Summary
Blood-central nervous system (CNS) barriers partition neural tissues from the blood, providing a homeostatic environment for proper neural function. The endothelial cells that form blood-CNS barriers have specialized tight junctions and low rates of transcytosis to limit the flux of substances between blood and CNS. However, the relative contributions of these properties to CNS barrier permeability are unknown. Here, by studying functional blood-retinal barrier (BRB) formation in mice, we found that immature vessel leakage occurs entirely through transcytosis, as specialized tight junctions are functional as early as vessel entry into the CNS. A functional barrier forms only when transcytosis is gradually suppressed during development. Mutant mice with elevated or reduced levels of transcytosis have delayed or precocious sealing of the BRB, respectively. Therefore, the temporal regulation of transcytosis governs the development of a functional BRB and suppression of transcytosis is a principal contributor for functional barrier formation.
Keywords: blood-CNS barrier, blood-retinal barrier, blood-brain barrier, endothelial cells, transcytosis, tight junctions, Mfsd2a, Cav-1, retinal vasculature, pericytes
Introduction
CNS endothelial cells lining blood vessels form the blood-CNS barriers, which include the blood-brain barrier, blood-spinal cord barrier, and inner BRB (Engelhardt and Coisne, 2011). These cells have two properties that limit the passage of substances between the blood and the CNS parenchyma: 1) specialized tight junction complexes between CNS endothelial cells prevent paracellular flux; and 2) low rates of vesicular trafficking between the luminal and abluminal membrane, known as transcytosis, limit transcellular passage (Andreone et al., 2015; Chow and Gu, 2015; Raviola, 1977; Reese and Karnovsky, 1967; Zhao et al., 2015). Although tight junctions are thought to be the principal mechanism for establishing blood-CNS barriers, recent findings suggest that altered rates of transcytosis also influence barrier permeability (Ben-Zvi et al., 2014; Knowland et al., 2014a). Barrier properties are not intrinsic to CNS endothelial cells, but rather are acquired from the neural environment during development (Blanchette and Daneman, 2015; Hagan and Ben-Zvi, 2015; Stewart and Wiley, 1981). However, it is not understood when and how specialized tight junctions and suppression of transcytosis occur, and what their relative contributions are in establishing a functional CNS barrier during development. Loss of barrier function is a hallmark of some CNS degenerative diseases (Obermeier et al., 2013; Winkler et al., 2014; Zlokovic, 2008). Yet, a functional barrier is also a major obstacle for CNS drug delivery (Banks, 2016). Dissecting the relative roles of tight junctions and transcytosis in regulating barrier permeability and elucidating the basic principles governing the establishment of CNS barrier properties will enable targeted manipulations of the barrier during disease.
The retinal vasculature is well-suited to address many fundamental questions about CNS barriers. Physiologically analogous to the blood-brain barrier, the BRB regulates the optimal milieu for phototransduction. Unlike the complex brain vascular network, the retinal vasculature has a relatively simple, stereotypic development and architecture, consisting of a three-tiered, two-dimensional plexus (Stone et al., 1995). Mouse retinal angiogenesis occurs shortly after birth. CNS endothelial cells invade the optic nerve head and expand radially along the vitreal surface from the center towards the periphery, forming the entire primary plexus by postnatal day 8 (P8) (Figures 1A and 1B) (Fruttiger, 2007). Sprouts from the primary plexus then penetrate into the retina, forming the deeper plexus, followed by further sprouting to form the intermediate plexus (Stahl et al., 2010). Here, we use the BRB as a model system to elucidate the relative contributions of tight junctions and transcytosis in regulating CNS barrier permeability during development. To our surprise, we found that functional tight junctions are already present when vessels first entered the CNS so the gradual suppression of transcytosis in CNS endothelial cells governs the development of a functional barrier.
Figure 1. Spatio-temporal characterization of functional BRB formation, See also Figure S1, S2 and S5.
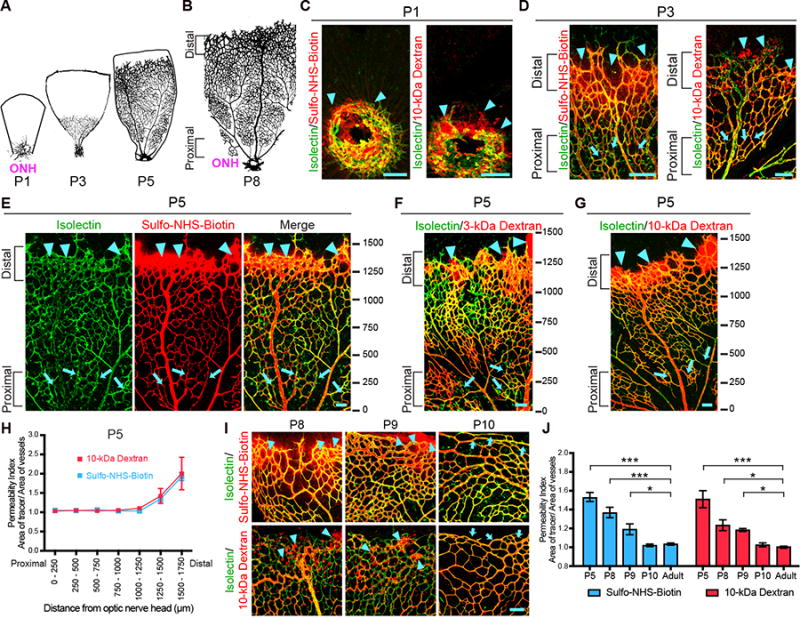
Intravenous injections of tracers in neonatal mice reveal that functional BRB is formed gradually from proximal to distal in the primary plexus. (A-B) Spatio-temporal development of the mouse retina primary plexus. (A)Vessel ingression at the optic nerve head (ONH) occurs at P1. Vessels then expand from the ONH to the retinal periphery during postnatal development. (B) Vessels reach the peripheral edge of the retina at P8. A spatio-temporal gradient of vessel maturation is observed: the vessels proximal to the ONH are more mature whereas the vessels distal to the ONH are nascent. (C) In P1 retinas, when blood vessels (isolectin; green) first enter the retina, Sulfo-NHS-Biotin (left) and 10-kDa dextran (right) tracer (red) leakage is observed around budding vessels (arrowheads). (D-G) In P3 (D) and P5 (E-G) retinas, a gradient of barrier functionality is observed. More mature vessels proximal to the ONH confine tracer (arrows) whereas nascent vessels distal to the ONH leak tracer (arrowheads). Ticks in (E-G) represent distance in microns from the ONH. 0 is slightly before the ONH. (H) Permeability index of P5 retinas from tracer-injected pups reveals the functional BRB is gradually formed in a proximal to distal fashion. The permeability index is measured by the ratio of tracer-positive area over isolectin-positive area. A ratio greater than 1 indicates that the BRB is permeable and immature. A ratio of 1 indicates that the BRB is impermeable and hence mature. 250 μm2 field of views were sampled and tiled starting from 0 to the angiogenic front. The average distance from the ONH to the angiogenic front at P5 is 1651 ± 288 μm. Data are mean ± s.e.m. (n = 5-6). (I) Higher magnification of distal vessels of the primary plexus show Sulfo-NHS-Biotin (top) and 10-kDa Dextran (bottom) tracer leakage at P8 and P9 (arrowheads). At P10, both tracers are confined in distal vessels (arrows). (J) Quantification of distal vessel permeability for Sulfo-NHS-Biotin and 10-kDa dextran throughout BRB development. Data are mean ± s.e.m. (n = 5-6 mice per age per tracer group, from 3 different litters). Statistical significance was determined by oneway ANOVA with a post-hoc Bonferroni multiple comparison adjustment, comparing the various neonatal ages with the adult in the respective Sulfo-NHS-Biotin and 10-kDa Dextran group. *, P < 0.05, ***, P < 0.001. Scale bar represents 100 μm for all panels.
Results
Functional BRB is formed gradually, and is fully acquired by P10
We first mapped the spatio-temporal formation of the functional BRB. To evaluate BRB permeability, mice were injected transcardially at several postnatal ages with Sulfo-NHS-Biotin (550 Da) or fluorescently-labeled, 10-kDa dextran tracer. A functional BRB, as in adults, completely confines both tracers within the vasculature (Figure S1A). However at P1, when blood vessels first enter the retina through the optic nerve head and are enshealthed by pericytes (Figure S2A), leakage of both tracers into the retinal parenchyma from the budding vessels was evident (Figure 1C), indicating that these developing vessels do not intrinsically have a functional barrier. In P3 and P5 retinas, both tracers leaked into the parenchyma and were taken up by non-vascular cells near nascent vessels at the angiogenic front, located distal to the optic nerve head (Figure 1D-G; arrowheads), while both tracers were confined in the more mature vessels located proximal to the optic nerve head (Figure 1D-G; arrows). Indeed, Sulfo-NHS-Biotin, 3-kDa and 10-kDa dextran tracers at P5 reveal that a functional barrier is formed gradually in a proximal to distal fashion (Figure 1E,1F,1G, and 1H). At P8 and P9, although the angiogenic front reaches the retinal periphery, the nascent, distal vessels still exhibited leakage (Figure 1I and 1J), whereas the mature, proximal vessels confined Sulfo-NHS-Biotin and 10-kDa dextran tracers (Figure S1B and S1C). At P10, both tracers were completely confined in all vessels of the primary plexus, including distal vessels (Figure 1I, 1J, S1B and S1C). Thus, these data demonstrate that developing vessels are initially leaky, a functional barrier is formed gradually from proximal to distal, and the primary plexus acquires a functional BRB by P10.
We next examined when the functional BRB is established in the deeper plexus, which sprouts from the primary plexus between P7 and P12. Sprouting vessels of the deeper plexus showed leakage of both Sulfo-NHS-Biotin and 10-kDa-dextran tracers at P8 and P9 (Figure S1D and S1E). However at P10, when the deeper plexus vessels continue to sprout, both tracers were confined in newly formed vessels (Figure S1D and S1E). Finally, we investigated when the BRB becomes functional in the intermediate plexus, which sprouts from the deeper plexus between P12 and P17. At P12, sprouting nascent vessels, surprisingly, already confined both tracers; no leakage was observed at any ages examined (Figure S1F and S1G). Together, these data demonstrate that the developing retinal vasculature does not intrinsically have a functional barrier but instead gradually acquires barrier properties. Once barrier properties are attained by P10, nascent vessels sprouting from vessels with a functional BRB inherit and maintain these barrier properties. Thus, the three-tiered retinal vasculature acquires a functional BRB remarkably by one distinctive age: P10 (Figure 1 and S1).
At P1, budding CNS endothelial cells possess functional tight junctions but display bulk transcytosis
We next addressed the subcellular mechanisms underlying functional BRB development. We asked whether CNS endothelial cells form functional tight junctions and acquire low rates of transcytosis synchronously or sequentially to establish the functional BRB, and what the relative contribution of each property is to the regulation of BRB permeability. To evaluate the functionality of tight junctions and suppression of transcytosis at subcellular resolution, we injected horseradish peroxidase (HRP) intravenously at several ages and imaged the retina with transmission electron microscopy (EM). In the lung and heart vasculature where barrier properties are absent, luminal HRP leaks into the basement membrane and parenchyma both by passing through junctions, observed as HRP-filled tight junctions, and by high rates of transcytosis, evident as numerous HRP-filled intracellular vesicles (Karnovsky, 1967; Schneeberger-Keeley and Karnovsky, 1968). However, in adult retinal endothelial cells, where barrier properties are present (Raviola, 1977; Reese and Karnovsky, 1967), we observed that luminal HRP is halted sharply at electron-dense “kissing points” between endothelial cells where adjacent membranes are tightly apposed (Figure 2A). Adult retinal endothelial cells also displayed few HRP-filled vesicles, indicating that transcytosis is suppressed (Figure 2B).
Figure 2. Gradual suppression of transcytosis governs the development of an impermeable, functional BRB. See also Figure S3.
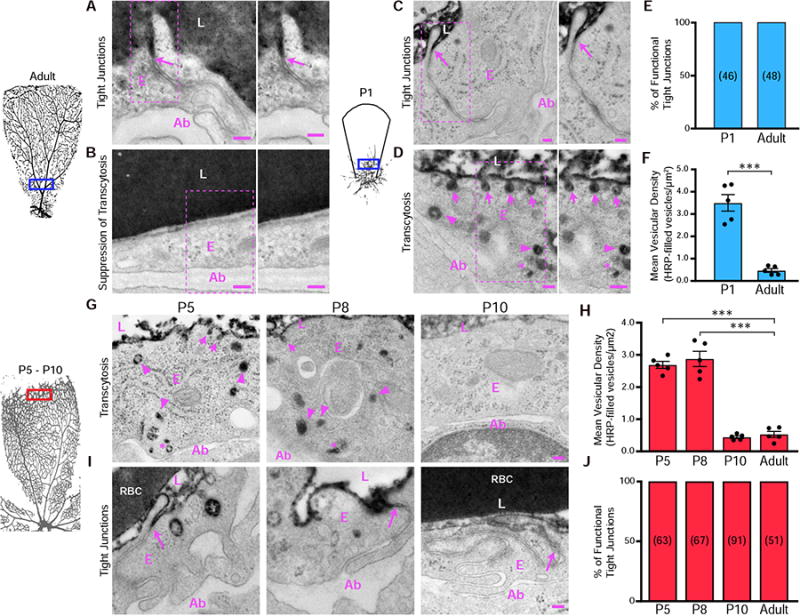
As early as P1, budding CNS endothelial cells possess functional tight junctions but display bulk transcytosis. (A and B) EM of endothelial cells in the proximal retina of an HRP-injected adult mouse. (A) Specialized tight junctions are functional and prevent electron dense 3-3' diaminobenzidine (DAB) reaction product in the lumen from invading through the intercellular cleft (arrows). (B) Transcytosis was suppressed in endothelial cells as seen by negligible numbers of tracer-filled vesicles. Also shown is a magnification of the boxed areas. (C and D) EM of endothelial cells in the proximal retina from an HRP-injected P1 pup. (C) Tracer invades through the intracellular cleft between the endothelial cells but stops at junctions without invading to the abluminal side (arrows). (D) DAB reaction product filled the vesicles attached to the luminal membrane (arrows), in the cytoplasm (arrowheads) and near the abluminal membrane (asterisk). Also shown is a magnification of the boxed areas. (E) The percentage of functional tight junctions from the EM images (n = 5 mice per age; 15-20 vessels analyzed per mouse; number of tight junctions analyzed are displayed in parenthesis). (F) Number of tracer-filled vesicles in endothelial cells in P1 and adult mice. Data are presented as mean ± s.e.m. (n = 5 mice per age, each circle represents the average vesicular density from 15 – 20 vessels per mouse). Statistical significance was assessed by unpaired t-test. L – lumen, E – endothelial cell, Ab – abluminal. **, P < 0.01. Scale bar represents 100 nm in all panels. (G) At P5 and P8, many tracer-filled vesicles are observed at the luminal membrane (arrows), cytoplasm (arrowheads) and at the abluminal membrane (*) in distal endothelial cells. At P10, distal endothelial cells contain negligible numbers of tracer-filled vesicles. (H) Number of tracer-filled vesicular densities in distal vessels at P5, P8 and P10. Data are shown as mean ± s.e.m. (n= 5 mice per age; each circle represents the average vesicular density from 15 – 20 vessels per mouse. Statistical significance was determined by one-way ANOVA with a post-hoc Bonferroni multiple comparison adjustment, comparing distal vessels of the various neonatal ages to distal vessels of adults. (I) EM of distal vessels from P5, P8 and P10 retinas reveals tracer product halts at tight junctions (arrows) at all ages. (J) Percentage of functional tight junctions from distal vessels. (n = 5 mice per age; 15-20 vessels analyzed per mouse; number of tight junctions analyzed are displayed in the bars). L – lumen, E – endothelial cells, Ab – abluminal, RBC- red blood cell. ***, P < 0.001. Scale bar represents 100 nm in all panels.
At P1, we observed tracer extravasation from budding vessels, indicating leaky vessels (Figure 1C). Surprisingly, under EM, these CNS endothelial cells already had specialized tight junctions that halted the HRP at the “kissing points” (Figure 2C and 2E), as observed in adult, mature CNS endothelial cells (Figure 2A). However, these CNS endothelial cells had many HRP-containing vesicles, including luminal and abluminal membrane associated vesicles and cytoplasmic vesicles (Figure 2D and 2F), similar to lung and heart endothelial cells where suppression of transcytosis is absent (Schneeberger-Keeley and Karnovsky, 1968). These results indicate that as early as vessel ingression at P1, functional tight junctions are present in budding CNS endothelial cells, but transcytosis has not been suppressed, suggesting that immature BRB leakiness is entirely due to uninhibited transcytosis.
Gradual suppression of transcytosis governs functional BRB formation
To test the hypothesis that suppression of transcytosis determines functional BRB development, we used EM to examine vesicular trafficking at later postnatal ages. At P5 and P8, in distal leaky vessels, many HRP-filled vesicles were observed (Figure 2G and 2H) indicating that transcytosis has not been suppressed. However, in the proximal vessels that are impermeable to tracer, very few HRP-filled vesicles were observed, suggesting that suppression of transcytosis had occurred (Figure S3A and S3C). By P10, when all the retinal vessels have a functional BRB, negligible HRP-filled vesicles were observed in both distal and proximal vessels (Figure 2G, 2H, S2A and S2C). Consistent with the observation at P1, functional tight junctions were present in all vessels, both proximal and distal, at all ages tested (Figure 2I, 2J, S3B and S3D). These data demonstrate that a leaky, immature barrier is due entirely to high rates of bulk transcytosis in endothelial cells. A functional barrier is established days later, only after transcytosis is suppressed, coinciding with functional BRB development by P10. Therefore, the spatio-temporal regulation of transcytosis governs the development of a functional BRB.
Mfsd2a is essential to suppress transcytosis at the functional BRB
Given the significance of transcytosis in functional BRB formation, we next examined whether Mfsd2a, a known transcytosis regulator in blood-brain barrier formation (Ben-Zvi et al., 2014), also plays a role in BRB formation. EM analysis of retinas from HRP-injected adult mice revealed increased HRP-filled vesicle density in the CNS endothelial cells of Mfsd2a−/− mice compared to Mfsd2a+/+ littermate controls (Figure 3A and 3B), whereas tight junctions remained functional (Figure 3C and 3D). Thus, Mfsd2a plays a similar role at the BRB in suppressing transcytosis.
Figure 3. Elevated levels of transcytosis deter functional BRB formation. See also Figure S4.
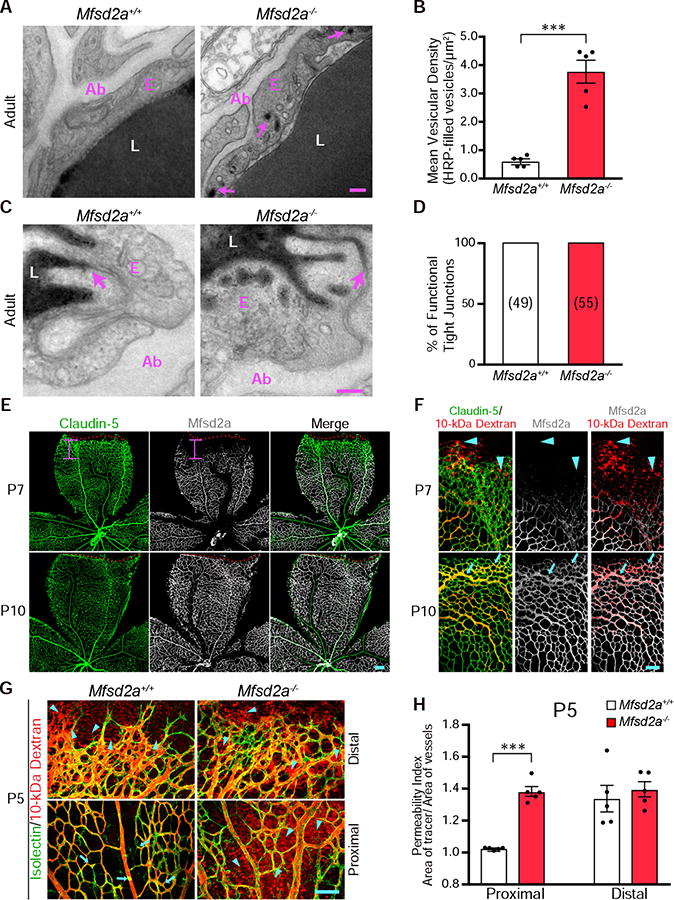
(A) Transcytosis is increased in Mfsd2a−/&minus mice. Many tracer-filled vesicles (arrows) were observed in adult retinal endothelial cells from Mfsd2a−/&minus but not Mfsd2a+/+ mice. (B) Quantification of HRP-filled vesicles in retinal endothelial cells from adult Mfsd2a+/+ and Mfsd2a−/&minus mice. Data are shown as mean ± s.e.m. (n= 5 mice per genotype; each circle represents the average vesicular density from 18 – 20 vessels per mouse). Statistical significance was determined by unpaired t-test. (C) EM of the adult retina confirms that specialized tight junctions halt tracer product in both Mfsd2a+/+ and Mfsd2a−/&minus adult mice (D) Quantification of functional tight junctions from Mfsd2a+/+ and Mfsd2a−/&minus adult mice (n = 5 mice per genotype; 18-20 vessels analyzed per mouse; number of tight junctions analyzed are displayed in parenthesis). (E) Immunostaining for Claudin-5 (green) and Mfsd2a (white) on P7 and P10 retinas shows the lack of Mfsd2a expression in nascent, distal vessels at P7. The red dash lines indicate the angiogenic front as determined by Claudin-5 expression, and the pink bar indicates the length from the angiogenic front to the appearance of Mfsd2a expression. In contrast to P7, Mfsd2a is expressed in the distal vessels at P10 (n= 5 mice per age). (F) Mfsd2a expression correlates with functional BRB formation. Immunostaining for Claudin-5 and Mfsd2a in retinas from 10-kDa dextran injected P7 and P10 mice shows that at P7, extravasation of tracer (arrowheads) occurs at nascent vessels where Mfsd2a is absent. At P10, tracer is confined (arrows) in distal vessels where Mfsd2a is expressed (n=5 mice per age). (G) Genetic ablation of Mfsd2a results in incomplete formation of the functional BRB. After intravenous injection of 10-kDa dextran in P5 Mfsd2a+/+ and Mfsd2a−/&minus pups, tracer was confined (arrow) in proximal vessels (bottom) but leaked from distal vessels (top) in Mfsd2a+/+ mice (arrowheads). In contrast, tracer leaked into the retinal parenchyma from both proximal and distal vessels in Mfsd2a−/&minus mice (arrowheads). (H) Permeability index from proximal and distal regions of P5 Mfsd2a−/&minus and Mfsd2a+/+ littermates. Data are shown as mean ± s.e.m. (n=5 mice per genotype; each circle represents the average permeability from each mouse). Statistical significance was determined by unpaired t-test. ***, P < 0.001. Scale bars represents 100 nm in (A and C) and 100 μm in (E,F, and G).
Mfsd2a expression correlates with functional BRB formation
We next examined Mfsd2a expression as a marker for suppressed transcytosis during BRB development by immunostaining retinas from dextran tracer-injected pups. At P7, Mfsd2a protein is only present in proximal, impermeable vessels but absent in distal, leaky vessels (Figure 3E and 3F). In contrast, claudin-5 is fully present at the distal vessels (Figure 3E and 3F). Importantly, Mfsd2a is absent from leaky, distal vessels during development (Figure 3F), and only by P10, when tracer is completely confined in the vessels, is Mfsd2a expression present at the distal vessels (Figure 3E and 3F). Thus, the spatio-temporal expression of Mfsd2a correlates with the gradual suppression of transcytosis and the development of a functional BRB.
Elevated levels of transcytosis deter functional BRB formation
If the timing of the gradual suppression of transcytosis governs the development of a functional BRB, then altered regulation of transcytosis should affect functional BRB development. To test this idea, we examined the time course of functional BRB formation in two mutant mice with either increased or decreased transcytosis. First, we injected 10-kDa dextran tracers into Mfsd2a−/− mice and wild type littermates at P5, P10 and adult ages. In P5 Mfsd2a+/+ wildtype mice, tracer leaked from distal vessels but was confined in proximal vessels (Figure 3G, H). However, in P5 Mfsd2a−/− mice, tracer leaked from both distal and proximal vessels (Figure 3G, 3H), similar to what we observed in P1 wildtype pups (Figure 1C). Even at P10 and adulthood, this leaky phenotype persisted, indicating that failure to suppress transcytosis results in incomplete formation of a functional BRB (Figure S4).
Precocious suppression of transcytosis accelerates functional BRB formation
We next tested whether precocious suppression of transcytosis during development accelerates functional BRB formation. Among various transcytotic pathways, peripheral endothelial cells frequently utilize the caveolae-pathway (Tuma and Hubbard, 2003). Caveolin-1 (Cav-1) knockout mice lack caveolae vesicles throughout the endothelium (Drab et al., 2001; Razani et al., 2001) but display normal tight junctions in CNS barriers (Knowland et al., 2014b). EM analysis of distal retinal vessels from P8 and P10 Cav-1+/+ wildtype retinas revealed decreased vesicular density from P8 to P10 (Figure 4A and 4B). However in Cav-1−/− mice, vesicular density was already low at P8 (Figure 4A and 4B), indicating a precocious suppression of transcytosis in the BRB. To examine the impact of precocious suppression of transcytosis on functional BRB formation, bovine serum albumin (BSA), known to be transported via caveolae vesicles in lung endothelial cells (Schubert et al., 2001), was injected intravenously in Cav-1+/+ and Cav-1−/− mice at P8. In Cav-1+/+ wildtype littermate mice, we observed BSA leakage into the retinal parenchyma from distal vessels (Figure 4C and 4D). However, in Cav-1−/− mice, BSA was confined throughout the vasculature, indicating that a functional BRB had formed at P8 instead of P10 (Figure 4C and 4D). This time shift in BRB impermeability demonstrates that precocious suppression of transcytosis results in an earlier formation of a functional BRB.
Figure 4. Precocious suppression of transcytosis accelerates functional BRB formation.
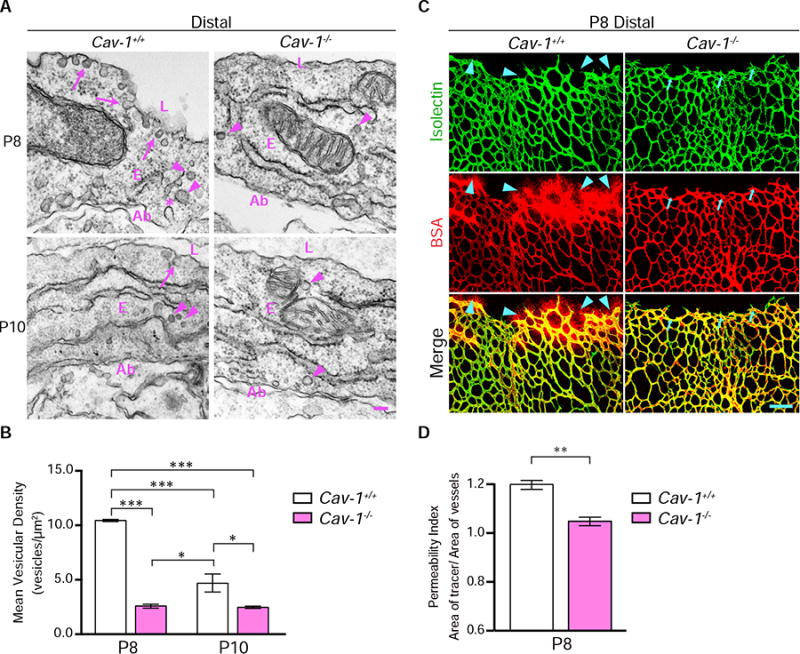
(A) Precocious suppression of transcytosis is observed in Cav-1−/&minus retinas. EM in distal vessels at P8 (top) reveals many vesicles associated with luminal (arrows) and abluminal membranes (*) and in the cytoplasm (arrowheads) in Cav-1+/+ endothelial cells. Cav-1−/&minus endothelial cells have drastically reduced numbers of vesicles. At P10 (bottom), few vesicles are observed in both Cav-1+/+ and Cav-1−/&minus distal vessels. (B) Quantifications of vesicles in distal vessels from Cav-1+/+ and Cav-1−/&minus mice at P8 and P10. Data are presented as mean ± s.e.m. (n= 3 mice per age per genotype, 15-20 vessels analyzed per mouse). Statistical significance was determined by two-way ANOVA with a post-hoc Bonferroni multiple comparison adjustment. (C) Intravenous injection of BSA (red) in P8 pups reveals tracer leakage in the retinal parenchyma from distal vessels of Cav-1+/+ (arrowheads) but not Cav-1−/&minus mice. (D) Permeability index from distal regions of P8 Cav-1+/+ and Cav-1−/&minus littermates. Data are presented as mean ± s.e.m. (n=3-4 mice per genotype). Statistical significance was determined by unpaired t-test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. L – lumen, E – endothelial cells, Ab – abluminal. Scale bar represents 100 nm in (A) and 100 μm in (C).
Discussion
This study provides the first spatio-temporal characterization of functional BRB formation and establishes the BRB as a tractable model system for future study of CNS barriers. Using this system, we unexpectedly found that suppression of transcytosis is a principal contributor in the normal development of a functional barrier. We demonstrated that during BRB development, budding CNS endothelial cells already display functional tight junctions as early as vessel ingression but have not yet suppressed transcytosis. Thus, the leakiness of the developing retinal vasculature is entirely due to transcytosis. A functional BRB is established days later, only after transcytosis is gradually suppressed in BRB endothelial cells (Figure S5). In contrast to the prevailing notion in the field emphasizing the role of tight junctions in barrier function, our findings support an emerging view that transcytosis also plays a key role in regulating CNS barrier permeability, and indicate that while specialized tight junctions are intrinsic to retinal endothelial cells, suppression of transcytosis is induced by CNS environmental cues during development. Our findings revealed that CNS endothelial cells have a developmental program that actively inhibits transcytosis to ensure barrier function. Finally, unregulated BRB dysfunction is a pathological feature of many ocular diseases (Klaassen et al., 2013). Our findings may offer insights to improving CNS drug delivery as manipulating transcytosis can transport different size cargos, including compounds less than 1kDa to large macromolecules. Furthermore, genes that regulate transcytosis can be targeted to repair CNS barriers to treat neurodegenerative diseases.
What are the molecular mechanisms that induce CNS endothelial cells to gradually suppress transcytosis to develop a functional BRB? Pericytes ensheathing CNS endothelial cells are the most promising candidates, as pericyte-deficient mice have leaky barriers in brain and retina, aberrant tight junctions, elevated transcytosis and reduced Mfsd2a protein at the blood-brain barrier (Armulik et al., 2010; Bell et al., 2010; Ben-Zvi et al., 2014; Daneman et al., 2010; Keskin et al., 2015; Sweeney et al., 2016; Winkler et al., 2011). Using NG2:DsRed reporter mice, which have labeled retinal mural cells, we examined pericyte coverage and density during BRB development and found that pericytes ensheath blood vessels at P1 (Figure S2A). In P5 retinas, we find about a 1:1 pericyte to endothelial cell ratio throughout the retina (Figure S2B-S2D), including distal, nascent leaky vessels where Mfsd2a protein is absent (Figure S2E). This suggests that the differentiation state of pericytes or inductive factors secreted from pericytes during development are likely the key mechanisms that induce CNS endothelial cells to suppress transcytosis for functional BRB formation. Indeed, the loss-of-function of two transcription factors, foxc1 and foxf2, in CNS pericytes actually increases CNS pericyte density but still results in blood-brain barrier breakdown (Reyahi et al., 2015; Siegenthaler et al., 2013), suggesting that pericyte differentiation state but not necessarily pericyte coverage or density per se is important for blood-brain barrier integrity. Therefore, it is critical for future work to identify the pericyte-secreted factors that mediate the gradual suppression of transcytosis for a functional BRB.
Canonical Wnt signaling is the best characterized signal for CNS angiogenesis and barrier formation (Stenman et al., 2008). In the retina, loss-of-function of the ligand (Norrin), receptor (Frizzled-4), co-receptors (Lrp5 and Tspan12) and downstream transcription factor (β-catenin), all result in impaired retinal angiogenesis and BRB breakdown (Junge et al., 2009; Wang et al., 2012; Ye et al., 2009; Zhou et al., 2014). Moreover, emerging evidence suggests that canonical Wnt signaling regulates tight junction function in CNS barriers, as loss-of-function of canonical Wnt signaling decreases Claudin-5 and increases PLVAP protein expression, resulting in opened junctions at the blood-brain barrier (Tran et al., 2016; Wang et al., 2012; Zhou et al., 2014). Whether canonical Wnt signaling also regulates the gradual suppression of transcytosis is not clear. On one hand, recent studies demonstrated canonical Wnt signaling is essential in the tip cells of nascent sprouts (Ulrich et al., 2016; Vanhollebeke et al., 2015) yet these nascent, distal vessels display bulk transcytosis and lack Mfsd2a during BRB development. But on the other hand, microarray analysis on retinas from canonical Wnt signaling deficient mice showed that Mfsd2a is downregulated (Chen et al., 2012). Therefore, while canonical Wnt signaling regulates functional tight junctions in BRB endothelial cells, it is imperative that future studies elucidate if canonical Wnt signaling also regulates the suppression of transcytosis.
It is intriguing that functional BRB formation and the shift in a homeostatic retinal microenvironment occurs by P10, a few days before mouse eyes open (Feller, 2009). Furthermore, it is notable that the timing of retinal barrier formation correlates with the development of the neural retina, particularly with retinal waves. Retinal waves are spontaneous neural activity in retinal ganglion cells during development, and are thought to refine visual circuitry prior to eye-opening and the onset of sensory-evoked neural activity (Huberman et al., 2008). It will be interesting to investigate whether the temporal correlation of BRB development and retinal waves implies cross-talk between functional BRB formation and neural function. While the acquisition of barrier properties is dependent on inductive cues from the neural environment, it is unclear if neural activity, especially spontaneous activity during development, is involved in transmitting these inductive cues for BRB formation. Alternatively, the maturation of the BRB could influence the spatio-temporal formation of the retinal waves. Addressing if there is causal relationship between BRB formation and retinal waves could shift thought paradigms in both vascular biology and neurobiology.
STAR Methods
Contact For Reagent and Resource Sharing
For further information and requests for reagents in this study, please contact Lead Contact: Chenghua Gu (chenghua_gu@hms.harvard.edu)
Experimental Model and Subject Details
Wild-type Swiss-Webster mice (Charles River Laboratory; Strain 024) were used for postnatal BRB functionality assays. Day of birth was defined as postnatal day zero (P0). Both males and females were used in this study. Mfsd2a−/− mice (Ben-Zvi et al., 2014)(MGI Cat# 5755244, RRID:MGI:5755244) were maintained on a C57Bl/129 background (currently backcrossed to C57Bl for the 5th generation) and used to determine incomplete BRB formation and for validation of the Mfsd2a antibody in retinas. Cav-1−/− mice (maintained on congenic C57Bl background) (Razani et al., 2001) (JAX; 007083, MGI Cat# 4418046, RRID:MGI:4418046) were maintained in C57BL/6J and used to determine precocious BRB development. NG2:DsRed mice (JAX; 008241, RRID:MGI:3796133) (maintained on congenic C57Bl background) were used to determine pericyte density and coverage during functional BRB development. Littermates of either sex were randomly assigned to experimental groups. A maximum of five adult mice were maintained in one cage. For postnatal mice, the litter and two adult mice were maintained in one cage. Mice were maintained on a 12 light/12 dark cycle. All animals were treated according to institutional and US National Institutes of Health (NIH) guidelines approved by the Institutional Animal Care and Use Committee (IACUC) at Harvard Medical School.
Method Details
BRB permeability assay and flatmount retina immunohistochemistry
Neonatal pups and adults (8 -10 weeks old) were deeply anesthetized. Sulfo-NHS-Biotin (Thermo Fisher Scientific; 21335), 3-kDa or 10-kDa dextran conjugated to Tetramethylrhodamine (Thermo Fisher Scientific; D3308 and D1868, respectively), and bovine serum albumin conjugated to Alexa Fluor 555 (Thermo Fisher Scientific; A34786) were injected at 0.5 mg/g body weight for Sulfo-NHS-Biotin and 0.1 mg/g body weight for the rest of tracers as previously described (Armulik et al., 2010) into the left-ventricle with a 31 gauge, 0.3 cc insulin syringe. The heartbeat was monitored for a steady heartbeat that was continuous for 5 minutes. If the heartbeat stopped during the 5 minutes circulation, the animals were excluded due to poor circulation of the tracer. After 10 minutes of tracer circulation of the tracer, both eyes were enucleated. Eyes were fixed briefly (5-10 minutes) in 4% PFA. Retinas were dissected out in 4% PFA to ensure dye fixation within the tissue. Retinas were fixed overnight in 4% PFA at 4°C and washed in PBS three times for 5 minutes each the next day. Retinas were incubated in blocking buffer (10% normal goat serum, 3% bovine serum albumin, and 0.5% Triton X-100 in PBS) for 1 hour at room temperature. Isolectin GS-IB4 conjugated to Alexa-Fluor® 488 (Thermo Scientific; I21411) or the following primary antibodies: rabbit α-ERG1/2/3 (Abcam Cat# ab92513, RRID:AB_2630401, 1:200), rabbit α-Mfsd2a (Cell Signaling Technology still under development, Cat# 14-87 Mfsd2a, RRID:AB_2617168, 1:200) and mouse α-Claudin-5, (Thermo Fisher Scientific Cat# 352588 Lot# RRID:AB_2532189, 1:400) were then incubated in blocking buffer overnight at 4°C. Retinas were then washed in PBS three times for 5 minutes and incubated with the Alexa-Fluor® conjugated secondary antibodies (Thermo Fisher Scientific; 1:300) for 4 hours at room temperature. Retinas were washed in PBS three times for 10 minutes and flat-mounted with vitreal surface facing up using Prolong Gold (Molecular Probe, 1747013).
Transmission electron microscopy
EM imaging of adult and neonatal HRP injected retinas was carried out as described previously (Ben-Zvi et al., 2014). HRP (0.5 mg/g body weight Sigma Aldrich, HRP type II) was dissolved in 0.4 ml of PBS and injected into the retro-orbital sinus of deeply anaesthetized mice. After 15 or 30 minutes of HRP circulation for pups or adults, respectively, the contralateral eye of the sinus injection was enucleated. The eye was dissected immediately in 4% PFA and fixed by immersion in a 0.1 M sodium-cacodylate-buffered mixture (5% glutaraldehyde and 4% PFA) for 1 hour at room temperature followed by overnight fixation in 4% PFA at 4°C. Following fixation, the tissue was washed three times and then overnight in 0.1 M sodium-cacodylate buffer. Retinas were incubated for 25 or 45 minutes for neonatal or adults, respectively, at room temperature in 0.05 M Tris-HCl pH 7.6 buffer, containing 5.0 mg per 10 ml of 3-39 diaminobenzidine (DAB, Sigma Aldrich) with 0.01% hydrogen peroxide. After DAB staining, the retinal vasculature could be visualized. The distal edge of the vasculature and proximal edge of optic nerve head were micro-dissected and samples were lightly nicked to provide orientation to cut ultrathin sections. Samples were then postfixed in 1% osmium tetroxide and 1.5% potassium ferrocyanide, dehydrated, and embedded in epoxy resin. P8 and P10 Cav-1−/− and wildtype littermate retinas were processed as stated above without HRP injections and fixed for 5 days in 0.5% glutaraldehyde in 4% PFA prepared in 0.1 mM phosphate buffer, pH 7.4 and without DAB staining. Retinas were washed three times and then overnight in 0.1 M sodium-cacodylate buffer. Ultrathin sections (80 nm) were then cut from the block surface, collected on copper grids, stained with Reynold's lead citrate, and examined under a 1200EX electron microscope (JEOL) equipped with a 2k CCD digital camera (AMT). The nick could be visualized on the ultrathin section.
Light microscopy
Olympus FluoView FV1000 and FV1200 laser scanning confocal microscopes (20×, 0.75 N.A. and 40×, 1.4 N.A.) and an Olympus VS 120 slide scanner (10×, 0.4 N.A.) were used for imaging flatmount retinas. Images were processed using Adobe Photoshop, Illustrator, Olympus Fluoview and FIJI (NIH).
Quantification and Statistical Analysis
Permeability Index quantifications. Related to Methods S1
All quantifications were performed blind with a self-written FIJI (NIH) macro. Six to eight 290 μm × 290 μm × 5 μm thick z-stack images of the vessels of distal and proximal regions of the primary plexus, six to eight 317 um × 317 um of deeper plexus and intermediate plexus at 3-5 μm thick z-stack images and six to eight 635 μm × 635 μm of Mfsd2a−/− and Cav-1−/− with their respective wildtype littermates per mouse retina were max Z- projected, filtered with a Gaussian blur and background subtracted via rolling ball method. Distal vessel images were acquired by aligning the field of view to capture the retinal tip cells and proximal images were acquired by aligning the field view slightly after the optic nerve head. The isolectin and tracer signals were auto-thresholded by the same method for all ages and genotypes. The area of the segmented vessel and the tracer were determined. The ratio of the area of the tracer and the area of the vessel was then calculated. A ratio of 1 means that the tracer and vessel area is the same, indicating a functional barrier. The average of the ratios from the six to eight images is considered one biological sample.
Mean Vesicular Density and functional tight junction quantification
15-20 vessels from retinas were imaged under electron microscopy. To quantify mean vesicular density, tracer-filled vesicles were counted and divided by the area of the cytoplasm of the endothelial cells (excluding the nucleus). To determine percentage of functional tight junctions, the number of tight junctions that halt the tracer at the luminal side without parenchymal leakage was counted and divided by the total number of tight junctions counted.
Pericyte density and coverage quantifications
Retinas from P5 NG2:DsRed mice were stained for ERG1/2/3 (endothelial nuclei marker) and isolectin. Images of four retinal leafs per retina were acquired. Images were max Z-projected, filtered with a Gaussian blur and background subtracted via rolling ball method. To quantify pericyte density, the NG2:DsRed signal was also applied with a minimum filter to reduce weak signal in the processes of pericytes but retain high signals in the soma. 250 μm × 250 μm field of views were analyzed from 0 μm (slightly before the optic nerve head) to the distal vessels (up to 1750 μm). Images were then segmented for NG2:DsRed and ERG1/2/3 signals and particles were then counted to determine pericyte density (NG2:DsRed+/ERG+ particles). To quantify pericyte coverage which is displayed as NG2:DsRed area/Isolectin area, a minimum filter was not applied but instead, images were simply segmented for NG2:DsRed signal and Isolectin signal.
Statistical analyses
Quantitative data are expressed as the mean ± s.e.m. The sample size, what each “n” represents, the statistical tests used and the result of the statistical test are indicated in each respective Figure Legend. No statistical methods were used to predetermine sample sizes, but our sample sizes are comparable to those reported in previous publications (Armulik et al., 2010; Ben-Zvi et al., 2014). Statistical analyses were performed using Prism 6 (GraphPad). Differences between two different groups (either age or genotype) were analyzed by unpaired t-test. Differences among different age groups were analyzed by one-way ANOVA followed by a Bonferroni's multiple comparisons correction. Differences among different age groups and genotype were analyzed by two-way ANOVA followed by a Bonferroni's multiple comparisons correction. Normality and equality of variances among the groups were analyzed by Brown-Forsythe tests. P-values less than 0.05 were considered significant.
Data and Software Availability
ImageJ macro for quantification of images to determine the permeability index is provided as Methods S1.
Supplementary Material
Highlights.
Retinal vessels at P1 have functional tight junctions but display bulk transcytosis
Immature vessel leakage is entirely due to transcytosis and not via tight junctions
Gradual suppression of transcytosis governs functional blood-retinal barrier formation
Retinal vasculature is a tractable system to study CNS barriers
In Brief.
Chow et al. characterized the spatio-temporal development of functional blood-retinal barrier formation and demonstrated that immature vessel leakage is entirely due to bulk transcytosis and not via tight junctions. Gradual suppression of transcytosis determines functional blood-retinal barrier formation.
Acknowledgments
We thank E. Raviola for advice and discussion; J. Cohen, P. D'Amore, D. Ginty, L. Goodrich, B. Lacoste and members of the Gu laboratory for comments on the manuscript; HMS Electron Microscopy Core Facility and the Neurobiology Imaging Facility for consultation and instrumentation that supported this work (this facility is supported in part by the Neural Imaging Center as part of NINDS P30 Core Center grant NS072030). This research was supported by NIH Training Grant 5T32MH20017-15 (B.W.C.); Fritz Hoffmann-La Roche Research Grant (C.G.); NIH DP1 NS092473 Pioneer Award (C.G.).
Footnotes
Authors Contributions: B.W.C and C.G. conceived and designed the project. B.W.C. performed experiments and data analysis. B.W.C. and C.G. wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreone BJ, Lacoste B, Gu C. Neuronal and Vascular Interactions Annu Rev Neurosci. 2015;38:25–46. doi: 10.1146/annurev-neuro-071714-033835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Banks WA. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov. 2016 doi: 10.1038/nrd.2015.21. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes Control Key Neurovascular Functions and Neuronal Phenotype in the Adult Brain and during Brain Aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette M, Daneman R. Formation and maintenance of the BBB. Mech Dev. 2015;138(Pt 1):8–16. doi: 10.1016/j.mod.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Chen J, Stahl A, Krah NM, Seaward MR, Joyal JS, Juan AM, Hatton CJ, Aderman CM, Dennison RJ, Willett KL, Sapieha P, Smith LEH. Retinal expression of Wnt-pathway mediated genes in low-density lipoprotein receptor-related protein 5 (Lrp5) knockout mice. PLoS ONE. 2012;7:e30203. doi: 10.1371/journal.pone.0030203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow BW, Gu C. The Molecular Constituents of the Blood-Brain Barrier. Trends in Neurosciences. 2015;38:598–608. doi: 10.1016/j.tins.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Coisne C. Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids Barriers CNS. 2011;8:4. doi: 10.1186/2045-8118-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller MB. Retinal waves are likely to instruct the formation of eye-specific retinogeniculate projections. Neural Development 2009. 2009;4:14–1. doi: 10.1186/1749-8104-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruttiger M. Development of the retinal vasculature. Angiogenesis. 2007;10:77–88. doi: 10.1007/s10456-007-9065-1. [DOI] [PubMed] [Google Scholar]
- Hagan N, Ben-Zvi A. The molecular, cellular, and morphological components of blood–brain barrier development during embryogenesis. Semin Cell Dev Biol. 2015;38:7–15. doi: 10.1016/j.semcdb.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge HJ, Yang S, Burton JB, Paes K, Shu X, French DM, Costa M, Rice DS, Ye W. TSPAN12 regulates retinal vascular development by promoting Norrin- but not Wnt-induced FZD4/beta-catenin signaling. Cell. 2009;139:299–311. doi: 10.1016/j.cell.2009.07.048. [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ. The ultrastructural basis of capillary permeability studied with peroxidase as a tracer. J Cell Biol. 1967;35:213–236. doi: 10.1083/jcb.35.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskin D, Kim J, Cooke VG, Wu CC, Sugimoto H, Gu C, De Palma M, Kalluri R, LeBleu VS. Targeting vascular pericytes in hypoxic tumors increases lung metastasis via angiopoietin-2. CellReports. 2015;10:1066–1081. doi: 10.1016/j.celrep.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen I, Van Noorden CJF, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Progress in Retinal and Eye Research. 2013;34:19–48. doi: 10.1016/j.preteyeres.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Knowland D, Arac A, Sekiguchi KJ, Hsu M, Lutz SE, Perrino J, Steinberg GK, Barres BA, Nimmerjahn A, Agalliu D. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron. 2014a;82:603–617. doi: 10.1016/j.neuron.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowland D, Arac A, Sekiguchi KJ, Hsu M, Lutz SE, Perrino J, Steinberg GK, Ben A Barres, Nimmerjahn A, Agalliu D. Stepwise Recruitment of Transcellular and Paracellular Pathways Underlies Blood-Brain Barrier Breakdown in Stroke. Neuron. 2014b;82:603–617. doi: 10.1016/j.neuron.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola G. The structural basis of the blood-ocular barriers. Experimental Eye Research. 1977;(25 Suppl):27–63. doi: 10.1016/s0014-4835(77)80009-2. [DOI] [PubMed] [Google Scholar]
- Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Harry Hou J, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 Null Mice Are Viable but Show Evidence of Hyperproliferative and Vascular Abnormalities. J Biol Chem. 2001a;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34:207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyahi A, Nik AM, Ghiami M, Gritli-Linde A, Pontén F, Johansson BR, Carlsson P. Foxf2 Is Required for Brain Pericyte Differentiation and Development and Maintenance of the Blood- Brain Barrier. Developmental Cell. 2015;34:19–32. doi: 10.1016/j.devcel.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Schneeberger-Keeley EE, Karnovsky MJ. THE ULTRASTRUCTURAL BASIS OF ALVEOLAR-CAPILLARY MEMBRANE PERMEABILITY TO PEROXIDASE USED AS A TRACER. J Cell Biol. 1968;37:781–793. doi: 10.1083/jcb.37.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert W, Frank PG, Razani B, Park DS, Chow CW, Lisanti MP. Caveolae-deficient Endothelial Cells Show Defects in the Uptake and Transport of Albumin in Vivo. J Biol Chem. 2001;276:48619–48622. doi: 10.1074/jbc.C100613200. [DOI] [PubMed] [Google Scholar]
- Siegenthaler JA, Choe Y, Patterson KP, Hsieh I, Li D, Jaminet SC, Daneman R, Kume T, Huang EJ, Pleasure SJ. Foxc1 is required by pericytes during fetal brain angiogenesis. Biology Open. 2013;2:647–659. doi: 10.1242/bio.20135009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, Seaward MR, Willett KL, Aderman CM, Guerin KI, Hua J, Löfqvist C, Hellström A, Smith LEH. The mouse retina as an angiogenesis model. Investigative Ophthalmology & Visual Science. 2010;51:2813–2826. doi: 10.1167/iovs.10-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- Stewart PA, Wiley MJ. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail--chick transplantation chimeras. Dev Biol. 1981;84:183–192. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- Stone J, Itin A, Alon T, Pe'er J, Gnessin H, Chan-Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. Journal of Neuroscience. 1995;15:4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci. 2016;19:771–783. doi: 10.1038/nn.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran KA, Zhang X, Predescu D, Huang X, Machado RF, Göthert JR, Malik AB, Valyi-Nagy T, Zhao YY. Endothelial β-Catenin Signaling Is Required for Maintaining Adult Blood-Brain Barrier Integrity and Central Nervous System Homeostasis. Circulation. 2016;133:177–186. doi: 10.1161/CIRCULATIONAHA.115.015982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma PL, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol Rev. 2003;83:871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- Ulrich F, Carretero-Ortega J, Menéndez J, Narvaez C, Sun B, Lancaster E, Pershad V, Trzaska S, Véliz E, Kamei M, Prendergast A, Kidd KR, Shaw KM, Castranova DA, Pham VN, Lo BD, Martin BL, Raible DW, Weinstein BM, Torres-Vázquez J. Reck enables cerebrovascular development by promoting canonical Wnt signaling. Development. 2016;143:147–159. doi: 10.1242/dev.123059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhollebeke B, Stone OA, Bostaille N, Cho C, Zhou Y, Maquet E, Gauquier A, Cabochette P, Fukuhara S, Mochizuki N, Nathans J, Stainier DY. Tip cell-specific requirement for an atypical Gpr124- and Reck-dependent Wnt/β-catenin pathway during brain angiogenesis. Elife. 2015;4 doi: 10.7554/eLife.06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rattner A, Zhou Y, Williams J, Smallwood PM, Nathans J. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell. 2012;151:1332–1344. doi: 10.1016/j.cell.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Sengillo JD, Sagare AP, Zhao Z, Ma Q, Zuniga E, Wang Y, Zhong Z, Sullivan JS, Griffin JH, Cleveland DW, Zlokovic BV. Blood-spinal cord barrier disruption contributes to early motor-neuron degeneration in ALS-model mice. Proc Natl Acad Sci USA. 2014;111:E1035–42. doi: 10.1073/pnas.1401595111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wang Y, Cahill H, Yu M, Badea TC, Smallwood PM, Peachey NS, Nathans J. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139:285–298. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and Dysfunction of the Blood-Brain Barrier. Cell. 2015;163:1064–1078. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wang Y, Tischfield M, Williams J, Smallwood PM, Rattner A, Taketo MM, Nathans J. Canonical WNT signaling components in vascular development and barrier formation. J Clin Invest. 2014;124:3825–3846. doi: 10.1172/JCI76431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


