ABSTRACT
Amyotrophic lateral sclerosis is a devastating neuromuscular degenerative disease characterized by a focal onset of motor neuron loss, followed by contiguous outward spreading of pathology including TAR DNA-binding protein of 43 kDa (TDP-43) aggregates. Previous work suggests that TDP-43 can move between cells. Here we used a novel flow cytometry technique (FloIT) to analyze TDP-43 inclusions and propagation. When cells were transfected to express either mutant G294A TDP-43 fused to GFP or wild type TDP-43fused to tomato red and then co-cultured, flow cytometry detected intact cells containing both fusion proteins and using FloIT detected an increase in the numbers of inclusions in lysates from cells expressing wild type TDP-43-tomato. Furthermore, in this same model, FloIT analyses detected inclusions containing both fusion proteins. These results imply the transfer of TDP-43 fusion proteins between cells and that this process can increase aggregation of wild-type TDP-43 by a mechanism involving co-aggregation with G294A TDP-43.
KEYWORDS: ALS, Flow cytometry, FloIT, Protein Aggregation, Propagation, Prion, TDP-43
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is an incurable neurodegenerative disorder characterized by the loss of both the upper and lower motor neurons in the brain and spinal cord respectively, resulting in the progressive paralysis of the muscles connected with speech, limbs, swallowing and respiration, due to the progressive degeneration of innervating motor neurons.1
The presence of aberrant protein aggregates in affected neurons is characteristic of most neurodegenerative diseases,2,3 however, the mechanisms that underlie pathology are not yet completely understood. Aberrant interactions of hydrophobic oligomeric protein aggregates with cellular structures has been proposed as one explanation for toxicity associated with protein aggregates.4 An increasing number of recent studies now support the prion-like propagation of a range of aggregated proteins associated with various neurodegenerative diseases [reviewed in5]. In addition to SOD1,6 TAR DNA-binding protein of 43 kDa (TDP-43) aggregate propagation has been compared with a prion-like seeding mechanism, with evidence of regional spreading of pathology occurring in a sequential pattern through the neuroaxis.7 Initially, evidence from HEK293 cells suggested that recombinantly produced TDP-43 could trigger aggregation of cell produced TDP-43 after its translocation into the cell,8 consistent with a seeding reaction. It has also been observed that insoluble TDP-43 from human ALS brain could seed further TDP-43 aggregation in SH-SY5Y cells after its artificial translocation into cells, and that these aggregates could transfer to naïve cells.9 Similarly, recent work has also observed that TDP-43 aggregates from ALS brain and spinal cord can seed cellular TDP-43 aggregation after protein transfection, and that aggregates can move cell to cell in a co-culture system using HEK293 cells.10 An additional study presented evidence to suggest that TDP-43 can transfer between HEK293 cells and primary cortical mouse neurons and interact with endogenous TDP-43 in recipient cells.11 More recently, it was observed that TDP-43 fibrils formed from short C-terminal derived peptides triggered the seed-dependent aggregation of wild type TDP-43 (TDP-43WT) or TDP-43 lacking a nuclear localization signal in SH-SY5Y cells resulting in different peptides sequences of TDP-43 producing fibrils with distinct biochemical properties reminiscent of prion strains.12
Recently a novel method for quantifying protein inclusions was described.13 We reasoned that this method might be used to provide a quick and robust measure of prion-like propagation. Here we describe the cell-to-cell propagation of TDP-43 using flow cytometric analysis combined with FloIT. We find that mutant TDP-43 fused to a fluorescent protein can move from cell-to-cell and cause an increase in TDP-43WT aggregation by a mechanism involving co-aggregation with TDP-43G294A. This technique may be useful for the quantification of protein aggregation propagation regardless of the pathological protein involved.
MATERIALS AND METHODS
Cell Lines
The mouse neuroblastoma x spinal cord hybrid cell line (NSC-34 cells14 were routinely cultured in DMEM/F-12 supplemented with 10% (v/v) fetal bovine serum and 2 mM GlutaMAX. Cells were maintained in an incubator at 37°C under a humidified atmosphere containing 5% (v/v) CO2.
Transient Transfections
The pCMV6-AC-tGFP expression vector containing TDP-43WT cDNA was obtained from Origene. TDP-43-tomato red (TDP-43-tdTomato) constructs were created by replacing the tGFP sequences with tdTomato (Genscript, USA); site-directed mutagenesis was also performed by Genscript (USA) to create the G294A mutant (TDP-43G294A). NSC-34 cells (2 – 3 × 104 cells/0.2 mL/chamber) were cultured in 8-chamber slides in complete culture medium and were incubated overnight at 37°C in a humidified atmosphere containing 5% (v/v) CO2. Cells were then incubated in DMEM-F-12 serum-free culture medium containing 2 μg pCMV6-TDP-43WT-tdTomato red plasmid DNA and Lipofectamine 2000 for 5 h under the same conditions. Cells were then washed once with serum-free medium and replenished with complete culture medium before being incubated for a further 24, 48, or 72 h.
Confocal Microscopy
An inverted microscope (DM IBRE) and a Leica TCS SP confocal imaging system were used to image transfected NSC-34 cells, collecting both tGFP and tdTomato fluorescence (tGFP: 488 nm excitation, 495–515 nm emission; tdTomato: 561 nm excitation, emission 590–630 nm). Fluorescence and bright field (differential interference contrast; DIC) images were captured using Leica confocal software.
Flow Cytometry (FloIT) Assay
The FloIT assay was performed as outlined in.13 Briefly, NSC‐34 cells were transiently transfected to express TDP-43 (TDP-43WT-tdTomato and/or TDP-43G294A‐tGFP) using Lipofectamine 2000. At 24, 48 and 72 h post-transfection, cells were harvested, washed x3 by centrifugation, and then resuspended in phosphate buffered saline (PBS; 0.5 mL/tube). An aliquot of cells (2 × 105 cells in 0.15 mL) were analyzed for transfection efficiency. The remaining cells (4 × 105 cells in 0.35mL) were washed as above and lysed in 0.5% (v/v) Triton X-100 in PBS before analysis. Cell lysates were then incubated with RedDot2 (1:1000) at RT for 2 min. Events were collected using a LSRFortessa X‐20 Cell Analyzer (BD Biosciences); excitation laser lines and emission band pass filters used were, respectively, 488 nm and 525/50 (tGFP), 561 and 586/15 (tdTomato), and 640 and 670/30 (RedDot2). All parameters collected were set to log10 during acquisition. The forward scatter (FSC) threshold was set to the minimum value (200 AU) to minimise exclusion of small protein inclusions. Nuclei were identified and enumerated based on RedDot2 fluorescence and forward scatter. The non-nuclear particles were analyzed for the presence of inclusions based on GFP/tdTomato fluorescence, forward scatter and comparison with lysates prepared from cells expressing only the corresponding fluorescent protein. The number of inclusions in the population of particles was normalized to the number of nuclei, and reported as inclusions/100 transfected cells (iFloIT) according to the equation
where ni represents the number of inclusions acquired, nnuc is the number of nuclei acquired, and γ is the transfection efficiency (expressed as a fraction). Analysis of all events was performed using FlowJo software version 10 (Tree Star, Ashland, OR). To account for expression level of the various constructs when comparing between fluorescent protein only controls and TDP-43 fusions, we adjusted the fluorescent protein control score by making the final score relative to the factor increase in fluorescence over the quantified mean cellular fluorescence of TDP-43 fusions collected before lysis.
Presentation of Data and Statistical Analyses
Data is presented as the mean ± SEM. ANOVA paired with Tukey's HSD multiple comparison post-tests were used to analyze and compare differences between multiple treatments. Unpaired student's t-tests were performed for single treatment comparisons. Prism 5 for Windows (Version 5.01) (GraphPad Software, San Diego, CA) was used to perform the statistical analyses. Differences were defined as significant for P < 0.05.
RESULTS
FloIT Quantifies TDP-43 Aggregation and Inclusion Formation in NSC-34 Cells
TDP-43 is normally localized mostly in the nucleus where it performs its biological functions, including RNA processing.15,16 Under certain conditions, however, TDP-43 is known to mislocalise to the cytosol and form insoluble aggregates,17 although the mechanisms underlying TDP-43 mislocalisation and aggregation are not completely understood. TDP-43 inclusions have been quantified by FloIT previously.13 Here, we quantified inclusion formation of either TDP-43WT or TDP-43G294A, fused to tdTomato or tGFP respectively, over a 72 h period. TDP-43 inclusions were found to be juxtanuclear cloud-like aggregates (Fig. 1A & D) as previously observed.18 The aggregates were dense with little diffusible component (Fig. S1) as previously found with M337V TDP-43 aggregates.18 Cells expressing TDP-43G294A had more large trappable material reactive with anti-TDP-43 antibody as judged by a filter trap assay (Fig. S2), consistent with aggregates being formed in those cells. In addition, although infrequent, aggregates of tGFP and tdTomato only controls also had little diffusable material as judged by FRAP (Fig. S1), and had various sized globular aggregates in the cytoplasm (Fig. S3). The cells were lysed in 0.5% (v/v) Triton X-100 in PBS and the lysate examined by flow cytometry. Particles in the lysate containing nucleic acid, including nuclei, were identified by RedDot2 staining and FSC and gated out during flow cytometric analysis (Fig. 1B & E). Inclusions were then distinguished from cellular debris by exploiting the fluorescent protein tag fused to TDP-4313 (Fig. 1C & F). Fluorescent particles large enough to be detected by the flow cytometer were then counted as inclusions. Given the previously measured transfection efficiency of the sample, the numbers of inclusions are then reported as inclusions counted per 100 transfected cells. Compared to cells expressing fluorescent proteins alone, there was a significant increase in the numbers of inclusions in cells expressing either TDP-43WT or TDP-43G294A by 72 h (Fig. 1G & H). Consistent with the G294A mutant TDP-43 being more aggregation-prone, cells expressing TDP-43G294A-tGFP formed inclusions more rapidly than cells expressing TDP-43WT-tdTomato. Similar results were obtained if inclusions were counted manually from microscopy images (Figs. S4-S8).
Figure 1.
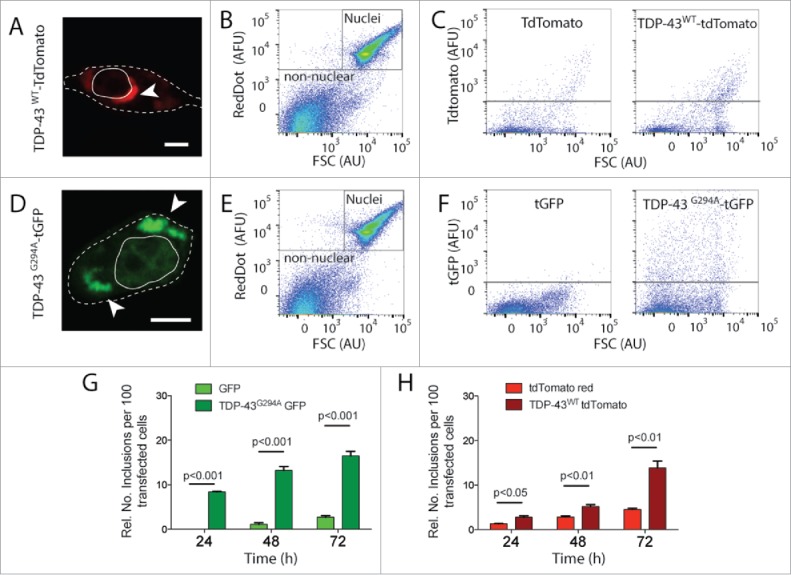
FloIT quantifies TDP-43 inclusions in NSC-34 cells. NSC‐34 cells were transiently transfected with either TDP-43WT–tdTomato or TDP-43G294A-tGFP fusion proteins and incubated for either 24, 48 or 72 h. (A, D) Confocal microscopy identifies extra nuclear TDP-43 that has formed distinct inclusions (white arrow head; Scale bar 5 μm). Dashed line represents cell border from transmission image enclosed line represents the nucleus. (B, E) Representative 2 parameter, pseudo-color flow cytometry plots showing identification of nuclei and non-nuclear particles (indicated) on the basis of forward scatter (FSC; arbitrary units, AU) and RedDot2 fluorescence (arbitrary fluorescence units, AFU). (C, F). Non-nuclear particles (gated as shown in representative plots (B, E)) analyzed for tdTomato (C) or tGFP (F) fluorescence versus FSC in lysates prepared from cells transfected with plasmids encoding TDP-43WT–tdTomato or TDP-43G294A-tGFP or fluorescent proteins alone (left). Inclusions are identified by their tdTomato or tGFP fluorescence. (G, H) Quantification of inclusions (FloIT) as number of inclusions per 100 transfected cells formed by TDP-43G294A-tGFP and TDP-43WT–tdTomato, compared with lysates prepared from cells expressing fluorescent proteins only. Results shown are the mean number of inclusions per 100 transfected cells ± SEM, n = 3 independent experiments. Significant differences indicated with p value represent unpaired T-test comparisons between treatments indicated by line.
TDP-43 Can Transfer from Cell-to-Cell
Next, the ability of TDP-43 to transfer cell-to-cell in culture was examined. We created 2 distinct cell populations by transiently transfecting with either TDP-43WT tdTomato or TDP-43G294A tGFP. Cells were co-cultured at a ratio of 1:1 and analyzed using flow cytometry on the intact cells (Fig. 2 B). Control cells were either transfected separately then mixed immediately before flow cytometry analysis (Fig. 2 A) or immediately co-transfected (positive control; Fig. 2 C). The co-transfection positive control (Fig. 2 C) aimed to show the maximum percentage of cells having both proteins. Transfer events were quantified as the percentage of transfected cells positive for both tGFP/tdTomato using a similar strategy previously published for Htt.19 While control cells showed negligible numbers of cells measured as 2 colored (Fig. 2 D), greater than 10% of transfected cells in the co-cultured cell populations contained both fusion proteins at 24 h. The number of cells containing both fusion proteins dropped at 72 h, suggesting that transfer of pathogenic proteins may be particularly toxic. Co-transfection of both TDP-43WT-tdTomato and TDP-43G294A-tGFP resulted in almost 50% of transfected cells being detected as containing both fusion proteins at 24 h, again these numbers dropped over time suggesting that expression of both proteins is detrimental to cell viability (Fig. 2 D). This was confirmed by following the cells lost from culture which showed that coculture or cotransfection of TDP-43WT tdTomato and TDP-43G294A tGFP increases the rate at which cells are lost (Fig. S9). Collectively, these data suggest that TDP-43 is capable of transferring between cells in culture.
Figure 2.
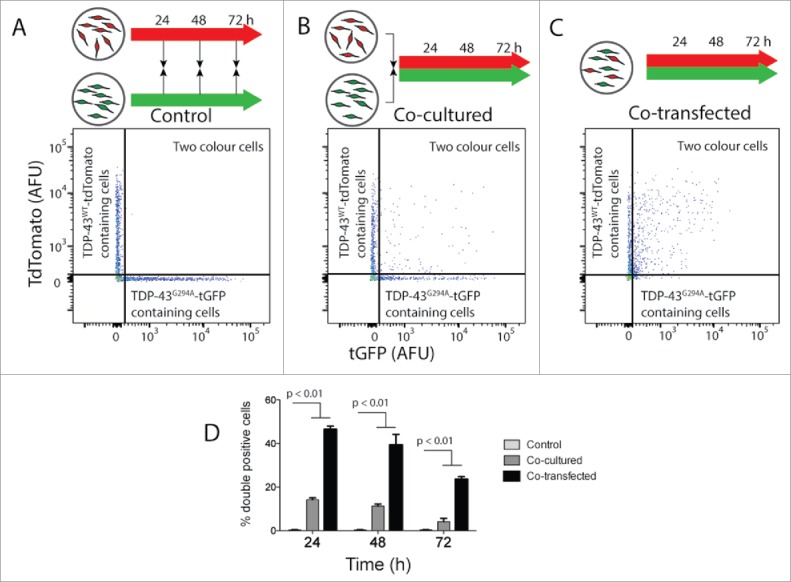
TDP-43WT or TDP-43G294A proteins can transfer from cell to cell in NSC-34 cells. NSC‐34 transiently transfected with TDP-43WT-tdTomato or TDP-43G294A-tGFP were either mixed (indicated by black arrows in schematic diagram) at the end of the incubation (A), immediately following transfection (B). Alternatively, cells were co-transfected to express both fusion proteins (C). After incubation for either 24, 48 or 72 h live intact cells were analyzed by flow cytometry. (A-C) Representative 2 parameter, pseudo-color flow cytometry plots showing identification of cells expressing TDP-43WT-tdTomato or TDP-43G294A-tGFP and cells containing both from the 48 h timepoint. (D) Histogram showing double positive cells as a percentage of all cells containing a fluorescent protein. Results shown as mean percentage of cells ± SEM, n = 3 independent experiments. Significant differences indicated with p value represent unpaired T-test comparisons between treatments indicated by line.
TDP-43G294A-tGFP Can Induce Inclusion Formation of TDP-43WT-tdTomato Protein in Co-Cultured NSC-34 Cells
Given the ability of TDP-43 to move from one cell to another, we next asked if TDP-43 transfer resulted in an increase in inclusion formation and co-aggregation of TDP-43WT-tdTomato and TDP-43G294A-tGFP. To test this, we used FloIT to quantify inclusions in cells that were either co-transfected with TDP-43WT-tdTomato and TDP-43G294A-tGFP, or transfected separately and the cell populations mixed immediately or just before analysis (Fig. 3). In particular, the number of the less aggregation prone TDP-43WT-tdTomato inclusions was used to determine whether the migration of TDP-43 from one cell population could induce inclusion formation in the other. A significant and almost 2-fold increase in number of TDP-43WT tdTomatoinclusions was detected in the co-transfected treatment (positive control; Fig. 3 C & D) compared with cells mixed just before analysis (Fig. 3 A & D) at each time point. This suggests that within individual cells mutant, TDP-43G294A-tGFP induced the aggregation of TDP-43WT-tdTomato and subsequent inclusion formation. Importantly, co-culture of cells expressing TDP-43G294A-tGFPand TDP-43WT-tdTomato also significantly increased the proportion of TDP-43WT-tdTomato expressing cells that contained inclusions (Fig. 3 B & D). This seeding effect was not observed when cells were co-cultured expressing the fluorescent proteins alone (Fig. S10). This is consistent with our observation that TDP-43 can move between cells and suggests that the cell to cell transfer of TDP-43 results in an increase in the aggregation of TDP-43WT.
Figure 3.
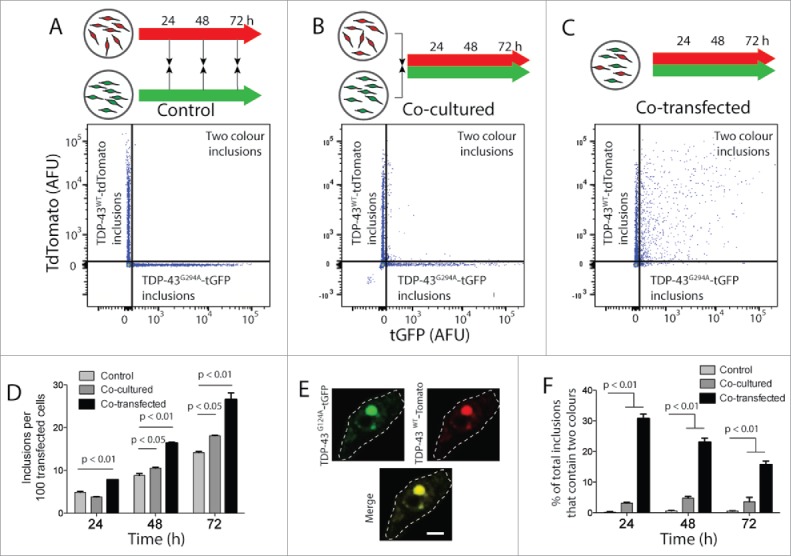
FloIT detects 2-color inclusions NSC-34 cell lysates. NSC‐34 transiently transfected with TDP-43WT-tdTomato or TDP-43G294A-tGFP were either mixed at the end of the incubation (A), or immediately following transfection (B). Alternatively, cells were co-transfected to express both fusion proteins (C). After incubation for either 24, 48 or 72 h cells were lysed and analyzed by FloIT. (A-C) Two-parameter, pseudo-color flow cytometry plots showing identification of inclusions containing TDP-43WT-tdTomato or TDP-43G294A-tGFP and inclusions containing both. (D) The total number of TDP-43WT-tdTomato inclusions including dual color inclusions were enumerated by FloIT and are shown as means ± SEM (n = 3). (E) Confocal microscopy of an NSC-34 cell with an inclusion containing both TDP-43WT-tdTomato and TDP-43G294A-tGFP. Dashed line represents cell border from transmission image. Scale bar 5 μm. (F) The percentage of TDP-43 inclusions formed in each treatment at time intervals that contained both TDP-43WT-tdTomato and TDP-43G294A-tGFP enumerated by FloIT are shown as means ± SEM (n = 3 independent experiments). Significant differences indicated with p value represent unpaired T-test comparisons between treatments indicated by line.
Our previous work demonstrates that mutant M337V TDP-43-tGFP co-aggregates inside cells with the WT form, TDP-43WT-tdTomato.18 Confocal microscopy shows co-localization of TDP-43WT-tdTomato and TDP-43G294A-tGFP when these are co-expressed in cells (Fig. 3 E; for lower magnification images see Figs. S11-S12). FloIT can quantify the proportion of inclusions containing 2 different proteins fused to distinct fluorescent protein tags.13 Here we took advantage of this and quantified the proportion of inclusions that contained both TDP-43WT-tdTomato and TDP-43G294A-tGFP. This measurement likely represents seeding and subsequent co-aggregation of TDP-43WT-tdTomato and TDP-43G294A-tGFP to forminclusions. When we count all inclusions together (red only, green only and inclusions that are red and green), from co-cultured cells we find that ∼5% of all inclusions contain both TDP-43WT-tdTomato and TDP-43G294A-tGFP (Fig. 3F). This is significantly greater than cells cultured separately and significantly less than the large proportion of 2 color aggregates when cells are co-transfected to express both TDP-43WT-tdTomato and TDP-43G294A-tGFP (Fig. 3F). Interestingly, the number of 2 color aggregates in co-transfected cells decreases with time, again consistent with the fact that expression of both proteins is detrimental to cell viability (Fig. S9).
DISCUSSION
Mutations in the TDP-43 gene are found in sporadic and non-SOD1 familial ALS, implicating TDP-43 as a contributing factor to disease.20,21 TDP-43 has previously been reported to spontaneously form inclusions in yeast and mammalian cell models that resemble TDP-43 deposits in degenerating neurons of ALS patients.18,22 Furthermore, previous studies have reported that in the TDP-43 protein sequence, the C-terminal domain is critical for spontaneous aggregation and that TDP-43 is intrinsically aggregation prone.22 In addition, previous work indicates that ALS associated mutations in TDP-43 can increase the number of discrete aggregates in the cytoplasm of yeast22 and mammalian cells18 suggesting that ALS mutations increase the aggregation propensity of TDP-43. The work presented here is consistent with these data, as using FloIT, we observed significant levels of inclusion formation by TDP-43G294A-tGFP from as early as 24 hours, while TDP-43WT-tdTomato required 72 hours to produce lower levels of inclusions.
We also describe the use of the rapid flow cytometry technique FloIT13 to quantify seeding and co-aggregation of proteins expressed in 2 separate populations of cells. In previous co-culture experiments using SH-SY5Y (human neuroblastoma) cells, it was shown that TDP-43 can transfer between cells and that phosphorylated TDP-43 aggregates can be released from donor cells and taken up into recipient cells.9 Using flow cytometry, in the current study we have also demonstrated movement of TDP-43 from one NSC-34 cell population to another. We also used FloIT to quantify seeding of inclusions in cells expressing TDP-43WT-tdTomato and to quantify the contribution of any co-aggregation between the 2 different TDP-43 fusion proteins to this phenomenon. In experiments in which separately transfected cells expressing either of the 2 TDP-43 fusion proteins were co-cultured, we observed that, those cells expressing TDP-43WT-tdTomato had increased numbers of inclusions relative to the same cells incubated in monoculture.
We suggest that TDP-43G294A-tGFP has been released from cells and subsequently taken up by cells expressing TDP-43WT-tdTomato to seed the formation of inclusions in the latter cell type, although our data does not conclusively show which TDP-43 fusion has been transferred. The detection of dual-colored inclusions in a fraction of co-cultured cells originally transfected to express TDP-43WT-tdTomato is entirely consistent with this conclusion. These results suggest that aggregates or inclusions formed by mutant TDP-43 are able to escape from cells and can be taken up by other cells. Previous work has shown that TDP-43 aggregates can be taken up by NSC-34 cells via macropinocytosis.23 It is likely that this mode of uptake is not specific to TDP-43 but to protein aggregates in general.24,25 Similarly, both conditioned media containing TDP-43 from cultured cells, and homogenates from ALS patient brains, have been shown to interact with cell produced TDP-43 using a split luciferase reporter11 and transduced TDP-43 aggregates from brain material has been shown to increase cell produced TDP-43 aggregation via western blot.8,9 It is interesting to note that the transmission rates are low in these experiments. However, others10 also find low proportions of cell-to-cell transfer of TDP-43 aggregates in 2D culture. Moreover, similar experiments with Htt polyQ showed 3.7% of cells had both donor and recipient constructs,19 indicating that in general these types of experiments have low rates of transfer, likely due to the fact that the culture is 2D and that the cells interface with media and the plastic more than adjacent cells.
In conclusion, our findings support the idea that TDP-43 aggregation can be propagated in a cell-to-cell fashion. Further, we also demonstrate the utility of FloIT for quantifying this process and suggest that this technique may be useful to quantify the cell-to-cell propagation of protein aggregation regardless of the individual pathogenic protein involved.
Supplementary Material
ABBREVIATIONS
- ALS
Amyotrophic Lateral Sclerosis
- FloIT
flow cytometric analysis of inclusions and trafficking
- FBS
Fetal Bovine Serum
- GFP
Green Fluorescent Protein
- PBS
Phosphate Buffered Saline
- SOD1
Cu/Zn Superoxide Dismutase
- TDP-43
TAR DNA Binding Protein
- WT
Wild-type
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflict of interest was reported by the authors.
FUNDING
This work was supported by the NHMRC under Grant 1084144 and 1095215.
REFERENCES
- [1].Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: Deciphering selective motor neuron death in ALS. Nature Reviews 2001; 2:806-819; PMID:11715057; https://doi.org/ 10.1038/35097565 [DOI] [PubMed] [Google Scholar]
- [2].Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 2006; 75:333-66; PMID:16756495; https://doi.org/ 10.1146/annurev.biochem.75.101304.123901 [DOI] [PubMed] [Google Scholar]
- [3].Yerbury JJ, Ooi L, Dillin A, Saunders DN, Hatters DM, Beart PM, Cashman NR, Wilson MR, and Ecroyd H. Walking the tightrope: proteostasis and neurodegenerative disease. J Neurochem 2016; 137(4):489-505; PMID:26872075; https://doi.org/ 10.1111/jnc.13575 [DOI] [PubMed] [Google Scholar]
- [4].Bolognesi B, Kumita JR, Barros TP, Esbjorner EK, Luheshi LM, Crowther DC, Wilson MR, Dobson CM, Favrin G, Yerbury JJ. ANS binding reveals common features of cytotoxic amyloid species. ACS Chem Biol 2010; 5(8):735-40; PMID:20550130; https://doi.org/ 10.1021/cb1001203 [DOI] [PubMed] [Google Scholar]
- [5].Marciniuk K, Taschuk R, Napper S. Evidence for prion-like mechanisms in several neurodegenerative diseases: potential implications for immunotherapy. Clin Dev Immunol 2013; 2013:473706; PMID:24228054; https://doi.org/ 10.1155/2013/473706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Grad LI, Yerbury JJ, Turner BJ, Guest WC, Pokrishevsky E, O'Neill MA, Yanai A, Silverman JM, Zeineddine R, Corcoran L, et al.. Intercellular propagated misfolding of wild-type Cu/Zn superoxide dismutase occurs via exosome-dependent and -independent mechanisms. Proc Natl Acad Sci U S A 2014; 111(9):3620-5; PMID:24550511; https://doi.org/ 10.1073/pnas.1312245111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, Suh E, Van Deerlin VM, Wood EM, Baek Y, et al.. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol 2013; 74(1):20-38; PMID:23686809; https://doi.org/ 10.1002/ana.23937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Furukawa Y, Kaneko K, Watanabe S, Yamanaka K, Nukina N. A seeding reaction recapitulates intracellular formation of Sarkosyl-insoluble transactivation response element (TAR) DNA-binding protein-43 inclusions. J Biol Chem 2011; 286(21):18664-72; PMID:21454603;https://doi.org/ 10.1074/jbc.M111.231209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nonaka T, Masuda-Suzukake M, Arai T, Hasegawa Y, Akatsu H, Obi T, Yoshida M, Murayama S, Mann DM, Akiyama H, et al.. Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep 2013; 4(1):124-34; PMID:23831027; https://doi.org/ 10.1016/j.celrep.2013.06.007 [DOI] [PubMed] [Google Scholar]
- [10].Smethurst P, Newcombe J, Troakes C, Simone R, Chen YR, Patani R, Sidle K. In vitro prion-like behaviour of TDP-43 in ALS. Neurobiol Dis 2016; 96:236-247; PMID:27590623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Feiler MS, Strobel B, Freischmidt A, Helferich AM, Kappel J, Brewer BM, Li D, Thal DR, Walther P, Ludolph AC, et al.. TDP-43 is intercellularly transmitted across axon terminals. J Cell Biol 2015; 211(4):897-911; PMID:26598621; https://doi.org/ 10.1083/jcb.201504057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shimonaka S, Nonaka T, Suzuki G, Hisanaga S, Hasegawa M. Templated Aggregation of TAR DNA-binding Protein of 43 kDa (TDP-43) by Seeding with TDP-43 Peptide Fibrils. J Biol Chem 2016; 291(17):8896-907; PMID:26887947; https://doi.org/ 10.1074/jbc.M115.713552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Whiten DR, San Gil R, McAlary L, Yerbury JJ, Ecroyd H, Wilson MR. Rapid flow cytometric measurement of protein inclusions and nuclear trafficking. Sci Rep 2016; 6:31138; PMID:27516358; https://doi.org/ 10.1038/srep31138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cashman NR, Durham HD, Blusztajn JK, Oda K, Tabira T, Shaw IT, Dahrouge S, Antel JP. Neuroblastoma x spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev Dynam 1992; 194:209-221; https://doi.org/ 10.1002/aja.1001940306 [DOI] [PubMed] [Google Scholar]
- [15].Lee EB, Lee VMY, Trojanowski JQ. Gains or losses: Molecular mechanisms of TDP43-mediated neurodegeneration. Nature Reviews Neuroscience 2012; 13(1):38-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Buratti E, Baralle FE. Characterization and functional implications of the RNA binding properties of Nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J Biol Chem 2001; 276(39):36337-36343 [DOI] [PubMed] [Google Scholar]
- [17].Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, et al.. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. J Cell Sci 2006; 314:130-133 [DOI] [PubMed] [Google Scholar]
- [18].Farrawell NE, Lambert-Smith IA, Warraich ST, Blair IP, Saunders DN, Hatters DM, Yerbury JJ. Distinct partitioning of ALS associated TDP-43, FUS and SOD1 mutants into cellular inclusions. Sci Rep 2015; 5:13416; PMID:26293199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Costanzo M, Abounit S, Marzo L, Danckaert A, Chamoun Z, Roux P, Zurzolo C. Transfer of polyglutamine aggregates in neuronal cells occurs in tunneling nanotubes. J Cell Sci 2013; 126(Pt 16):3678-85; PMID:23781027 [DOI] [PubMed] [Google Scholar]
- [20].Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Velde CV, Bouchard JP, Lacomblez L, Pochigaeva K, Salachas F, et al.. TARDBP Mutations in Individuals with Sporadic and Familial Amyotrophic Lateral Sclerosis. Nature Genetics 2008; 40(5):572-574; PMID:18372902 [DOI] [PubMed] [Google Scholar]
- [21].Sreedharan J, Blair LP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, et al.. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Journal of Cell Science 2008; 319:1668-1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Johnson BS, Snead D, Lee JJ, McCaffery JM, Shorter J, Gitler AD. TDP-43 is intrinsically aggregation-prone, and amyotrophic lateral sclerosis-linked mutations accelerate aggregation and increase toxicity. J Biol Chem 2009; 284(30):20329-39; PMID:19465477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zeineddine R, Pundavela JF, Corcoran L, Stewart EM, Do-Ha D, Bax M, Guillemin G, Vine KL, Hatters DM, Ecroyd H, et al.. SOD1 protein aggregates stimulate macropinocytosis in neurons to facilitate their propagation. Mol Neurodegener 2015; 10:57; PMID:26520394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yerbury JJ. Protein aggregates stimulate macropinocytosis facilitating their propagation. Prion 2016; 10(2):119-26; PMID:26963158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zeineddine R, and Yerbury JJ, The role of macropinocytosis in the propagation of protein aggregation associated with neurodegenerative diseases. Front Physiol 2015; 6:277; PMID:26528186 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


