Abstract
Varicella zoster virus (VZV) is a ubiquitous alphaherpesvirus that establishes latency in ganglionic neurons throughout the neuraxis after primary infection. Here, we show that VZV infection induces a time-dependent significant change in mitochondrial morphology, an important indicator of cellular health, since mitochondria are involved in essential cellular functions. VZV immediate-early protein 63 (IE63) was detected in mitochondria-rich cellular fractions extracted from infected human fetal lung fibroblasts (HFL) by Western blotting. IE63 interacted with cytochrome c oxidase in bacterial 2-hybrid analyses. Confocal microscopy of VZV-infected HFL cells at multiple times after infection revealed the presence of IE63 in the nucleus, mitochondria, and cytoplasm. Our data provide the first evidence that VZV infection induces alterations in mitochondrial morphology, including fragmentation, which may be involved in cellular damage and/or death during virus infection.
Keywords: Varicella zoster virus, IE63, Mitochondria, Mitochondria morphology, Mitochondria swelling
Introduction
Mitochondria are cellular organelles present in the cytoplasm of eukaryotic cells and essential for cell survival. In addition to production of ATP, mitochondria regulate many cellular functions, such as redox balance (Korenaga et al. 2005; Chu et al. 2011), calcium ion dynamics (Korenaga et al. 2005; Chu et al. 2011) and apoptosis (Brazeau et al. 2010). The morphology of mitochondria provides a reliable indicator of overall cellular health, and changes in the morphology are critically involved in cell signaling, maintenance of cellular developmental stages, aging, and antiviral responses. Perturbation of mitochondrial morphology frequently occurs during viral disease. For example, changes in mitochondria morphology are prevalent in hepatitis C virus infection of human hepatoma cells altering mitochondrial calcium homeostasis and resulting in excess mitochondrial-generated reactive oxygen species (Korenaga et al. 2005; Chu et al. 2011). Disruption of mitochondrial membrane potential and subsequent mitochondrial fragmentation is a mechanism by which human immunodeficiency virus (HIV) induces cell death (Huang et al. 2012). Canine parvovirus localizes to mitochondrial membranes during virus replication, causing membrane disruption that leads to mitochondrial swelling (blebbing), loss of membrane integrity, and elevated reactive oxygen species in initial stages of infection (Nykky et al. 2014). Herpes viruses also affect mitochondria structure and function. Early in Herpes simplex virus type 1 (HSV-1) infection, a virus encoded alkaline nuclease (UL12.5 gene product) degrades mitochondria DNA (Saffran et al. 2007). While elimination of mitochondrial DNA is not essential for virus replication (Duguay et al. 2014), virus-induced mitochondria DNA instability enhances interferon-stimulated gene expression that promotes an antiviral state through STING activation (West et al. 2015). Pseudorabies virus, another alphaherpesvirus, alters mitochondrial motility and increases action potential firing rates, increasing intracellular Ca2+ that enhances virus pathogenesis (Kramer and Enquist 2012). Human cytomegalovirus (HCMV) infection increases fatty acid synthesis for virus membrane formation and this energy-demanding process is supplied by virus-induced increase in mitochondrial biogenesis (Kaarbø et al. 2011). To counteract the stress accompanying increased energy consumption, the viral mitochondrion-localized inhibitor of apoptosis (vMIA) enters mitochondria from endoplasmic reticulum via mitochondrion-associated membrane to inhibit Bax-mediated apoptosis (Williamson and Colberg-Poley 2009; Zhang et al. 2013).
Varicella zoster virus (VZV) is a ubiquitous human neurotrophic alphaherpesvirus that becomes latent in neurons of cranial nerve ganglia, dorsal root ganglia, and autonomic ganglia following primary infection (varicella). As cell-mediated immunity to VZV declines in elderly or immunocompromised individuals, virus reactivates to cause zoster (shingles) and other serious neurologic and ocular diseases (Mueller et al. 2010). During productive infection, VZV expresses approximately 70 genes including the immediate-early protein 63 (IE63), one of many proteins critical for efficient production of progeny virions (Baiker et al. 2004). The cellular location of VZV IE63 during productive infection is predominately nuclear, but also cytoplasmic (Mueller et al. 2009, 2012). Since mitochondrial morphology is an established indicator of cellular physiology, we assessed mitochondria morphology after VZV infection, using VZV IE63 to identify virus-infected cells. Multiple microscopic imaging analyses revealed diffuse localization of IE63 in both mitochondria and the cytosol, suggesting that the presence of the protein in the mitochondria is due to passive diffusion. Over the course of 48 h, mitochondria in VZV-infected cells gradually fragmented and expanded, consistent with localized damage and altered morphology.
Materials and methods
Cells and virus
Human fetal lung cells (HFL) (ATCC, Manassas, VA) were propagated as described (Baird et al. 2014). To detect VZV IE63 in infected cells, the enhanced green fluorescent protein (eGFP) was fused to the C-terminus of the protein via two-step red-mediated en passant mutagenesis (Tischer et al. 2006, 2007). VZV IE63 is encoded by open reading frame (ORF 63), which maps within the inverted repeat region of the unique short segment of VZV DNA and is present as ORFs 63 and 70 encoding IE63 and IE70, respectively (Davison and Scott 1986). The construction was verified by PCR, DNA sequencing, and multiple restriction fragment length polymorphism analyses and showed no growth defects compared to wild-type parental virus (Goodwin et al. 2013). Upon propagation of the dual-labeled virus, plaques developed that fluoresced only green and that yielded, upon 3×-plaque purification, recombinant virus containing PCR-verified eGFP fused to the 3′ end of ORFs 63 and 70. This virus was used at an infectivity ratio of 1 VZV-infected cell to 100 uninfected cells.
VZV IE63 bacterial 2-hybrid analysis
To identify proteins that bind VZV IE63, a bacterial rather than a yeast 2-hybrid analysis was used, since full-length IE63 fused to the DNA binding domain of GAL4 transactivates eukaryotic gene expression (Ambagala and Cohen 2007). DNA sequencing verified that fusion of VZV ORF 63 to the λcI repressor of pBT (BacterioMatch; Stratagene, La Jolla, CA) did not transactivate prokaryotic genes. This construct was co-transformed with a mouse brain cDNA library fused to the C-terminus of E. coli RNA polymerase II into E. coli KJ1C (hisB-null) and histidine auxotrophs selected on defined media lacking histidine (Tse et al. 2009).
Cell fractionation and Western blot analysis
HFL cells were infected with VZV as described (Baird et al. 2014). Mitochondria were extracted from HFL cells 48 h after infection as per the manufacturer's instructions (Qproteome Mitochondria Isolation Kit; Qiagen, Germantown, MD). Cell fractions (mitochondria, M; cytosolic, C; nuclear, N; and microsomal, μ) were separated by SDS-PAGE, transferred to PVDF membranes, and antigens were detected with antibodies against voltage-dependent anion channel 1 (VDAC1; Abcam, Cambridge, MA), mitogen-activated protein kinase (p38; Cell Signaling, Danvers, MA), proliferating cell nuclear antigen (PCNA; Abcam), calnexin (Abcam), and VZV IE63 (Mahalingam et al. 1996). Secondary HRP-labeled goat anti-rabbit or goat anti-mouse (Life Technologies, Carisbad, CA) antibodies were used and detected with ECL detection reagent (Pierce, Hallsborough, NC). Blots were imaged using a BioRad ChemiDoc XRS+ (Hercules, CA).
Confocal microscopy
HFL cells were fixed and stained at 12, 24, 36, and 48 h post-infection. At the indicated times, medium was aspirated and cells were washed twice with phosphate-buffered saline (PBS), fixed in 4 % paraformaldehyde (PFA, Fisher Scientific) for 30 min at 37 °C, permeabilized in 0.01 % Triton X for 30 min, and blocked with 10 % fetal bovine serum (FBS) in PBS for 25 min at 37 °C. Mitochondrial outer membranes were identified by incubating cells with monoclonal anti-Tom20 antibody (Santa Cruz Biotechnology, CA) in 5 % FBS for 1 h at 37 °C followed by Alexafluor 546 (Life Technologies) secondary antibody (anti-mouse) for 1 h at 37 °C. Nuclei were stained with DAPI (Life Technologies) for 10 min at room temperature. Coverslips were mounted and imaged on a Leica SP8 confocal microscope. Images were obtained using a ×40 oil immersion objective. Image size was 1024×1024 pixels, with a pixel size of 180.4 nm.
Image analysis
Fluorescent signal colocalization analysis was conducted with the Leica LAS-X, version 1.8.1.13759 software, which generated Pearson's correlations based on the detection and analysis of fluorescence at two wavelengths. Mitochondrial morphology was assessed using a fully automated software technique developed by P.M. McClatchey (McClatchey et al. 2015; Keller et al. 2016). Briefly, the software applies adaptive image enhancement algorithms to identify mitochondria (Otsu 1979; Peng et al. 2010).Changes in mitochondrial morphology were assessed based on the number of mitochondrial bodies detected, total mitochondrial projected area, and total mitochondrial perimeter, all adjusted for signal-to-noise ratio and focus effects (McClatchey et al. 2015). Bias due to microscope point-spread function (PSF) was not accounted for in this analysis, likely resulting in an overestimation of mean mitochondrion length by ∼150 nm. After separation of green (VZV IE63), red (mitochondria, Tom20), and blue (nucleus, DAPI) channels, the amount of VZV IE63 that localized to specific areas (cytosol, mitochondria, or nucleus) was assessed based on the mean GFP intensity within each compartment. Mean pixel intensities were normalized to overall cytoplasm intensity, thereby preventing detection of spurious differences due to sample variations in total virus content.
Statistical analysis
Pearson's correlations and changes in mitochondrial length and width between time points or treatment groups were assessed for statistical significance using the Student's t test or one-way ANOVA using Tukey's post hoc correction for multiple comparisons to assess individual group differences; differences of p< 0.05 were considered statistically significant.
Results
VZV IE63 is present in mitochondrial fractions from virus-infected HFL cells
Bacterial 2-hybrid analysis was performed to identify candidate proteins encoded by a mouse brain cDNA that bound IE63. Selection of bacteria in minimal medium lacking histidine and supplemented with 100 μg/ml streptomycin yielded 80 colonies, 10 (12.5 %) of which contained sequences that mapped to mouse cytochrome c oxidase (Table 1), an integral protein in the inner mitochondrial membrane (Kadenbach et al. 2013). Western blot analysis of mitochondria extracted from VZV-infected HFL cells revealed the presence of VZV IE63 (Fig. 1a). In addition, the mitochondria-enriched fraction contained mitochondrial membrane-associated VDAC1, but not nuclear PCNA or diffuse cytoplasmic p38, and also contained calnexin (Fig. 1b), a calcium-binding chaperone protein that facilitates protein assembly. The presence of calnexin in both mitochondria and microsomal extracts of VZV-infected and control HFL cells is indicative of endoplasmic reticulum (ER) fragments, commonly present in mitochondrial preparations due to ER/mitochondrial contacts (Bozidis et al. 2007; Lee and Yoon 2014).
Table 1. VZV IE63 binding proteins identified by bacterial 2-hybrid analysis.
| Number of clones | Protein | Accession number |
|---|---|---|
| 10 | Cytochrome c oxidase | AB042432 |
| 4 | mKIAA | AK122299 |
| 2 | Tubulin a2 | XM 486246 |
| 2 | eIF5a | AK011306 |
| 2 | eIF2B3 | XM 131572 |
| 1 | Calmodulin I | NM 009790.3 |
| 1 | Nicolin I | AJ299741 |
| 1 | Enolase I | AK088788 |
| 1 | APEXpI (nuclease) | AB084238 |
| 1 | Protein tyrosine phosphatase 4a2 | BC087551 |
| 1 | Mg-dependent phosphatase I | BC046613 |
| 1 | Serine proteinase inhibitor | AF426024.1 |
| 1 | Cold-inducible cDNA binding protein | AK014655 |
| 1 | Ring finger protein (Rnf 10) | AB026621 |
| 1 | Zinc finger protein (Mkr 5) | M36516 |
| 1 | Myelin basic protein | AK079621 |
| 1 | Eukaryotic translation elongation factor (Eef 1a1) | BC004067 |
| 48 | No protein identified |
Fig. 1.

Presence of VZV IE63 in mitochondria-enriched fractions of VZV-infected human lung fibroblasts. Mitochondria (M) were extracted from VZV-infected and uninfected HFL cells and protein contents resolved on a 12 % polyacrylamide gel along with 1:10 dilutions of nuclear (N), cytoplasmic (C), and microsomal (μ) fractions. VZV-infected and uninfected mitochondria strips were probed sequentially for VZV IE63 (panel a), VDAC1 PCNA, P38, and calnexin (panel b). Residual bands of previous staining are evident on VDAC and calnexin blots. Arrowheads indicate location of specific proteins
Presence of VZV IE63 in the nucleus and mitochondria during infection
Confocal microscopy showed VZV IE63 (green) in mitochondria (red) of VZV-infected cells as infection progressed over time (Fig. 2). Pearson's correlations, determined for each pair of conditions for all time points imaged, showed no correlation between mitochondria and nuclei throughout infection (Table 2, column 1), validating our parameter for negative localization. VZV IE63 correlated positively with nuclei and mitochondria throughout virus infection (Table 2, columns 2 and 3), but its localization to mitochondria and nuclei was significantly different only at 24 h (Table 2, p < 0.05). Additionally, accumulation of IE63 in both nuclei and mitochondria, determined by Pearson's correlations across a time course of infection (Fig. 3) doubled at 36 h as compared to the amount at earlier time points. At 48 h, the amount of IE63 in the nucleus decreased significantly from that at 36 h (Fig. 3, p <0.05), while the amount of IE63 in mitochondria remained constant.
Fig. 2.
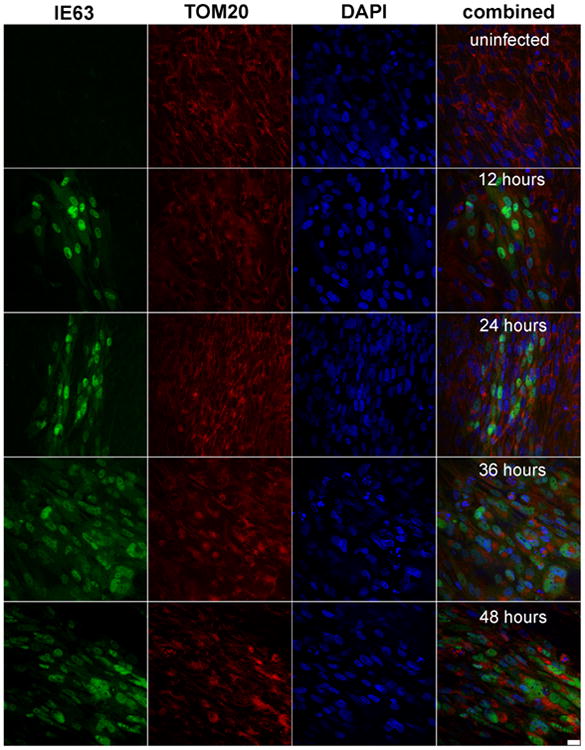
Representative images of human fetal lung cells. Images show GFP-IE63 (green), mitochondrial TOM-20 (red), and nucleus (DAPI; blue) over the course of viral infection (uninfected, 12, 24, 36, and 48 h post-infection). Scale bar represents 25 μm
Table 2. Pearson's correlations of VZV IE63, nucleus, and mitochondria fluorescence.
| Mitochondria+ nucleusa p value average ± SEM | IE63 vs. nucleusa p value average ± SEM | IE63 vs. mitochondriaa p value average ± SEM | |
|---|---|---|---|
| 12 h | −0.180±0.048 | 0.172±0.031 | 0.083±0.040** |
| 24 h | −0.244±0.015 | 0.135±0.022 | 0.051±0.017*,** |
| 36 h | −0.043±0.052 | 0.388±0.017 | 0.215±0.069 |
| 48 h | −0.101±0.027 | 0.275±0.023 | 0.230 ±0.060 |
Total n = 6 for each measurement
p < 0.05 as compared with mitochondria + nucleus, one-way ANOVA, Tukey post hoc comparison;
p < 0.05 as compared with IE63 + nucleus, oneway ANOVA, Tukey post hoc comparison
Fig. 3.
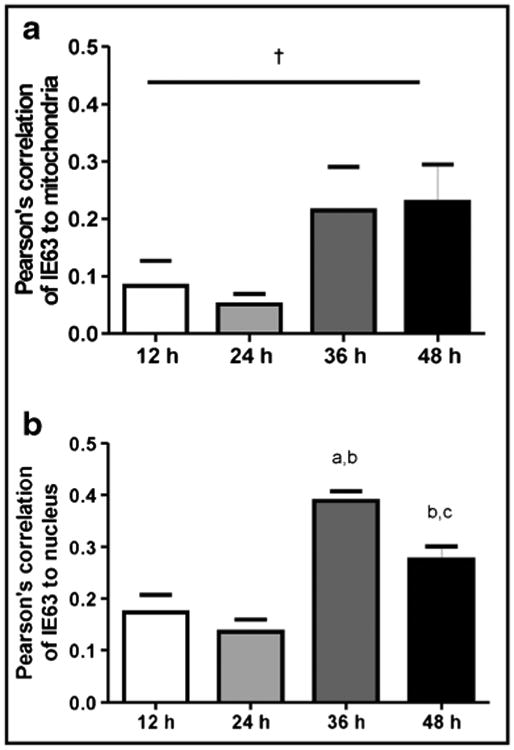
Association of IE63 with the mitochondria a and nucleus b. Confocal microscopy analysis was used to detect the presence of IE63 in the mitochondria (stained with TOM-20) or nucleus (stained with DAPI). Pearson's correlation for each wavelength was generated. Three photos were taken from two separate staining experiments for a total n = 6 for each measurement. Data are mean±SEM. ap < 0.05as compared with the 12-h time point, bp < 0.05 as compared with the 24-h time point, cp < 0.05 as compared with the 36-h time point, †p < 0.08, one-way ANOVA, Tukey post hoc comparison. At 36 h, IE63 increased in both mitochondria and nucleus
VZV infection increases mitochondrial width and decreases length
Mitochondria morphology was analyzed for computer-assisted digitized mitochondria structure, with VZV-infected cells identified by the presence of IE63 (Fig. 4a).Mitochondrion width (Fig. 4b) remained constant from 12–48 h in both infected and uninfected cells, but was significantly greater in infected than in uninfected cells at all time points (p < 0.05). Mitochondrion length was significantly less at 48 h compared to 12 h (p < 0.05, data not shown).
Fig. 4.
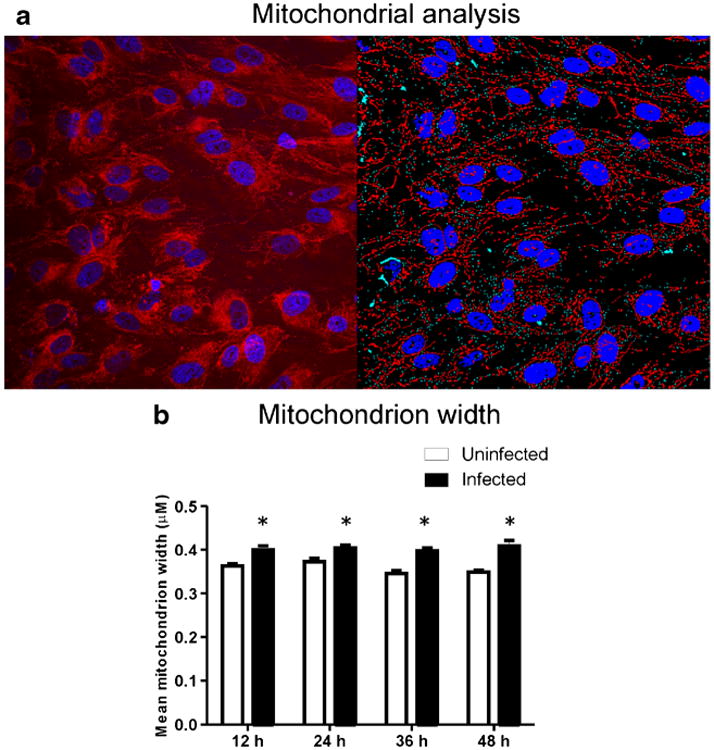
Mitochondrial morphology metrics and virus localization. a Representative microscopy analysis showing mitochondria (red) and nucleus (blue) in uninfected cells (left) and computer identification of mitochondria and nuclei (right). b Mitochondrion width remained stable in both uninfected and infected cells, but was significantly (p < 0.05) greater at all time points in VZV-infected cells, indicative of mitochondrial swelling
Discussion
We observed the diffuse presence of VZV IE63 throughout the virus-infected cell but particularly in the nucleus, consistent with previous reports of VZV IE63 localization in the nucleus (Mueller et al. 2010; Ambagala et al. 2009). Nuclear functions attributed to VZV IE63 include regulation of viral and cellular gene transcription (Jackers et al. 1992; Zuranski et al. 2005; Habran et al. 2007; Khalil et al. 2013), perhaps by modulating the host anti-silencing factor (Ambagala et al. 2009), by enhancing the function of VZV IE62, the major virus immediate-early regulator of gene transcription (Lynch et al. 2002), or by modifying how cellular RNA polymerase II recognized virus and cell gene promoters (Di Valentin et al. 2005). In the cytoplasm, VZV IE63 acts to disarm cellular antiviral defenses by blocking eIF-2 phosphorylation (Ambagala et al. 2009). In agreement with previous reports (Ambagala et al. 2009; Lyman and Enquist 2009; Che et al. 2011), we also found VZV IE63 diffusely distributed in the cytoplasm. Along with indicating VZV IE63 associated with cytochrome c oxidase, the yeast two-hybrid results detected four other candidate IE63 binding partners; mKIAA, two members of the eIF2 complex, and tubulin. mKIAA is the murine homolog of human KIAA, a set of 2000 sequence genes of unknown function identified by whole genome sequencing (Okazaki et al. 2003). VZV IE63 binding eIF2B3 and eIF5a may help provide a mechanism through which IE63 inhibits eIF-2 phosphorylation (Ambagala and Cohen 2007). VZV IE63 is a tegument protein (Kinchington et al. 1995) and its binding tubulin may assist virus migration through the cytoplasm during pathogenesis. The yeast two-hybrid results suggests intriguing VZV IE63 functions during productive infection, however conformation by an independent means such as protein identification by mass spectrophotometry following co-immunoprecipitation is first required.
Our findings demonstrated mitochondrial fragmentation and swelling that persisted throughout the time course of VZV infection, suggesting that VZV-induced mitochondrial swelling occurs within 12 h after infection and providing the first evidence that VZV modifies mitochondria in virus-infected cells. The change in mitochondrial morphology in infected cells is indicative of mitochondrial fission, which together with mitochondrial swelling, may indicate inner membrane disruptions secondary to viral disruption of membrane potential or excess reactive oxygen species, indicators of autophagy. Mitochondrial fission and membrane disruptions are morphological occurrences known to be associated with the decline of oxidative phosphorylation-generated ATP. While VZV has not been shown to perturb mitochondrial functions, it induces apoptosis in productively infected cells through the intrinsic (mitochondria-associated) pathway (Brazeau et al. 2010; Pugazhenthi et al. 2011), and VZV IE63 inhibits virus-induced apoptosis in infected human neurons (Hood et al. 2006). Because VZV IE63 increases in mitochondria with time after infection, VZV infection may be modulating apoptosis.
VZV may be present in the mitochondrial membrane during later stages of infection, as observed with other viral infections (Nykky et al. 2014). Since only HFL cells were investigated, we cannot comment on the universality of VZV-induced mitochondrial changes. Nonetheless, one explanation for VZV interaction with mitochondria may rest in the commandeering of glycolytic endpoints. Known as the Warburg effect, such commandeering is common in cancer biology and after viral infection (Guo et al. 2014; Hsieh et al. 2015). Further studies that measure respiration, glycolysis enzymes and products, and mitochondrial complex activity are needed to target the mechanisms by which VZV modulates mitochondrial morphology. The effect of VZV infection on intercellular ATP stores is not currently known, but the potential loss of mitochondrial ATP production would undoubtedly contribute to virus-induced autophagy observed both in VZV-infected human melanoma cells in tissue culture and skin biopsies containing zoster vesicles (Takahashi et al. 2009).
Acknowledgments
This work was supported by Public Health Service grants AG093716 (D.G.), AG032958 (D.G. and R.J.C.), and NS082228 (R.J.C.) from the National Institutes of Health, and VA Merit grant, CCTSI (UL1RR025780) and the Center for Women's Health Research (J.E.B.R.). Drs. H. Badani and N. L. Baird were supported by training grant NS007321 to Dr. Gilden from the National Institutes of Health. Dr. Keller was supported by training fellowship 1P01HL14985 from the National Institutes of Health. Mr. McClatchey is supported by a VA Merit grant (J.E.B.R.).
Footnotes
Compliance with ethical standards: Conflict of interest The authors declare that they have no conflict of interest.
References
- Ambagala APN, Cohen JI. Varicella-zoster virus IE63, a major viral latency protein, is required to inhibit the alpha interferon-induced antiviral response. J Virol. 2007;81:7844–7851. doi: 10.1128/JVI.00325-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambagala AP, Bosma T, Ali MA, Poustovoitov M, Chen JJ, Gershon MD, Adams PD, Cohen JI. Varicella-zoster virus immediate-early 63 protein interacts with human antisilencing function 1 protein and alters its ability to bind histones h3.1 and h3.3. J Virol. 2009;83:200–209. doi: 10.1128/JVI.00645-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiker A, Fabel K, Cozzio A, Zerboni L, Fabel K, Sommer M, Uchida N, He D, Weissman I, Arvin AM. Varicella-zoster virus infection of human neural cells in vivo. Proc Natl Acad Sci U S A. 2004;101:10792–10797. doi: 10.1073/pnas.0404016101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird NL, Bowlin JL, Cohrs RJ, Gilden D, Jones KL. Comparison of varicella-zoster virus RNA sequences in human neurons and fibroblasts. J Virol. 2014;88:5877–5880. doi: 10.1128/JVI.00476-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozidis P, Williamson CD, Colberg-Poley AM. Isolation of endoplasmic reticulum, mitochondria, and mitochondria-associated membrane fractions from transfected cells and from human cytomegalovirus-infected primary fibroblasts. Curr Protoc Cell Biol. 2007 doi: 10.1002/0471143030.cb0327s37. Chapter 3: Unit 3.27. [DOI] [PubMed] [Google Scholar]
- Brazeau E, Mahalingam R, Gilden D, Wellish M, Kaufer BB, Osterrieder N, Pugazhenthi S. Varicella-zoster virus-induced apoptosis in MeWo cells is accompanied by down-regulation of Bcl-2 expression. J Neurovirol. 2010;16:133–140. doi: 10.3109/13550281003682547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che X, Oliver SL, Sommer MH, Rajamani J, Reichelt M, Arvin AM. Identification and functional characterization of the Varicella zoster virus ORF11 gene product. Virology. 2011;412:156–166. doi: 10.1016/j.virol.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu VC, Bhattacharya S, Nomoto A, Lin J, Zaidi SK, Oberley TD, Weinman SA, Azhar S, Huang TT. Persistent expression of hepatitis C virus non-structural proteins leads to increased autophagy and mitochondrial injury in human hepatoma cells. PLoS One. 2011;6:e28551. doi: 10.1371/journal.pone.0028551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison AJ, Scott JE. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Di Valentin E, Bontems S, Habran L, Jolois O, Markine-Goriaynoff N, Vanderplasschen A, Sadzot-Delvaux C, Piette J. Varicella-zoster virus IE63 protein represses the basal transcription machinery by disorganizing the pre-initiation complex. Biol Chem. 2005;386:255–267. doi: 10.1515/BC.2005.031. [DOI] [PubMed] [Google Scholar]
- Duguay BA, Saffran HA, Ponomarev A, Duley SA, Eaton HE, Smiley JR. Elimination of mitochondrial DNA is not required for herpes simplex virus 1 replication. J Virol. 2014;88:2967–2976. doi: 10.1128/JVI.03129-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin TJ, McCarthy M, Osterrieder N, Cohrs RJ, Kaufer BB. Three-dimensional normal human neural progenitor tissue-like assemblies: a model of persistent varicella-zoster virus infection. PLoS Pathog. 2013;9:e1003512. doi: 10.1371/journal.ppat.1003512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Meng X, Ma J, Zheng Y, Wang Q, Wang Y, Shang H. Human papillomavirus 16 E6 contributes HIF-1α induced Warburg effect by attenuating the VHL-HIF-1±; interaction. Int J Mol Sci. 2014;15:7974–7986. doi: 10.3390/ijms15057974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habran L, El Mjiyad N, Di Valentin E, Sadzot-Delvaux C, Bontems S, Piette J. The varicella-zoster virus immediate-early 63 protein affects chromatin-controlled gene transcription in a cell-type dependent manner. BMC Mol Biol. 2007;8:99. doi: 10.1186/1471-2199-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood C, Cunningham AL, Slobedman B, Arvin AM, Sommer MH, Kinchington PR, Abendroth A. Varicella-zoster virus ORF63 inhibits apoptosis of primary human neurons. J Virol. 2006;80:1025–1031. doi: 10.1128/JVI.80.2.1025-1031.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh YC, Chen YM, Li CY, Chang YH, Liang SY, Lin SY, Lin CY, Chang SH, Wang YJ, Khoo KH, Aoki T, Wang HC. To complete its replication cycle, a shrimp virus changes the population of long chain fatty acids during infection via the PI3K-Akt-mTOR-HIF1α pathway. Dev Comp Immunol. 2015;53:85–95. doi: 10.1016/j.dci.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Huang CY, Chiang SF, Lin TY, Chiou SH, Chow KC. HIV-1 Vpr triggers mitochondrial destruction by impairing Mfn2-mediated ER-mitochondria interaction. PLoS One. 2012;7:e33657. doi: 10.1371/journal.pone.0033657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackers P, Defechereux P, Baudoux L, Lambert C, Massaer M, Merville-Louis MP, Rentier B, Piette J. Characterization of regulatory functions of the varicella-zoster virus gene 63-encoded protein. J Virol. 1992;66:3899–3903. doi: 10.1128/jvi.66.6.3899-3903.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadenbach B, Ramzan R, Vogt S. High efficiency versus maximal performance—the cause of oxidative stress in eukaryotes: a hypothesis. Mitochondrion. 2013;13:1–6. doi: 10.1016/j.mito.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Kaarbø M, Ager-Wick E, Osenbroch PØ, Kilander A, Skinnes R, Müller F, Eide L. Human cytomegalovirus infection increases mitochondrial biogenesis. Mitochondrion. 2011;11:935–945. doi: 10.1016/j.mito.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Keller AC, Knaub LA, McClatchey PM, Connon CA, Bouchard R, Miller MW, Geary KE, Walker LA, Klemm DJ, Reuch JE. Differential mitochondrial adaptation in primary vascular smooth muscle cells from a diabetic rat model. Oxid Med Cell Longev. 2016 doi: 10.1155/2016/8524267. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil MI, Sommer M, Arvin A, Hay J, Ruyechan WT. Regulation of the varicella-zoster virus ORF3 promoter by cellular and viral factors. Virology. 2013;440:171–181. doi: 10.1016/j.virol.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchington PR, Bookey D, Turse SE. The transcriptional regulatory proteins encoded by varicella-zoster virus open reading frames (ORFs) 4 and 63, but not ORF 61, are associated with purified virus particles. J Virol. 1995;69:4274–4282. doi: 10.1128/jvi.69.7.4274-4282.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenaga M, Wang T, Li Y, Showalter LA, Chan T, Sun J, Weinman SA. Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production. J Biol Chem. 2005;280:37481–37488. doi: 10.1074/jbc.M506412200. [DOI] [PubMed] [Google Scholar]
- Kramer T, Enquist LW. Alphaherpesvirus infection disrupts mito-chondrial transport in neurons. Cell Host Microbe. 2012;11:504–514. doi: 10.1016/j.chom.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Yoon Y. Mitochondrial fission: regulation and ER connection. Mol Cells. 2014;37:89–94. doi: 10.14348/molcells.2014.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman MG, Enquist LW. Herpesvirus interactions with the host cytoskeleton. J Virol. 2009;83:2058–2066. doi: 10.1128/JVI.01718-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JM, Kenyon TK, Grose C, Hay J, Ruyechan WT. Physical and functional interaction between the varicella zoster virus IE63 and IE62 proteins. Virology. 2002;302:71–82. doi: 10.1006/viro.2002.1555. [DOI] [PubMed] [Google Scholar]
- Mahalingam R, Wellish M, Cohrs R, Debrus S, Piette J, Rentier B, Gilden DH. Expression of protein encoded by varicella-zoster virus open reading frame 63 in latently infected human ganglionic neurons. Proc Natl Acad Sci U S A. 1996;93:2122–2124. doi: 10.1073/pnas.93.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchey PM, Keller AC, Bouchard R, Knaub LA, Reusch JEB. Fully automated software for quantitative measurements of mitochondrial morphology. Mitochondrion. 2015;26:58–71. doi: 10.1016/j.mito.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller NH, Graf LL, Orlicky D, Gilden D, Cohrs RJ. Phosphorylation of the nuclear form of varicella-zoster virus immediate-early protein 63 by casein kinase II at serine 186. J Virol. 2009;83:12094–12100. doi: 10.1128/JVI.01526-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller NH, Walters MS, Marcus RA, Graf LL, Prenni J, Gilden D, Silverstein SJ, Cohrs RJ. Identification of phosphorylated residues on varicella-zoster virus immediate-early protein ORF63. J Gen Virol. 2010;91:1133–1137. doi: 10.1099/vir.0.019067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller NH, Bos NL, Seitz S, Wellish M, Mahalingam R, Gilden D, Cohrs RJ. Recombinant monoclonal antibody recognizes a unique epitope on varicella-zoster virus immediate-early 63 protein. J Virol. 2012;86:6345–6349. doi: 10.1128/JVI.06814-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykky J, Vuento M, Gilbert L. Role of mitochondria in parvovirus pathology. PLoS One. 2014;9:e86124. doi: 10.1371/journal.pone.0086124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki N, Kikuno R, Ohara R, Inamoto S, Aizawa H, Yuasa S, Nakajima D, Nagase T, Ohara O, Koga H. Prediction of the coding sequences of mouse homologues of KIAA gene: II. The complete nucleotide sequences of mouse KIAA-homologous cDNAs identified by screening of terminal sequences of cDNA clones randomly sampled from size-fractionated libraries. DNA Res. 2003;10:35–48. doi: 10.1093/dnares/10.1.35. [DOI] [PubMed] [Google Scholar]
- Otsu NA. A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern. 1979;9:62–66. [Google Scholar]
- Peng JY, Lin CC, Hsu CN. 2010 International Conference on Technologies and Applications of Artificial Intelligence. IEEE Computer Soc; 2010. Adaptive image enhancement for fluorescence microscopy; pp. 9–16. [DOI] [Google Scholar]
- Pugazhenthi S, Nair S, Velmurugan K, Liang Q, Mahalingam R, Cohrs RJ, Nagel MA, Gilden D. Varicella-zoster virus infection of differentiated human neural stem cells. J Virol. 2011;85:6678–6686. doi: 10.1128/JVI.00445-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffran HA, Pare JM, Corcoran JA, Weller SK, Smiley JR. Herpes simplex virus eliminates host mitochondrial DNA. EMBO Rep. 2007;8:188–193. doi: 10.1038/sj.embor.7400878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi MN, Jackson W, Laird DT, Culp TD, Grose C, Haynes JI, Benetti L. Varicella-zoster virus infection induces autophagy in both cultured cells and human skin vesicles. J Virol. 2009;83:5466–5476. doi: 10.1128/JVI.02670-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischer BK, von Einem J, Kaufer B, Osterrieder N. Two-step red-mediated recombination for versatile high-efficiency markerless DNA manipulation in Escherichia coli. Biotechniques. 2006;40:191–197. doi: 10.2144/000112096. [DOI] [PubMed] [Google Scholar]
- Tischer BK, Kaufer BB, Sommer M, Wussow F, Arvin AM, Osterrieder N. A self-excisable infectious bacterial artificial chromosome clone of varicella-zoster virus allows analysis of the essential tegument protein encoded by ORF9. J Virol. 2007;81:13200–13208. doi: 10.1128/JVI.01148-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse MCL, Lane C, Mott K, Onlamoon N, Hsiao HM, Perng GC. ICAM-5 modulates cytokine/chemokine production in the CNS during the course of herpes simplex virus type 1 infection. J Neuroimmunol. 2009;213:12–19. doi: 10.1016/j.jneuroim.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, Bestwick M, Duguay BA, Raimundo N, MacDuff DA, Kaech SM, Smiley JR, Means RE, Iwasaki A, Shadel GS. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553–557. doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson CD, Colberg-Poley AM. Access of viral proteins to mitochondria via mitochondria-associated membranes. Rev Med Virol. 2009;19:147–164. doi: 10.1002/rmv.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Hildreth RL, Colberg-Poley AM. Human cytomegalovirus inhibits apoptosis by proteasome-mediated degradation of Bax at endoplasmic reticulum-mitochondrion contacts. J Virol. 2013;87:5657–5668. doi: 10.1128/JVI.00145-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuranski T, Nawar H, Czechowski D, Lynch JM, Arvin A, Hay J, Ruyechan WT. Cell-type-dependent activation of the cellular EF-1alpha promoter by the varicella-zoster virus IE63 protein. Virology. 2005;338:35–42. doi: 10.1016/j.virol.2005.05.005. [DOI] [PubMed] [Google Scholar]


