overall rates of change in ascending BrAC are associated with greater reductions in subjective intoxication over the course of a single exposure to alcohol. This association does not appear to be due to faster overall increases in BrAC corresponding to greater initial levels of intoxication, as has been previously proposed (Ramsay and Woods, 1997). Further research, using both oral and IV methods of administration, is necessary to determine the mechanisms that underlie the association between the overall rate of change in rising BrAC and acute tolerance in subjective intoxication.
Acknowledgments
SOURCE OF FUNDING
This research was supported by grants (R01 AA019546; T32 AA013526) from the National Institute of Alcohol Abuse and Alcoholism, Rockville, Maryland, USA. Dr. Amlung’s role was supported, in part, by the Peter Boris Centre for Addictions Research.
Footnotes
Results from this larger project revealed that participants’ in-the-moment appraisals of the dangers of driving after drinking were markedly reduced from the ascending to descending limb, providing clear evidence that acute tolerance affects judgments about driving after drinking.
CONFLICTS OF INTEREST
None
References
- Amlung MT, Morris DH, McCarthy DM. Effects of acute alcohol tolerance on perceptions of danger and willingness to drive after drinking. Psychopharmacology. 2014;231:4271–4279. doi: 10.1007/s00213-014-3579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraona E, Abittan CS, Dohmen K, Moretti M, Pozzato G, Chayes ZW, Lieber C, et al. Gender differences in pharmacokinetics of alcohol. Alcohol Clin Exp Res. 2001;25:502–507. doi: 10.1111/j.1530-0277.2001.tb02242.x. [DOI] [PubMed] [Google Scholar]
- Bartels C, Wegrzyn M, Wiedl A, Ackerman V, Ehrenreich H. Practice effects in healthy adults: A longitudinal study on frequent repetitive cogntive testing. BMC Neuroscience. 2010;11:1–12. doi: 10.1186/1471-2202-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MR, Bornstein RA, Lang JM. Practice effects on commonly used measures of executive function across twelve months. Clin Neuropsychol. 1999;13:283–292. doi: 10.1076/clin.13.3.283.1743. [DOI] [PubMed] [Google Scholar]
- Beirness D, Vogel-Sprott M. The development of alcohol tolerance: Acute recovery as a predictor. Psychopharmacology. 1984;84:398–401. doi: 10.1007/BF00555220. [DOI] [PubMed] [Google Scholar]
- Calamia M, Markon K, Tranel D. Scoring higher the second time around: Meta-analysis of practice effects in neuropsychological assessment. The Clinical Neuropsychologist. 2012;26:543–570. doi: 10.1080/13854046.2012.680913. [DOI] [PubMed] [Google Scholar]
- Collie A, Maruff P, Darby DG, McStephen M. The effecs of practice on the cognitive test performance of neurologically normal individuals assessed at brief test-retest intervals. J Int Neuropsychol Soc. 2003;9:419–428. doi: 10.1017/S1355617703930074. [DOI] [PubMed] [Google Scholar]
- Cromer JR, Cromer JA, Maruff P, Snyder PJ. Perception of alcohol intoxication shows acute tolerance while executive functions remain impaired. Exp Clin Psychopharm. 2010;18:329–339. doi: 10.1037/a0019591. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, Fairchild BA. Alcohol and cognitive control: Implications for regulation of behavior during response conflict. J Abnorm Psychol. 2003;112:424–436. doi: 10.1037/0021-843X.112.3.424. [DOI] [PubMed] [Google Scholar]
- Evans SM, Levin FR. Differential response to alcohol in light and moderate female social drinkers. Behav Pharmacol. 2004;15:167–181. Retrieved from http://journals.lww.com/behaviouralpharm/Abstract/2004/05000/Differential_response_to_alcohol_in_light_and.1.aspx. [PubMed] [Google Scholar]
- Fein G, Meyerhoff DJ. Ethanol in human brain by magnetic resonance spectroscopy: Correlation with blood and breath levels, relaxation, and magnetization transfer. Alcohol Clin Exp Res. 2000;24:1227–1235. doi: 10.1111/j.1530-0277.2000.tb02088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Dixon MJ, Schweizer TA. Alcohol Affects Processing of Ignored Stimuli in a Negative Priming Paradigm. J Stud Alcohol Drugs. 2000;61:571–579. doi: 10.15288/jsa.2000.61.571. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Behavioral impairment under alcohol: cognitive and pharmacokinetic factors. Alcohol Clin Exp Res. 1998;22:1476–1482. doi: 10.1111/j.1530-0277.1998.tb03938.x. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Weafer J. Acute tolerance to alcohol in at-risk binge drinkers. Psychol Addict Behav. 2012;26:693–702. doi: 10.1037/a0026110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A, Rosalki S, Gamble G, Pounder R. Inter-individual and intra-individual variability of ethanol concentration-time profiles: comparison of ethanol ingestion before or after an evening meal. Brit J Clin Pharmaco. 1995;40:387–392. doi: 10.1111/j.1365-2125.1995.tb04561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola PR. Difficult temperature, acute alcohol intoxication, and aggressive behavior. Drug and Alcohol Dependence. 2004;74:135–145. doi: 10.1016/j.drugalcdep.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Giancola PR. Influence of subjective intoxciation, breath alcohol concentration, and expectancies on the alcohol-aggression relation. Alcohol Clin Exp Res. 2006;30:844–850. doi: 10.1111/j.1530-0277.2006.00099.x. [DOI] [PubMed] [Google Scholar]
- Hendershot CS, Wardell JD, Strang NM, Markovich MSD, Claus ED, Ramchandani VA. Application of an alcohol clamp paradigm to examine inhibitory control, subjective responses, and acute tolerance in late adolescence. Alcohol Clin Exp Res. 2015;23:147–158. doi: 10.1037/t04185-000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen AJ. Acute alcohol tolerance in social drinkers: Changes in subjective effects dependent on the alcohol dose and prior alcohol experience. Alcohol. 1997;14:373–378. doi: 10.1016/S0741-8329(96)00186-3. [DOI] [PubMed] [Google Scholar]
- Horowitz M, Maddox A, Bochner M, Wishart J, Bratasiuk R, Collins P, Shearman D. Relationships between gastric emptying of solid and caloric liquid meals and alcohol absorption. Am J Physiol Gastrointest Liver Physiol. 1989;257:291–298. doi: 10.1152/ajpgi.1989.257.2.G291. Retrieved from http://ajpgi.physiology.org/content/257/2/G291. [DOI] [PubMed] [Google Scholar]
- Johnston L, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings, 2010. Ann Arbor: Institute for Social Research, The University of Michigan; 2011. [Google Scholar]
- Kalant H. What neurobiology cannot tell us about addition. Addiction. 2010;105:780–789. doi: 10.1111/j.1360-0443.2009.02739.x. [DOI] [PubMed] [Google Scholar]
- Kaplan HL, Sellers EM, Hamilton C, Naranjo CA, Dorian P. Is there acute tolerance to alcohol at steady state? J Stud Alcohol. 1985;46:253–256. doi: 10.15288/jsa.1985.46.253. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug Abuse: Hedonic Homeostatic Dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335-52. [DOI] [PubMed] [Google Scholar]
- Lê AD, Kalant H. Influence of intoxicated practice on the development of acute tolerance to the motor impairment effect of ethanol. J Stud Alcohol. 1992;106:572–576. doi: 10.1007/BF02244833. [DOI] [PubMed] [Google Scholar]
- LeBlanc AE, Kalant H, Gibbins RJ. Acute tolerance to ethonal in the rat. Psychopharmacologia. 1975;41:43–46. doi: 10.1007/BF00421304. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Acute alcohol tolerance on subjective intoxication and simulated driving performance in binge drinkers. Psychol Addict Behav. 2009;23:238–47. doi: 10.1037/a0014633. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M. Ascending and descending rates of change in blood alcohol concentration and subjective intoxication ratings. J Subst Abuse. 1990;2:345–352. doi: 10.1016/S0899-3289(10)80006-9. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Martin CS, Moss H. Measurement of acute tolerance to alcohol in human subjects. Alcohol Clin Exp Res. 1993;17:211–216. doi: 10.1111/j.1530-0277.1993.tb00751.x. [DOI] [PubMed] [Google Scholar]
- Mellanby E. Alcohol: Its absorption into and disappearance from the blood under different conditions (Medical Research Council Special Report Series No 31) London, England: Medical Research Council; 1919. [Google Scholar]
- Morris DM, Treloar HT, Niculete ME, McCarthy DM. Perceived danger while intoxicated uniquely contributes to driving after drinking. Alcohol Clin Exp Res. 2013;38:521–528. doi: 10.1111/acer.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morzorati SL, Ramchandani VA, Flury L, Li TK, O’Connor S. Self-reported subjective perception of intoxication reflects family history of alcoholism when breath alcohol levels are constant. Alcohol Clin Exp Res. 2002;26:1299–1306. doi: 10.1097/01.ALC.0000025886.41927.83. [DOI] [PubMed] [Google Scholar]
- Nagoshi CT, Wilson JR. Long-term repeatability of human alcohol metabolism, sensitivity and acute tolerance. J Stud Alcohol. 1989;50:162–169. doi: 10.15288/jsa.1989.50.162. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular neurobiology of addiction. Am J Addiction. 2001;10:201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- NIAAA. National Advisory Council on Alcohol Abuse and Alcoholism - Recommended Council Guidelines on Ethyl Alcohol Administration in Human Experimentation. NIAAA; Bethedsa, MD: 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor S, Morzorati S, Christian J, Li TK. Clamping breath alcohol concentration reduces experimental variance: Application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcohol Clin Exp Res. 1998;22:202–210. doi: 10.1111/j.1530-0277.1998.tb03639.x. [DOI] [PubMed] [Google Scholar]
- O’Neill B, Williams AF, Dubowski KM. Variability in blood alcohol concentration: Implications for estimating individual results. J Stud Alcohol. 1983;44:222–230. doi: 10.15288/jsa.1983.44.22. [DOI] [PubMed] [Google Scholar]
- Peterson JB, Rothfleisch J, Zelazo PD, Pihl RO. Acute alcohol intoxication and cognitive functioning. J Stud Alcohol. 1990;51:114–122. doi: 10.15288/jsa.1990.51.114. [DOI] [PubMed] [Google Scholar]
- Plawecki MH, Zimmermann US, Vitvitskiy V, Doerschuk PC, Crabb D, O’Connor S. Alcohol exposure rate control through physiologically based pharmacokinetic modeling. Alcohol Clin Exp Res. 2012;36:1042–1049. doi: 10.1111/j.1530-0277.2011.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portans I, White JM, Staiger PK. Acute tolerance to alcohol: Changes in subjective effects among social drinkers. Psychopharmacology. 1989;97:365–369. doi: 10.1007/BF00439452. [DOI] [PubMed] [Google Scholar]
- Poulos CX, Cappell H. Homeostatic theory of drug tolerance: A general model of physiological adaptation. Psychol Rev. 1991;98:390–408. doi: 10.1037/0033-295X.98.3.390. [DOI] [PubMed] [Google Scholar]
- Radlow R. A quantitative theory of acute tolerance to alcohol. Psychopharmacology. 1994;114:1–8. doi: 10.1007/BF02245438. [DOI] [PubMed] [Google Scholar]
- Radlow R. A mathematical theory for temporal changes in tolerance to the behavioral effects of alcohol. Pharmacol Bochem Behav. 2006;85:370–383. doi: 10.1016/j.pbb.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Radlow R, Hurst PM. Temporal relations between blood alcohol concentration and alcohol effect: An experiment with human subjects. Psychopharmacology. 1985;85:260–266. doi: 10.1007/BF00428184. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Flury L, Morzorati SL, Kareken D, Blekher T, Foroud T, O’Connor S, et al. Recent drinking history: Association with family history of alcoholism and the acute response to alcohol during a 60 mg% clamp. J Stud Alcohol. 2002;63:734–744. doi: 10.15288/jsa.2002.63.734. [DOI] [PubMed] [Google Scholar]
- Ramsay DS, Woods SC. Biological consequences of drug administration: Implications for acute and chronic tolerance. Psychol Rev. 1997;104:170–193. doi: 10.1037/0033-295X.104.1.170. [DOI] [PubMed] [Google Scholar]
- Schweizer TA, Vogel-Sprott M. Alcohol-impaired speed and accuracy of cognitive functons: A review of acute tolerance and recovery of cognitive performance. Exp Clin Psychopharm. 2008;16:240–250. doi: 10.1037/1064-1297.16.3.240. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. II. Cigarette addiction. J Abnorm Psychol. 1973;81:158–71. doi: 10.1037/h0034534. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Rothstein JD. Biology of tolerance and dependence. In: Tabakoff B, Sutker PB, Randall CL, editors. Medical and Social Aspects of Alcohol Abuse. New York: Plenum Press; 1983. pp. 187–220. [Google Scholar]
- Weafer J, Fillmore MT. Acute tolerance to alcohol impairment of behavioral and cognitive mechanisms related to driving: Drinking and driving on the descending limb. Psychopharmacology. 2012;220:697–706. doi: 10.1007/s00213-011-2519-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill L, Morzorati SL, Foroud T, Windisch K, Darlington T, Zimmerman US, O’Connor SJ, et al. Subjective perceptions associated with the ascending and descending slopes of breath alcohol exposure vary with recent drinking history. Alcohol Clin Exp Res. 2012;36:1050–1057. doi: 10.1111/j.1530-0277.2011.01642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


