Abstract
Purpose
National Comprehensive Cancer Network guidelines recommend 18F-FDG-PET/CT, in addition to standard staging procedures, for systemic staging of newly diagnosed stage III breast cancer patients. However factors in addition to stage may influence PET/CT utility. As breast cancers that are negative for estrogen receptor, progesterone receptor, and human epidermal growth factor receptor (triple-negative breast cancer, or TNBC) are more aggressive and metastasize earlier than other breast cancers, we hypothesized that receptor expression may be one such factor. This study assesses 18F-FDG-PET/CT for systemic staging of newly diagnosed TNBC.
Methods
In this Institutional Review Board-approved retrospective study, our Healthcare Information System was screened for patients with TNBC who underwent 18F-FDG-PET/CT in 2007–2013 prior to systemic or radiation therapy. Initial stage was determined from mammography, ultrasound, magnetic resonance imaging, and/or surgery, if performed prior to 18F-FDG-PET/CT. 18F-FDG-PET/CT was evaluated to identify unsuspected extra-axillary regional nodal and distant metastases, as well as unsuspected synchronous malignancies. Kaplan Meier survival estimates were calculated for initial stage IIB patients stratified by whether or not stage 4 disease was detected by 18F-FDG-PET/CT.
Results
A total of 232 patients with TNBC met inclusion criteria. 18F-FDG-PET/CT revealed unsuspected distant metastases in 30 (13%): 0/23 initial stage I, 4/82 (5%) stage IIA, 13/87 (15%) stage IIB, 4/23 (17%) stage IIIA, 8/14 (57%) stage IIIB, and 1/3 (33%) stage IIIC. Twenty six of 30 patients upstaged to IV by 18F-FDG-PET/CT were confirmed by pathology, with the remaining 4 confirmed by follow-up imaging. In addition, 7 unsuspected synchronous malignancies were identified in 6 patients. Initial stage 2B patients who were upstaged to 4 by 18F-FDG-PET/CT had significantly shorter survival compared to initial stage 2B patients who were not (3 year Kaplan Meier estimate 0.33, 95% CI 0.13–0.55 versus 0.97, CI 0.76–0.93, p<.0001).
Conclusion
18F-FDG-PET/CT revealed distant metastases in 15% of patients with stage IIB TNBC. Stage IIB patients upstaged to 4 by 18F-FDG-PET/CT had significantly shorter survival than those who were not, consistent with 18F-FDG-PET/CT detecting an increased burden of disease. This study provides further evidence that populations of patients with stage IIB breast cancer, such as TNBC, should be considered for systemic staging with 18F-FDG-PET/CT at the time of initial diagnosis.
Keywords: estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2, triple negative, breast cancer, 18F-FDG PET/CT
INTRODUCTION
Fluorine-18 Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography (18F-FDG-PET/CT) is useful for systemic staging of selected patients with newly diagnosed breast cancer, as the detection of unsuspected extra-axillary regional nodal metastases and distant metastases affects staging, treatment, and prognosis [1]. The question of which patients will benefit from systemic staging with 18F-FDG-PET/CT is a matter of active debate [2–5]. The 2015 National Comprehensive Cancer Network (NCCN) guidelines recommend systemic staging with 18F-FDG-PET/CT, in addition to standard staging procedures, for patients with stage III breast cancer [6]. However, additional factors besides initial clinical stage may be important when determining whether a staging 18F-FDG-PET/CT is clinically indicated. Tumor histology may be one such factor, given evidence that 18F-FDG-PET/CT has a higher yield in patients with invasive ductal carcinomas (IDC) than in patients with invasive lobular carcinomas (ILC) [7, 8]. Patient age may be another factor, as younger patients with breast cancer have more aggressive malignancies [9, 10], and 18F-FDG-PET/CT demonstrates a high yield for unsuspected distant metastases in younger patients [11].
Another potential factor for determining whether staging 18F-FDG-PET/CT may be of value is the receptor phenotype of the primary breast malignancy. Molecular genetic classifications of breast cancer distinguish subclasses of breast cancer [12–15]. While full molecular genetic classifications are still unlikely for most breast cancer patients, surrogate categorizations based on immunohistochemical receptor phenotypes is clinically standard [16, 17]. Expression of estrogen receptor (ER) and/or progesterone receptor (PR) in breast cancer cells predicts response to hormonal therapies. ER/PR positive breast cancers tend to be lower grade than comparable ER negative tumors and have a relatively better prognosis [18]. Overexpression of human epidermal growth factor 2 (HER2) in breast cancer cells is associated with aggressive biology and poorer prognosis [19]; although, HER2 targeted antibody therapies have prolonged survival in both the metastatic and adjuvant settings [20–22]. Triple-negative breast cancers (TNBC) are a heterogenous group of breast cancers that do not express ER, PR, or HER2. Approximately 15% of breast cancers are classified as triple-negative, and these tumors are very aggressive, with early metastases and poor prognosis [23, 24]. The time from diagnosis to metastasis in TNBC is very short (7–12 months) compared to other breast cancers [23], and the most dramatic difference in survival between patients with TNBC and other breast cancers is seen within two years of diagnosis [24]. Given the propensity of TNBC for early metastases, we hypothesized that systemic staging with 18F-FDG-PET/CT may be valuable in breast cancers with a triple-negative receptor phenotype earlier than clinical stage III. In this retrospective study, we evaluate the impact of systemic staging with 18F-FDG-PET/CT in patients with clinical stage I-III TNBC.
MATERIALS AND METHODS
Study Design and Patients
This retrospective single-institution study was performed in compliance with the Health Insurance Portability and Accountability Act (HIPAA) and with Institutional Review Board (IRB) approval. The requirement to obtain informed consent was waived. The Memorial Sloan Kettering Cancer Center Healthcare Information System (HIS) was screened for patients with stage I to IIIC TNBC who underwent 18F-FDG-PET/CT between January 2007 and December 2013 prior to beginning treatment with chemotherapy, hormonal therapy, or radiation. Electronic medical records (EMR) were reviewed and patients with the following characteristics were excluded: Known stage IV disease for the current malignancy prior to 18F-FDG-PET/CT, symptoms suspicious for metastatic disease, prior or concurrent malignancies (except non-melanoma skin cancer), systemic therapy or radiation prior to 18F-FDG-PET/CT. Surgical management of the primary breast lesion and axillary nodes was allowed. Age at diagnosis, race, and time of follow up and final survival status (alive, deceased) were recorded for each patient. Histology and grade were recorded for each tumor.
Determination of Initial Stage
Initial stages were determined according to the American Joint Committee on Cancer (AJCC) Staging Manual [25]. Initial clinical stage was determined from physical exam, mammography, breast ultrasound, and, if available, breast magnetic resonance imaging (mri) and/or surgical findings.
PET/CT Imaging and Interpretation
All patients in this retrospective study had a staging 18F-FDG-PET/CT. Prior to 18F-FDG injection for PET/CT, patients fasted for at least six hours. Each patient was injected intravenously with 444–555 MBq of 18F-FDG when plasma glucose was less than 200 mg/dL. After 18F-FDG injection patients rested for a scheduled 60-minute uptake period followed by image acquisition on one of several GE Healthcare Discovery PET/CT systems. PET/CT scans were acquired supine from the mid-skull to the mid-thigh. In most cases, low-dose CT scans with oral contrast were obtained. Occasionally, intravenous contrast was administered. In all cases, attenuation-corrected images were reviewed on a picture-archiving and communication system workstation (PACS, GE Healthcare), displaying a maximum-intensity-projection image and multiplanar PET, CT, and PET/CT fusion images. According to standard 18F-FDG-PET/CT reporting, uptake was considered abnormal when it was focal, was not considered physiologic or inflammatory, and had intensity greater than the local background. Suspicion for malignancy was based on the integration of metabolic information from the PET images, anatomic information from the CT images, and the fused PET/CT images. PET/CT studies were reinterpreted by a radiologist (G.U.) dually boarded in diagnostic radiology and nuclear medicine with nine years of PET/CT experience, blinded to the original PET/CT report and the results of other imaging modalities. Unsuspected local extra-axillary nodal metastases (internal mammary and supraclavicular) and distant metastases were recorded and if they were noted on imaging, a new stage was assigned.
Verification of Metastases
Histology was the preferred method to verify malignancy for 18F-FDG-PET/CT findings. When histology was not available, follow-up imaging was used. Lesions had to show typical features of metastatic disease on initial imaging and show progression or response to treatment on follow-up imaging.
Statistical Analysis
Medians and ranges were used to summarize continuous variables and frequencies, and percentages were used to summarize categorical variables, including distant metastases and upstaging. Race was grouped into African American, Asian, Caucasian, and other. The relationships between patient characteristics and upstaging were assessed using Fisher’s exact test for categorical variables and the Wilcoxon Rank Sum test for continuous variables. Kaplan Meier curves as well as 3 year Kaplan Meier survival estimates were provided for initial stage IIB patients stratified by whether or not stage 4 disease was detected by 18F-FDG-PET/CT, and the log rank test was used to assess the difference in survival.
P-values less than 0.05 were considered statistically significant and all analyses were performed using SAS 9.4 (The SAS Institute, Cary, NC).
RESULTS
Patients with TNBC
A search of the Memorial Sloan Kettering Cancer Center Healthcare Information System identified 649 patients with newly diagnosed stage I-IIIC TNBC from January 2007 to December 2013. From these, 351 were excluded due to systemic or radiation therapy prior to 18F-FDG-PET/CT and 66 were excluded for synchronous or prior malignancies. Thus, the final cohort was composed of 232 patients with stage I to IIIC TNBC who underwent 18F-FDG-PET/CT prior to systemic therapy or radiation between 2007 and 2013 and met all eligibility criteria. The median age of this cohort of patients with TNBC was 51 years (range 25–93 years). Before 18F-FDG-PET/CT imaging, 23 (10%) patients were stage I, 82 (35%) were stage IIA, 87 (38%) were stage IIB, 23 (10%) were stage IIIA, 14 (6%) were stage IIIB, and 3 (1%) were stage IIIC. The vast majority of TNBC was of invasive ductal histology and was high-grade. Patient characteristics are summarized in Table 1.
TABLE 1.
Characteristics of patients and tumors with triple negative breast cancer (TNBC)
| N | % | ||
|---|---|---|---|
| Total patients with TNBC | 23 | 100 | |
| Stage prior to PET/CT* | |||
| I | 23 | 10 | |
| IIA | 82 | 35 | |
| IIB | 87 | 38 | |
| IIIA | 23 | 10 | |
| IIIB | 14 | 6 | |
| IIIC | 3 | 1 | |
| 100 | |||
| Race | |||
| Caucasian | 153 | 66 | |
| African American | 49 | 21 | |
| Asian | 21 | 9 | |
| Other/Unknown | 9 | 4 | |
| 100 | |||
| Histology | |||
| IDC | 217 | 94 | |
| ILC | 2 | 1 | |
| Mixed IDC/ILC | 5 | 2 | |
| Other | 8 | 3 | |
| 100 | |||
| Tumor Grade | |||
| High | 217 | 94 | |
| Intermediate | 8 | 3 | |
| Unknown | 7 | 3 | |
| 100 | |||
IDC = invasive ductal cancer; ILC = invasive lobular cancer.
Clinical classification according to the seventh edition of the American Joint Committee on Cancer Staging Manual [25].
Upstaging by 18F-FDG-PET/CT
Distant metastases
18F-FDG-PET/CT demonstrated unsuspected metastases upstaging to stage IV in 30 of 232 patients with TNBC (Figures. 1, 2, 3). 18F-FDG-PET/CT demonstrated metastatic disease in the bone in eleven, liver in eight, distant nodes in eight, lung in seven, and pleura in one patient. Five patients had metastatic disease involving more than one organ site. In 26 of 30 patients, metastases were confirmed by pathology. In four patients (one liver, one bone, two distant nodes), metastases were confirmed by follow-up imaging. Stratified by initial clinical stage, 18F-FDG-PET/CT demonstrated metastases in 0/23 (0%) initial stage I, 4/82 (5%) stage IIA, 13/87 (15%) stage IIB, 4/23 (17%) stage IIIA, 8/14 (57%) stage IIIB, and 1/3 (33%) stage IIIC patients with TNBC (Table 2).
FIGURE 1.
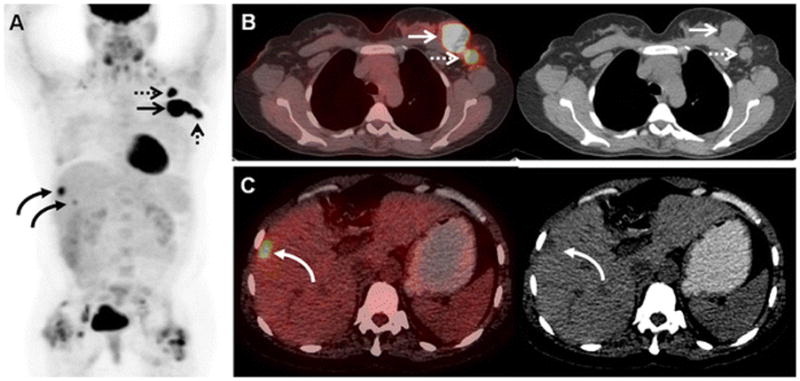
43-year-old woman with initial stage IIB TNBC upstaged to stage IV on 18F-FDG-PET/CT. (A) 18F-FDG MIP image demonstrates the previously known primary left breast malignancy (arrow), the previously known left axillary nodal metastases (dashed arrows), and 18F-FDG -avid foci overlying the liver (curved arrows). Avidity adjacent to both hip joints was benign and related to bilateral hip prostheses. (B) Axial fused and CT image on soft tissue window demonstrate the previously known primary left breast malignancy (arrows) and the previously known left axillary nodal metastases (dashed arrows). (C) Axial fused and CT image on liver window localize an 18F-FDG-avid focus to a low attenuation liver lesion (curved arrows), subsequently biopsy-proven to be a liver metastasis.
FIGURE 2.
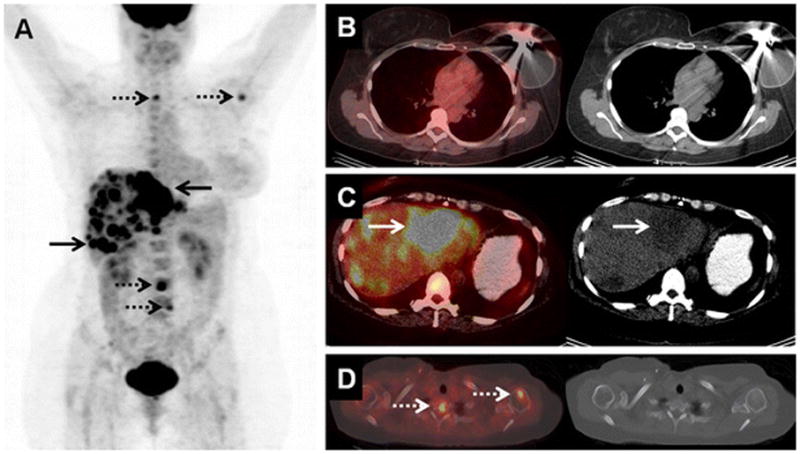
43-year-old woman with initial stage IIA TNBC upstaged to stage IV on 18F-FDG-PET/CT. (A) 18F-FDG MIP image demonstrates the 18F-FDG-avidity overlying the liver (arrows) and osseous structures (dashed arrows). (B) Axial fused and CT image on soft tissue window demonstrate the left-sided tissue expander following left mastectomy six weeks prior to PET/CT. (C) Axial fused and CT image on liver window localize the 18F-FDG-avid foci to low attenuation liver lesions (arrows), subsequently biopsy-proven to be liver metastases. (D) Axial fused and CT image on bone window localize additional 18F-FDG-avid foci to the spine and left humerus, without clear CT correlates. These were presumed to be osseous metastases, although no osseous biopsy was performed.
FIGURE 3.
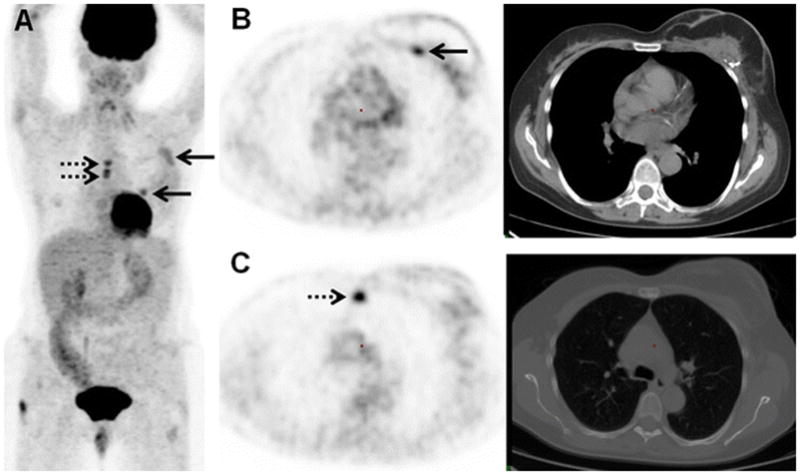
61-year-old woman with initial stage IIB TNBC upstaged to stage IV on 18F-FDG-PET/CT. (A) 18F-FDG MIP image demonstrates the 18F-FDG-avidity overlying the breast breast surgical bed (arrows) and midline chest (dashes arrows). (B) Axial PET and axial CT image on soft tissue window demonstrate the FDG-avid post-surgical changes association with mastectomy and reconstruction 2 weeks before 18F-FDG-PET/CT (arrow). (C) Axial PET and CT image on bone window localize 18F-FDG-avid foci to the sternum without clear CT correlate (dashes arrow), subsequently biopsy-proven to be an osseous metastasis.
TABLE 2.
Summary of patients upstaged by PET/CT categorized by initial clinical stage
| Post PET/CT Stage | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Initial Clinical Stage | Total | I | IIA | IIB | IIIA | IIIB | IIIC | IV (%) |
| I | 23 | 23 | 0 (0) | |||||
| IIA | 82 | 73 | 1 | 4 | 4 (5) | |||
| IIB | 87 | 67 | 1 | 6 | 13 (15) | |||
| IIIA | 23 | 18 | 1 | 4 (17) | ||||
| IIIB | 14 | 6 | 8 (57) | |||||
| IIIC | 3 | 2 | 1 (33) | |||||
| Total: | 232 | 23 | 73 | 68 | 19 | 6 | 13 | 30 (13) |
There were no proven false positives in this study, although only 26 of 30 patients demonstrating unsuspected distant metastases on 18F-FDG-PET/CT were confirmed by pathology. Patients in this study were not down-staged by PET/CT results.
Regional nodal metastases
Thirteen patients were upstaged by the identification of unsuspected local nodal metastases (Figure 4). Three of these nodal lesions were confirmed by pathology and the remainder by follow-up imaging.
FIGURE 4.
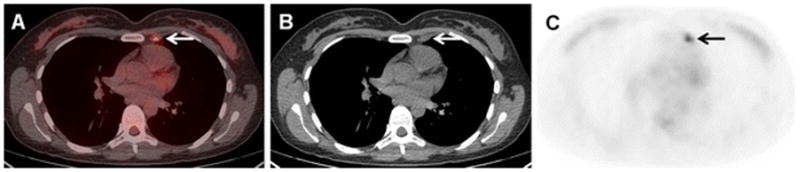
40-year-old woman with initial stage IIB TNBC upstaged to stage IIIA on 18F-FDG-PET/CT. (A) Axial fused, (B) axial CT, and (C) axial 18F-FDG-PET images demonstrate a previously unknown left internal mammary node, subsequently biopsy-proven to be a nodal metastasis. This increased the tumor staging from T3N0 to T3N1, and upstaged the malignancy to stage IIIA.
Associations Between Clinical Parameters and Upstaging
No associations were found between patients that were upstaged to stage IV compared to those that were not upstaged to stage IV for age (median 58 years (range: 33–93 years) vs. 50 years (range: 25–90 years), p=0.07), race (Caucasian 79% (22/28) vs. 66% (131/299), p=0.20), histology (IDC 97% (29/30) vs. 93% (188/202) p=0.62), or tumor grade (high grade 93% (27/29) vs. 97% (190/196), p=0.28). Similarly, no associations were found between patients with any upstaging compared to those that without any upstaging and age (median 54 years (range:33–93 years) vs. 51 years (25–87 years), p=0.78), race (Caucasian 73% (30/41) vs. 66% (123/186), p=0.46), histology (IDC 95% (41/43) vs. 93% (176/199) p=0.28), or tumor grade (high grade 93% (39/42) vs. 97% (178/183) p=0.17).
Synchronous Malignancies
18F-FDG-PET/CT demonstrated seven unsuspected synchronous malignancies in six patients, including one non-small cell lung cancer, one renal cell carcinoma of the kidney, one grade 3 astrocytoma, one basal cell skin cancer, and three papillary thyroid cancers. One patient had two unsuspected primary malignancies, a non-small cell lung cancer, and a papillary thyroid cancer. All seven unsuspected synchronous malignancies were proven by pathology.
Survival of initial stage 2B patients stratified by 18F-FDG-PET/CT results
Thirteen of 87 (15%) of initial stage 2B patients were upstaged to stage 4 by results of 18F-FDG-PET/CT. Initially stage 2B patients who were upstaged to stage 4 by 18F-FDG-PET/CT had significantly shorter survival compared to initial stage 2B patients who were not staged to 4 (3 year Kaplan Meier estimate 0.33, 95% CI 0.13–0.55 versus 0.97, CI 0.76–0.93, p<.0001). Kaplan Meier curves for initial stage 2B patients stratified by whether or not stage 4 disease was detected by 18F-FDG-PET/CT is shown in Figure 5.
FIGURE 5.
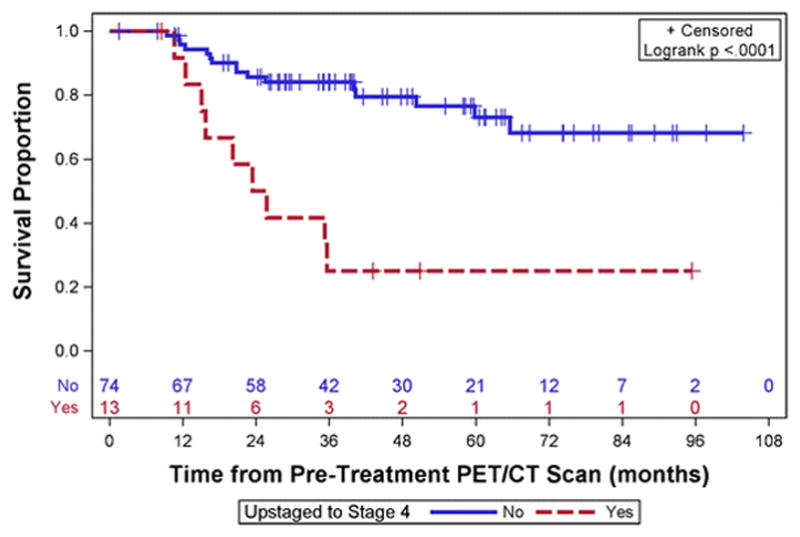
Kaplan Meier curves for initial stage 2B patients stratified by whether or not stage 4 disease was detected by 18F-FDG-PET/CT
DISCUSSION
18F-FDG-PET/CT has proven valuable in the systemic staging of patients with breast cancer, as the detection of unsuspected distant metastases will alter patient management from surgery with or without neoadjuvant systemic therapy to palliative systemic therapy without surgery. The 2015 National Comprehensive Cancer Network guidelines recommend systemic staging with 18F-FDG-PET/CT, in addition to standard staging procedures, for patients stage III breast cancer [6]. However, initial stage may not be the only criterion that affects 18F-FDG-PET/CT yield. TNBC, a highly aggressive subtype of breast cancer, metastasizes early, and may benefit from 18F-FDG-PET/CT at earlier initial stages. This study demonstrated that a substantial percentage (15%) of patients with initial stage IIB TNBC are upstaged to stage IV by 18F-FDG-PET/CT, and provides evidence of value in this patient population.
The survival of initially stage 2B patients who were upstaged to stage 4 by 18F-FDG-PET/CT was significantly shorter than other initially stage 2B patients who were not upstaged to 4. This is consistent with the increased disease burden of stage 4 disease. The more accurate initial staging provided by 18F-FDG-PET/CT will move patients with more advanced disease out of the 2B stage and may make the remaining stage 2B patients look like they have better survival rates than stage 2B patients in the past that did not benefit from 18F-FDG-PET/CT imaging. This apparent improvement in survival due to better initial classification of disease stage has been called stage migration [26].
Of note, the majority of patients in this study were early stage, including stage I (23/232, 10%), stage IIA (82/232, 35%) or stage IIB (87/232, 38%) at initial diagnosis. Clinicians may already be using triple negative receptor status as a prompt to request systemic staging with 18F-FDG-PET/CT at early initial stages. As none of the 23 initially stage I patients were shown to have unsuspected distant or nodal metastases on staging 18F-FDG-PET/CT, this report provides reassurance that patients with stage I TNBC will be unlikely to benefit from systemic staging with 18F-FDG-PET/CT. Avoiding the use of 18F-FDG-PET/CT in stage I patients will help maximize the yield of 18F-FDG-PET/CT studies. There was a low rate of detection of distant metastases in patients with stage IIA TNBC (4/82, 5%), which may cause debate on the use of 18F-FDG-PET/CT in these patients. While the impact may be very high in patients with initial stage IIA disease being upstaged to IV, the relatively low rate of upstaging would not lead us to advocate the use of 18F-FDG-PET/CT in these patients without additional findings, such as suspicious clinical signs or symptoms.
The value of systemic staging with 18F-FDG-PET/CT was demonstrated in Figure 1. With an initial stage of IIB, treatment would have been surgical with neoadjuvant or adjuvant chemotherapy. However, after demonstration of stage IV disease by 18F-FDG-PET/CT, treatment became palliative chemotherapy without surgery. Breast surgery has not been shown to improve survival in stage IV breast malignancy [27], thus 18F-FDG-PET/CT may prevent unnecessary breast surgeries, as well as unnecessary toxicities from intensive, curative-intent neoadjuvant or adjuvant chemotherapy. The patient in Figure 2 underwent mastectomy six weeks prior to the 18F-FDG-PET/CT that revealed a substantial volume of distant metastases. If 18F-FDG-PET/CT had been performed during the initial workup, perhaps surgery may have been avoided.
This study did not include a comparison of TNBC to ER-positive or HER2-positve breast cancers. Two other studies included this comparison. In Groheux et al, the rates of distant metastases did not differ between TNBC (16%), HER2-positive (26%), and ER-positive (22%) breast cancers (p = 0.42) [28]. Likewise, in Riedl et al, there were no statistically significant relationships between upstaging and receptor phenotype (p = 0.52) [11]. Statistical power in these studies was not reported.
Other studies have reported low rates (13/225, 6%) of 18F-FDG-avid false-positives for distant metastases on 18F-FDG-PET/CT [29]. There were no proven false positives in this study, although only 26 of 30 patients demonstrating unsuspected distant metastases on 18F-FDG-PET/CT were confirmed by pathology. It is possible that the four patients considered to have unsuspected distant metastases based on subsequent imaging could have been false positives.
While 18F-FDG-PET/CT demonstration of unsuspected regional nodal disease will not have the same impact as the demonstration of unsuspected distant metastases, upstaging due to unsuspected nodal disease may still alter the course of treatment, such as extension of planned radiotherapy or surgery. 18F-FDG-PET/CT demonstrated 18F-FDG-avid unsuspected nodal disease in five of 82 (6%) initially stage IIA and seven of 87 (8%) initially stage IIB patients with TNBC.
18F-FDG-PET/CT has also been known to detect unsuspected synchronous malignancies during the workup of an initial malignancy [30, 31]. In this study, six of 232 (2.6%) patients had pathologically proven synchronous malignancies discovered on the staging 18F-FDG-PET/CT, a percentage similar to prior reports.
A strength of this study is the large number of TNBC patients evaluated. The triple-negative receptor phenotype is a less common, more aggressive, phenotype of breast cancer, and the large cohort in this retrospective study allows an evaluation of 18F-FDG-PET/CT for systemic staging of patients with TNBC.
The study has several limitations. The retrospective single institution study design lends itself to selection biases that are difficult to control. We have mentioned that a high percentage of patients in the study had stage I and II disease, resulting in an apparent selection bias for patients with lower stage disease. The reasons that patients with TNBC underwent systemic staging with 18F-FDG-PET/CT at rates skewed more towards lower stages than for other groups of breast cancer are not clear, but we suggest that referring clinicians may be using the triple-negative receptor phenotype as a rationale to request 18F-FDG-PET/CT. Most patients (26 of 30) classified as having distant metastases were histologically proven, which is a strong percentage for a retrospective study. Still, the lack of histological proof in four patients with distant metastases is a limitation. Further, most patients classified with unsuspected regional nodal metastases were not confirmed histologically. In general, upstaging for locoregional disease has less of an impact on clinical management, and thus less commonly prompts histologic confirmation. The lack of histologic verification of nodal disease limits the utility of the regional nodal 18F-FDG-PET/CT findings. As most TNBC was IDC in histology and high in tumor grade (Table 1), these comparisons may have been lower in statistical power.
CONCLUSION
Fifteen percent of patients with initial stage IIB TNBC demonstrated unsuspected distant metastases, and were upstaged to stage IV, on 18F-FDG-PET/CT. Although NCCN guidelines recommend against systemic staging with 18F-FDG-PET/CT in patients with stage II breast cancer, our data suggest that patients with the aggressive triple negative receptor phenotype benefit from 18F-FDG-PET/CT staging at least as early as stage IIB. Prospective evaluation of the impact that the receptor phenotype has on the utility of 18F-FDG-PET/CT for systemic staging of patients with breast cancer is warranted.
Acknowledgments
Financial Support: Susan G. Komen for the Cure Research Grant KG110441 (GAU and MG). MSKCC Biostatistics Core (P30 CA008748).
Funding: This work was supported by a Susan G. Komen for the Cure Research Grant KG110441 (GAU), as well as the MSKCC Biostatistics Core (P30 CA008748).
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
Conflicts of interest: None
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was waived by the institutional review board for this retrospective study.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
References
- 1.Groheux D, Espie M, Giacchetti S, Hindie E. Performance of FDG PET/CT in the clinical management of breast cancer. Radiology. 2013;266:388–405. doi: 10.1148/radiol.12110853. radiol.12110853 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Gilardi L, Fumagalli L, Paganelli G. Preoperative PET/CT in early-stage breast cancer: is the TNM classification enough? Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2013;24:852. doi: 10.1093/annonc/mdt004. mdt004 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Bernsdorf M. Reply to ‘preoperative PET/CT in early-stage breast cancer: is the TNM classification enough?’. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2013;24:852–3. doi: 10.1093/annonc/mdt006. mdt006 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Groheux D, Hindie E. Breast Cancer Staging: To Which Women Should 18F-FDG PET/CT Be Offered? J Nucl Med. 2015;56:1293. doi: 10.2967/jnumed.115.160945. jnumed.115.160945 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Ulaner GA, Riedl CC. Reply: Breast Cancer Staging: To Which Women Should 18F-FDG PET/CT Be Offered? J Nucl Med. 2015;56:1293–4. doi: 10.2967/jnumed.115.161042. jnumed.115.161042 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. Breast Cancer Version 2.2015. Journal of the National Comprehensive Cancer Network: JNCCN. 2015;13:448–75. doi: 10.6004/jnccn.2015.0060. 13/4/448 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Hogan MP, Goldman DA, Dashevsky B, Riedl CC, Gonen M, Osborne JR, et al. Comparison of 18F-FDG PET/CT for Systemic Staging of Newly Diagnosed Invasive Lobular Carcinoma Versus Invasive Ductal Carcinoma. J Nucl Med. 2015;56:1674–80. doi: 10.2967/jnumed.115.161455. jnumed.115.161455 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dashevsky BZ, Goldman DA, Parsons M, Gonen M, Corben AD, Jochelson MS, et al. Appearance of untreated bone metastases from breast cancer on FDG PET/CT: importance of histologic subtype. Eur J Nucl Med Mol Imaging. 2015 doi: 10.1007/s00259-015-3080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gnerlich JL, Deshpande AD, Jeffe DB, Sweet A, White N, Margenthaler JA. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg. 2009;208:341–7. doi: 10.1016/j.jamcollsurg.2008.12.001. S1072-7515(08)01633-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sidoni A, Cavaliere A, Bellezza G, Scheibel M, Bucciarelli E. Breast cancer in young women: clinicopathological features and biological specificity. Breast. 2003;12:247–50. doi: 10.1016/s0960-9776(03)00095-x. S096097760300095X [pii] [DOI] [PubMed] [Google Scholar]
- 11.Riedl CC, Slobod E, Jochelson M, Morrow M, Goldman DA, Gonen M, et al. Retrospective analysis of 18F-FDG PET/CT for staging asymptomatic breast cancer patients younger than 40 years. J Nucl Med. 2014;55:1578–83. doi: 10.2967/jnumed.114.143297. jnumed.114.143297 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 13.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi: 10.1073/pnas.191367098. 98/19/10869 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–23. doi: 10.1073/pnas.0932692100. 0932692100 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciriello G, Gatza ML, Beck AH, Wilkerson MD, Rhie SK, Pastore A, et al. Comprehensive Molecular Portraits of Invasive Lobular Breast Cancer. Cell. 2015;163:506–19. doi: 10.1016/j.cell.2015.09.033. S0092-8674(15)01195-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–95. doi: 10.1200/JCO.2009.25.6529. JCO.2009.25.6529 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. JCO.2013.50.9984 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Cadoo KA, Fornier MN, Morris PG. Biological subtypes of breast cancer: current concepts and implications for recurrence patterns. Q J Nucl Med Mol Imaging. 2013;57:312–21. R39Y2013N04A0312 [pii] [PubMed] [Google Scholar]
- 19.Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. The oncologist. 2009;14:320–68. doi: 10.1634/theoncologist.2008-0230. theoncologist.2008-0230 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/nejm200103153441101. [DOI] [PubMed] [Google Scholar]
- 21.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 22.Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–34. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin NU, Vanderplas A, Hughes ME, Theriault RL, Edge SB, Wong YN, et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118:5463–72. doi: 10.1002/cncr.27581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Shaughnessy J, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011;364:205–14. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 25.Edge S, Byrd D, Compton C. AJCC Cancer Staging Manual. 7. New York, NY: Springer; 2010. [Google Scholar]
- 26.Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312:1604–8. doi: 10.1056/NEJM198506203122504. [DOI] [PubMed] [Google Scholar]
- 27.King TA, Lyman JP, Gonen M. TBCRC 013: a prospective analysis of the role of surgery in stage IV breast cancer. Poster presented at: 2013 San Antonio Breast Cancer Symposium; December 10–14, 2013; San Antonio, TX. Abstract P2-18-09. [Google Scholar]
- 28.Groheux D, Hindie E, Delord M, Giacchetti S, Hamy AS, de Bazelaire C, et al. Prognostic impact of (18)FDG-PET-CT findings in clinical stage III and IIB breast cancer. Journal of the National Cancer Institute. 2012;104:1879–87. doi: 10.1093/jnci/djs451. djs451 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niikura N, Costelloe CM, Madewell JE, Hayashi N, Yu TK, Liu J, et al. FDG-PET/CT compared with conventional imaging in the detection of distant metastases of primary breast cancer. The oncologist. 2011;16:1111–9. doi: 10.1634/theoncologist.2011-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agress H, Jr, Cooper BZ. Detection of clinically unexpected malignant and premalignant tumors with whole-body FDG PET: histopathologic comparison. Radiology. 2004;230:417–22. doi: 10.1148/radiol.2302021685. 2302021685 [pii] [DOI] [PubMed] [Google Scholar]
- 31.Sebro R, Aparici CM, Pampaloni MH. Frequency and clinical implications of incidental new primary cancers detected on true whole-body 18F-FDG PET/CT studies. Nucl Med Commun. 2013;34:333–9. doi: 10.1097/MNM.0b013e32835f163f. [DOI] [PubMed] [Google Scholar]


