Abstract
Diabetes increasingly afflicts our aging and dysmetabolic population. Type 2 diabetes (T2D) and the antecedent metabolic syndrome (MetS) represent the vast majority of the disease burden –increasingly prevalent in children as well as older adults. However, type 1 diabetes (T1D) is also advancing in preadolescent children. As such, a crushing wave of cardiometabolic disease burden now faces our society. Arteriosclerotic calcification is increased in MetS, T2D, and T1D – impairing conduit vessel compliance and function, thereby increasing the risk for dementia, stroke, heart attack, limb ischemia, renal insufficiency and lower extremity amputation. Preclinical models of these dysmetabolic settings have provided insights into the pathobiology of arterial calcification. Osteochondrogenic morphogens in the BMP-Wnt signaling relay and transcriptional regulatory programs driven by Msx and Runx gene families are entrained to innate immune responses – responses activated by the dysmetabolic state – to direct arterial matrix deposition and mineralization. Recent studies implicate the endothelial-mesenchymal transition (EndMT) in contributing to the phenotypic drift of mineralizing vascular progenitors. In this brief overview, we discuss preclinical disease models that provide mechanistic insights – and point to the emerging potential for translating these insights into new therapeutic strategies for our patients challenged with diabetes and its arteriosclerotic complications.
Keywords: Vascular calcification, Arteriosclerosis, Diabetes mellitus, Metabolic syndrome, Animal models of human disease
I. Introduction
In westernized societies, the arterial macrovasculature is the second most extensively calcified vertebrate tissue beyond the skeleton1, 2. In response to diabetes, advanced age, dyslipidemia, uremia, hypertension – and certain primary inflammatory disorders – the arterial vasculature accrues a substantial mineral load, impairing valve and arterial compliance necessary for smooth distal tissue perfusion1, 2. Once considered only a passive process of dead and dying cells, arterial calcification has now emerged as an actively regulated form of tissue biomineralization. Primary and/or secondary vascular inflammatory signals are common to all type of arterial mineral deposition, evident in both genetic and metabolic causes of disease1, 2. Reflecting upon this, Demer and colleagues were the first to note that this likely represents an important role for tissue calcification in certain forms of innate immunity – such as the calcified granuloma that wall off mycobacterial or fungal infections in the lung2. However, arterial mineralization has devastating consequences, increasing myocardial workload, decreasing diastolic perfusion, and compromising perfusion thereby increasing risk of stroke, dementia, myocardial infarction, heart failure, renal failure, and lower extremity amputation1–9. Currently, no effective pharmacotherapies exist to reduce arterial calcification and restore conduit vessel compliance – although useful insights may be forthcoming from the biology of progeria10, 11. Robust preclinical models of disease are necessary to better understand disease biology and validate and vet potential therapeutic approaches that might mitigate disease in our increasingly aging and dysmetabolic population.
Type 2 diabetes (T2D), Type 1 diabetes (T1D), and the metabolic syndrome (MetS) have emerged as particularly important causes of arterial calcification12–14 (Figure 1). Indeed, at every level of declining renal function – another key contributor to vascular mineral load3 – glycemic control augments the risk for the severity and extent of arterial calcification15. In this overview, we consider the preclinical disease models of T1D and the T2D-MetS spectrum that help inform our understanding of vascular mineral metabolism in these clinical settings. Strengths, mechanistic insights, and potential shortcomings of the models are briefly discussed to help frame future studies in ways that might lead to new therapeutic approaches for our patients afflicted with arteriosclerotic disease.
Figure 1. Overlapping yet distinct metabolic milieus of T1D and the T2D-MetS spectrum shape cardiovascular disease.
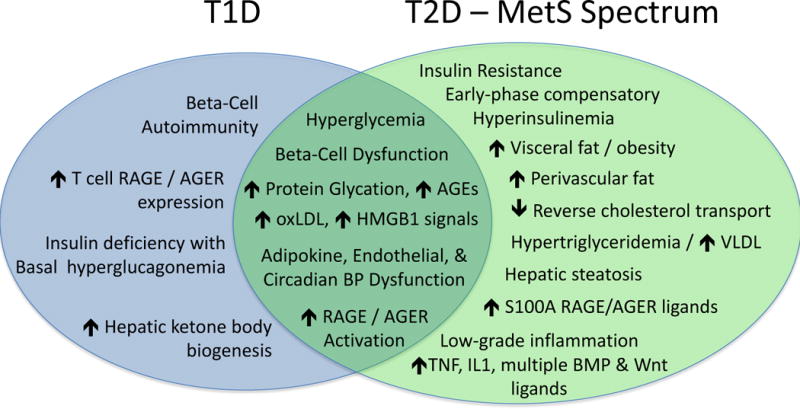
T1D and T2D share many cardiometabolic risk factors – including elevated levels of multiple ligands169–174 capable of activating the RAGE/AGER and toll like receptors (TLRs) of the innate immune system that can drive arterial calcification100, 101, 175 along with prototypic inflammatory cytokines (TNF, IL1beta, etc.)70, 90, 176, 177. Note that expanded visceral and perivascular (aortic, coronary) fat depots1, 178–182 and hepatic steatosis183–186 – harbingers of valve and vascular calcification – are key features of the “diabesity” phenotype along the T2D-MetS spectrum12, 187–189. T2D also impairs reverse cholesterol transport190, and VLDL metabolism is an independent contributor to arterial calcification risk191. AGE, advanced glycation end product; BMP, bone morphogenetic protein; BP, blood pressure; HMGB1, high mobility group protein B1; oxLDL, oxidized LDL cholesterol.
II. Rodent Models Of Arterial Calcification In Type I Diabetes
While much less common than T2D, the cardiovascular consequences of T1D are protean, and most definitely encompass arterial calcification16–18. It’s interesting to note, however, that when insulin resistance19 and hypertension20 are quantified in the setting of T1D that the severity and extent of coronary artery calcification (CAC) is in fact further increased19. This points to common cardiometabolic underpinnings of arteriosclerotic calcification in T1D and T2D. In this section, we consider the better-studied preclinical models of arteriosclerotic calcification and vascular stiffening in T1D.
II.1. Streptozotocin models
Streptozotocin (STZ), the β-cell toxin, is the most frequently utilized method for inducing T1D in rodent models(Table 1). Its relative tissue selectivity relates to uptake by Glut2 with subsequent DNA alkylation, poly ADP-ribosylation, and depletion of cellular NAD and ATP21. While known for decades, only recently has the capacity of STZ-induced T1D been utilized in detailed studies of arterial calcification and vascular stiffness. Chen and colleagues established that the 5 day low-dose STZ model is sufficient to induce diabetes, upregulate arterial Akt-Runx2 osteochondrogenic signaling, enhance arterial mineral deposition, and increase arterial stiffness in C57BL/6 mice22. Additional studies comparing the arterial mineralized matrix ultrastructure in this model vs. other diabetes models promises to be enlightening. It’s important to note that cardiovascular sclerosis often relates more to changes in Runx2 and osteogenic protein accumulation that mRNA accumulation22; indeed, both Runx223–25 and type I collagen biogenesis26 are prodigiously regulated at the post-transcriptional level. Using a rat model, Moreau and colleagues27, 28 implemented a combination of STZ with warfarin – the latter an inhibitor of vitamin K – dependent protein γ-carboxylation that impairs the anti-calcific actions of matrix Gla protein (MGP) partially mediated by BMP2/4 inhibition29, 30. In both models, dysglycemic components common to the clinical setting are important, with intracellular (non-enzymatic protein glycation and enzymatic O-GlcNacylation) and extracellular (advanced glycation end product / AGE formation and inflammatory cytokine) signals contributing to calcific vascular disease. Osteochondrogenic differentiation programs dependent upon the transcription factor Runx231, 32 are recruited to direct VSM mineralization. Importantly, protein-protein interactions between Runx2 and BMP-regulated osteogenic Smads figure prominently in orthotopic mineralization as well33, dependent upon the stable sub-nuclear targeting of Runx2 complexes in the nuclear matrix34. Whether sub-nuclear targeting and turnover of Runx2 in VSM might offer therapeutic approaches has yet to be determined.
Table 1.
Preclinical Models of Arterial Calcification in T1D and the T2D-MetS Spectrum
| Model | Comments | References |
|---|---|---|
| C57BL/6J Mouse with Streptozotocin (STZ) Treatment | T1D. Aortic calcification and stiffness both quantified and increased. Osteogenic programs identified in other models activated. Comparison of mineralized vascular matrix ultrastructure with other models promises to be enlightening. |
Ref. 22 |
| Wistar rat with STZ Treatment + Co-inducer - Warfarin+vitamin K (WVK) - Vitamin D and nicotine (VDN) |
T1D. Femoral artery calcification noted as well as aortic calcification. Peripheral artery calcification often assessed in preclinical models but relevant to peripheral arterial disease. Disease responsive to modulation of RAGE/AGER and NADPH oxidase / Nox signaling. |
Ref. 27, 28, 39, 40, 196 |
| ApoE−/− mouse with STZ treatment | T1D. Severe hypercholesterolemia Robust arterial calcification responses are observed. ApoE-null VSM cells are given to chondroid metaplasia. S100A12 transgene accelerates calcification in ApoE-null mice. |
Ref. 45, 47, 100 |
| Ins2Akita/+ Mouse | T1D. Streptozotocin administration is not required to induce arterial calcification in this T1D model. Aortic calcification increased, stiffness to be characterized. Neuropathy is also induced. On LDLR−/− background diet-induced diabetes, hypercholesterolemia, & hypertriglyceridemia severe in both male & female animals, but arteriosclerosis has yet to be quantified in Ins2Akita/+;LDLR−/− mice. |
Ref.30, 66, 197 |
| Male LDLR−/− Mouse with STZ Treatment | T1D. Atherosclerosis lesion more extensive after 2 weeks of diabetes. Aortic calcification and stiffness remain to be characterized. |
Ref.198, 199 |
| Golden Syrian Hamster with STZ + High Fat Diet (HFD) | T1D. Nephropathy also arises in this model. Few publications, little physiological phenotypic data available. |
Ref.200, 201 |
| Male LDLR−/− Mouse Fed High Fat Diet (HFD) | T2D – MetS spectrum. Severe hypercholesterolemia, modest fasting hypertriglyceridemia (~200 mg/dL), elevated free fatty acids, increased visceral and periaortic fat accumulation, hyperglycemia & hyperinsulinemia, increased HOMA-IR, and oxidative stress signaling regulated by OPN and Nox activity. Induced by high fat Western diet (Teklad 88137; 42% calories from fat). Aortic calcification and stiffness both quantified and increased. Regulated by canononical and noncanonical Wnt signals. Disease is induced in response to clinically relevant stimulus that promote VSM chondroid metaplasia and RAGE/AGER co-localization. Chondroid metaplasia is restrained by VSM OPN. Renal fibrosis induced via oxidized LDL signals. Modifiable with 5/6 nephrectomy to phenocopy arteriosclerotic disease in setting of T2D + renal insufficiency (chronic kidney disease – mineral and bone disorder, CKD-MBD). Latter is sensitive to renal and dietary phosphate metabolism. S100A/calgranulin proteins, key RAGE/AGER ligands that drive ectopic calcification, are increased by high fat diet. |
Ref. 42, 67–69, 71, 72, 86, 89, 100, 101, 113, 121, 137, 202–207 |
| LDLR−/−;ApoB100/100;RIP1-IGF2Tg mouse | T2D-MetS spectrum. Similar to male LDLR−/− but more significant fasting hyperglycemia in both male and female mice, Western diet challenge performed in old animals. Severe hypercholesterolemia arises since all ApoB containing lipoproteins are now B100 only – and the LDLR is missing. Transgenic IGF2 expression in pancreatic beta cell from rat insulin promoter (RIP1) accelerates T2D with beta-cell failure upon dietary challenge. Extent of aortic calcification appears to track insulin resistance > hypercholesterolemia, fasting hyperglycemia. |
Ref.107, 112 |
| db/db Mouse | T2D-MetS spectrum. Modest hypercholesterolemia, severe hypertriglyceridemia and visceral fat accumulation on Teklad rodent diet 8604 (14% calories from fat). In addition to nephropathy these leptin receptor –deficient mice also develop neuropathy (either on the C57BKS background, or on C57BL/6J with high fat diet to sustain hyperglycemia). Intriguing recent report of arterial stiffening without calcification in aged diabetic db/db mouse; genetic dietary conditions, composition, and basic metabolic profiling important to report to enable comparisons. |
Ref.30, 63, 64, 126 |
| Ossabaw Island Hog | T2D – MetS spectrum. Modest hypercholesterolemia, hypertriglyceridemia, increased visceral fat accumulation, impaired glucose tolerance, and insulin-resistant. Early stages of coronary artery microcalcification by histology co-registered with 18F - PET/CT and 41Ca uptake. Epicardial fat surgically demonstrated to impact coronary disease processes. |
Ref. 130, 131, 208–211 |
| Male Rhesus Macaque on High-Fat High-Sucrose Diet | T2D – MetS spectrum. Modest hypercholesterolemia, hypertriglyceridemia, increased visceral fat, hyperglycemia & hyperinsulinemia, increased HOMA-IR. Arterial calcification and stiffness are both increased, but arterial calcification assessed only histologically. Resveratrol supplementation over 2 years protective at doses of 80 mg to 480 mg daily (glass of red wine contains ~ 1 mg of resveratrol). |
Ref. 134 |
| Watanabe (WHHL) Rabbit Fed High-Fructose High-Fat Diet | T2D – MetS spectrum. Severe hypercholesterolemia (in-frame LDLR deletion). Metabolic syndrome features similar to the male LDLR−/− model, e.g. hypertriglyceridemia, increased visceral fat, impaired glucose tolerance / increased HOMA-IR. While calcification is histologically increased, not rigorously quantified. |
Ref. 212 |
Of note, as a DNA alkylating agent, STZ chemically induces the DNA damage response in multiple cell and tissue types35. In both osteoblasts and vascular smooth muscle, DNA damage is a potent stimulus for the osteochondrogenic program when elicited by either chemical means, nuclear senescence36, or irradiation37, 38. Thus, while not perfectly mimicking the pathogenesis of human T1D and its complications, the STZ models can rapidly elicit vascular injury and biomineralization responses that reproducibly recapitulate key features of human disease. The cellular toxicities of STZ above and beyond β-cell death may contribute to the vascular disease process in these important preclinical models, not unlike the addition of nicotine to rat model of vitamin D – induced vasculopathy discussed below.
The STZ-treated Wistar rat has been used for studies of arterial calcification that arises in T1D. In this model, calcific vasculopathy is accentuated by either (a) warfarin treatment with vitamin K-mediated, hepatocyte-selective rescue of coagulation factor γ-carboxylation (WVK)27; or (b) vitamin D3 intoxication with nicotine (VDN) administration39, 40 (Table 1). Elastinolysis is a prominent histopathologic contributor to the accentuated vascular toxicity of these secondary treatments that drive arterial mineralization with STZ. Many of the same signaling cascades observed in the STZ+VDN model – including hyperphosphatemia-driven by vitamin D3 intoxication, nicotine-induced elastin fragmentation, and advanced glycation end product receptor (RAGE/AGER) signaling – are observed in other preclinical arterial calcification models that more closely reflect common clinical settings1, 41, 42. Pioglitazone-mediated activation of PPAR-ϒ, an inhibitor of non-canonical Wnt signaling43, reduces arteriosclerotic calcification in VDN models44. In the STZ-WVK model, RAGE/AGER signaling is also increased and very important; inhibition of AGE ligand formation with pyridoxamine or AGE cross-link destruction with alagebrium inhibited femoral artery calcification in the STZ-WVK model (primarily medial calcification)28. The adventitial inflammation including TNF expression27 – so characteristic of human diabetic macrovascular disease41 – is also observed in STZ models27. The rapidity with which robust disease arises with STZ-induced T1D and its co-modifiers has lead to its increasing use in preclinical models of arteriosclerotic calcification. The combination of STZ and high-fat diet induced hypercholesterolemia in the apolipoprotein E −/− (ApoE−/−) mouse appears to be particularly effective in inducing arterial calcification45, accentuating the propensity for ApoE-null VSM to undergo chondroid metaplasia46, 47 (Table 1).
II.2. The Ins2Akita Mouse
Recently, Bostrom, Yao, and colleagues48 addressed one of the potential shortcomings of the STZ mouse model by characterizing arterial mineralization in the Akita mouse (Ins2Akita heterozygous mice) 48. Due to an insulin codon mutation that impairs secretion and induces β-cell endoplasmic reticulum stress, the Akita mouse develops an age-dependent evolution of T1D. In this model, arterial calcium deposition is increased with time, driven by BMP2/4 –Smad signal relays – and mediated part via endothelial dysfunction that promote the endothelial-mesenchymal transition (EndMT)30, 48. In this process, endothelial cells (EC) lose their normal differentiated EC barrier phenotype, and adopt a mesenchymal phenotype capable of osteogenic differentiation with mineralized matrix deposition. EndMT processes have emerged as one potential source of arterial osteogenic cells in the diseased vasculature48 as well as in heterotopic bone formation49. However, the commonly used “endothelial-specific” transgenic Cre lines used to track the EC lineage also express within myeloid lineage – cells can adopt the EC fate50 and contribute to arterial calcification in T2D51, 52. Moreover, co-culture of endothelial progenitors with mesenchymal progenitors have pointed to paracrine interactions between multipotent Sox2-positive populations in both lineages that promote osteogenic mineralization53. Thus, a mechanistically more important consequence of EC de-differentiation during the EndMT likely relates to alterations in paracrine endothelial –mesenchymal signals that program an osteogenic fate of adjacent VSM and mesenchymal progenitors.54 Nevertheless, lineage tracing studies suggest that the sources of osteogenic cells in this T1D model quantitatively differ from those of pure atherosclerotic models such as the ApoE – null mouse; in the latter, approximately 80% of vascular osteogenic cells appear to arise from the vascular smooth muscle (VSM) lineage55, 56. The Akita mouse model has also revealed a critical role of Sox2-regulated serine proteases in the EndoMT48. The latter has important translational implications, since recombinant serine protease inhibitors (serpins) have been successfully deployed in other clinical settings to ameliorate disease57. While predicted to be altered, the arterial compliance and conduit vessel function of the Akita mouse has not been characterized (Table 1).
Of note, neuropathy is emerging as once key contributor to arterial calcification in humans58. Indeed, when neurectomy had been utilized in the past to address intractable ischemia rest pain in individuals afflicted with peripheral arterial disease (PAD), profound ipsilateral medial artery calcification blossoms in those so treated59. Intriguingly, models of heterotopic ossification have suggested neural progenitors might be capable of adopting osteogenic fates in response to injury60 – and, as such, resemble the mineralizing phenotypic plasticity of Sox2-positive neural crest cells61, 62. Because of its broader general toxicity, higher-dose STZ administration is effective in simultaneously inducing neuropathy63. However, the less-toxic, low-dose regimens that are more β-cell selective do not appear to induce neuropathic disease64. Neurogenic contributions to ectopic mineralization in other settings are just now being investigated60, 65, and this is an important pathogenic feature to consider moving forward. Importantly, the Akita mouse develops impairment in nerve conduction velocity (NCV)63 as well as abnormal parasympathetic control of heart rate and sinoatrial node function66.
III. Preclinical Models Of Arterial Calcification In The Setting Of Type II Diabetes and the Metabolic Syndrome
Obesity is epidemic in westernized societies, driving a crushing wave of metabolic syndrome (MetS) and type II diabetes (T2D)4. Preclinical model of diet-induced obesity, insulin resistant diabetes, and arteriosclerotic disease67–71 predicted the relationships between MetS, T2D, and valve and vascular calcification as subsequently established in MESA (Multiethnic Study of Atherosclerosis)12, 13. Numerous preclinical studies detail arterial atherosis – primarily lipid-laden lesion area. However, by comparison, relatively little attention has been given to quantifying the sclerotic component – extracellular matrix metabolism, calcification, and stiffness - of cardiovascular disease in MetS and T2D models. In this section, we focus upon those models where sclerosis – calcification, fibrosis, and/or vascular stiffening – has been characterized as related to conduit artery disease and dysfunction (Figure 2) in this clinically important setting.
Figure 2. Consequences of arterial stiffening and impaired Windkessel physiology.
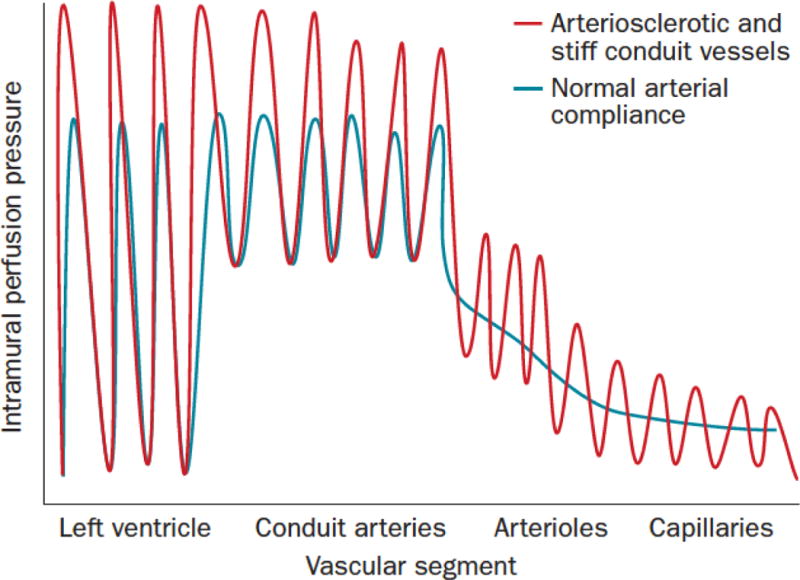
During systole, some kinetic energy is stored as potential energy in elastic conduit arteries. This stored energy permits smooth distal tissue perfusion during diastole (blue tracing). With arteriosclerotic stiffening (red tracing), less potential energy is stored during systole, giving rise to more erratic and pulsatile flow during diastole. Diabetes and aging significantly increase arteriosclerotic conduit vessel stiffening192. Reproduced from reference1 with permission. See also references193, 194,158, 195 for detailed discussion.
III.1. Male LDLR−/− mice fed high fat diets
When fed high fat diets characteristic of westernized societies, the male LDLR−/− mouse develops hypercholesterolemia, hypertriglyceridemia, and insulin-resistant diabetes with profound accumulation of visceral fat67, 68, 71. LeBoeuf and colleagues72 identified that the weight gain and hyper-leptinemia of LDLR-deficient mice was greater than that of ApoE-deficient mice, tracking the evolution of insulin-resistant diabetes in the former and not the latter. As LeBoeuf highlights, male LDLR−/− mice – particularly on the C57Bl/6 background73 - are relatively susceptible to diet-induced obesity73. Male LDLR−/− mice also develop more severe hypertriglyceridemia74 and hepatic steatosis when challenged with high fat diets75; whether these differences72–74 are responsible for gender-specific responses to atheroma modulation by PPAR-gamma agonists remains to be determined74. The enhanced myeloid adipo-inflammatory response of male mice to high fat diet feeding may be responsible, and these sexually dimorphic metabolic responses have been recently reviewed in detail76.
Of note, in the absence of hyperglycemia, androgen treatment increases arterial calcification in both male and female ApoE-null mice77 – and conditional deletion of the androgen receptor reduces VSM calcification in vitro78. However, the effects of androgens on arterial calcification differ between the aortic sinus (unresponsive) and the innominate artery (responsive/worsened)77. Moreover, differences between male and female ApoE-null mice with respect to arterial calcification have been observed following genetic challenge79. While osteopontin (OPN)-deficiency decreases atheroma size (atherosis) in female ApoE−/− mice with minimal calcification, OPN-deficiency triples lesion calcification area (sclerosis) in male ApoE−/− mice79. We have noted that following high fat diet challenge, male LDLR−/− mice develop more significant arterial calcification and lipofuscin accumulation (unpublished). Likewise, male mice transgenic for VSM TNAP (bone alkaline phosphatase) are also more susceptible to arterial calcification and early lethality as compared to their female TNAP transgenic siblings80.
In the male LDLR−/− model, high fat diets (HFD) upregulate aortic expression of Msx1 and Msx267, two osteogenic transcription factors important in mineralization of the craniofacial skeleton81, 82. Bone morphogenetic protein 2 (BMP2) and osteopontin (OPN) are also upregulated by diet-induced disease67, reflecting contributions first identified in human calcified vessels83 and valves84. The pivotal role of these osteogenic programs in the arteriosclerotic calcification arising in diet-induced diabetes was recently demonstrated. Deletion of Msx1 and Msx2 within the VSM lineage (SM22-Cre transgene85) reduced arterial calcification and vascular stiffness without altering diet-induced obesity, diabetes, or dyslipidemia in the LDLR-deficient mouse86. Importantly, pro-calcific canonical and noncanonical Wnt signaling cascades entrained to Msx269 were also reduced in VSM with Msx gene deletion or knockdown86. Since Msx1 and Msx2 are genomic targets and secondary mediators of BMP287, their actions may serve to link paracrine BMP – and Wnt- dependent osteochondrogenic programming of mesenchymal progenitors in the diseased vessel wall88 as well as in the craniofacial skeleton87.
Additional studies in LDLR−/− mouse model have revealed that inflammatory cues, provided in part by the prototypic inflammatory cytokine TNF70, 89, 90, are proximal signals in the initiation of diabetic arteriosclerosis. TNF-dependent mural (intima-media and adventitial) inflammation upregulates aortic BMP2, Msx2, OPN, and Wnt signaling programs in vivo, and VSM-specific augmentation of TNF expression is sufficient to mimic early phases of diet-induced disease70, 90. However, other inflammatory signals initiated by (a) IL1β90, 91; (b) agonists of the overlapping yet distinct damage/danger – and pathogen- associated molecular pathogen pathways92 (viz. S100A/calgranulin family93, HMGB1, AGEs, oxLDL); and (c) hyperphosphatemia are also capable of driving these programs94–97. As Demer, Tintut and colleagues have pointed out2, oxidatively damaged molecules such as oxidized LDL92 (oxLDL) likely mimic the foreign lipidaceous signatures of mycobacterial and fungal pathogens that are controlled in part via sclerotic innate immune responses that contain organisms in calcified granuloma. When activated in the elastin-rich arterial wall with diabetes and dyslipidemia, these sclerotic responses are maladaptive for conduit vessel functions.
Importantly, diabetes – with our without uremia – increases the expression of S100A1298 (a.k.a. EN-RAGE), a powerful marker of cardiovascular risk in humans99. The relevance of these findings with respect to diabetic arterial calcification becomes readily apparent when considering the significant impact of altering S100A12 signaling tone in atherosclerosis models independent of glucose homeostasis. In a series of elegant studies by Hofmann Bowman and colleagues, a “humanized” transgenic mouse was created wherein VSM expression of S100A12 was shown to augment arterial calcification in atherosclerotic ApoE-null background100 and in mice with renal failure due to ureteral ligation101. Importantly, administration of paquinimod, a S100A neutralizing antagonist102, mitigated arterial calcification in the S100A12Tg;ApoE−/− mouse103. More recently, the Hofmann Bowman lab has created an hBAC transgenic line for the entire human S100A gene cluster (S100A8/S100A9/S100A12); myeloid expression of the hS100A cluster accentuated valve and vascular calcification in the ureteral ligation model of CKD104. Since S100A12 levels strongly correlate with cardiovascular outcome in CKD – outperforming soluble RAGE prognostication105, 106 – strategies targeting S100A signaling in diabetic arteriosclerosis promise to be extremely fruitful (Figure 1).
In 2007, Heinonen et al107 interbred ApoB100-only;LDLR−/− mice108 and the rat insulin promoter – IGF2 transgenic mouse109 to create a new model of arterial calcification in the setting of T2D (Table 1). The IGF2 transgene engenders the beta-cell ER stress response and functional decline that phenocopies an accelerated T2D progression110. This model may prove useful for studies of impaired ventricular-vascular coupling111 in diabetic arteriosclerosis112 – similar to the S100A8/S100A9/S100A12 transgenic mouse model of Hofmann Bowman104.
Hruska and colleagues significantly advanced our understanding of arterial calcification of chronic kidney disease – mineral and bone disorder (CKD-MBD) by introducing 5/6 nephrectomy onto the LDLR−/− model113, 114 (Table 1). CKD-MBD denotes the global perturbations in mineral metabolism that convey cardiovascular risk and skeletal fragility115, most frequently arising in the setting of the T2D-MetS spectrum. They demonstrated that low turnover bone disease arises along with arterial calcification in the nephrectomized LDLR−/− model – and that stimulating bone formation with the unique BMP7 ligand mitigated both bone disease and vascular calcification113, 114. This is highly relevant to the human setting; London and colleagues demonstrated that low turnover bone disease arising from either parathyroid hormone (PTH) resistance or deficiency correlates with vascular calcium load116 and arterial stiffness or PAD117 in CKD-MBD. Serum phosphate levels at any level of renal function convey cardiovascular risk, and exert pro-inflammatory actions upon VSM that promote vesicle-mediated vascular mineralization118, 119. It has been proposed that the skeleton represent an important “buffer” for phosphate in CKD120, and that orthotopic osteoblast –mediated calcium phosphate (as hydroxyapatite) deposition in bone may be responsible for the vascular benefits of normal bone formation. As compared to ApoE-null models, this model has relatively modest arterial calcium loads. As such, it is highly sensitive to physiological changes in serum phosphate121, and even modest drug-related improvements in renal function might improve vasculopathy. However, both BMP7 and PTH have direct salutary actions on the VSM68, 69, 71, 122. Moreover, T2D reduces blood flow to bone123 via a process regulated in part by PTH signaling at the principal nutrient artery124. The relative contributions of skeletal vs. vascular osteotropic hormone signals with respect to cardiovascular health have yet to be elucidated.
III.2. The male db/db mouse
Bostrom, Yao, and colleagues demonstrated that the db/db mouse model of T2D also accrues arterial calcium with progression of obesity and diabetes arising from leptin resistance30. They demonstrated that activation of the BMP2/4 signaling cascade was a key pathogenic feature of arteriosclerotic calcification30 as in the LDLR−/− mouse88. The db/db mouse has an additional advantage in that progressive nephropathy125 and neuropathy64, contributing features to diabetic arteriosclerosis in both T1D and T2D (Table 1). Dependent upon the strain background (BKS vs. B6), high fat diet feeding may be required to sustain hyperglycemia, however64. Using this model, studies from Tsao and colleagues126 have highlighted that Runx2-dependent arterial fibrosis and stiffening can progress somewhat independent of calcium deposition. Although the dietary intervention was not specified, this finding has important implications. Extracellular matrix stiffness is a “mechanocrine” regulator of osteogenic potential of mesenchymal progenitors, with a ~25 kPa stiffness optimal for osteogenic differentiation127; thus, a stiffening matrix environment arising from non-enzymatic glycated cross-linking, elastolysis, or fibrosis may impact subsequent osteogenic calcium deposition. Since calcium phosphate is proinflammatory128,52, feed-forward mechanisms are likely to offer strategies that differentially target disease during initiation and progression. Since dysglycemia is present for an estimated 6 years prior to T2D diagnosis in humans129, time-dependent vascular matrix remodeling may be rate-limiting in strategies that prove successful in reversing arteriosclerotic calcification and vascular stiffness in patients with T2D.
III.3. Insights from larger animal models of the T2D-MetS spectrum
A variety of large animal models have been recently implemented to study the T2D-MetS spectrum and its relationship to arteriosclerotic calcification130. Studies using the Ossabaw Island Hog (Table 1) have permitted characterization of calcium – rich diets on arteriosclerotic calcification, and demonstrated that deposition is not significantly influenced by calcium intake in the absence of renal dysfunction131; whether this differs in the setting of CKD132 remains to be assessed. A high fructose high salt diet induces arteriosclerotic calcification with insulin resistance and elevated oxLDL in the LDLR(R94/C94) Heterozygous Hog, but the model has yet to be completely characterized133. One of the more interesting preclinical models recently reported is the male rhesus macaque fed a high fat high sucrose diet134. In this nonhuman primate model, histological evidence of arteriosclerotic calcification was co-registered with non-invasive assessment of vascular stiffness by Doppler-assessed carotid-femoral pulse wave velocity134. It’s interesting to note that β-catenin and TGM2 (transglutaminase 2, a ligand for activation of Wnt signaling cascades in VSM135, 136), USF1 (a novel mediator of noncanonical Wnt signaling during VSM calcification137), and Gas6 (a vitamin K –dependent γ-carboxylated protein that inhibits osteogenic differentiation of vascular pericytes138, 139) were all upregulated with diet-induced disease, and downregulated with restoration of vascular compliance with high dose resveratrol134. Since fructose derived from diet or dietary sucrose metabolism induces insulin resistance with de novo lipogenesis in multiple species – including humans140 – findings from such diet-induced disease models promise to faithfully recapitulate the pathogenic features of arteriosclerotic calcification most important to address in therapeutic translation to patients afflicted with T2D or MetS.
IV. Translational Implications and Future Directions
In this overview, we’ve very briefly touched upon the preclinical models of arteriosclerosis that are beginning to inform the metabolic origins of arterial calcification in the setting of diabetes (Table 1). As of yet, no single model has emerged as perfectly recapitulating all aspects of human disease in clinically relevant settings. This arises, in part, because of (a) the multifactorial contributions – hyperglycemia, insulin resistance, hypertension, dyslipidemia, neuropathy – to arterial disease biology (Figure 1); (b) an imperfect understanding of bone-vascular interactions in cardiometabolic disease; and (c) the absence of therapeutic tools validated in preclinical models that have been successfully assessed in humans with robust clinical and/or physiological phenotypic responses. These limitations, in many ways, mirror obstacles that were eventually overcome during the nearly century-long process required to prove Anitschkow’s lipid hypothesis141
To date, the only drugs clearly impacting arterial calcification risk have been aminobisphosphonates142, phosphate binders (calcium vs. non-calcium based143–145), and statins146 – and with impact highly dependent upon subject age, metabolic milieu, and duration of treatment. For example, in MESA (Multiethnic Study of Atherosclerosis), aminobisphosphonate use was associated with reduced vascular calcification in elderly woman – but increased vascular calcium load in younger women142. However, in the setting of metabolic pyrophosphate deficiency, bisphosphonates can inhibit vascular mineralization147, 148 and prolong life. Statins – like bisphosphonates – inhibit bone resorbing osteoclast-like cell function149 while additionally limiting inflammatory osteogenic programs in VSM150. The former may explain why longer-term statin use (healthy survivors) may accrue increased coronary calcification with time146, while the latter has been used to argue for early intervention in preclinical porcine disease models151. Thus, physiological context and metabolic milieu significantly impacts the net impact of any intervention that directly or indirectly targets vascular mineralization. The only randomized trial of bisphosphonate treatment in arterial calcification was very small, very short (18 months), and emphasized CKD3-CKD4152; vascular calcium load was unchanged and only a non-significant trend (p = 0.07) for improved arterial compliance was observed. Notably, none of the clinical data currently available for consideration prospectively interrogate the impact of intervention as a function of T1D, T2D, MetS contributions, glycemic control and duration, renal function, baseline arterial disease, arterial anatomic venue, or gender. The recent NIH-AARP Diet and Health Study reinforces the lesson that gender-specific outcomes must be considered when assessing the cardiovascular consequences of manipulating calcium metabolism153 – even if only by dietary supplements. The elegant genetic studies of St. Hilaire and colleagues teach us that lower extremity peripheral artery calcification is genetically distinct from coronary, aortic, and valvular calcification154. Additionally, it has become abundantly clear that advanced aortic valve calcification, whether arising in the presence or absence of diabetes, is physiologically and pharmacologically unique155, 156.
Moving forward, it will be important to systematically phenotype (i.e., in both preclinical and clinical models) not only conduit vessel compliance but also end-organ perfusion and physiology157 –- an integrated function of the arterial Windkessel (Figure 2), vessel patency, resistance arteriole neuroregulation, microvascular integrity and density, and efferent vessel anatomy7, 121, 158, 159. It is reassuring that many of the molecular signatures identified as mechanistically important in pre-clinical models of arteriosclerosis are also manifest in humans83, 160–164. The development of preclinical models that are “humanized” for clinically relevant molecular mediators, dietary compositions, and metabolic milieus have contributed to this important but small success. Furthermore, the capacity of calcium-independent phosphate binders in reducing serum phosphate and limit arterial calcification while maintaining PTH levels in both preclinical and clinical CKD-MBD settings132, 165 will very likely extend to diabetic patients with less severe166 or no167 renal insufficiency; this should be explicitly examined with consideration of the contextual modifiers discussed above. We must increasingly seek to exploit every opportunity to validate and vet our understanding of human vascular mineralized matrix metabolism – opportunities afforded by drug repurposing10, 57, 103, phase IV or similar studies142, and by coordinating human molecular genetics164, 168, metabolomics, and physiological phenotyping with preclinical model development137, 168. Not every approach will be successful. Therefore, given finite research resources and the disease burden and its consequences4–6, we must insist upon experimental design that embraces specific and relevant patient-oriented clinical settings to prioritize our pre-clinical research – with the goal of improving cardiovascular health and independence in our diabetic patients afflicted with arteriosclerotic disease.
Highlights.
Arteriosclerotic calcification is actively regulated in the settings of type 1 and Type 2 diabetes.
Preclinical disease models have revealed important roles for innate immune signaling and osteogenic morphogens in vascular mineralization.
Successful translation of an improved understanding of arteriosclerosis disease biology will require experimental design that symmetrically develops detailed arterial phenotyping and function in both preclinical and clinical research studies.
Acknowledgments
None.
Sources of Funding – Supported by NIH grants HL069229 and HL114806, the J.D. & Maggie E. Wilson Distinguished Chair in Biomedical Research, the Jean D. Wilson Center for Biomedical Research, and the Louis V. Avioli Professorship in Mineral Metabolism Research.
Footnotes
Disclosures – None.
References
- 1.Thompson B, Towler DA. Arterial calcification and bone physiology: Role of the bone-vascular axis. Nat Rev Endocrinol. 2012;8:529–543. doi: 10.1038/nrendo.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demer LL, Tintut Y. Inflammatory, metabolic, and genetic mechanisms of vascular calcification. Arterioscler Thromb Vasc Biol. 2014;34:715–723. doi: 10.1161/ATVBAHA.113.302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hruska KA, Seifert M, Sugatani T. Pathophysiology of the chronic kidney disease-mineral bone disorder. Curr Opin Nephrol Hypertens. 2015;24:303–309. doi: 10.1097/MNH.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanter JE, Bornfeldt KE. Impact of diabetes mellitus. Arterioscler Thromb Vasc Biol. 2016;36:1049–1053. doi: 10.1161/ATVBAHA.116.307302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juutilainen A, Lehto S, Suhonen M, Ronnemaa T, Laakso M. Thoracoabdominal calcifications predict cardiovascular disease mortality in type 2 diabetic and nondiabetic subjects: 18-year follow-up study. Diabetes Care. 2010;33:583–585. doi: 10.2337/dc09-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehto S, Niskanen L, Suhonen M, Ronnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 7.Maillard P, Mitchell GF, Himali JJ, Beiser A, Tsao CW, Pase MP, Satizabal CL, Vasan RS, Seshadri S, DeCarli C. Effects of arterial stiffness on brain integrity in young adults from the framingham heart study. Stroke. 2016;47:1030–1036. doi: 10.1161/STROKEAHA.116.012949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pase MP, Himali JJ, Mitchell GF, Beiser A, Maillard P, Tsao C, Larson MG, DeCarli C, Vasan RS, Seshadri S. Association of aortic stiffness with cognition and brain aging in young and middle-aged adults: The framingham third generation cohort study. Hypertension. 2016;67:513–519. doi: 10.1161/HYPERTENSIONAHA.115.06610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinstein G, Maillard P, Himali JJ, Beiser AS, Au R, Wolf PA, Seshadri S, DeCarli C. Glucose indices are associated with cognitive and structural brain measures in young adults. Neurology. 2015;84:2329–2337. doi: 10.1212/WNL.0000000000001655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon LB, Massaro J, D’Agostino RB, Sr, Campbell SE, Brazier J, Brown WT, Kleinman ME, Kieran MW, Progeria Clinical Trials C Impact of farnesylation inhibitors on survival in hutchinson-gilford progeria syndrome. Circulation. 2014;130:27–34. doi: 10.1161/CIRCULATIONAHA.113.008285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon LB, Kleinman ME, Miller DT, et al. Clinical trial of a farnesyltransferase inhibitor in children with hutchinson-gilford progeria syndrome. Proc Natl Acad Sci U S A. 2012;109:16666–16671. doi: 10.1073/pnas.1202529109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz R, Wong ND, Kronmal R, Takasu J, Shavelle DM, Probstfield JL, Bertoni AG, Budoff MJ, O’Brien KD. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the multi-ethnic study of atherosclerosis. Circulation. 2006;113:2113–2119. doi: 10.1161/CIRCULATIONAHA.105.598086. [DOI] [PubMed] [Google Scholar]
- 13.Katz R, Budoff MJ, O’Brien KD, Wong ND, Nasir K. The metabolic syndrome and diabetes mellitus as predictors of thoracic aortic calcification as detected by non-contrast computed tomography in the multi-ethnic study of atherosclerosis. Diabet Med. 2015;33:912–919. doi: 10.1111/dme.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiani AN, Magder LS, Post WS, Szklo M, Bathon JM, Schreiner PJ, O’Leary D, Petri M. Coronary calcification in sle: Comparison with the multi-ethnic study of atherosclerosis. Rheumatology (Oxford) 2015;54:1976–1981. doi: 10.1093/rheumatology/kev198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishimura E, Okuno S, Kitatani K, Kim M, Shoji T, Nakatani T, Inaba M, Nishizawa Y. Different risk factors for peripheral vascular calcification between diabetic and non-diabetic haemodialysis patients–importance of glycaemic control. Diabetologia. 2002;45:1446–1448. doi: 10.1007/s00125-002-0920-8. [DOI] [PubMed] [Google Scholar]
- 16.Olson JC, Edmundowicz D, Becker DJ, Kuller LH, Orchard TJ. Coronary calcium in adults with type 1 diabetes: A stronger correlate of clinical coronary artery disease in men than in women. Diabetes. 2000;49:1571–1578. doi: 10.2337/diabetes.49.9.1571. [DOI] [PubMed] [Google Scholar]
- 17.Ix JH, Miller RG, Criqui MH, Orchard TJ. Test characteristics of the ankle-brachial index and ankle-brachial difference for medial arterial calcification on x-ray in type 1 diabetes. J Vasc Surg. 2012;56:721–727. doi: 10.1016/j.jvs.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maser RE, Wolfson SK, Jr, Ellis D, Stein EA, Drash AL, Becker DJ, Dorman JS, Orchard TJ. Cardiovascular disease and arterial calcification in insulin-dependent diabetes mellitus: Interrelations and risk factor profiles. Pittsburgh epidemiology of diabetes complications study-v. Arterioscler Thromb. 1991;11:958–965. doi: 10.1161/01.atv.11.4.958. [DOI] [PubMed] [Google Scholar]
- 19.Schauer IE, Snell-Bergeon JK, Bergman BC, Maahs DM, Kretowski A, Eckel RH, Rewers M. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: The cacti study. Diabetes. 2011;60:306–314. doi: 10.2337/db10-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodrigues TC, Canani LH, Schvartzman P, Gross JL. Hypertension is the metabolic syndrome component most strongly associated with microvascular complications and coronary artery calcification in type 1 diabetes. J Endocrinol Invest. 2011;34:e58–63. doi: 10.1007/BF03347077. [DOI] [PubMed] [Google Scholar]
- 21.Szkudelski T. The mechanism of alloxan and streptozotocin action in b cells of the rat pancreas. Physiol Res. 2001;50:537–546. [PubMed] [Google Scholar]
- 22.Heath JM, Sun Y, Yuan K, Bradley WE, Litovsky S, Dell’Italia LJ, Chatham JC, Wu H, Chen Y. Activation of akt by o-linked n-acetylglucosamine induces vascular calcification in diabetes mellitus. Circ Res. 2014;114:1094–1102. doi: 10.1161/CIRCRESAHA.114.302968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai Z, Ding Y, Zhang M, Lu Q, Wu S, Zhu H, Song P, Zou MH. Ablation of adenosine monophosphate-activated protein kinase alpha1 in vascular smooth muscle cells promotes diet-induced atherosclerotic calcification in vivo. Circ Res. 2016;119:422–433. doi: 10.1161/CIRCRESAHA.116.308301. [DOI] [PubMed] [Google Scholar]
- 24.Shimazu J, Wei J, Karsenty G. Smurf1 inhibits osteoblast differentiation, bone formation, and glucose homeostasis through serine 148. Cell Rep. 2016;15:27–35. doi: 10.1016/j.celrep.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon WJ, Cho YD, Kim WJ, Bae HS, Islam R, Woo KM, Baek JH, Bae SC, Ryoo HM. Prolyl isomerase pin1-mediated conformational change and subnuclear focal accumulation of runx2 are crucial for fibroblast growth factor 2 (fgf2)-induced osteoblast differentiation. J Biol Chem. 2014;289:8828–8838. doi: 10.1074/jbc.M113.516237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forlino A, Marini JC. Osteogenesis imperfecta. Lancet. 2016;387:1657–1671. doi: 10.1016/S0140-6736(15)00728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouvet C, Peeters W, Moreau S, DeBlois D, Moreau P. A new rat model of diabetic macrovascular complication. Cardiovasc Res. 2007;73:504–511. doi: 10.1016/j.cardiores.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Brodeur MR, Bouvet C, Bouchard S, Moreau S, Leblond J, Deblois D, Moreau P. Reduction of advanced-glycation end products levels and inhibition of rage signaling decreases rat vascular calcification induced by diabetes. PLoS One. 2014;9:e85922. doi: 10.1371/journal.pone.0085922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao Y, Bennett BJ, Wang X, Rosenfeld ME, Giachelli C, Lusis AJ, Bostrom KI. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res. 2010;107:485–494. doi: 10.1161/CIRCRESAHA.110.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bostrom KI, Jumabay M, Matveyenko A, Nicholas SB, Yao Y. Activation of vascular bone morphogenetic protein signaling in diabetes mellitus. Circ Res. 2011;108:446–457. doi: 10.1161/CIRCRESAHA.110.236596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin ME, Chen T, Leaf EM, Speer MY, Giachelli CM. Runx2 expression in smooth muscle cells is required for arterial medial calcification in mice. Am J Pathol. 2015;185:1958–1969. doi: 10.1016/j.ajpath.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun Y, Byon CH, Yuan K, Chen J, Mao X, Heath JM, Javed A, Zhang K, Anderson PG, Chen Y. Smooth muscle cell-specific runx2 deficiency inhibits vascular calcification. Circ Res. 2012;111:543–552. doi: 10.1161/CIRCRESAHA.112.267237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang YW, Yasui N, Ito K, Huang G, Fujii M, Hanai J, Nogami H, Ochi T, Miyazono K, Ito Y. A runx2/pebp2alpha a/cbfa1 mutation displaying impaired transactivation and smad interaction in cleidocranial dysplasia. Proc Natl Acad Sci U S A. 2000;97:10549–10554. doi: 10.1073/pnas.180309597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, Montecino M, Hassan MQ, Gaur T, Lengner CJ, Young DW. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:1–16. doi: 10.1007/s11154-006-9001-5. [DOI] [PubMed] [Google Scholar]
- 35.Bennett RA, Pegg AE. Alkylation of DNA in rat tissues following administration of streptozotocin. Cancer Res. 1981;41:2786–2790. [PubMed] [Google Scholar]
- 36.Liu Y, Drozdov I, Shroff R, Beltran LE, Shanahan CM. Prelamin a accelerates vascular calcification via activation of the DNA damage response and senescence-associated secretory phenotype in vascular smooth muscle cells. Circ Res. 2013;112:e99–109. doi: 10.1161/CIRCRESAHA.111.300543. [DOI] [PubMed] [Google Scholar]
- 37.Havelek R, Soukup T, Cmielova J, Seifrtova M, Suchanek J, Vavrova J, Mokry J, Muthna D, Rezacova M. Ionizing radiation induces senescence and differentiation of human dental pulp stem cells. Folia Biol (Praha) 2013;59:188–197. [PubMed] [Google Scholar]
- 38.Ong DS, Aertker RA, Clark AN, Kiefer T, Hughes GC, Harrison JK, Bashore TM. Radiation-associated valvular heart disease. J Heart Valve Dis. 2013;22:883–892. [PubMed] [Google Scholar]
- 39.Ren X, Wei Q, Shao H, Sun Z, Liu N. A rat model of diabetic artery calcification. J Endocrinol Invest. 2012;35:497–503. doi: 10.3275/7865. [DOI] [PubMed] [Google Scholar]
- 40.Wei Q, Ren X, Jiang Y, Jin H, Liu N, Li J. Advanced glycation end products accelerate rat vascular calcification through rage/oxidative stress. BMC Cardiovasc Disord. 2013;13:13. doi: 10.1186/1471-2261-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shao JS, Cheng SL, Sadhu J, Towler DA. Inflammation and the osteogenic regulation of vascular calcification: A review and perspective. Hypertension. 2010;55:579–592. doi: 10.1161/HYPERTENSIONAHA.109.134205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen N, Naik V, Speer MY. Diabetes mellitus accelerates cartilaginous metaplasia and calcification in atherosclerotic vessels of ldlr mutant mice. Cardiovasc Pathol. 2013;22:167–175. doi: 10.1016/j.carpath.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woldt E, Terrand J, Mlih M, et al. The nuclear hormone receptor ppargamma counteracts vascular calcification by inhibiting wnt5a signalling in vascular smooth muscle cells. Nat Commun. 2012;3:1077. doi: 10.1038/ncomms2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaillard V, Casellas D, Seguin-Devaux C, Schohn H, Dauca M, Atkinson J, Lartaud I. Pioglitazone improves aortic wall elasticity in a rat model of elastocalcinotic arteriosclerosis. Hypertension. 2005;46:372–379. doi: 10.1161/01.HYP.0000171472.24422.33. [DOI] [PubMed] [Google Scholar]
- 45.Wang Z, Jiang Y, Liu N, Ren L, Zhu Y, An Y, Chen D. Advanced glycation end-product nepsilon-carboxymethyl-lysine accelerates progression of atherosclerotic calcification in diabetes. Atherosclerosis. 2012;221:387–396. doi: 10.1016/j.atherosclerosis.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 46.Qiao JH, Fishbein MC, Demer LL, Lusis AJ. Genetic determination of cartilaginous metaplasia in mouse aorta. Arterioscler Thromb Vasc Biol. 1995;15:2265–2272. doi: 10.1161/01.atv.15.12.2265. [DOI] [PubMed] [Google Scholar]
- 47.Tse J, Martin-McNaulty B, Halks-Miller M, Kauser K, DelVecchio V, Vergona R, Sullivan ME, Rubanyi GM. Accelerated atherosclerosis and premature calcified cartilaginous metaplasia in the aorta of diabetic male apo e knockout mice can be prevented by chronic treatment with 17 beta-estradiol. Atherosclerosis. 1999;144:303–313. doi: 10.1016/s0021-9150(98)00325-6. [DOI] [PubMed] [Google Scholar]
- 48.Yao J, Guihard PJ, Blazquez-Medela AM, Guo Y, Moon JH, Jumabay M, Bostrom KI, Yao Y. Serine protease activation essential for endothelial-mesenchymal transition in vascular calcification. Circ Res. 2015;117:758–769. doi: 10.1161/CIRCRESAHA.115.306751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bailey AS, Willenbring H, Jiang S, Anderson DA, Schroeder DA, Wong MH, Grompe M, Fleming WH. Myeloid lineage progenitors give rise to vascular endothelium. Proc Natl Acad Sci U S A. 2006;103:13156–13161. doi: 10.1073/pnas.0604203103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fadini GP, Albiero M, Menegazzo L, et al. Widespread increase in myeloid calcifying cells contributes to ectopic vascular calcification in type 2 diabetes. Circ Res. 2011;108:1112–1121. doi: 10.1161/CIRCRESAHA.110.234088. [DOI] [PubMed] [Google Scholar]
- 52.New SE, Goettsch C, Aikawa M, Marchini JF, Shibasaki M, Yabusaki K, Libby P, Shanahan CM, Croce K, Aikawa E. Macrophage-derived matrix vesicles: An alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ Res. 2013;113:72–77. doi: 10.1161/CIRCRESAHA.113.301036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wen L, Wang Y, Wen N, Yuan G, Wen M, Zhang L, Liu Q, Liang Y, Cai C, Chen X, Ding Y. Role of endothelial progenitor cells in maintaining stemness and enhancing differentiation of mesenchymal stem cells by indirect cell-cell interaction. Stem Cells Dev. 2016;25:123–138. doi: 10.1089/scd.2015.0049. [DOI] [PubMed] [Google Scholar]
- 54.Lin CH, Lilly B. Endothelial cells direct mesenchymal stem cells toward a smooth muscle cell fate. Stem Cells Dev. 2014;23:2581–2590. doi: 10.1089/scd.2014.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, Frutkin A, Dichek D, Giachelli CM. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–741. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Naik V, Leaf EM, Hu JH, Yang HY, Nguyen NB, Giachelli CM, Speer MY. Sources of cells that contribute to atherosclerotic intimal calcification: An in vivo genetic fate mapping study. Cardiovasc Res. 2012;94:545–554. doi: 10.1093/cvr/cvs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Towler DA. Arteriosclerotic calcification: A serpi(n)ginous path to cardiovascular health? Circ Res. 2015;117:744–746. doi: 10.1161/CIRCRESAHA.115.307407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Papanas N, Ziegler D. Risk factors and comorbidities in diabetic neuropathy: An update 2015. Rev Diabet Stud. 2015;12:48–62. doi: 10.1900/RDS.2015.12.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goebel FD, Fuessl HS. Monckeberg’s sclerosis after sympathetic denervation in diabetic and non-diabetic subjects. Diabetologia. 1983;24:347–350. doi: 10.1007/BF00251822. [DOI] [PubMed] [Google Scholar]
- 60.Lazard ZW, Olmsted-Davis EA, Salisbury EA, Gugala Z, Sonnet C, Davis EL, Beal E, 2nd, Ubogu EE, Davis AR. Osteoblasts have a neural origin in heterotopic ossification. Clin Orthop Relat Res. 2015;473:2790–2806. doi: 10.1007/s11999-015-4323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gopinathan G, Kolokythas A, Luan X, Diekwisch TG. Epigenetic marks define the lineage and differentiation potential of two distinct neural crest-derived intermediate odontogenic progenitor populations. Stem Cells Dev. 2013;22:1763–1778. doi: 10.1089/scd.2012.0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clewes O, Narytnyk A, Gillinder KR, Loughney AD, Murdoch AP, Sieber-Blum M. Human epidermal neural crest stem cells (hepi-ncsc)–characterization and directed differentiation into osteocytes and melanocytes. Stem Cell Rev. 2011;7:799–814. doi: 10.1007/s12015-011-9255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Brien PD, Sakowski SA, Feldman EL. Mouse models of diabetic neuropathy. ILAR J. 2014;54:259–272. doi: 10.1093/ilar/ilt052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sullivan KA, Hayes JM, Wiggin TD, Backus C, Su Oh S, Lentz SI, Brosius F, 3rd, Feldman EL. Mouse models of diabetic neuropathy. Neurobiol Dis. 2007;28:276–285. doi: 10.1016/j.nbd.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Genet F, Kulina I, Vaquette C, Torossian F, Millard S, Pettit AR, Sims NA, Anginot A, Guerton B, Winkler IG, Barbier V, Lataillade JJ, Le Bousse-Kerdiles MC, Hutmacher DW, Levesque JP. Neurological heterotopic ossification following spinal cord injury is triggered by macrophage-mediated inflammation in muscle. J Pathol. 2015;236:229–240. doi: 10.1002/path.4519. [DOI] [PubMed] [Google Scholar]
- 66.Krishnaswamy PS, Egom EE, Moghtadaei M, Jansen HJ, Azer J, Bogachev O, Mackasey M, Robbins C, Rose RA. Altered parasympathetic nervous system regulation of the sinoatrial node in akita diabetic mice. J Mol Cell Cardiol. 2015;82:125–135. doi: 10.1016/j.yjmcc.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 67.Towler DA, Bidder M, Latifi T, Coleman T, Semenkovich CF. Diet-induced diabetes activates an osteogenic gene regulatory program in the aortas of low density lipoprotein receptor-deficient mice. J Biol Chem. 1998;273:30427–30434. doi: 10.1074/jbc.273.46.30427. [DOI] [PubMed] [Google Scholar]
- 68.Shao JS, Cheng SL, Charlton-Kachigian N, Loewy AP, Towler DA. Teriparatide (human parathyroid hormone (1–34)) inhibits osteogenic vascular calcification in diabetic low density lipoprotein receptor-deficient mice. J Biol Chem. 2003;278:50195–50202. doi: 10.1074/jbc.M308825200. [DOI] [PubMed] [Google Scholar]
- 69.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine wnt signals. J Clin Invest. 2005;115:1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Al-Aly Z, Shao JS, Lai CF, Huang E, Cai J, Behrmann A, Cheng SL, Towler DA. Aortic msx2-wnt calcification cascade is regulated by tnf-alpha-dependent signals in diabetic ldlr−/− mice. Arterioscler Thromb Vasc Biol. 2007;27:2589–2596. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 71.Cheng SL, Shao JS, Halstead LR, Distelhorst K, Sierra O, Towler DA. Activation of vascular smooth muscle parathyroid hormone receptor inhibits wnt/beta-catenin signaling and aortic fibrosis in diabetic arteriosclerosis. Circ Res. 2010;107:271–282. doi: 10.1161/CIRCRESAHA.110.219899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schreyer SA, Vick C, Lystig TC, Mystkowski P, LeBoeuf RC. Ldl receptor but not apolipoprotein e deficiency increases diet-induced obesity and diabetes in mice. Am J Physiol Endocrinol Metab. 2002;282:E207–214. doi: 10.1152/ajpendo.2002.282.1.E207. [DOI] [PubMed] [Google Scholar]
- 73.Schreyer SA, Chua SC, Jr, LeBoeuf RC. Obesity and diabetes in tnf-alpha receptor- deficient mice. J Clin Invest. 1998;102:402–411. doi: 10.1172/JCI2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li AC, Brown KK, Silvestre MJ, Willson TM, Palinski W, Glass CK. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in ldl receptor-deficient mice. J Clin Invest. 2000;106:523–531. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Norheim F, Hui ST, Kulahcioglu E, Mehrabian M, Cantor RM, Pan C, Parks BW, Lusis AJ. Genetic and hormonal control of hepatic steatosis in female and male mice. J Lipid Res. 2016 Nov 3; doi: 10.1194/jlr.M071522. pii: jlr.M071522. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Griffin C, Lanzetta N, Eter L, Singer K. Sexually dimorphic myeloid inflammatory and metabolic responses to diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2016;311:R211–216. doi: 10.1152/ajpregu.00136.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McRobb L, Handelsman DJ, Heather AK. Androgen-induced progression of arterial calcification in apolipoprotein e-null mice is uncoupled from plaque growth and lipid levels. Endocrinology. 2009;150:841–848. doi: 10.1210/en.2008-0760. [DOI] [PubMed] [Google Scholar]
- 78.Zhu D, Hadoke PW, Wu J, Vesey AT, Lerman DA, Dweck MR, Newby DE, Smith LB, MacRae VE. Ablation of the androgen receptor from vascular smooth muscle cells demonstrates a role for testosterone in vascular calcification. Sci Rep. 2016;6:24807. doi: 10.1038/srep24807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matsui Y, Rittling SR, Okamoto H, Inobe M, Jia N, Shimizu T, Akino M, Sugawara T, Morimoto J, Kimura C, Kon S, Denhardt D, Kitabatake A, Uede T. Osteopontin deficiency attenuates atherosclerosis in female apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1029–1034. doi: 10.1161/01.ATV.0000074878.29805.D0. [DOI] [PubMed] [Google Scholar]
- 80.Sheen CR, Kuss P, Narisawa S, Yadav MC, Nigro J, Wang W, Chhea TN, Sergienko EA, Kapoor K, Jackson MR, Hoylaerts MF, Pinkerton AB, O’Neill WC, Millan JL. Pathophysiological role of vascular smooth muscle alkaline phosphatase in medial artery calcification. J Bone Miner Res. 2015;30:824–836. doi: 10.1002/jbmr.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilkie AO, Oldridge M, Tang Z, Maxson RE., Jr Craniosynostosis and related limb anomalies. Novartis Found Symp. 2001;232:122–133. doi: 10.1002/0470846658.ch9. discussion 133–143. [DOI] [PubMed] [Google Scholar]
- 82.Alappat S, Zhang ZY, Chen YP. Msx homeobox gene family and craniofacial development. Cell Res. 2003;13:429–442. doi: 10.1038/sj.cr.7290185. [DOI] [PubMed] [Google Scholar]
- 83.Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–1809. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.O’Brien KD, Kuusisto J, Reichenbach DD, Ferguson M, Giachelli C, Alpers CE, Otto CM. Osteopontin is expressed in human aortic valvular lesions. Circulation. 1995;92:2163–2168. doi: 10.1161/01.cir.92.8.2163. [DOI] [PubMed] [Google Scholar]
- 85.Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, Hammer RE, Herz J, Kuhn M. Smooth muscle-selective deletion of guanylyl cyclase-a prevents the acute but not chronic effects of anp on blood pressure. Proc Natl Acad Sci U S A. 2002;99:7142–7147. doi: 10.1073/pnas.102650499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheng SL, Behrmann A, Shao JS, Ramachandran B, Krchma K, Bello Arredondo Y, Kovacs A, Mead M, Maxson R, Towler DA. Targeted reduction of vascular msx1 and msx2 mitigates arteriosclerotic calcification and aortic stiffness in ldlr-deficient mice fed diabetogenic diets. Diabetes. 2014;63:4326–4337. doi: 10.2337/db14-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun J, Ishii M, Ting MC, Maxson R. Foxc1 controls the growth of the murine frontal bone rudiment by direct regulation of a bmp response threshold of msx2. Development. 2013;140:1034–1044. doi: 10.1242/dev.085225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shao JS, Cai J, Towler DA. Molecular mechanisms of vascular calcification: Lessons learned from the aorta. Arterioscler Thromb Vasc Biol. 2006;26:1423–1430. doi: 10.1161/01.ATV.0000220441.42041.20. [DOI] [PubMed] [Google Scholar]
- 89.Lai CF, Seshadri V, Huang K, Shao JS, Cai J, Vattikuti R, Schumacher A, Loewy AP, Denhardt DT, Rittling SR, Towler DA. An osteopontin-nadph oxidase signaling cascade promotes pro-matrix metalloproteinase 9 activation in aortic mesenchymal cells. Circ Res. 2006;98:1479–1489. doi: 10.1161/01.RES.0000227550.00426.60. [DOI] [PubMed] [Google Scholar]
- 90.Lai CF, Shao JS, Behrmann A, Krchma K, Cheng SL, Towler DA. Tnfr1-activated reactive oxidative species signals up-regulate osteogenic msx2 programs in aortic myofibroblasts. Endocrinology. 2012;153:3897–3910. doi: 10.1210/en.2012-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Awan Z, Denis M, Roubtsova A, Essalmani R, Marcinkiewicz J, Awan A, Gram H, Seidah NG, Genest J. Reducing vascular calcification by anti-il-1beta monoclonal antibody in a mouse model of familial hypercholesterolemia. Angiology. 2016;67:157–167. doi: 10.1177/0003319715583205. [DOI] [PubMed] [Google Scholar]
- 92.Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, Montano E, Shaw PX, Tsimikas S, Binder CJ, Witztum JL. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oesterle A, Bowman MA. S100a12 and the s100/calgranulins: Emerging biomarkers for atherosclerosis and possibly therapeutic targets. Arterioscler Thromb Vasc Biol. 2015;35:2496–2507. doi: 10.1161/ATVBAHA.115.302072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Suga T, Iso T, Shimizu T, Tanaka T, Yamagishi S, Takeuchi M, Imaizumi T, Kurabayashi M. Activation of receptor for advanced glycation end products induces osteogenic differentiation of vascular smooth muscle cells. J Atheroscler Thromb. 2011;18:670–683. doi: 10.5551/jat.7120. [DOI] [PubMed] [Google Scholar]
- 95.Taylor J, Butcher M, Zeadin M, Politano A, Shaughnessy SG. Oxidized low-density lipoprotein promotes osteoblast differentiation in primary cultures of vascular smooth muscle cells by up-regulating osterix expression in an msx2-dependent manner. J Cell Biochem. 2011;112:581–588. doi: 10.1002/jcb.22948. [DOI] [PubMed] [Google Scholar]
- 96.Alesutan I, Feger M, Tuffaha R, et al. Augmentation of phosphate-induced osteo-/chondrogenic transformation of vascular smooth muscle cells by homoarginine. Cardiovasc Res. 2016;110:408–418. doi: 10.1093/cvr/cvw062. [DOI] [PubMed] [Google Scholar]
- 97.Yao L, Sun YT, Sun W, Xu TH, Ren C, Fan X, Sun L, Liu LL, Feng JM, Ma JF, Wang LN. High phosphorus level leads to aortic calcification via beta-catenin in chronic kidney disease. Am J Nephrol. 2015;41:28–36. doi: 10.1159/000370250. [DOI] [PubMed] [Google Scholar]
- 98.Basta G, Sironi AM, Lazzerini G, Del Turco S, Buzzigoli E, Casolaro A, Natali A, Ferrannini E, Gastaldelli A. Circulating soluble receptor for advanced glycation end products is inversely associated with glycemic control and s100a12 protein. J Clin Endocrinol Metab. 2006;91:4628–4634. doi: 10.1210/jc.2005-2559. [DOI] [PubMed] [Google Scholar]
- 99.Ligthart S, Sedaghat S, Ikram MA, Hofman A, Franco OH, Dehghan A. En-rage: A novel inflammatory marker for incident coronary heart disease. Arterioscler Thromb Vasc Biol. 2014;34:2695–2699. doi: 10.1161/ATVBAHA.114.304306. [DOI] [PubMed] [Google Scholar]
- 100.Hofmann Bowman MA, Gawdzik J, Bukhari U, Husain AN, Toth PT, Kim G, Earley J, McNally EM. S100a12 in vascular smooth muscle accelerates vascular calcification in apolipoprotein e-null mice by activating an osteogenic gene regulatory program. Arterioscler Thromb Vasc Biol. 2011;31:337–344. doi: 10.1161/ATVBAHA.110.217745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gawdzik J, Mathew L, Kim G, Puri TS, Hofmann Bowman MA. Vascular remodeling and arterial calcification are directly mediated by s100a12 (en-rage) in chronic kidney disease. Am J Nephrol. 2011;33:250–259. doi: 10.1159/000324693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bjork P, Bjork A, Vogl T, Stenstrom M, Liberg D, Olsson A, Roth J, Ivars F, Leanderson T. Identification of human s100a9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol. 2009;7:e97. doi: 10.1371/journal.pbio.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yan L, Bjork P, Butuc R, Gawdzik J, Earley J, Kim G, Hofmann Bowman MA. Beneficial effects of quinoline-3-carboxamide (abr-215757) on atherosclerotic plaque morphology in s100a12 transgenic apoe null mice. Atherosclerosis. 2013;228:69–79. doi: 10.1016/j.atherosclerosis.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yan L, Mathew L, Chellan B, Gardner B, Earley J, Puri TS, Hofmann Bowman MA. S100/calgranulin-mediated inflammation accelerates left ventricular hypertrophy and aortic valve sclerosis in chronic kidney disease in a receptor for advanced glycation end products-dependent manner. Arterioscler Thromb Vasc Biol. 2014;34:1399–1411. doi: 10.1161/ATVBAHA.114.303508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lindholm B. Serum s100a12: A risk marker or risk factor of vascular calcification in chronic kidney disease. Am J Nephrol. 2015;42:1–3. doi: 10.1159/000438873. [DOI] [PubMed] [Google Scholar]
- 106.Isoyama N, Leurs P, Qureshi AR, Bruchfeld A, Anderstam B, Heimburger O, Barany P, Stenvinkel P, Lindholm B. Plasma s100a12 and soluble receptor of advanced glycation end product levels and mortality in chronic kidney disease stage 5 patients. Nephrol Dial Transplant. 2015;30:84–91. doi: 10.1093/ndt/gfu259. [DOI] [PubMed] [Google Scholar]
- 107.Heinonen SE, Leppanen P, Kholova I, Lumivuori H, Hakkinen SK, Bosch F, Laakso M, Yla-Herttuala S. Increased atherosclerotic lesion calcification in a novel mouse model combining insulin resistance, hyperglycemia, and hypercholesterolemia. Circ Res. 2007;101:1058–1067. doi: 10.1161/CIRCRESAHA.107.154401. [DOI] [PubMed] [Google Scholar]
- 108.Farese RV, Jr, Veniant MM, Cham CM, Flynn LM, Pierotti V, Loring JF, Traber M, Ruland S, Stokowski RS, Huszar D, Young SG. Phenotypic analysis of mice expressing exclusively apolipoprotein b48 or apolipoprotein b100. Proc Natl Acad Sci U S A. 1996;93:6393–6398. doi: 10.1073/pnas.93.13.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Devedjian JC, George M, Casellas A, Pujol A, Visa J, Pelegrin M, Gros L, Bosch F. Transgenic mice overexpressing insulin-like growth factor-ii in beta cells develop type 2 diabetes. J Clin Invest. 2000;105:731–740. doi: 10.1172/JCI5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Casellas A, Mallol C, Salavert A, Jimenez V, Garcia M, Agudo J, Obach M, Haurigot V, Vila L, Molas M, Lage R, Morro M, Casana E, Ruberte J, Bosch F. Insulin-like growth factor 2 overexpression induces beta-cell dysfunction and increases beta-cell susceptibility to damage. J Biol Chem. 2015;290:16772–16785. doi: 10.1074/jbc.M115.642041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Athyros VG, Pagourelias ED, Gossios TD, Vasilikos VG. Treating heart failure with preserved ejection fraction related to arterial stiffness. Can we kill two birds with one stone? Curr Vasc Pharmacol. 2015;13:368–380. doi: 10.2174/1570161112666141126150948. [DOI] [PubMed] [Google Scholar]
- 112.Heinonen SE, Merentie M, Hedman M, Makinen PI, Loponen E, Kholova I, Bosch F, Laakso M, Yla-Herttuala S. Left ventricular dysfunction with reduced functional cardiac reserve in diabetic and non-diabetic ldl-receptor deficient apolipoprotein b100-only mice. Cardiovasc Diabetol. 2011;10:59. doi: 10.1186/1475-2840-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mathew S, Davies M, Lund R, Saab G, Hruska KA. Function and effect of bone morphogenetic protein-7 in kidney bone and the bone-vascular links in chronic kidney disease. Eur J Clin Invest. 2006;36(Suppl 2):43–50. doi: 10.1111/j.1365-2362.2006.01663.x. [DOI] [PubMed] [Google Scholar]
- 114.Davies MR, Lund RJ, Mathew S, Hruska KA. Low turnover osteodystrophy and vascular calcification are amenable to skeletal anabolism in an animal model of chronic kidney disease and the metabolic syndrome. J Am Soc Nephrol. 2005;16:917–928. doi: 10.1681/ASN.2004100835. [DOI] [PubMed] [Google Scholar]
- 115.Moe SM, Drueke T, Lameire N, Eknoyan G. Chronic kidney disease-mineral-bone disorder: A new paradigm. Adv Chronic Kidney Dis. 2007;14:3–12. doi: 10.1053/j.ackd.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 116.London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, de Vernejoul MC. Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol. 2004;15:1943–1951. doi: 10.1097/01.asn.0000129337.50739.48. [DOI] [PubMed] [Google Scholar]
- 117.London GM, Marchais SJ, Guerin AP, de Vernejoul MC. Ankle-brachial index and bone turnover in patients on dialysis. J Am Soc Nephrol. 2015;26:476–483. doi: 10.1681/ASN.2014020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kapustin AN, Shanahan CM. Emerging roles for vascular smooth muscle cell exosomes in calcification and coagulation. J Physiol. 2016;594:2905–2914. doi: 10.1113/JP271340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kapustin AN, Chatrou ML, Drozdov I, et al. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ Res. 2015;116:1312–1323. doi: 10.1161/CIRCRESAHA.116.305012. [DOI] [PubMed] [Google Scholar]
- 120.Nordholm A, Mace ML, Gravesen E, Olgaard K, Lewin E. A potential kidney-bone axis involved in the rapid minute-to-minute regulation of plasma ca2+ BMC Nephrol. 2015;16:29. doi: 10.1186/s12882-015-0019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mathew S, Lund RJ, Strebeck F, Tustison KS, Geurs T, Hruska KA. Reversal of the adynamic bone disorder and decreased vascular calcification in chronic kidney disease by sevelamer carbonate therapy. J Am Soc Nephrol. 2007;18:122–130. doi: 10.1681/ASN.2006050490. [DOI] [PubMed] [Google Scholar]
- 122.Dorai H, Vukicevic S, Sampath TK. Bone morphogenetic protein-7 (osteogenic protein-1) inhibits smooth muscle cell proliferation and stimulates the expression of markers that are characteristic of smc phenotype in vitro. J Cell Physiol. 2000;184:37–45. doi: 10.1002/(SICI)1097-4652(200007)184:1<37::AID-JCP4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 123.Stabley JN, Prisby RD, Behnke BJ, Delp MD. Type 2 diabetes alters bone and marrow blood flow and vascular control mechanisms in the zdf rat. J Endocrinol. 2015;225:47–58. doi: 10.1530/JOE-14-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Benson T, Menezes T, Campbell J, Bice A, Hood B, Prisby R. Mechanisms of vasodilation to pth 1-84, pth 1-34, and pthrp 1-34 in rat bone resistance arteries. Osteoporos Int. 2016;27:1817–1826. doi: 10.1007/s00198-015-3460-z. [DOI] [PubMed] [Google Scholar]
- 125.Tesch GH, Lim AK. Recent insights into diabetic renal injury from the db/db mouse model of type 2 diabetic nephropathy. Am J Physiol Renal Physiol. 2011;300:F301–310. doi: 10.1152/ajprenal.00607.2010. [DOI] [PubMed] [Google Scholar]
- 126.Raaz U, Schellinger IN, Chernogubova E, et al. Transcription factor runx2 promotes aortic fibrosis and stiffness in type 2 diabetes mellitus. Circ Res. 2015;117:513–524. doi: 10.1161/CIRCRESAHA.115.306341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 128.Nadra I, Mason JC, Philippidis P, Florey O, Smythe CD, McCarthy GM, Landis RC, Haskard DO. Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase c and map kinase pathways: A vicious cycle of inflammation and arterial calcification? Circ Res. 2005;96:1248–1256. doi: 10.1161/01.RES.0000171451.88616.c2. [DOI] [PubMed] [Google Scholar]
- 129.Porta M, Curletto G, Cipullo D, Rigault de la Longrais R, Trento M, Passera P, Taulaigo AV, Di Miceli S, Cenci A, Dalmasso P, Cavallo F. Estimating the delay between onset and diagnosis of type 2 diabetes from the time course of retinopathy prevalence. Diabetes Care. 2014;37:1668–1674. doi: 10.2337/dc13-2101. [DOI] [PubMed] [Google Scholar]
- 130.Wastney M, Lee W, Jackson GS, Alloosh M, Sturek M, Lachcik P, Peacock M, Martin B, Weaver CM. Soft tissue calcification in the ossabaw miniature pig: Experimental and kinetic modeling studies. Osteoporos Int. 2013;24:2123–2126. doi: 10.1007/s00198-012-2229-x. [DOI] [PubMed] [Google Scholar]
- 131.Phillips-Eakley AK, McKenney-Drake ML, Bahls M, Newcomer SC, Radcliffe JS, Wastney ME, Van Alstine WG, Jackson G, Alloosh M, Martin BR, Sturek M, Weaver CM. Effect of high-calcium diet on coronary artery disease in ossabaw miniature swine with metabolic syndrome. J Am Heart Assoc. 2015;4:e001620. doi: 10.1161/JAHA.114.001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Raggi P, James G, Burke SK, Bommer J, Chasan-Taber S, Holzer H, Braun J, Chertow GM. Decrease in thoracic vertebral bone attenuation with calcium-based phosphate binders in hemodialysis. J Bone Miner Res. 2005;20:764–772. doi: 10.1359/JBMR.041221. [DOI] [PubMed] [Google Scholar]
- 133.Nichols TC, Merricks EP, Bellinger DA, Raymer RA, Yu J, Lam D, Koch GG, Busby WH, Jr, Clemmons DR. Oxidized ldl and fructosamine associated with severity of coronary artery atherosclerosis in insulin resistant pigs fed a high fat/high nacl diet. PLoS One. 2015;10:e0132302. doi: 10.1371/journal.pone.0132302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mattison JA, Wang M, Bernier M, et al. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 2014;20:183–190. doi: 10.1016/j.cmet.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Beazley KE, Deasey S, Lima F, Nurminskaya MV. Transglutaminase 2-mediated activation of beta-catenin signaling has a critical role in warfarin-induced vascular calcification. Arterioscler Thromb Vasc Biol. 2012;32:123–130. doi: 10.1161/ATVBAHA.111.237834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Faverman L, Mikhaylova L, Malmquist J, Nurminskaya M. Extracellular transglutaminase 2 activates beta-catenin signaling in calcifying vascular smooth muscle cells. FEBS Lett. 2008;582:1552–1557. doi: 10.1016/j.febslet.2008.03.053. [DOI] [PubMed] [Google Scholar]
- 137.Cheng SL, Ramachandran B, Behrmann A, Shao JS, Mead M, Smith C, Krchma K, Bello Arredondo Y, Kovacs A, Kapoor K, Brill LM, Perera R, Williams BO, Towler DA. Vascular smooth muscle lrp6 limits arteriosclerotic calcification in diabetic ldlr−/− mice by restraining noncanonical wnt signals. Circ Res. 2015;117:142–156. doi: 10.1161/CIRCRESAHA.117.306712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kaesler N, Immendorf S, Ouyang C, Herfs M, Drummen N, Carmeliet P, Vermeer C, Floege J, Kruger T, Schlieper G. Gas6 protein: Its role in cardiovascular calcification. BMC Nephrol. 2016;17:52. doi: 10.1186/s12882-016-0265-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Collett G, Wood A, Alexander MY, Varnum BC, Boot-Handford RP, Ohanian V, Ohanian J, Fridell YW, Canfield AE. Receptor tyrosine kinase axl modulates the osteogenic differentiation of pericytes. Circ Res. 2003;92:1123–1129. doi: 10.1161/01.RES.0000074881.56564.46. [DOI] [PubMed] [Google Scholar]
- 140.Stanhope KL, Bremer AA, Medici V, Nakajima K, Ito Y, Nakano T, Chen G, Fong TH, Lee V, Menorca RI, Keim NL, Havel PJ. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, ldl-cholesterol, and apolipoprotein-b in young men and women. J Clin Endocrinol Metab. 2011;96:E1596–1605. doi: 10.1210/jc.2011-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Steinberg D. In celebration of the 100th anniversary of the lipid hypothesis of atherosclerosis. J Lipid Res. 2013;54:2946–2949. doi: 10.1194/jlr.R043414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Elmariah S, Delaney JA, O’Brien KD, Budoff MJ, Vogel-Claussen J, Fuster V, Kronmal RA, Halperin JL. Bisphosphonate use and prevalence of valvular and vascular calcification in women mesa (the multi-ethnic study of atherosclerosis) J Am Coll Cardiol. 2010;56:1752–1759. doi: 10.1016/j.jacc.2010.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sekercioglu N, Thabane L, Diaz Martinez JP, Nesrallah G, Longo CJ, Busse JW, Akhtar-Danesh N, Agarwal A, Al-Khalifah R, Iorio A, Guyatt GH. Comparative effectiveness of phosphate binders in patients with chronic kidney disease: A systematic review and network meta-analysis. PLoS One. 2016;11:e0156891. doi: 10.1371/journal.pone.0156891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438–441. doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 145.Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, Raggi P. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68:1815–1824. doi: 10.1111/j.1523-1755.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 146.Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR, Tuzcu EM, Nissen SE. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65:1273–1282. doi: 10.1016/j.jacc.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 147.Towler DA. Inorganic pyrophosphate: A paracrine regulator of vascular calcification and smooth muscle phenotype. Arterioscler Thromb Vasc Biol. 2005;25:651–654. doi: 10.1161/01.ATV.0000158943.79580.9d. [DOI] [PubMed] [Google Scholar]
- 148.Rutsch F, Boyer P, Nitschke Y, et al. Hypophosphatemia, hyperphosphaturia, and bisphosphonate treatment are associated with survival beyond infancy in generalized arterial calcification of infancy. Circ Cardiovasc Genet. 2008;1:133–140. doi: 10.1161/CIRCGENETICS.108.797704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Staal A, Frith JC, French MH, Swartz J, Gungor T, Harrity TW, Tamasi J, Rogers MJ, Feyen JH. The ability of statins to inhibit bone resorption is directly related to their inhibitory effect on hmg-coa reductase activity. J Bone Miner Res. 2003;18:88–96. doi: 10.1359/jbmr.2003.18.1.88. [DOI] [PubMed] [Google Scholar]
- 150.Lin CP, Huang PH, Lai CF, Chen JW, Lin SJ, Chen JS. Simvastatin attenuates oxidative stress, nf-kappab activation, and artery calcification in ldlr−/− mice fed with high fat diet via down-regulation of tumor necrosis factor-alpha and tnf receptor 1. PLoS One. 2015;10:e0143686. doi: 10.1371/journal.pone.0143686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li Y, Fuchimoto D, Sudo M, et al. Development of human-like advanced coronary plaques in low-density lipoprotein receptor knockout pigs and justification for statin treatment before formation of atherosclerotic plaques. J Am Heart Assoc. 2016;5:e002779. doi: 10.1161/JAHA.115.002779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Toussaint ND, Lau KK, Strauss BJ, Polkinghorne KR, Kerr PG. Effect of alendronate on vascular calcification in ckd stages 3 and 4: A pilot randomized controlled trial. Am J Kidney Dis. 2010;56:57–68. doi: 10.1053/j.ajkd.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 153.Xiao Q, Murphy RA, Houston DK, Harris TB, Chow WH, Park Y. Dietary and supplemental calcium intake and cardiovascular disease mortality: The national institutes of health-aarp diet and health study. JAMA Intern Med. 2013;173:639–646. doi: 10.1001/jamainternmed.2013.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.St Hilaire C, Ziegler SG, Markello TC, et al. Nt5e mutations and arterial calcifications. N Engl J Med. 2011;364:432–442. doi: 10.1056/NEJMoa0912923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O’Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM. Calcific aortic valve disease: Not simply a degenerative process: A review and agenda for research from the national heart and lung and blood institute aortic stenosis working group. Executive summary: Calcific aortic valve disease-2011 update. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yutzey KE, Demer LL, Body SC, Huggins GS, Towler DA, Giachelli CM, Hofmann-Bowman MA, Mortlock DP, Rogers MB, Sadeghi MM, Aikawa E. Calcific aortic valve disease: A consensus summary from the alliance of investigators on calcific aortic valve disease. Arterioscler Thromb Vasc Biol. 2014;34:2387–2393. doi: 10.1161/ATVBAHA.114.302523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Geijselaers SL, Sep SJ, Schram MT, van Boxtel MP, van Sloten TT, Henry RM, Reesink KD, Kroon AA, Koster A, Schaper NC, Dagnelie PC, van der Kallen CJ, Biessels GJ, Stehouwer CD. Carotid stiffness is associated with impairment of cognitive performance in individuals with and without type 2 diabetes. The maastricht study. Atherosclerosis. 2016;253:186–193. doi: 10.1016/j.atherosclerosis.2016.07.912. [DOI] [PubMed] [Google Scholar]
- 158.Coutinho T, Turner ST, Kullo IJ. Aortic pulse wave velocity is associated with measures of subclinical target organ damage. JACC Cardiovasc Imaging. 2011;4:754–761. doi: 10.1016/j.jcmg.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tsao CW, Himali JJ, Beiser AS, Larson MG, DeCarli C, Vasan RS, Mitchell GF, Seshadri S. Association of arterial stiffness with progression of subclinical brain and cognitive disease. Neurology. 2016;86:619–626. doi: 10.1212/WNL.0000000000002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tyson KL, Reynolds JL, McNair R, Zhang Q, Weissberg PL, Shanahan CM. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol. 2003;23:489–494. doi: 10.1161/01.ATV.0000059406.92165.31. [DOI] [PubMed] [Google Scholar]
- 161.Koleganova N, Piecha G, Ritz E, Schirmacher P, Muller A, Meyer HP, Gross ML. Arterial calcification in patients with chronic kidney disease. Nephrol Dial Transplant. 2009;24:2488–2496. doi: 10.1093/ndt/gfp137. [DOI] [PubMed] [Google Scholar]
- 162.Laurila PP, Soronen J, Kooijman S, et al. Usf1 deficiency activates brown adipose tissue and improves cardiometabolic health. Sci Transl Med. 2016;8:323ra313. doi: 10.1126/scitranslmed.aad0015. [DOI] [PubMed] [Google Scholar]
- 163.Breyne J, Juthier F, Corseaux D, Marechaux S, Zawadzki C, Jeanpierre E, Ung A, Ennezat PV, Susen S, Van Belle E, Le Marec H, Vincentelli A, Le Tourneau T, Jude B. Atherosclerotic-like process in aortic stenosis: Activation of the tissue factor-thrombin pathway and potential role through osteopontin alteration. Atherosclerosis. 2010;213:369–376. doi: 10.1016/j.atherosclerosis.2010.07.047. [DOI] [PubMed] [Google Scholar]
- 164.Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, Lifton RP. Lrp6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jamal SA, Vandermeer B, Raggi P, Mendelssohn DC, Chatterley T, Dorgan M, Lok CE, Fitchett D, Tsuyuki RT. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: An updated systematic review and meta-analysis. Lancet. 2013;382:1268–1277. doi: 10.1016/S0140-6736(13)60897-1. [DOI] [PubMed] [Google Scholar]
- 166.Vlassara H, Uribarri J, Cai W, Goodman S, Pyzik R, Post J, Grosjean F, Woodward M, Striker GE. Effects of sevelamer on hba1c, inflammation, and advanced glycation end products in diabetic kidney disease. Clin J Am Soc Nephrol. 2012;7:934–942. doi: 10.2215/CJN.12891211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Cholesterol Recurrent Events Trial I. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 168.Wang S, Song K, Srivastava R, Fathzadeh M, Li N, Mani A. The protective effect of transcription factor 7-like 2 risk allele rs7903146 against elevated fasting plasma triglyceride in type 2 diabetes: A meta-analysis. J Diabetes Res. 2015;2015:468627. doi: 10.1155/2015/468627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta. 2014;1842:446–462. doi: 10.1016/j.bbadis.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Leung SS, Forbes JM, Borg DJ. Receptor for advanced glycation end products (rage) in type 1 diabetes pathogenesis. Curr Diab Rep. 2016;16:100. doi: 10.1007/s11892-016-0782-y. [DOI] [PubMed] [Google Scholar]
- 171.Malmstedt J, Karvestedt L, Swedenborg J, Brismar K. The receptor for advanced glycation end products and risk of peripheral arterial disease, amputation or death in type 2 diabetes: A population-based cohort study. Cardiovasc Diabetol. 2015;14:93. doi: 10.1186/s12933-015-0257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dong N, Shi H, Xu B, Cai Y. Increased plasma s100a12 levels are associated with diabetic retinopathy and prognostic biomarkers of macrovascular events in type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2015;56:4177–4185. doi: 10.1167/iovs.15-16470. [DOI] [PubMed] [Google Scholar]
- 173.Liu X, Wang Y, Ming Y, Song Y, Zhang J, Chen X, Zeng M, Mao Y. S100a9: A potential biomarker for the progression of non-alcoholic fatty liver disease and the diagnosis of non-alcoholic steatohepatitis. PLoS One. 2015;10:e0127352. doi: 10.1371/journal.pone.0127352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bornfeldt KE. 2013 russell ross memorial lecture in vascular biology: Cellular and molecular mechanisms of diabetes mellitus-accelerated atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:705–714. doi: 10.1161/ATVBAHA.113.301928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hofmann Bowman MA, McNally EM. Genetic pathways of vascular calcification. Trends Cardiovasc Med. 2012;22:93–98. doi: 10.1016/j.tcm.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tintut Y, Patel J, Parhami F, Demer LL. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the camp pathway. Circulation. 2000;102:2636–2642. doi: 10.1161/01.cir.102.21.2636. [DOI] [PubMed] [Google Scholar]
- 177.Lee HL, Woo KM, Ryoo HM, Baek JH. Tumor necrosis factor-alpha increases alkaline phosphatase expression in vascular smooth muscle cells via msx2 induction. Biochem Biophys Res Commun. 2010;391:1087–1092. doi: 10.1016/j.bbrc.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 178.Patil HR, Patil NT, King SI, O’Keefe E, Chhabra R, Ansari S, Kennedy KF, Dey D, O’Keefe JH, Helzberg JH, Thompson RC. Increased intrathoracic and hepatic visceral adipose tissue independently correlates with coronary artery calcification in asymptomatic patients. J Nucl Cardiol. 2014;21:880–889. doi: 10.1007/s12350-014-9946-9. [DOI] [PubMed] [Google Scholar]
- 179.Tonbul HZ, Turkmen K, Kayikcioglu H, Ozbek O, Kayrak M, Biyik Z. Epicardial adipose tissue and coronary artery calcification in diabetic and nondiabetic end-stage renal disease patients. Ren Fail. 2011;33:770–775. doi: 10.3109/0886022X.2011.599913. [DOI] [PubMed] [Google Scholar]
- 180.Bos D, Shahzad R, van Walsum T, van Vliet LJ, Franco OH, Hofman A, Niessen WJ, Vernooij MW, van der Lugt A. Epicardial fat volume is related to atherosclerotic calcification in multiple vessel beds. Eur Heart J Cardiovasc Imaging. 2015;16:1264–1269. doi: 10.1093/ehjci/jev086. [DOI] [PubMed] [Google Scholar]
- 181.Fox CS, Massaro JM, Schlett CL, Lehman SJ, Meigs JB, O’Donnell CJ, Hoffmann U, Murabito JM. Periaortic fat deposition is associated with peripheral arterial disease: The framingham heart study. Circ Cardiovasc Imaging. 2010;3:515–519. doi: 10.1161/CIRCIMAGING.110.958884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lehman SJ, Massaro JM, Schlett CL, O’Donnell CJ, Hoffmann U, Fox CS. Peri-aortic fat, cardiovascular disease risk factors, and aortic calcification: The framingham heart study. Atherosclerosis. 2010;210:656–661. doi: 10.1016/j.atherosclerosis.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Osawa K, Miyoshi T, Yamauchi K, Koyama Y, Nakamura K, Sato S, Kanazawa S, Ito H. Nonalcoholic hepatic steatosis is a strong predictor of high-risk coronary-artery plaques as determined by multidetector ct. PLoS One. 2015;10:e0131138. doi: 10.1371/journal.pone.0131138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mantovani A, Pernigo M, Bergamini C, Bonapace S, Lipari P, Valbusa F, Bertolini L, Zenari L, Pichiri I, Dauriz M, Zoppini G, Barbieri E, Byrne CD, Bonora E, Targher G. Heart valve calcification in patients with type 2 diabetes and nonalcoholic fatty liver disease. Metabolism. 2015;64:879–887. doi: 10.1016/j.metabol.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 185.Fargion S, Porzio M, Fracanzani AL. Nonalcoholic fatty liver disease and vascular disease: State-of-the-art. World J Gastroenterol. 2014;20:13306–13324. doi: 10.3748/wjg.v20.i37.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fracanzani AL, Tiraboschi S, Pisano G, Consonni D, Baragetti A, Bertelli C, Norata D, Valenti L, Grigore L, Porzio M, Catapano A, Fargion S. Progression of carotid vascular damage and cardiovascular events in non-alcoholic fatty liver disease patients compared to the general population during 10 years of follow-up. Atherosclerosis. 2016;246:208–213. doi: 10.1016/j.atherosclerosis.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 187.Wong ND, Nelson JC, Granston T, Bertoni AG, Blumenthal RS, Carr JJ, Guerci A, Jacobs DR, Jr, Kronmal R, Liu K, Saad M, Selvin E, Tracy R, Detrano R. Metabolic syndrome, diabetes, and incidence and progression of coronary calcium: The multiethnic study of atherosclerosis study. JACC Cardiovasc Imaging. 2012;5:358–366. doi: 10.1016/j.jcmg.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Blaha MJ, DeFilippis AP, Rivera JJ, Budoff MJ, Blankstein R, Agatston A, Szklo M, Lakoski SG, Bertoni AG, Kronmal RA, Blumenthal RS, Nasir K. The relationship between insulin resistance and incidence and progression of coronary artery calcification: The multi-ethnic study of atherosclerosis (mesa) Diabetes Care. 2011;34:749–751. doi: 10.2337/dc10-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Katz R, Budoff MJ, Takasu J, Shavelle DM, Bertoni A, Blumenthal RS, Ouyang P, Wong ND, O’Brien KD. Relationship of metabolic syndrome with incident aortic valve calcium and aortic valve calcium progression: The multi-ethnic study of atherosclerosis (mesa) Diabetes. 2009;58:813–819. doi: 10.2337/db08-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Apro J, Tietge UJ, Dikkers A, Parini P, Angelin B, Rudling M. Impaired cholesterol efflux capacity of high-density lipoprotein isolated from interstitial fluid in type 2 diabetes mellitus-brief report. Arterioscler Thromb Vasc Biol. 2016;36:787–791. doi: 10.1161/ATVBAHA.116.307385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Prenner SB, Mulvey CK, Ferguson JF, Rickels MR, Bhatt AB, Reilly MP. Very low density lipoprotein cholesterol associates with coronary artery calcification in type 2 diabetes beyond circulating levels of triglycerides. Atherosclerosis. 2014;236:244–250. doi: 10.1016/j.atherosclerosis.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Greenwald SE. Ageing of the conduit arteries. J Pathol. 2007;211:157–172. doi: 10.1002/path.2268. [DOI] [PubMed] [Google Scholar]
- 193.Westerhof N, Lankhaar JW, Westerhof BE. The arterial windkessel. Med Biol Eng Comput. 2009;47:131–141. doi: 10.1007/s11517-008-0359-2. [DOI] [PubMed] [Google Scholar]
- 194.Hatsuda S, Shoji T, Shinohara K, Kimoto E, Mori K, Fukumoto S, Koyama H, Emoto M, Nishizawa Y. Regional arterial stiffness associated with ischemic heart disease in type 2 diabetes mellitus. J Atheroscler Thromb. 2006;13:114–121. doi: 10.5551/jat.13.114. [DOI] [PubMed] [Google Scholar]
- 195.Kubozono T, Ohishi M. Prognostic significance of regional arterial stiffness for stroke in hypertension. Pulse (Basel) 2015;3:98–105. doi: 10.1159/000381795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Doyon M, Mathieu P, Moreau P. Decreased expression of gamma-carboxylase in diabetes-associated arterial stiffness: Impact on matrix gla protein. Cardiovasc Res. 2013;97:331–338. doi: 10.1093/cvr/cvs325. [DOI] [PubMed] [Google Scholar]
- 197.Zhou C, Pridgen B, King N, Xu J, Breslow JL. Hyperglycemic ins2akitaldlr(−)/(−) mice show severely elevated lipid levels and increased atherosclerosis: A model of type 1 diabetic macrovascular disease. J Lipid Res. 2011;52:1483–1493. doi: 10.1194/jlr.M014092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Willecke F, Yuan C, Oka K, Chan L, Hu Y, Barnhart S, Bornfeldt KE, Goldberg IJ, Fisher EA. Effects of high fat feeding and diabetes on regression of atherosclerosis induced by low-density lipoprotein receptor gene therapy in ldl receptor-deficient mice. PLoS One. 2015;10:e0128996. doi: 10.1371/journal.pone.0128996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Goldberg IJ, Hu Y, Noh HL, Wei J, Huggins LA, Rackmill MG, Hamai H, Reid BN, Blaner WS, Huang LS. Decreased lipoprotein clearance is responsible for increased cholesterol in ldl receptor knockout mice with streptozotocin-induced diabetes. Diabetes. 2008;57:1674–1682. doi: 10.2337/db08-0083. [DOI] [PubMed] [Google Scholar]
- 200.Costache G, Popov D, Georgescu A, Cenuse M, Jinga VV, Simionescu M. The effects of simultaneous hyperlipemia-hyperglycemia on the resistance arteries, myocardium and kidney glomeruli. J Submicrosc Cytol Pathol. 2000;32:47–58. [PubMed] [Google Scholar]
- 201.Simionescu M, Popov D, Sima A, Hasu M, Costache G, Faitar S, Vulpanovici A, Stancu C, Stern D, Simionescu N. Pathobiochemistry of combined diabetes and atherosclerosis studied on a novel animal model. The hyperlipemic-hyperglycemic hamster. Am J Pathol. 1996;148:997–1014. [PMC free article] [PubMed] [Google Scholar]
- 202.Shao JS, Sierra OL, Cohen R, Mecham RP, Kovacs A, Wang J, Distelhorst K, Behrmann A, Halstead LR, Towler DA. Vascular calcification and aortic fibrosis: A bifunctional role for osteopontin in diabetic arteriosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:1821–1833. doi: 10.1161/ATVBAHA.111.230011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Davies MR, Lund RJ, Hruska KA. Bmp-7 is an efficacious treatment of vascular calcification in a murine model of atherosclerosis and chronic renal failure. J Am Soc Nephrol. 2003;14:1559–1567. doi: 10.1097/01.asn.0000068404.57780.dd. [DOI] [PubMed] [Google Scholar]
- 204.Dai Y, Palade P, Wang X, Mercanti F, Ding Z, Dai D, Mehta JL. High fat diet causes renal fibrosis in ldlr-null mice through mapk-nf-kappab pathway mediated by ox-ldl. J Cardiovasc Pharmacol. 2014;63:158–166. doi: 10.1097/FJC.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 205.Awan Z, Denis M, Bailey D, Giaid A, Prat A, Goltzman D, Seidah NG, Genest J. The ldlr deficient mouse as a model for aortic calcification and quantification by micro-computed tomography. Atherosclerosis. 2011;219:455–462. doi: 10.1016/j.atherosclerosis.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 206.Srivastava R, Zhang J, Go GW, Narayanan A, Nottoli TP, Mani A. Impaired lrp6-tcf7l2 activity enhances smooth muscle cell plasticity and causes coronary artery disease. Cell Rep. 2015;13:746–759. doi: 10.1016/j.celrep.2015.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.de Munter W, Blom AB, Helsen MM, Walgreen B, van der Kraan PM, Joosten LA, van den Berg WB, van Lent PL. Cholesterol accumulation caused by low density lipoprotein receptor deficiency or a cholesterol-rich diet results in ectopic bone formation during experimental osteoarthritis. Arthritis Res Ther. 2013;15:R178. doi: 10.1186/ar4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Neeb ZP, Edwards JM, Alloosh M, Long X, Mokelke EA, Sturek M. Metabolic syndrome and coronary artery disease in ossabaw compared with yucatan swine. Comp Med. 2010;60:300–315. [PMC free article] [PubMed] [Google Scholar]
- 209.Hill Gallant KM, Weaver CM, Towler DA, Thuppal SV, Bailey RL. Nutrition in cardioskeletal health. Adv Nutr. 2016;7:544–555. doi: 10.3945/an.115.011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.McKenney-Drake ML, Territo PR, Salavati A, Houshmand S, Persohn S, Liang Y, Alloosh M, Moe SM, Weaver CM, Alavi A, Sturek M. (18)f-naf pet imaging of early coronary artery calcification. JACC Cardiovasc Imaging. 2016;9:627–628. doi: 10.1016/j.jcmg.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 211.McKenney ML, Schultz KA, Boyd JH, Byrd JP, Alloosh M, Teague SD, Arce-Esquivel AA, Fain JN, Laughlin MH, Sacks HS, Sturek M. Epicardial adipose excision slows the progression of porcine coronary atherosclerosis. J Cardiothorac Surg. 2014;9:2. doi: 10.1186/1749-8090-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ning B, Wang X, Yu Y, Waqar AB, Yu Q, Koike T, Shiomi M, Liu E, Wang Y, Fan J. High-fructose and high-fat diet-induced insulin resistance enhances atherosclerosis in watanabe heritable hyperlipidemic rabbits. Nutr Metab (Lond) 2015;12:30. doi: 10.1186/s12986-015-0024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


