Abstract
The enzymatic β-C–H hydroxylation of the feedstock chemical isobutytic acid has enabled the asymmetric synthesis of a wide variety of polyketides. The analogous transitionmetal catalyzed enantioselective β-C–H functionalization of isobutyric acid-derived substrates should provide a versatile method for constructing useful building blocks with enantioenriched α-chiral centers from this abundant C-4 skeleton. However, the desymmetrization of ubiquitous isopropyl moieties by organometallic catalysts has remained an unanswered challenge. Herein, we report the design of chiral mono-protected aminomethyl oxazoline (MPAO) ligands that enable desymmetrization of isopropyl groups via palladium insertion into the C(sp3)-H bonds of one of the prochiral methyl groups. We detail the enantioselective β-arylation, -alkenylation and -alkynylation of isobutyric acid/2-amino-isobutyric acid derivatives, which may serve as a platform for the construction of α-chiral centers.
C–H activation reactions enable the use of minimally functionalized bulk chemicals as building blocks for the synthesis of complex molecules, thereby shortening synthetic sequences, reducing waste, and improving the sustainability of chemical synthesis. In order for the logic of C–H activation to be fully realized in the synthesis of chiral molecules, enantioselective C–H activation reactions — whereby stereochemistry is controlled in the C–H cleavage step by a metal catalyst — must be developed (1). The biosynthetic pathways to a myriad of natural products proceed through the enantioselective β-C–H hydroxylation of isobutyric acid (2, 3); further, this process has been adapted for the industrial preparation of the Roche ester, which has served as an invaluable source of α-center chirality in the synthesis of complex molecules (4–7; Fig. 1A). Inspired by the bulk preparation of the Roche ester and the central role of α-chiral centers in retrosynthetic analysis (8), our group has long sought to develop methodologies that desymmetrize the isopropyl group of isobutyric acid derivatives through transition metal catalyzed C–H functionalization, in turn expanding the existing chiral pool (Fig. 1B).
Fig. 1. Construction of α-chiral centers via C–H activation.
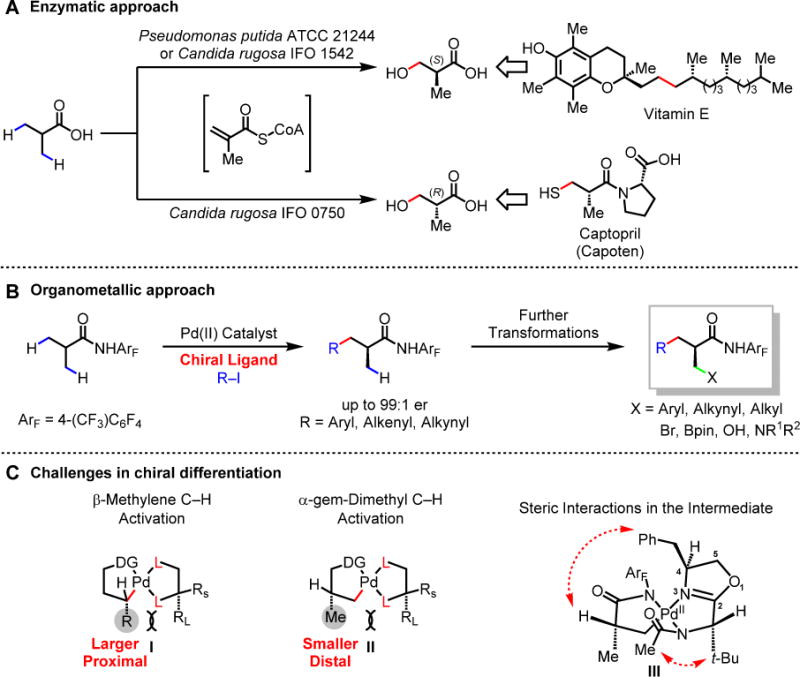
(A) Enzymatic approach. (B) Organometallic approach. (C) Challenges in chiral differentiation. DG, directing group; Me, methyl group; t-Bu, tert-butyl group; CoA, Coenzyme A; R, alkyl or aryl group; pin, pinacolato group.
Although enantioselective β-methylene C–H arylation has recently been realized (9), desymmetrization of isopropyl groups is a fundamentally different challenge. This challenge is reflected in an analysis of the steric environment associated with respective C–H insertion intermediates I and II (Fig. 1C). In methylene C–H activation (I), steric differentiation between a bulky alkyl group and a methylene C–H bond is required, whereas in the desymmetrization of isopropyl moieties (II) the α-hydrogen atom must be differentiated from a relatively small α-methyl substituent. Moreover, the prochiral center (the α-carbon) in isopropyl desymmetrization is too far away to interact directly with the ligated transition metal catalyst, making asymmetric induction considerably more challenging.
To date, intermolecular desymmetrization of α-dialkyl groups has only been possible with cyclopropane, cyclobutane or substrates containing large α-substituents, which serve to amplify the chiral recognition in the transition state (10–13). Related studies on desymmetrization of α-dialkyl groups via C–H activation from other laboratories have been limited to intramolecular Pd(0)-catalyzed C–H arylations (14–22). An inspiring formal enantioselective intermolecular β-arylation of isobutyric esters has been demonstrated with moderate enantioselectivity (67:33 to 77:23 er) (23). In this reaction, α-lithiation precedes α-palladation, which is followed by β-hydride elimination to give the corresponding olefin intermediate. Subsequent enantioselective carbopalladation of the double bond, rather than chiral recognition of geminal methyl groups, is responsible for chiral induction.
Herein we report the design of chiral mono-protected aminomethyl oxazoline (MPAO) ligands that enable Pd(II)-catalyzed enantioselective β-arylation, -alkenylation, and -alkynylation of isobutyric amides (Fig. 1B). The remaining α-methyl group can then undergo further C–H functionalizations to afford greater product diversity. Using a chiral benzoyl-protected aminomethyl oxazoline ligand, desymmetrization of 2-aminoisobutyric acid-derived amide (3) is also showcased to provide straightforward access to a wide range of enantioenriched α,α-dialkyl α-amino acids that may be used as building blocks for peptide based drug synthesis (24, 25). Systematic ligand modification suggests that the additional interaction of the chiral center on the oxazoline ring with the substrate is crucial to the successful desymmetrization of isopropyl groups.
Our early efforts to desymmetrize isopropyl groups of isobutyric acid-derived substrates focused on the use of chiral auxiliaries (10, 11). These studies, through isolation of well-defined chiral C–H insertion intermediates and computational analysis (26, 27), improved our understanding of how chiral differentiation of α-gem-dimethyl groups could potentially be achieved (Fig. 1C). The difficulty of desymmetrizing the gem-dimethyl groups in isobutyric acid-derived substrates is demonstrated by the fact that even when employing a bulky oxazoline as a chiral auxiliary, no diastereoselectivity is obtained (10). Furthermore, desymmetrization of α-dimethyl groups using a chiral amino acid-derived ligands is only successful (90:10 er) when the α-hydrogen atom is replaced by an extremely bulky α-tert-butyl group (12). The use of chiral phosphoric acid ligands has also failed to enantioselectively differentiate the gem-dimethyl groups in isobutyric acid-derived substrates (28–31).
Our recent development of bidentate chiral acetyl-protected aminoethyl quinoline (APAQ, L1) ligands for enantioselective methylene C–H activation prompted us to test whether these ligands would prove useful for the desymmetrization of isopropyl groups in isobutyric acid-derived amides (9). With isobutyric amide substrate 1, L1 gave nearly racemic products (Fig. 2A). Furthermore, extensive modification of APAQ ligands did not lead to a noticeable improvement in enantiomeric ratio (er). This result highlights the difference between the desymmetrization of isopropyl groups and enantioselective methylene C–H activation. To amplify steric interactions between substrate and catalyst, we replaced the quinoline motif with oxazoline, thereby enabling introduction of additional chiral bias to the catalyst. Ligand L2 — consisting of an achiral oxazoline moiety, a bulky isopropyl stereocenter (derived from valine), and an N-acetyl-protected amine — failed to achieve significant stereoinduction. Further, the chiral oxazoline ligand L3 derived from N-acetyl glycine — and consequently lacking a stereocenter on the ligand backbone — also failed to afford enantioenriched products. Intriguingly, the combination of the two chiral centers at C-4 on the oxazoline and the side chain in L22 afforded a promising er of 85:15. Increasing the steric bulkiness on the side chain (i.e. from isopropyl to tert-butyl, L23) further improved the selectivity to 98:2 er (For extensive optimizations of chiral ligands, Pd source, additives, and reaction concentrations, see tables S1-S5). The synergistic effect of the mutual relationship between these two stereocenters is evident from the complete loss of enantioselectivity when the absolute stereochemistry of one of the chiral centers is reversed as in L24. Although full rationalizations of the observed asymmetric induction require extensive computational and kinetic analysis, the absolute configuration of the product and the observed influence of each chiral center on the effectiveness of the ligands are consistent with the steric interactions indicated in the C–H insertion intermediate III (Fig. 1C). We propose that the N-acetyl group on the coordinating nitrogen is oriented on the top face of the palladium square plane due to steric repulsion from the chiral center on the side chain. The 4-benzyl group on the oxazoline ring further shields the top face of intermediate III. These combined steric interactions may reinforce the orientation of the α-methyl group in III thereby establishing the stereochemistry of the C–H cleavage step.
Fig. 2. Enantioselective C–H arylation of isobutyric acid and 2-aminoisobutyric acid.
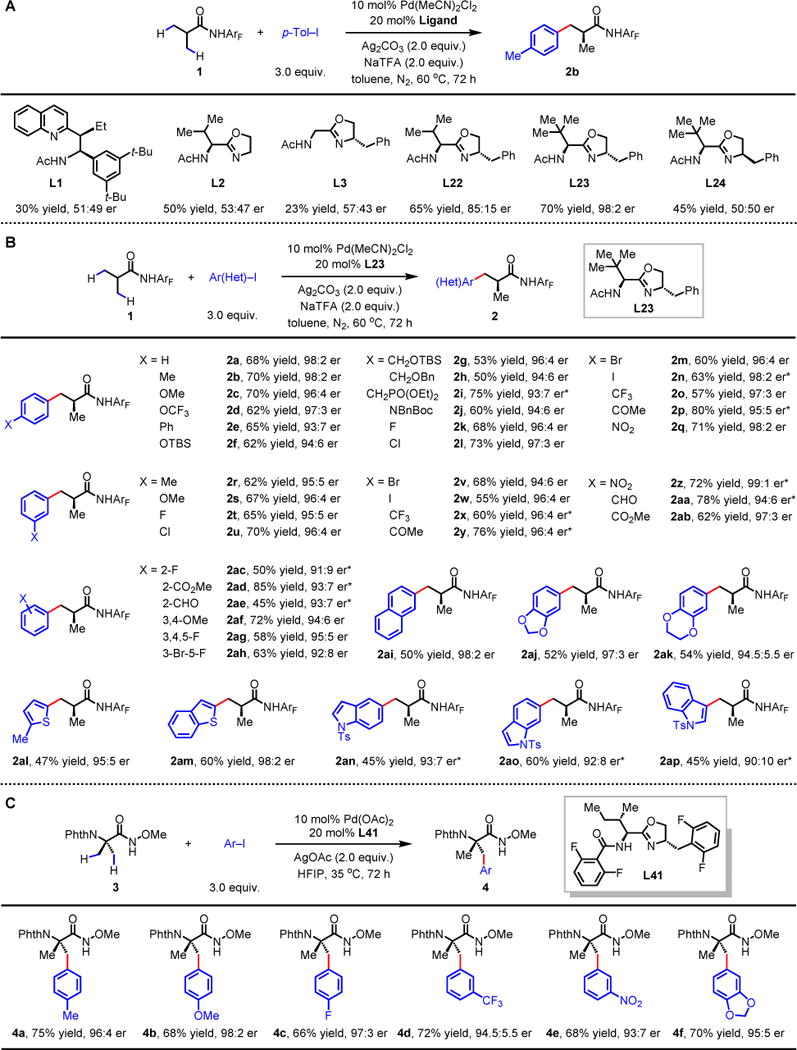
(A) Ligand optimization. (B) Arylation of isobutyric acid. (C) Arylation of 2-aminoisobutyric acid. For each entry number (in boldface), data are reported as isolated yield. See supplementary materials for experimental details. *10 mol% Pd(OAc)2, 20 mol% L30, Ar-I, Ag2CO3, toluene, 50 °C, 72 h. ArF, 4-(CF3)C6F4; p-Tol-I, para-tolyl iodide; equiv., equivalent; Et, ethyl group; Ph, phenyl group; Ac, acetyl group; Ar(Het), (hetero)aryl group; TBS, fert-butyldimethylsilyl group; Bn, benzyl group; Boc, tert-butyloxycarbonyl group; Ts, tosyl group; Phth, phthalimido group; HFIP, hexafluoro-2-propanol.
With the optimized chiral ligand and conditions in hand, we screened the enantioselective β-C–H arylation of isobutyric amide 1 with a wide range of aryl iodides (Fig. 2B). A range of para-substituents on the aryl iodides from electron-donating (OMe, NBnBoc) to electron-withdrawing (halides, CF3, keto and nitro) groups are tolerated, providing enantioselectivity up to 98:2 er (2a–q). The scope of meta-substituted aryl iodides is also broad, and enantiomeric ratios greater than 94:6 (2r–ab) were obtained. The above scope of ortho-, meta- and para-substituted aryl iodides features either halogens (as in 21-2n, 2u-2w and 2ah) or reactive groups (phosphonate moiety as in 2i and aldehyde functionality as in 2aa and 2ae) that can serve as useful synthetic handles for subsequent chemical manipulation. A number of heteroaryl iodides, including thiophene, benzothiophene and indole moieties are also compatible, affording products with high enantioselectivity in synthetically useful yields (2al–ap). The simplicity of deprotecting the amide auxiliary using BF3·Et2O was also demonstrated with product 2q without erosion in enantioselectivity (see supplementary materials for experimental details).
The unique pharmacological and conformational properties of α,α-disubstituted α-amino acids display in peptide drug molecules (24, 25) prompted us to apply these conditions to N-phthaloyl-protected 2-amino-isobutyric acid derivatives (Fig. 2C). However, the analogous amide substrate prepared by condensing the N-Phth-protected-2-aminoisobutyric acid with 2,3,5,6-tetrafluoro-4-(trifluoromethyl)aniline is not reactive under these conditions, presumably due to the steric bulkiness of this substrate which may prevent coordination. To reduce the steric hindrance of the substrate, we prepared the N-methoxy amide 3 of N-phthaloyl-protected 2-amino-isobutyric acid (32, 33; Fig. 2C). Although 3 showed substantial decomposition under the conditions established for substrate 1, we were able to find milder conditions that allowed the β-arylation to proceed at 35 °C. Although ligand L23 gave satisfactory enantioselectivity (91:9 er), the yield remained low despite extensive optimizations (ca. 40% yield). We next elected to screen MPAO ligands. Whereas modification of the oxazoline ring and the amino acid side chain afforded only moderate improvement in yields (see supplementary materials for experimental details), replacement of the N-acetyl moiety by an ortho-difluorobenzoyl group (L41) increased the yield of 4a to 75% while maintaining high enantioselectivity (Fig. 2C, 96:4 er). C–H coupling with a variety of aryl iodides afforded difficult-to-access enantioenriched α,α-disubstituted α-amino acids containing chiral α-quaternary centers (4a–f, for coupling with a wide range of aryl iodides, see Fig. S1 in supplementary materials). The facile conversion of the N-methoxy amide auxiliary to an ester renders this method easy to use (32). On gram scale, both the N-methoxy amide auxiliary and the phth protecting group were removed and converted the Fmoc-protected amino acid as a building block for peptide synthesis (See supplementary materials).
One potential advantage of an organometallic approach to enantioselective functionalizations of C(sp3)-H bonds is that the metallated intermediate can undergo reactivity with various coupling partners. Unfortunately, Pd-catalyzed enantioselective C(sp3)-H activation reactions have been limited to arylation thus far. To fully realize the potential of asymmetric metal insertion processes, we next developed enantioselective C–H alkenylation, and alkynylation reactions of 1. β-Styrenylation of amide 1 using chiral ligand L23 afforded 88:12 er (Fig. 3). The use of L30 containing an additional chiral center at C-5 improved the enantioselectivity to 94:6 er. A number of (E)-styrenyl iodides are compatible coupling partners (5a–s) including the disubstituted (E)-styrenyl iodide (5t). The use of other simple (E)-alkenyl iodides also gave good enantioselectivity, albeit in lower yields (5u, 5v). Upon minor alteration of reaction conditions, L30 has also allowed the enantioselective β-alkynylation to proceed in 94.5:5.5 er (6). To further broaden the diversity of the chiral centers accessed through these three enantioselective transformations, we also performed subsequent diverse β-functionalizations of the remaining methyl group including arylation (7a–f), alkynylation (8), alkylation (9), bromination (10), and borylation (11) (Fig. 4). Thus the sequential C–H functionalizations of the isopropyl groups with a wide range of coupling partners afford a myriad of α-chiral carboxylic acids.
Fig. 3. Enantioselective C–H alkenylation and alkynylation of isobutyric acid.
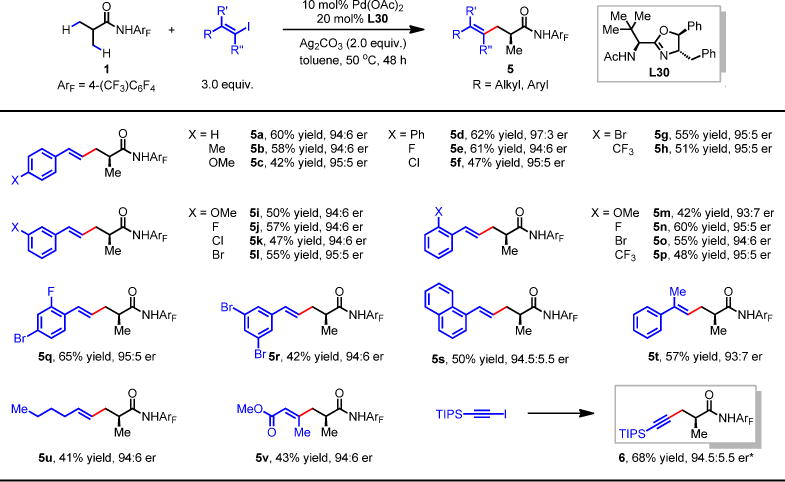
For each entry number (in boldface), data are reported as isolated yield. See supplementary materials for experimental details. *10 mol% Pd(MeCN)2Ch2 was used instead of Pd(OAc)2. NaOAc was used as the additive. TIPS, triisopropylsilyl group.
Fig. 4. Diverse functionalizations of the remaining methyl group.
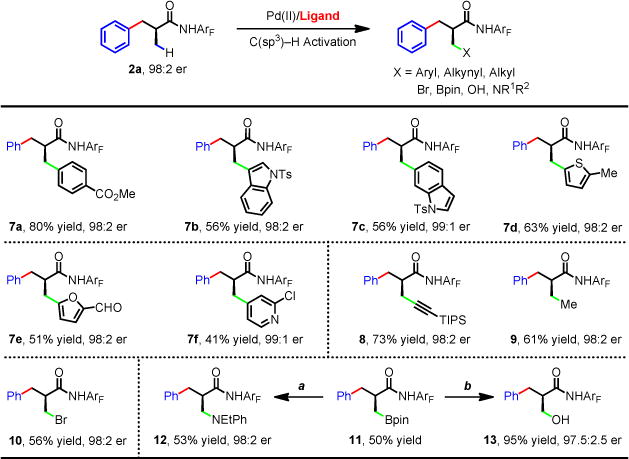
For each entry number (in boldface), data are reported as isolated yield. See supplementary materials for experimental details. Reagents and conditions: (a) 10 mol% Cu(OAc)2, NHEtPh, Ag2CO3, toluene, 100 °C (b) H2O2, aq. buffer (pH = 7), THF, rt.
Our success in the steric differentiation of a hydrogen atom and a relatively small methyl group by an organometallic catalyst provides invaluable insight for the further development of desymmetrizing enantioselective C–H activation reactions. The enantioselective β-C–H activation reactions reported herein provide a versatile platform for constructing α-chiral centers in asymmetric synthesis.
Supplementary Material
One Sentence Summary.
Pd-catalyzed enantioselective C–H arylation, alkenylation, and alkynylation of isobutyric acid/2-amino-isobutyric acid derivatives enables the construction of α-tertiary and α-quaternary centers from bulk chemicals.
Acknowledgments
We gratefully acknowledge The Scripps Research Institute, the NIH (NIGMS, 2R01GM084019) and Bristol-Myers Squibb for their financial support. Tyler Gordon Saint-Denis, Marcus E. Farmers and Anh Tran are thanked for editorial assistance. J.-Q. Y. conceived the concept. Q.-F. W. developed the chiral ligands, optimized the reaction conditions and surveyed the substrate scope. P.-X. S. performed the sequential C–H functionalization reactions. J. H., X.-B. W., F. Z., Q. S. optimized the chiral ligands. R.-Y. Z. performed the C–H bromination reaction. C. M., J. X. Q., M. A. P. scaled up the C–H activation reaction for the preparation of β-arylated 2-amino-isobutyric acids. J.-Q. Y. directed the project. Additional data supporting the conclusions are in supplementary material. Metrical parameters for the structures of L23 and 4c are available free of charge from the Cambridge Crystallographic Data Centre under reference number CCDC- 1518967 and CCDC- 1518968, respectively.
Footnotes
Supplementary Materials:
NMR Spectra
HPLC Traces
X-Ray Crystallographic Data
References (34–42)
References and Notes
- 1.Giri R, Shi BF, Engle KM, Maugel N, Yu JQ. Chem Soc Rev. 2009;38:3242–3272. doi: 10.1039/b816707a. [DOI] [PubMed] [Google Scholar]
- 2.Goodhue CT, Schaeffer JR. Biotechnol Bioeng. 1971;13:203–214. doi: 10.1002/bit.260130204. [DOI] [PubMed] [Google Scholar]
- 3.Hasegawa J, Ogura M, Hamaguchi S, Shimazaki M, Kawaharada H, Watanabe K. J Ferment Technol. 1981;59:203–208. [Google Scholar]
- 4.Bode JW, Fraefel N, Muri D, Carreira EM. Angew Chem Int Ed. 2001;40:2082–2085. [PubMed] [Google Scholar]
- 5.Paterson I, Britton R, Delgado O, Meyer A, Poullennec KG. Angew Chem Int Ed. 2004;43:4629–4633. doi: 10.1002/anie.200460589. [DOI] [PubMed] [Google Scholar]
- 6.Lawhorn BG, Boga SB, Wolkenberg SE, Colby DA, Gauss CM, Swingle MR, Amable L, Honkanen RE, Boger DL. J Am Chem Soc. 2006;128:16720–16732. doi: 10.1021/ja066477d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng J, Kasun ZA, Krische MJ. J Am Chem Soc. 2016;138:5467–5478. doi: 10.1021/jacs.6b02019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corey EJ, Kürti L, editors. Enantioselective Chemical Synthesis: Methods, Logic and Practice. Direct Book Publishing; Dallas, TX: 2010. [Google Scholar]
- 9.Chen G, Gong W, Zhuang Z, Andrä MS, Chen Y-Q, Hong X, Yang Y-F, Liu T, Houk KN, Yu J-Q. Science. 2016;353:1023–1027. doi: 10.1126/science.aaf4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giri R, Chen X, Yu JQ. Angew Chem Int Ed. 2005;44:2112–2115. doi: 10.1002/anie.200462884. [DOI] [PubMed] [Google Scholar]
- 11.Giri R, Liang J, Lei JG, Li JJ, Wang DH, Chen X, Naggar IC, Guo C, Foxman BM, Yu JQ. Angew Chem Int Ed. 2005;44:7420–7424. doi: 10.1002/anie.200502767. [DOI] [PubMed] [Google Scholar]
- 12.Xiao KJ, Lin DW, Miura M, Zhu RY, Gong W, Wasa M, Yu JQ. J Am Chem Soc. 2014;136:8138–8142. doi: 10.1021/ja504196j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He C, Gaunt M. Angew Chem Int Ed. 2015;54:15840–15844. doi: 10.1002/anie.201508912. [DOI] [PubMed] [Google Scholar]
- 14.Nakanishi M, Katayev D, Besnard C, Kündig EP. Angew Chem Int Ed. 2011;50:7438–7441. doi: 10.1002/anie.201102639. [DOI] [PubMed] [Google Scholar]
- 15.Anas S, Cordi A, Kagan HB. Chem Comm (Camb) 2011;47:11483–11485. doi: 10.1039/c1cc14292e. [DOI] [PubMed] [Google Scholar]
- 16.Martin N, Pierre C, Davi M, Jazzar R, Baudoin O. Chem Eur J. 2012;18:4480–4484. doi: 10.1002/chem.201200018. [DOI] [PubMed] [Google Scholar]
- 17.Saget T, Lemouzy SJ, Cramer N. Angew Chem Int Ed. 2012;51:2238–2242. doi: 10.1002/anie.201108511. [DOI] [PubMed] [Google Scholar]
- 18.Larionov E, Nakanishi M, Katayev D, Besnard C, Kündig EP. Chem Sci. 2013;4:1995–2005. [Google Scholar]
- 19.Holstein PM, Vogler M, Larini P, Pilet G, Clot E, Baudoin O. ACS Catal. 2015;5:4300–4308. [Google Scholar]
- 20.Murai M, Takeshima H, Morita H, Kuninobu Y, Takai K. J Org Chem. 2015;80:5407–5414. doi: 10.1021/acs.joc.5b00920. [DOI] [PubMed] [Google Scholar]
- 21.Yang L, Melot R, Neuburger M, Baudoin O. Chem Sci. doi: 10.1039/C6SC04006C. advance article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holstein PM, Dailler D, Vantourout J, Shaya J, Millet A, Baudoin O. Angew Chem Int Ed. 2016;55:2805–2809. doi: 10.1002/anie.201511457. [DOI] [PubMed] [Google Scholar]
- 23.Renaudat A, Jean-Gérard L, Jazzar R, Kefalidis CE, Clot E, Baudoin O. Angew Chem Int Ed. 2010;49:7261–7265. doi: 10.1002/anie.201003544. [DOI] [PubMed] [Google Scholar]
- 24.Venkatraman J, Shankaramma SC, Balaram P. Chem Rev. 2001;101:3131–3152. doi: 10.1021/cr000053z. [DOI] [PubMed] [Google Scholar]
- 25.Sagan S, Karoyan P, Lequin O, Chassaing G, Lavielle S. Curr Med Chem. 2004;11:2799–2822. doi: 10.2174/0929867043364108. [DOI] [PubMed] [Google Scholar]
- 26.Musaev DG, Kaledinm AL, Shi BF, Yu JQ. J Am Chem Soc. 2012;134:1690–1698. doi: 10.1021/ja208661v. [DOI] [PubMed] [Google Scholar]
- 27.Giri R, Lan Y, Liu P, Houk KN, Yu JQ. J Am Chem Soc. 2012;134:14118–14126. doi: 10.1021/ja304643e. [DOI] [PubMed] [Google Scholar]
- 28.Engle KM, Yu JQ. J Org Chem. 2013;78:8927–8955. doi: 10.1021/jo400159y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan SB, Zhang S, Duan WL. Org Lett. 2015;17:2458–2461. doi: 10.1021/acs.orglett.5b00968. [DOI] [PubMed] [Google Scholar]
- 30.Jain P, Verma P, Xia G, Yu JQ. Nat Chem. 2016;134:14118–14126. [Google Scholar]
- 31.Wang H, Tong HR, He G, Chen G. Angew Chem Int Ed. 2016;55:15387–15391. doi: 10.1002/anie.201609337. [DOI] [PubMed] [Google Scholar]
- 32.Chen G, Shigenari T, Jain P, Zhang Z, Jin Z, He J, Li S, Mapelli C, Miller MM, Poss MA, Scola PM, Yeung KS, Yu JQ. J Am Chem Soc. 2015;137:3338–3351. doi: 10.1021/ja512690x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu RY, Farmer ME, Chen YQ, Yu JQ. Angew Chem Int Ed. 2016;55:10578–10599. doi: 10.1002/anie.201600791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wasa M, Engle KM, Yu JQ. Pd(II)-catalyzed olefination of sp3C–H bonds. J Am Chem Soc. 2010;132:3680–3681. doi: 10.1021/ja1010866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li K, Tan G, Huang J, Song F, You J. Iron-catalyzed oxidative C–H/C–H cross-coupling: an efficient route to α-quaternary α-amino acid derivatives. Angew Chem Int Ed. 2013;52:12942–12945. doi: 10.1002/anie.201306181. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura M, Hatakeyama T, Hara K, Nakamura E. Enantioselective synthesis of α-substituted ketones by asymmetric addition of chiral zinc enamides to 1-alkenes. J Am Chem Soc. 2003;125:6362–6363. doi: 10.1021/ja035091p. [DOI] [PubMed] [Google Scholar]
- 37.Reetz MT, Drewes MW, Schmitz A. Stereoselective synthesis of β-Amino alcohols from optically active α-amino acids. Angew Chem Int Ed. 1987;26:1141–1143. [Google Scholar]
- 38.Wu Y, Liu J, Li X, Chan ASC. Vanadium-catalyzed asymmetric oxidation of sulfides using schiff base ligands derived from β-amino alcohols with two stereogenic centers. Eur J Org Chem. 2009:2607–2610. [Google Scholar]
- 39.Sun X, Chen Y, Wu N, Kang CS, Song HA, Jin S, Fu Y, Bryant H, Frank JA, Chong HS. RSC Adv. 2015;5:94571–94581. doi: 10.1039/C5RA11306G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kazmierczak M, Koroniak H. J Fluorine Chem. 2012;139:23–27. [Google Scholar]
- 41.Lee JY, Miller JJ, Hamilton SS, Sigman MS. Stereochemical diversity in chiral ligand design: discovery and optimization of catalysts for the enantioselective addition of allylic halides to aldehydes. Org Lett. 2005;7:1837–1839. doi: 10.1021/ol050528e. [DOI] [PubMed] [Google Scholar]
- 42.Nerdel F, John U. Untersuchungen in der reihe der hydratropasäure, v. mitteil.: optisch aktive m- und p- substituierte homohydratropasäuren. Chem Ber. 1956;89:1945–1950. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


