Abstract
A new approach was developed for the rapid detection and identification of Brazilian alphaviruses and flaviviruses. The methodology involves the genus-specific detection of Alphavirus and Flavivirus by a duplex reverse transcription-PCR (D-RT-PCR), followed by multiplex nested PCR (M-N-PCR) or nested PCR (N-PCR) assays for species-specific identification. By this protocol, 25 arboviruses were specifically detected and identified. Detection levels between 101.3 and 103.5 50% tissue culture infective doses (TCID50)/ml of Flavivirus and Alphavirus strains were achieved by D-RT-PCR, and levels of <1 TCID50/ml were achieved by M-N-PCR assays. To assess the suitability and clinical application of this methodology, a total of 101 human or animal stored samples were analyzed. Results obtained suggest that this technique could be applied as a rapid diagnostic tool in clinical samples in which arbovirus infection is suspected and differential diagnosis is required, avoiding the need to test specimens by separate PCR methods.
Arboviruses are frequently associated with outbreaks in humans and represent a serious public health problem with economic and social impact. In Brazil, most of the arboviruses causing human disease belong to the Togaviridae (Alphavirus genus) and Flaviviridae (Flavivirus genus) families. Alphaviruses eastern equine encephalitis virus (EEEV), western equine encephalitis virus (WEEV), Aura virus (AURAV), Venezuelan equine encephalitis virus (VEEV, subtype I, variety F), Mucambo virus (MUCV, subtype III), Pixuna virus (PIXV, subtype IV), Mayaro virus (MAYV), Una virus, and flaviviruses dengue virus (DENV, subtypes 1 to 4), Saint Louis encephalitis virus (SLEV), Bussuquara virus (BSQV), Cacipacore virus, Iguape virus, Ilheus virus (ILHV), Rocio virus (ROCV), and yellow fever virus (YFV) have been isolated from mosquitoes, animals, or humans (1, 15, 37). These arboviruses cause a variety of diseases, such as acute febrile illness, hemorrhagic fever, and encephalitis. The differential clinical diagnosis between arboviruses can be difficult, principally in the acute phase of the infections where symptoms are similar. The diagnosis of arbovirus infection has been done by virus isolation or serological testing. False-negative serological tests in the first days of disease and cross-reaction of antibodies with antigens of viruses belonging to the same genus are common. To overcome such problems, some reverse transcription-PCR (RT-PCR) assays using species-specific primers have been described for certain alphaviruses (3, 19, 20, 25, 28, 33, 38, 39) and certain flaviviruses (7, 8, 9, 12, 13, 16, 21, 23, 27, 35). Since there are no specific clinical symptoms for arbovirus infection and different arboviruses are present in the same area, a universal genus-specific diagnosis by RT-PCR would be a useful tool for differential diagnosis of these infections. Although RT-PCR protocols using universal primers for Alphavirus (22, 29, 31) and Flavivirus (2, 5, 10, 14, 17, 22, 26, 30, 32, 36) detection have been developed, a multiplex RT-PCR protocol including the detection of more than one genus in a single PCR has not been performed.
In the present study, genus-specific universal primers were used simultaneously in a duplex RT-PCR (D-RT-PCR) for detection of Alphavirus and Flavivirus isolates collected in Brazil. Based on the amplicons obtained from the first amplification, species-specific primers were selected and tested in multiplex nested PCR (M-N-PCR) and nested PCR (N-PCR) assays for virus identification. These methodologies were validated with both cultured virus and human or animal clinical samples.
MATERIALS AND METHODS
Virus and RNA extraction.
The viral strains used in this study were supplied from Evandro Chagas, Adolfo Lutz, and Oswaldo Cruz Institutes in Brazil or the University of Texas or were isolated in our laboratory (Table 1). The viruses were propagated by intracerebral inoculation of 1-day-old suckling mice or by C6/36 Aedes albopictus cell cultures as previously described (11, 34). mouse brain tissue extract infected with Bujaru virus (BUJV) BeAn 47693 (Bunyaviridae; genus Phlebovirus), uninfected cell culture supernatant, and uninfected mouse brain tissue extract were used as negative controls to test the specificity of the assays. Virus RNAs were extracted from 140 μl of a 1/20 dilution of mouse brain tissue macerated suspensions or from cell culture supernatant with the QIAamp Viral RNA Mini kit (QIAGEN, Inc.) according to the manufacturer's instructions and yielded a 60-μl final volume.
TABLE 1.
Brazilian arboviruses used in this study
| Family | Genus | Virus (abbreviation) | Strain (abbreviation) |
|---|---|---|---|
| Togaviridae | Alphavirus | Venezuelan equine encephalitis virus (VEEV) | BeAr 40403 |
| 78V 3531 | |||
| Mucambo (MUCV) BeAn 8 | |||
| Pixuna (PIXV) BeAr 35645 | |||
| Eastern equine encephalitis virus (EEEV) | SpAn 14723 | ||
| Western equine encephalitis virus (WEEV) | Rio 1257 | ||
| Aura virus (AURAV) | BeAr 10315 | ||
| Mayaro virus (MAYV) | BeAr 20290 | ||
| Flaviviridae | Flavivirus | Dengue virus (DENV) | DENV 1 RibH 830 |
| DENV 1 RioH 289731 | |||
| DENV 2 Cea 24622 | |||
| DENV 2 SpH 125367 | |||
| DENV 2 Toc 213 | |||
| DENV 3 RibH 1 | |||
| DENV 3 Rio | |||
| DENV 4 Boa Vista | |||
| St. Louis encephalitis virus (SLEV) | BeH 355964 | ||
| BeAn 421498 | |||
| BeAr 417704 | |||
| SpAn 11916 | |||
| Bussuquara virus (BSQV) | BeAn 4073 | ||
| Ilheus virus (ILHV) | BeH 7445 | ||
| Rocio virus (ROCV) | SpH 34675 | ||
| Yellow fever virus (YFV) | 17D (vaccine) | ||
| BeAn 131 |
D-RT-PCR primers.
Genus-specific primers were selected for annealing in conserved regions of Alphavirus and Flavivirus genomes producing specific PCR products of easily distinguishable sizes. The forward M2W (YAGAGCDTTTTCGCAYSTRGCHW) and reverse cM3W (ACATRAANKGNGTNGTRTCRAANCCDAYCC) primer set anneals to the nonstructural protein 1 gene of Alphavirus, producing amplicons of approximately 434 bp (29). The forward FG1 (TCAAGGAACTCCACACATGAGATGTACT) and reverse FG2 (GTGTCCCATCCTGCTGTGTCATCAGCATACA) primer set anneals to the NS5 gene of Flavivirus, producing amplicons of approximately 958 bp (17).
D-RT-PCR assay.
The RT mixture contained 8 μl of RNA template, 4 μl of 5× first strand buffer (250 mM Tris-HCl [pH 8.3], 375 mM KCl, 15 mM MgCl2), 1.5 μl of dithiothreitol (0.1 M), 1 μl of each reverse primer cM3W and FG2 (100 and 15 μM, respectively), 1 μl of deoxynucleoside triphosphate (dNTP) mixture (250 μM each dNTP), 20 U of RNase inhibitor (RNaseOUT; Invitrogen), 200 U of reverse transcriptase (Superscript; Invitrogen), and water to complete a 20-μl volume. The mixture was incubated at 42°C for 50 min and at 95°C for 5 min to inactivate the reverse transcriptase. The PCR mixture contained 8 μl of cDNA, 5 μl of 10× PCR buffer (200 mM Tris-HCl [pH 8.4], 500 mM KCl), 2 μl of MgCl2 (50 mM), 1 μl of forward primers M2W and FG1 (50 and 15 μM, respectively), 1 μl of dNTP mixture (250 μM each dNTP), 1 U of Taq DNA polymerase (Platinum Taq DNA polymerase; Invitrogen), and water to complete a 50-μl volume. The mixture was submitted to 30 cycles of 94°C for 1 min, 53°C for 1 min, and 72°C for 2 min, followed by a final extension step at 72°C for 5 min. The thermal cycling was performed with a Mini Cycler machine (MJ Research). Ten microliters of the PCR products was electrophoresed on a 2% (wt/vol) agarose gel, stained with ethidium bromide, and visualized with a UV light.
Primers for virus identification.
In this study, 14 specific inner primers were selected for flavivirus and alphavirus identification at the species level by N-PCR methods. To select species-specific primers for flavivirus identification, the amplicons obtained from first amplification of DENV subtype 1 (DENV 1) RibH 830, DENV 1 RioH 289731, DENV 2 Cea 24622, DENV 2 SpH 125367, DENV 2 Toc 213, DENV 3 Rio, DENV 4 Boa Vista, SLEV BeH 355964, SLEV SpAn 11916, BSQV BeAn 4073, ILHV BeH 7445, ROCV SpH 34675, YFV 17D, and YFV BeAn 131 were sequenced and aligned. First, the amplicons were recovered from the gel and purified with the QIAquick gel extraction kit (QIAGEN), as recommended by the manufacturer. Sequencing reactions were performed with the Thermo Sequenase Cy5.5 Dye Terminator Cycle sequencing kit (Amersham Pharmacia Biotech) with the FG1-FG2 primer set and analyzed with the SEQ 4X4 Personal sequencing system (Amersham Pharmacia Biotech). Second, nucleotide sequences obtained from the 14 Flavivirus amplicons were aligned with ClustalW software (Informax). Thus, specific inner primers were selected in low-homology genome regions for DENV 1, DENV 2, DENV 3, and DENV 4; SLEV; BSQV; ILHV; ROCV; and YFV. Species-specific primers used for VEEV, EEEV, WEEV, AURAV, and MAYV identification were previously described (4).
M-N-PCR assays.
With the selected specific inner primers, two M-N-PCR assays were performed: the M-N-PCR flavivirus assay for identification of the most important Brazilian flaviviruses like DENV 1, DENV 2, DENV 3, and YFV and the M-N-PCR-alphavirus for VEEV, EEEV, WEEV, AURAV, and MAYV identification. In general, the reaction mixture contained 1 μl from the first amplification, 5 μl of PCR buffer (200 mM Tris-HCl [pH 8.4], 500 mM KCl), 2 μl of MgCl2 (50 mM), 1 μl of dNTP mixture (250 μM each dNTP), 1 U of Taq DNA polymerase (Platinum Taq DNA polymerase; Invitrogen) and water to complete a 50-μl volume. For the M-N-PCR flavivirus reaction mixture, the forward FG1 primer was added simultaneously with the inner specific primers for DENV 1, DENV 2, DENV 3, and YFV at a concentration of 15 μM. For the M-N-PCR alphavirus reaction mixture, the reverse cM3W primer (at 100 μM) was added simultaneously with the inner specific primers for VEEV, EEEV, WEEV, AURAV, and MAYV (at 50 μM). The mixture was subjected to 25 cycles of 94°C for 1 min, 53°C for 1 min, and 72°C for 2 min. A final extension step was carried out at 72°C for 5 min. Analysis of the amplicons was performed as previously described.
N-PCR assays.
Since the specific inner primers produce amplicons that are very similar in size, conventional N-PCRs were performed for DENV 4, SLEV, BSQV, ILHV, and ROCV identification. The PCR mixture and cycling conditions were performed as for M-N-PCR flavivirus assays. However, only a single primer pair was added to each reaction mixture (FG1 and the specific primer). Cross-reactivity tests were performed with specific primers and heterologous viruses.
Detection limits of the D-RT-PCR and M-N-PCR assays.
Stock seeds of MAYV and YFV 17D containing 106.5 and 103.3 50% tissue culture infective doses (TCID50)/ml, respectively, were serially (10-fold) diluted in phosphate-buffered saline or in human serum (MAYV only), to mimic viremic serum samples. The RNA was extracted from each virus dilution and submitted to D-RT-PCR and M-N-PCR analyses.
Clinical samples.
A total of 97 samples were taken from patients clinically suspected of having arbovirus infection, who had experienced symptoms for ≤5 days. Ninety-four serum samples were obtained from patients from Rio de Janeiro, Roraima, and São Paulo states, Brazil, during DENV epidemics in 2001 and 2003. Three tissue samples were recovered from YFV patients from Goiás state and Brasília, Brazil, in 1999 and 2001. Four other viremic serum samples were recovered from suckling mice experimentally infected with MUCV, EEEV, WEEV, and MAYV. All these samples were previously examined by virus isolation in cell culture or in suckling mice and were identified by virus neutralization or immunofluorescence assays. These samples were stored at −70 or −20°C until tested by D-RT-PCR and M-N-PCR.
Nucleotide sequence accession numbers.
The NS5 gene nucleotide sequences of the following viruses were submitted to GenBank (accession numbers are in parentheses): DENV 1 RibH 830 (AY498844), DENV 1 RioH 289731 (AY498845), DENV 2 Cea 24622 (AY498846), DENV 2 SpH 125367 (AY498847), DENV 2 Toc 213 (AY498848), DENV 3 Rio (AY368702), DENV 4 Boa Vista (AY498849), SLEV BeH 355964 (AY498852), SLEV SpAn 11916 (AY498853), BSQV BeAn 4073 (AY498843), ILHV BeH 7445 (AY498850), ROCV SpH 34675 (AY498851), and YFV BeAn 131 (AY498854). The YFV 17D sequence was not submitted.
RESULTS
D-RT-PCR assay.
Some relevant RT-PCR parameters, such as primer choice and determination of primer concentration and annealing temperatures were initially optimized to achieve a maximum level of sensitivity and specificity for the D-RT-PCR. Thus, the test showed clearly distinguishable amplicons with specific expected sizes for all tested Alphavirus (434 bp) and Flavivirus (958 bp) isolates; no amplicons were obtained with negative controls (Fig. 1A and 2A). The D-RT-PCR was capable of detecting MAYV and YFV at levels of 103.5 and 101.3 TCID50/ml, respectively.
FIG. 1.
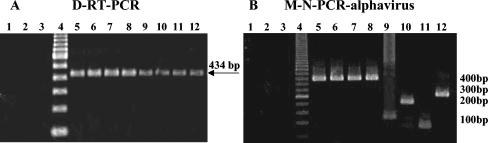
Agarose gel electrophoresis of amplicons from D-RT-PCR (A) and M-N-PCR (B) for Alphavirus. Lanes 1 to 3, negative controls (RNA extract from uninfected mouse brain tissue, BUJV, and water); lane 4, molecular size marker (DNA ladder, 100 or 50 bp); lanes 5 to 12, Alphavirus VEEV BeAr 40403, VEEV 78V 3531, MUCV BeAn 8, PIXV BeAr 35645, EEEV SPAn 14723, WEEV Rio 1257, AURAV BeAr 10315, and MAYV BeAr 20290.
FIG. 2.
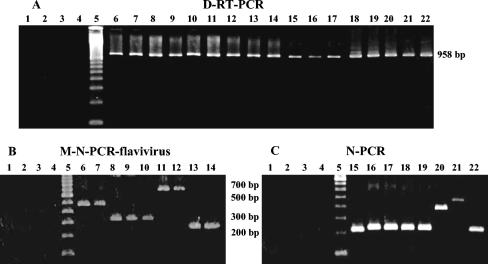
Agarose gel electrophoresis of amplicons from D-RT-PCR (A), M-N-PCR (B), and N-PCR (C) for Flavivirus. Lanes 1 to 4, negative controls (RNA extract from uninfected mouse brain tissue, uninfected cell culture supernatant, BUJV, and water); lane 5, molecular size marker (DNA ladder, 100 bp); lanes 6 to 22, Flavivirus DENV 1 RibH 830, DENV 1 RioH 289731, DENV 2 Cea 24622, DENV 2 SpH 125367, DENV 2 Toc 213, DENV 3 Rio, DENV 3 RibH 1, YFV 17D, YFV BeAnm131, DENV 4 Boa Vista, SLEV BeH 355964, SLEV BeAn 421498, SLEV SpAn 11916, SLEV BeAr 417704, BSQV BeAn 4073, ILHV BeH 7445, and ROCV SpH 34675.
Primers for virus identification.
The 14 specific inner primers used for flavivirus and alphavirus identification by N-PCR assay are described in Table 2.
TABLE 2.
Species-specific primers used in the nested-PCR assays
| Assay | Primera | Sequence (5′-3′) | Amplicon (bp) |
|---|---|---|---|
| M-N-PCR alphavirus | nVEE (+) | ACGGAGGTAGACCCATCCGA | 400b |
| nEEE (+) | CCACGGTACCGTTGCC | 124b | |
| nWEE (+) | GGCGGCAGACCTGCTGGAA | 208b | |
| nAURA (+) | TCAATGCACCTTCGACCA | 86b | |
| nMAY (+) | GGAAGTTGGCCAAGGC | 270b | |
| M-N-PCR flavivirus | nDEN1 (−) | CGTTTTGCTCTTGTGTGCGC | 472c |
| nDEN2 (−) | GAACCAGTTTGTTTDRTTTCATAGCTGCCd,e | 316c | |
| nDEN3 (−) | TTCCTCGTCCTCAACAGCAGCTCTCGCACT | 659c | |
| nYF (−) | TCAGAAGACCAAGAGGTCATGTd | 253c | |
| N-PCR | nDEN4 (−) | GCAATCGCTGAAGCCTTCTCCC | 222c |
| nSLE (−) | ATTCTTCTCTCAATCTCCGTd | 232c | |
| nBSQ (−) | AAGTGACACCTGTTCAGGGTA | 388c | |
| nILH (−) | TCCACCGCTGATCTGAGCCCGTGA | 474c | |
| nROC (−) | TCACTCTTCAGCCTTTCG | 230c |
(+), forward; (−), reverse.
With cM3W primer.
With FG1 primer.
Consensus primer.
Degenerated primer.
M-N-PCR and N-PCR assays.
With the selected specific inner primers, amplicons with predicted and distinct sizes were observed for VEEV (400 bp), EEEV (124 bp), WEEV (208 bp), AURAV (86 bp), and MAYV (270 bp) by the M-N-PCR for alphaviruses (Fig. 1B). By the M-N-PCR flavivirus assay, amplicons with predicted sizes were observed for DENV 1 (472 bp), DENV 2 (316 bp), DENV 3 (659 bp), and YFV (253 bp), as shown in Fig. 2B. Amplicons with predicted sizes were also observed for DENV 4 (222 bp), SLEV (232 bp), BSQV (388 bp), ILHV (474 bp), and ROCV (230 bp) by N-PCR (Fig. 2C). Cross-reactivity between specific primers and heterologous viruses in the same genus was not observed, and no amplicons were obtained from negative controls, indicating that these assays were virus species specific. The M-N-PCRs detected virus at levels <1 TCID50/ml for MAYV and YFV.
Clinical samples.
Results obtained by D-RT-PCR and M-N-PCR were compared to those obtained by virus isolation. Viruses were isolated from 95 samples. Of these samples, 61 were found to be positive by D-RT-PCR; 57 were classified as Flavivirus isolates, and 4 were classified as Alphavirus isolates. Six samples for which virus isolation was not possible were also negative by D-RT-PCR. The sensitivity and specificity of D-RT-PCR were 64 and 100%, respectively.
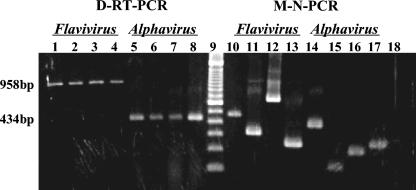
Of the 95 samples for which virus isolation was performed, 94 were also positive by M-N-PCR, and 88 were identified as DENV (63 were DENV 1, 4 were DENV 2, and 21 were DENV 3), 3 were identified as YFV, and 4 were identified as MUCV, EEEV, WEEV, or MAYV. One sample identified as DENV 2 by virus isolation was negative by the M-N-PCR assay for flaviviruses. Five of the six samples that did not allow virus isolation were negative by M-N-PCR. One sample that did not have virus isolated had DENV 2 detected by M-N-PCR for flaviviruses. The sensitivity and specificity of the M-N-PCR were 99 and 83%, respectively. The profile of results is shown in Table 3; representative results obtained from clinical samples analyzed by D-RT-PCR, followed by M-N-PCR, are shown in Fig. 3.
TABLE 3.
Results obtained by D-RT-PCR, M-N-PCR, and virus isolation (VI)
| Genus | D-RT-PCR | Virus | M-N-PCR | VI |
|---|---|---|---|---|
| Flavivirus | 57 | DENV 1 | 63 | 63 |
| DENV 2 | 4 | 4a | ||
| DENV 3 | 21 | 21 | ||
| YFV | 3 | 3 | ||
| Alphavirus | 4 | MUCV | 1 | 1 |
| EEEV | 1 | 1 | ||
| WEEV | 1 | 1 | ||
| MAYV | 1 | 1 | ||
| 6 | Negative | 6 | 6b |
One sample that had DENV 2 isolated was negative by the M-N-PCR flavivirus assay.
One sample that did not have virus isolated had DENV 2 detected by the M-N-PCR flavivirus assay.
FIG. 3.
D-RT-PCR detection, followed by M-N-PCR identification of flaviviruses and alphaviruses in clinical samples. Lanes 1 to 4, Flavivirus genus; lanes 5 to 8, Alphavirus genus; lane 9, molecular size marker (DNA ladder, 100 bp); lanes 10 to 13, M-N-PCR flavivirus assay of DENV 1, DENV 2, DENV 3, and YFV; lanes 14 to 17, M-N-PCR alphavirus assay of MUCV, EEEV, WEEV, and MAYV; lane 18, water.
DISCUSSION
In Brazil, arboviruses have a wide geographic distribution, produce several diseases, and represent an important health problem. Thus, efficient epidemiologic surveillance programs are necessary to monitor different arbovirus activity in the regions where these arboviruses are endemic or enzootic. These programs should contain viral dissemination and reduce the socioeconomic impact caused by these diseases. For this, fast, sensitive, specific, and low-cost methods of diagnosing arbovirus diseases are important tools. Therefore, we developed a new diagnosis strategy involving two steps: in the first step, two sets of genus-specific primers were used in a multiplex system (D-RT-PCR) for Alphavirus or Flavivirus detection. In the second step, nested assays were used for virus identification. For this, the results obtained by D-RT-PCR directed the choice of the nested assay: for the Alphavirus genus, the M-N-PCR for alphaviruses was used; for the Flavivirus genus, M-N-PCR or N-PCR for flaviviruses was used.
One factor that influences greatly the sensitivity of RT-PCR is RNA extraction. In this study, we used a silica gel membrane spin column (QIAamp Viral RNA Mini kit; QIAGEN, Inc.). In agreement with previous reports, this method offered the technical simplicity, sensitivity, and speed that are essential in this new PCR strategy, as well as being more strongly indicated for alphavirus and flavivirus RNA extraction (6, 22).
With the D-RT-PCR, a careful choice of primers was critically important to obtain distinct and specific amplification of the part of the virus genome belonging to each of the genera studied. Selection of suboptimal primers is the cause of many undesirable results, such as primer dimer formation, hairpin formation, false-negative or false-positive results, and generation of spurious products (22). In this study, primer sequences with similar hybridization kinetics, length, and G-C content were selected. In addition, some parameters were optimized, such as the best primer concentrations, to obtain uniform amplification signals of the fragments, and selection of an optimal annealing temperature, to obtain a desired specific product. Furthermore, cross-reactivity of one genus primer with viruses from other genus or lack of reactivity to some strains of a given genus may affect the quality of RT-PCR assays. By using FG1 and FG2 primers, we were able to detect different flaviviruses without cross-reaction with other arboviruses. However, test sensitivity may be adversely affected by mismatches in these primer sequences. These mismatches normally occur because relevant sequence data are not available for multiple sequence alignment to select genus-specific primers (22). In fact, the FG1 and FG2 primers were selected based on 13 flavivirus sequences that represented only approximately 20% of all flaviviruses (17). In a recent study, the performance of FG1 and FG2 primers was evaluated, showing good results in the detection of the most common human flaviviruses, despite the fact that these primers did not detect some strains of Japanese encephalitis virus (Nakayama), West Nile virus (E101), and YFV (FNV) (32). The occurrence of mismatches, some of them close to the 3′ prime binding site of FG1 and FG2 primers, may explain the lesser efficiency of amplification with these strains and possibly with other flaviviruses. However, all flaviviruses analyzed in the present study, including two YFV strains, had the genome amplified by these primers.
Sensitivity of the D-RT-PCR assay was suitable, allowing Alphavirus and Flavivirus detection at 103.5 and 101.3 TCID50/ml, respectively; the presence of human serum did not reduce the test sensitivity. Besides, the D-RT-PCR results were comparable with those for RT-PCR performed for each genus by other authors (29, 32). M-PCR has been used for the detection of DENV (18), as well as EEEV, La Crosse virus, and SLEV (24); it is as sensitive as the D-RT-PCR developed in this work. However, these studies used species-specific primers, which detect only a few viruses. In contrast, genus-specific primers in a multiplex PCR system are able to diagnosis a larger amount of viruses in a single reaction mixture.
In the N-PCR assays, species-specific primers selected were effective for Brazilian alphavirus and flavivirus identification. The M-N-PCR alphavirus and M-N-PCR flavivirus assays showed amplicons with different sizes that allowed a sensitive and specific diagnosis of each virus. In the N-PCR assays, species-specific primers selected were effective for Brazilian alphavirus and flavivirus identification. However, to select these virus-specific primers, we only used sequence data obtained from strains isolated in Brazil. Viruses of different genotypes can come from other geographical areas, principally from other areas in the Americas where arbovirus is endemic, causing outbreaks of the disease. In these cases, the primers must be able to identify the viruses with high efficiency. Especially in the case of DENV-specific primers, some mismatches were observed when these primers were aligned with homologous virus sequences from other geographical areas obtained from the GenBank database. This can produce false-negative results and decrease the test sensitivity. In some cases, the optimization of annealing temperatures can allow a primer with some mismatched bases to bind efficiently. In this study, a suitable annealing temperature allowed a successful amplification of viral isolates incorporating up to three mismatches (the nden2 primer with DENV strains). Nevertheless, other viral strains may contain additional mismatches that may affect primer annealing and amplification. In these cases, further studies are necessary to evaluate the efficiency of our selected primers; if necessary, new species-specific primers should be created to identify distinct viruses from other geographical areas.
Results obtained by D-RT-PCR and M-N-PCR were compared to those obtained by virus isolation with clinical samples submitted to analysis by both methods. The D-RT-PCR was able to detect and to classify different arboviruses isolated in Brazil from clinical samples into Alphavirus and Flavivirus genera. The assay showed 64% sensitivity when compared to virus isolation. This low sensitivity could be explained by the previous storage conditions of the samples analyzed by D-RT-PCR. These samples were obtained from reference laboratories like the Evandro Chagas and Oswaldo Cruz Institutes, where they were collected and processed for virus isolation on the same day and thereafter stored for a long time (more than 3 years), some of them at temperatures of −20°C. In addition, these samples were thawed two or more times for use by other researchers. These inadequate storage conditions could have led to the loss of RNA integrity with consequent reduction of D-RT-PCR sensitivity. Further studies submitting fresh samples to multiple freezing and thawing procedures, followed by arbovirus detection, could be performed to check the effects of these adverse conditions on virus isolation and on D-RT-PCR. On the other hand, the limit of detection obtained for Flavivirus isolates (101.3 TCID50/ml) suggests that D-RT-PCR should be at least as sensitive as virus isolation.
The arboviruses detected in the clinical samples by D-RT-PCR were successfully identified at the species level by the M-N-PCRs (M-N-PCR alphavirus or M-N-PCR flavivirus assay). The apparently false-positive sample, which was negative by virus isolation and which had DENV 2 detected by M-N-PCR flavivirus assay, suggests that although virus infectivity was compromised, viral RNA could still be detected. Other studies show that the virus genome can be amplified even after virus neutralization by antibodies (6).
The strategy using D-RT-PCR, followed by M-N-PCR or N-PCR, was sensitive, specific, fast, and easy to perform (the test can be realized in 8 h, and multiple samples can be tested simultaneously with minimal technical effort). Furthermore, this methodology presents some advantages when compared to the conventional methods of RT-PCR. (i) The use of genus-specific primers for initial amplification allows the detention of all members of each genus, including virus for which diagnosis is still nonroutine, besides being useful for detecting a new related virus. (ii) This first multiplex PCR can detect a range of possible etiologic agents and make a previous genus classification, reducing the time and the cost of successive tests for virus identification. (iii) The use of species-specific primers in a system of multiplex PCR or N-PCR assays improves the relatively low sensitivity of single-round PCR and allows the specific identification of each virus, providing important information for both epidemiological analysis and evolutionary studies. (iv) Multiplex PCR methods are less laborious and more economical than single PCRs. (v) In cases of nonspecific clinical symptoms of arbovirus infection, this methodology offers the potential for very rapid detection with a single clinical sample against a large number of potential pathogens. In short, this diagnosis strategy can be used as a reliable alternative for routine diagnostic and epidemiological surveillance of arboviruses isolated in Brazil.
Acknowledgments
We are grateful to Pedro Vasconcelos (Evandro Chagas Institute, Belém, Brazil), Rita Nogueira (Oswaldo Cruz Foundation, Rio de Janeiro, Brazil), Terezinha Coimbra (Adolfo Lutz Institute, São Paulo, Brazil), and Robert E. Shope (University of Texas, Galveston, Tex.) for supplying viruses and clinical samples. We are also grateful to Victor Hugo Aquino Quintana (Universidade de São Paulo, São Paulo, Brazil) and Aramis Augusto Pinto (Universidade Estadual Paulista, São Paulo, Brazil) for reviewing the manuscript.
We are grateful to Fundação de Amparo à Pesquisa no Estado de São Paulo (FAPESP) (99/08207-4 and 01/01935-6) for supporting this study.
REFERENCES
- 1.Alice, F. J. 1956. Infecção humana pelo vírus leste de encefalite eqüina. Bol. Inst. Biol. (Bahia) 3:3-9. [Google Scholar]
- 2.Baleotti, F. G., M. L. Moreli, and L. T. M. Figueiredo. 2003. Brazilian Flavivirus phylogeny base on NS5. Mem. Inst. Oswaldo Cruz 98:379-382. [DOI] [PubMed] [Google Scholar]
- 3.Brightwell, G., J. M. Brown, and D. M. Coates. 1998. Genetic targets for the detection and identification of Venezuelan equine encephalitis viruses. Arch. Virol. 143:731-742. [DOI] [PubMed] [Google Scholar]
- 4.Bronzoni, R. V. M., M. L. Moreli, A. C. R. Cruz, and L. T. M. Figueiredo. Multiplex nested-PCR for Brazilian Alphavirus diagnosis. Trans. R. Soc. Trop. Med. Hyg. 98:456-461. [DOI] [PubMed]
- 5.Chow, V. T. K., C. L. K. Seah, and Y. C. Chan. 1993. Use of NS3 consensus primers for the polymerase chain reaction amplification and sequencing of dengue viruses and other flaviviruses. Arch. Virol. 133:157-170. [DOI] [PubMed] [Google Scholar]
- 6.De Paula, S. O., C. Nunes, R. Matos, Z. M. Oliveira, D. M. Lima, and B. A. L. Fonseca. 2001. Comparison of techniques for extracting viral RNA from isolation-negative serum for dengue diagnosis by the polymerase chain reaction. J. Virol. Methods 98:119-125. [DOI] [PubMed] [Google Scholar]
- 7.De Paula, S. O., D. M. Lima, and B. A. L. Fonseca. 2002. Detection and identification of dengue-1 virus in clinical samples by a nested-PCR followed by restriction enzyme digestion of amplicons. J. Med. Virol. 66:529-534. [DOI] [PubMed] [Google Scholar]
- 8.Deubel, V., M. Laille, J. P. Hugnot, E. Chungue, J. L. Guesdom, M. T. Drouet, S. Bassot, and D. Chevrier. 1990. Identification of dengue sequences by genomic amplification: rapid diagnosis of dengue virus serotypes in peripheral blood. J. Virol. Methods 30:41-54. [DOI] [PubMed] [Google Scholar]
- 9.Deubel, V., M. Huerre, G. Cathomas, M. T. Drouet, N. Wuscher, B. LeGuenno, and A. F. Widmer. 1997. Molecular detection and characterization of yellow fever virus in blood and liver specimens of a non-vaccinated fatal human case. J. Med. Virol. 53:212-217. [PubMed] [Google Scholar]
- 10.Eldadah, Z. A., D. M. Asher, M. S. Godee, K. L. Pomeroy, L. G. Goldfarb, S. M. Feinstone, H. Levitan, C. J. Gibbs, and D. C. Gajdusek. 1991. Detection of flavivirus by reverse-transcriptase polymerase chain reaction. J. Med. Virol. 33:260-267. [DOI] [PubMed] [Google Scholar]
- 11.Figueiredo, L. T. M. 1990. Uso de células de Aedes albopictus C6/36 na propagação e classificação de arbovírus das famílias Togaviridae, Bunyaviridae, Flaviviridae e Rhabdoviridae. Rev. Soc. Bras. Med. Trop. 23:13-18. [DOI] [PubMed] [Google Scholar]
- 12.Figueiredo, L. T. M., W. C. Batista, and A. Igarashi. 1997. Detection and identification of dengue virus isolates from Brazil by a simplified reverse transcription-polymerase chain reaction (RT-PCR) method. Rev. Inst. Med. Trop. (São Paulo) 39:95-99. [DOI] [PubMed] [Google Scholar]
- 13.Figueiredo, L. T. M., W. C. Batista, and A. Igarashi. 1997. A simple reverse transcription-polymerase chain reaction for dengue type 2 virus identification. Mem. Inst. Oswaldo Cruz 92:395-398. [DOI] [PubMed] [Google Scholar]
- 14.Figueiredo, L. T. M., W. C. Batista, S. Kashima, and E. S. Nassar. 1998. Identification of Brazilian flaviviruses by a simplified reverse transcription-polymerase chain reaction method using Flavivirus universal primers. Am. J. Trop. Med. Hyg. 59:357-362. [DOI] [PubMed] [Google Scholar]
- 15.Figueiredo, L. T. M. 2000. The Brazilian flaviviruses. Microbes Infect. 2:1-7. [DOI] [PubMed] [Google Scholar]
- 16.Filippis, A. M. B., R. M. R. Nogueira, H. G. Schatzmayr, D. S. Tavares, A. V. Jabor, and S. C. M. Diniz. 2002. Outbreak of jaundice and hemorrhagic fever in the southeast of Brazil in 2001: detection and molecular characterization of yellow fever virus. J. Med. Virol. 68:620-627. [DOI] [PubMed] [Google Scholar]
- 17.Fulop, L., A. D. T. Barrett, R. Phillpotts, K. Martin, D. Leslie, and R. W. Titball. 1993. Rapid identification of flaviviruses based conserved NS5 gene sequences. J. Virol. Methods 44:179-188. [DOI] [PubMed] [Google Scholar]
- 18.Harris, E., T. G. Roberts, L. Smith, J. Selle, L. D. Kramer, S. Valle, E. Sandoval, and A. Balmaseda. 1998. Typing of dengue viruses in clinical specimens and mosquitoes by single-tube multiplex reverse transcriptase PCR. J. Clin. Microbiol. 36:2634-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasebe, F., M. C. Parquet, B. D. Pandey, E. G. M. Mathenge, K. Morita, V. Balasubramaniam, Z. Saat, A. Yusop, M. Sinniah, S. Natkunam, and A. Iguarashi. 2002. Combined detection and genotyping of chikungunya virus by a specific reverse transcription-polymerase chain reaction. J. Med. Virol. 67:370-374. [DOI] [PubMed] [Google Scholar]
- 20.Hörling, J., S. Vene, C. Franzén, and B. Niklasson. 1993. Detection of Ockelbo virus RNA in skin biopses by polymerase chain reaction. J. Clin. Microbiol. 31:2004-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howe, D. K., M. H. Vodkin, R. J. Novak, R. E. Shope, and G. L. Mclaughlin. 1992. Use of the polymerase chain reaction of the sensitive detection of St. Louis encephalitis viral RNA. J. Virol. Methods 36:101-110. [DOI] [PubMed] [Google Scholar]
- 22.Kuno, G. 1998. Universal diagnostic RT-PCR protocol for arboviroses. J. Virol. Methods 72:27-41. [DOI] [PubMed] [Google Scholar]
- 23.Lanciotti, R. S., C. H. Calisher, D. J. Gubler, G. L. Chang, and V. Vorndam. 1992. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 30:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, J. H., K. Tennessen, B. G. Lilley, and T. R. Unnasch. 2002. Simultaneous detection of three mosquito-borne encephalitis viruses (Eastern equine, La Crosse, and St. Louis) with a single-tube multiplex reverse transcriptase polymerase chain reaction assay. J. Am. Mosq. Control Assoc. 18:26-31. [PubMed] [Google Scholar]
- 25.Linssen, B., R. M. Kinney, P. Aguilar, K. L. Russell, D. M. Watts, O. R. Kaaden, and M. Pfeffer. 2000. Development of reverse transcription-PCR assays specific for detection of equine encephalitis viruses. J. Clin. Microbiol. 38:1527-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meiyu, F., C. Huosheng, C. Cuihua, T. Xiaodong, J. Lianhua, P. Yifei, C. Weijun, and G. Huiyu. 1997. Detection of flaviviruses by reverse transcriptase-polymerase chain reaction with the universal primer set. Microbiol. Immunol. 41:209-213. [DOI] [PubMed] [Google Scholar]
- 27.Miagostovich, M. P., F. B. Santos, E. S. M. Araújo, J. Dias, H. G. Schatzmayr, and R. M. Nogueira. 1997. Diagnosis of dengue by using reverse transcriptase-polymerase chain reaction. Mem. Inst. Oswaldo Cruz 92:595-600. [DOI] [PubMed] [Google Scholar]
- 28.Monroy, A. M., T. W. Scott, and B. A. Webb. 1996. Evaluation of reverse transcriptase polymerase chain reaction for the detection of Eastern equine encephalomyelitis virus during vector surveillance. J. Med. Entomol. 33:449-457. [DOI] [PubMed] [Google Scholar]
- 29.Pfeffer, M., B. Proebster, R. M. Kinney, and O. R. Kaaden. 1997. Genus-specific detection of alphaviruses by semi-nested reverse transcription-polymerase chain reaction. Am. J. Trop. Med. Hyg. 57:709-718. [DOI] [PubMed] [Google Scholar]
- 30.Pierre, V., M. T. Drouet, and V. Deubel. 1994. Identification of mosquito-borne flavivirus sequences using universal primers and reverse transcription/polymerase chain reaction. Res. Virol. 145:93-104. [DOI] [PubMed] [Google Scholar]
- 31.Sánchez-Seco, M. P., D. Rosario, E. Quiroz, G. Guzmán, and A. Tenório. 2001. A generic nested-RT-PCR followed by sequencing for detection and identification of members of the alphavirus genus. J. Virol. Methods 95:153-161. [DOI] [PubMed] [Google Scholar]
- 32.Scaramozzino, N., J. M. Crance, A. Jouan, D. A. Debriel, F. Stoll, and D. Garin. 2001. Comparison of Flavivirus universal primer pairs and development of a rapid, highly sensitive heminested reverse transcription-PCR assay for detection of flaviviruses targeted to a conserved region of the NS5 gene sequences. J. Clin. Microbiol. 39:1922-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sellner, L. N., R. J. Coelen, and J. S. Mackenzie. 1994. Sensitive detection of Ross river virus-—a one-tube nested RT-PCR. J. Virol. Methods 49:47-58. [DOI] [PubMed] [Google Scholar]
- 34.Shope, R. E., and G. E. Sather. 1979. Arboviruses, p. 767-814. In F. H. Lennet and N. J. Schimidt (ed.), Diagnostic procedures for viral, rickettsial and clamydial Infections, 2nd ed. American Public Health Association, Washington, D.C.
- 35.Sudiro, T. M., H. Ishiko, S. Green, D. W. Vaughn, A. Nisalak, S. Kalayanarooj, A. L. Rothman, B. Raengsakulrach, J. Janus, I. Kurane, and F. A. Ennis. 1997. Rapid diagnosis of dengue viremia by reverse transcriptase-polymerase chain reaction using 3′-noncoding region universal primers. Am. J. Trop. Med. Hyg. 56:424-429. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka, M. 1993. Rapid identification of Flavivirus using the polymerase chain reaction. J. Virol. Methods 41:311-322. [DOI] [PubMed] [Google Scholar]
- 37.Vasconcelos, P. F. C., A. P. A. Travassos da Rosa, F. P. Pinheiro, R. E. Shope, J. F. S. Travassos da Rosa, S. G. Rodrigues, N. Dégallier, and E. S. Travassos da Rosa. 1998. Arboviruses pathogenic for man in Brazil, p. 72-99. In A. P. A. Travassos da Rosa, P. F. C. Vasconcelos, and J. F. S. Travassos da Rosa (ed.), An overview of arbovirology in Brazil and neighbouring countries. Evandro Chagas Institute, Belém, Brazil.
- 38.Vodkin, M. H., G. L. McLaughlin, J. P. Day, R. E. Shope, and R. J. Novak. 1993. A rapid diagnostic assay for Eastern equine encephalomyelitis viral RNA. Am. J. Trop. Med. Hyg. 49:772-776. [DOI] [PubMed] [Google Scholar]
- 39.Vodkin, M. H., T. Streit, C. J. Mitchell, G. L. Mclaughlin, and R. J. Novak. 1994. PCR-based detection of arboviral RNA from mosquitoes homogenized in detergent. BioTechniques 17:114-116. [PubMed] [Google Scholar]