Abstract
An immunocompromised patient with an invasive soft tissue infection due to Scedosporium apiospermum was successfully treated with voriconazole and surgical debridement. After transition from intravenous to oral therapy, successive adjustments of the oral dose were required to achieve complete resolution. For soft tissue infections due to molds characterized by thin, septate hyphae branching at acute angles, voriconazole should be considered a first-line antifungal agent. The potential usefulness of plasma voriconazole levels for guiding optimal therapy should be investigated.
CASE REPORT
A 58-year-old woman who had been treated chronically for Behçet's disease with prednisone, at doses ranging from 10 to 40 mg per day over 10 years, developed pain, swelling, and erythema of the left wrist. These symptoms were felt by her rheumatologist to represent an unusually severe exacerbation of arthritis, and a single dose of infliximab at 3 mg/kg was given intravenously. In addition, prednisone was increased from 20 to 100 mg per day, and amoxicillin-clavulanic acid at 875 mg twice daily was empirically started. Due to continued pain and erythema, the patient underwent exploratory surgery of her left wrist 10 days later at a local community hospital. Intraoperatively, thickening and early mucinous degeneration of the tenosynovium of an extensor tendon was noted from the wrist to the distal end of the third metacarpal. Surgically obtained tissue grew rare methicillin-sensitive Staphylococcus aureus but was negative for fungi and mycobacteria based on both cultures and histopathology. Antibiotic therapy was changed from oral amoxicillin-clavulanic acid to intravenous oxacillin at 2 g every 4 h, and her oral prednisone dosage was reduced gradually from 100 mg daily to 20 mg daily over the following week.
The patient's symptoms continued to progress, however, with erythema spreading from the dorsum of the left wrist to the elbow. At 4 weeks after the exploratory surgery, the patient was admitted to Stanford University Medical Center. Oxacillin was discontinued, and intravenous vancomycin, given as 1 g every 12 h, was initiated. The following day, surgical exploration and debridement of the left wrist and forearm were performed, and necrosis involving multiple extensor tendons of the wrist was noted intraoperatively. Specimens from her wrist joint and tendons were sent for bacterial, mycobacterial, and fungal cultures. Small, gray colonies of a mold grew in a culture derived from an extensor tendon. Early examination of this mold revealed thin, septate hyphae branching at acute angles. On the basis of this morphology, intravenous voriconazole was initiated at a loading dose of 6 mg/kg every 12 h for two doses, followed by 4 mg/kg given every 12 h; prednisone was continued at 20 mg daily. Two days later, diminished pain and erythema of the patient's forearm and elbow were noted. The mold was ultimately identified as Scedosporium apiospermum. The patient showed continued improvement over a 10-day course of intravenous voriconazole. Three days prior to discharge, vancomycin was discontinued and prednisone was decreased to 15 mg daily. She was discharged from the hospital on prednisone 10 mg daily and oral voriconazole at the manufacturer's recommended dose of 200 mg twice per day. Vancomycin was not continued after hospital discharge.
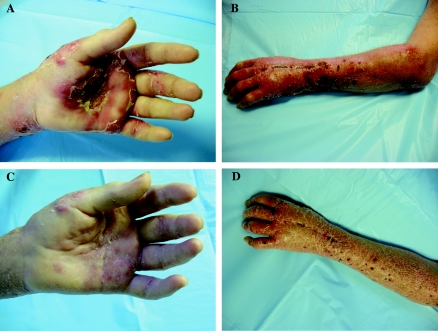
One week later, the patient was noted to have significant worsening of her infection despite reporting strict adherence to the oral antifungal regimen. There were no changes in her prescribed medications, including her immunosuppressive therapy, and she denied using any new over-the-counter medications or supplements. Extensive erythema and swelling of her left wrist, forearm, and elbow were again evident, along with the development of discrete subcutaneous nodules and pustules over her left hand and forearm in a distribution consistent with lymphangitic spread (Fig. 1A and B). Purulent material expressed from these nodules yielded S. apiospermum in pure culture in an outside laboratory, as well as 1 week later in the clinical microbiology laboratory at Stanford University Medical Center.
FIG. 1.
Left hand (A) and arm (B) of patient 1 week after transition from intravenous to oral voriconazole. The nodules and pustules form a pattern characteristic of lymphangitic spread. At 2 weeks after reinstitution of intravenous voriconazole therapy, the purulent nodules had disappeared from the hand (C) and arm (D).
Antifungal susceptibility testing of the isolate revealed MICs at 48 h of 16 μg/ml for amphotericin B, 1 μg/ml for itraconazole, and 0.25 μg/ml for voriconazole. The minimum effective concentration of caspofungin at 24 h was >4 μg/ml. Antifungal drugs tested in various combinations (amphotericin B plus voriconazole, caspofungin plus voriconazole, caspofungin plus itraconazole, and voriconazole plus terbinafine) displayed indifference. In none of the combinations tested was synergy or an additive effect seen. (All antifungal susceptibility tests were performed by the Fungus Testing Laboratory, University of Texas Health Science Center at San Antonio.)
Given the patient's prior response to parenteral therapy, intravenous voriconazole was resumed at a dose of 4 mg/kg every 12 h. No antibacterial agents were given, and no change was made to the patient's prednisone dose. Two weeks later, resolution of the nodules was observed (Fig. 1C and D). A second attempt was made to transition the patient to oral therapy, this time at an increased dose (300 mg twice per day) in light of the clinical failure observed at a dose of 200 mg twice per day. Her infection continued to improve on this higher oral dose. Three weeks later, however, she reported nausea, difficulty concentrating, and visual hallucinations, complaints consistent with known voriconazole side effects. The voriconazole dose was then decreased to 250 mg twice per day. On this regimen her central nervous system symptoms abated, and the infection continued to improve clinically. The patient ultimately completed a total of 5 months of oral voriconazole while on an oral prednisone dose ranging from 10 to 25 mg per day. She remained free of any signs of recurrence 14 months after the completion of therapy.
S. apiospermum, previously known as Monosporium apiospermum, is the anamorph or asexual form of Pseudallescheria boydii, previously known as Petriellidium or Allescheria boydii (24). Usual sites of infection include the central nervous system, eyes, lungs, sinuses, bones, joints, and soft tissues (45). The diagnosis of invasive fungal infection is ideally based on a combination of histopathologic, microbiologic, and clinical findings. Although histopathology was not available for our patient, S. apiospermum was isolated in pure culture from three independently collected samples over a 1-month period, including one specimen obtained intraoperatively. In addition, the patient's relapse responded to voriconazole in the absence of antibacterial therapy, and her clinical presentation was consistent with an S. apiospermum soft tissue infection as described in the literature (see below). This evidence strongly supports the diagnosis of invasive S. apiospermum infection.
A search of the MEDLINE database (1966 to 2004) for reports published in the English literature of localized, culture-proven S. apiospermum soft tissue infection (excluding mycetoma) found 27 cases (Table 1). Infections were described more commonly in immunosuppressed patients (3-5, 7, 9, 13, 17, 21, 23, 25, 27, 28, 30-32, 35, 38, 41, 42, 46) but also occurred in immunocompetent patients (20, 21, 26, 40, 43, 44). Cases have been reported to occur secondary to inoculation from environmental sources (21, 40), after medical procedures such as surgery or intravenous catheter placement (5, 26), and in the absence of identifiable trauma. In our patient, the source and timing of exposure to S. apiospermum is unknown.
TABLE 1.
Summary of patient characteristics and clinical interventions in case reports published in English of localized S. apiospermum soft tissue infections, listed in chronologic order of publication
| Case no. | Age (yr) | Sexa | Predisposing conditionb | Infection location | Antifungal(s) usedc | Surgical treatment | Lymphangitic spread | Treatment success | Source of reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 24 | M | Surgical wound | Scalp | MIC | N | N | + | 26 |
| 2 | 50 | F | None identified | Leg | MIC, KTC | Y | N | + | 44 |
| 3 | 58 | M | Cardiac transplant | Knee | MIC | Y | N | + | 38 |
| 4 | 51 | M | Traumatic injury | Foot | AMB, MIC, ITC | Y | N | + | 40 |
| 5 | 45 | M | Renal transplant | Knee | ITC | Y | N | + | 28 |
| 6 | 63 | M | AML and chemotherapy | Leg | ITC | N | Y | + | 46 |
| 7 | 71 | M | AML and chemotherapy | Foot | FLC, AMB, ITC | N | N | + | 9 |
| 8 | 63 | M | AML and chemotherapy | Foot | ITC | Y | N | + | 42 |
| 9 | 59 | M | Corticosteroids (nephrotic syndrome) | Arm | ITC | N | Y | + | 17 |
| 10 | 67 | M | Corticosteroids (asthma) | Hand | FLC | N | N | + | 23 |
| 11 | 42 | M | Corticosteroids (Addison's disease) | Face | ITC | N | N | + | 27 |
| 12 | 66 | F | None identified | Arm | ITC | N | N | + | 43 |
| 13 | 69 | M | Corticosteroids (polymyositis) | Hand | Topical antifungal | N | N | − | 31 |
| 14 | 81 | M | Corticosteroids (pulmonary fibrosis) | Arm | ITC | N | Y | − | 4 |
| 15 | 65 | M | Cardiac transplant | Arm | ITC, MIC | Y | N | + | 13 |
| 16 | 65 | F | Corticosteroids (osteoarthritis) | Arm | ITC | Y | Y | + | 21 |
| 17 | 48 | M | Traumatic injury | Hand | ITC | Y | N | + | 21 |
| 18 | 64 | M | Corticosteroids (sarcoidosis) | Arm | KTC, ITC | N | N | + | 25 |
| 19 | 69 | F | Corticosteroids (rheumatoid arthritis) | Arm | ITC | Y | Y | + | 5 |
| 20 | 60 | M | Renal transplant | Foot | ITC | Y | N | − | 30 |
| 21 | 58 | M | Renal transplant | Leg | ITC, VRC | N | Y | + | 32 |
| 22 | 49 | M | Bone marrow transplant | Foot | AMB, MIC, ITC | N | N | − | 35 |
| 23 | 59 | M | Renal transplant | Hand | ITC, VRC | N | N | + | 3 |
| 24 | 84 | M | Corticosteroids (COPD) | Arm | VRC | N | N | + | 3 |
| 25 | 63 | F | Corticosteroids, cyclosporine (rheumatoid arthritis) | Arm | ITC | N | Y | + | 7 |
| 26 | 27 | F | None identified | Foot | TRB (plus topical CLT) | N | N | + | 20 |
| 27 | 58 | M | Renal transplant | Foot | FLC, ITC, MIC | Y | N | + | 41 |
| 28 | 58 | F | Corticosteroids (Behçet's disease) | Arm | VRC | Y | Y | + | This report |
M, male; F, female.
AML, acute myelogenous leukemia; COPD, chronic obstructive pulmonary disease.
MIC, miconazole; KTC, ketoconazole; AMB, amphotericin B; ITC, itraconazole; FLC, fluconazole; VRC, voriconazole; TRB, terbinafine; CLT, clotrimazole.
In approximately one-fourth of cases a nodular lymphangitic pattern of spread was noted (4, 5, 7, 17, 21, 32, 46), a pattern classically associated with organisms such as Sporothrix schenckii, Mycobacterium marinum, Francisella tularensis, Nocardia brasiliensis, and Leishmania brasiliensis (15). S. apiospermum should be considered in the differential diagnosis of patients who present with this clinical finding, particularly if they are immunocompromised. In patients with invasive fungal infections, immunosuppressive medications should be minimized to the extent that clinical circumstances allow. In addition, surgical intervention has been described as an important element of successful treatment of soft tissue S. apiospermum infections in 11 of 27 published case reports (5, 13, 21, 28, 30, 38, 40-42, 44). In our patient, reduction of the prednisone dose, discontinuation of the infliximab, and surgical debridement of the wrist likely contributed to her successful outcome.
Amphotericin B has historically proven to be ineffective in the treatment of S. apiospermum infection, and most isolates exhibit resistance in vitro (8, 11, 12). Intravenous miconazole has been used despite limited efficacy, but this agent is currently unavailable in the United States. Itraconazole has also been used for S. apiospermum soft tissue infections, with some case reports demonstrating successful outcomes (Table 1). There are fewer published reports describing successful outcomes with itraconazole for the treatment of disseminated S. apiospermum infection in immunocompromised hosts (2, 37).
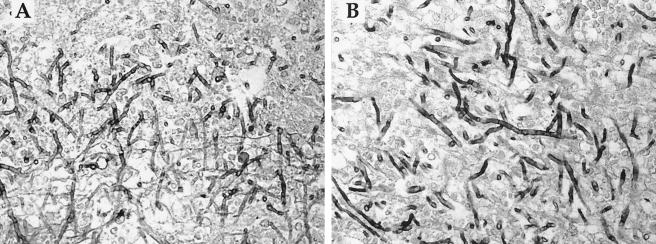
Because Aspergillus spp. and S. apiospermum are often difficult to distinguish by histopathology (Fig. 2), the fact that most isolates of S. apiospermum are resistant to amphotericin B is a critical consideration in selecting an antifungal prior to final species identification. Lopez et al., for example, emphasize the importance of avoiding mistaken assumptions regarding the identity of fungal isolates based on histopathology or early cultures, especially in an acutely ill patient (29).
FIG. 2.
Histopathologic sections demonstrating the similarity between S. apiospermum (A) and A. fumigatus (B) in autopsy sections of cerebral tissues from immunosuppressed patients (Gomori-methenamine silver stain, ×600 magnification). Both fungi display thin, delicate, septate hyphae branching at acute angles. Note that these sections are provided for illustrative purposes and are not from the patient discussed in this case report.
Voriconazole has a broad spectrum of antifungal activity in vitro, including S. apiospermum, Aspergillus spp., and Fusarium spp., but generally poor activity against the class Zygomycetes (8, 12, 18, 19). Voriconazole has also shown promise in the treatment of disseminated S. apiospermum infection in a neutropenic mouse model (6). In humans, the successful use of voriconazole has been reported in S. apiospermum subcutaneous infection (3) and disseminated disease in immunocompromised adults (14, 33) and children (47). Other case reports of S. apiospermum infections successfully treated with voriconazole include keratitis (36), brain abscess (34), and pneumonia (22).
There is also a growing body of literature demonstrating the effectiveness of voriconazole against Aspergillus species, as well as against other clinically important molds. A multicenter, randomized trial of primary therapy for invasive aspergillosis showed a better outcome in patients randomized to receive voriconazole compared to those randomized to receive amphotericin B deoxycholate (16). Other investigations have demonstrated the efficacy of voriconazole for salvage therapy of invasive fungal infections in patient populations that historically have proven difficult to treat. In one study consisting primarily of immunocompromised patients, voriconazole resulted in a satisfactory response in 47% of patients whose infections failed to respond to previous antifungal therapy and in 68% of patients whose infections had no approved therapy (39). In a smaller study with a similar patient population, salvage therapy with voriconazole led to a complete or partial response in 58% of patients who failed or were intolerant of standard antifungal therapy (1).
In clinical practice, however, voriconazole can present obstacles to its effective use. First, voriconazole is known to have variable pharmacokinetics. In a study of voriconazole for the treatment of invasive aspergillosis, patients on standard doses (6 mg/kg every 12 h for two doses, followed by 4 mg/kg every 12 h for intravenous dosing and 200 mg twice per day for oral dosing) demonstrated levels in plasma ranging from <0.1 μg/ml to as high as 9.7 μg/ml (10). In our patient, there was significant clinical relapse after transition from intravenous to oral therapy despite following the manufacturer's recommended dosing. However, at a higher oral dose (300 mg twice per day), the patient again showed clinical improvement; this was presumably attributable to the achievement of higher levels in plasma.
Some data suggest, however, that elevated voriconazole levels in plasma may be associated with an increased likelihood of side effects. In the study cited above, 6 of 22 (27%) of patients with concentrations that were >6.0 μg/ml in plasma developed abnormal liver function tests (defined as greater than three times the upper limit of normal), whereas 14 of 100 (14%) of patients with levels that were <6.0 μg/ml did so (10). Whether these findings actually represent concentration-dependent side effects is unknown, and further studies are needed. Our patient experienced intolerable side effects at 300 mg twice per day of oral therapy, but at a decreased dose of 250 mg twice per day had a sustained clinical response without undue toxicity. Another potentially complicating factor is the significant potential for drug-drug interactions (18). This did not appear to be an issue in this case.
In summary, we describe a patient with Behçet's disease treated with infliximab and chronic prednisone therapy who developed a soft tissue S. apiospermum infection that was successfully treated with voriconazole and surgical debridement. For soft tissue infections due to molds characterized by thin, septate hyphae branching at acute angles (thereby rendering zygomycetes unlikely), voriconazole should be considered a first-line antifungal agent. Because the pharmacokinetics of voriconazole are variable and difficult to predict, the potential role of voriconazole levels in plasma in guiding optimal therapy should be investigated.
Acknowledgments
We greatly appreciate the help of our coworkers Ellen Jo Baron (Clinical Microbiology Laboratory, Stanford University School of Medicine) and Brady Feliz (Department of Pathology, Stanford University School of Medicine) in obtaining and photographing fungal specimens. We also thank Dora McCarthy for performing antifungal susceptibility testing.
REFERENCES
- 1.Baden, L. R., J. T. Katz, J. A. Fishman, C. Koziol, A. DelVecchio, M. Doran, and R. H. Rubin. 2003. Salvage therapy with voriconazole for invasive fungal infections in patients failing or intolerant to standard antifungal therapy. Transplantation 76:1632-1637. [DOI] [PubMed] [Google Scholar]
- 2.Barbaric, D., and P. J. Shaw. 2001. Scedosporium infection in immunocompromised patients: successful use of liposomal amphotericin B and itraconazole. Med. Pediatr. Oncol. 37:122-125. [DOI] [PubMed] [Google Scholar]
- 3.Bosma, F., A. Voss, H. W. van Hamersvelt, R. G. de Sevaux, J. Biert, B. J. Kullberg, W. G. Melchers, and P. E. Verweij. 2003. Two cases of subcutaneous Scedosporium apiospermum infection treated with voriconazole. Clin. Microbiol. Infect. 9:750-753. [DOI] [PubMed] [Google Scholar]
- 4.Bower, C. P., J. D. Oxley, C. K. Campbell, and C. B. Archer. 1999. Cutaneous Scedosporium apiospermum infection in an immunocompromised patient. J. Clin. Pathol. 52:846-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canet, J. J., X. Pagerols, C. Sanchez, P. Vives, and J. Garau. 2001. Lymphocutaneous syndrome due to Scedosporium apiospermum. Clin. Microbiol. Infect. 7:648-650. [DOI] [PubMed] [Google Scholar]
- 6.Capilla, J., C. Serena, F. J. Pastor, M. Ortoneda, and J. Guarro. 2003. Efficacy of voriconazole in treatment of systemic scedosporiosis in neutropenic mice. Antimicrob. Agents Chemother. 47:3976-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaveiro, M. A., R. Vieira, J. Cardoso, and A. Afonso. 2003. Cutaneous infection due to Scedosporium apiospermum in an immunosuppressed patient. J. Eur. Acad. Dermatol. Venereol. 17:47-49. [DOI] [PubMed] [Google Scholar]
- 8.Cuenca-Estrella, M., B. Ruiz-Diez, J. V. Martinez-Suarez, A. Monzon, and J. L. Rodriguez-Tudela. 1999. Comparative in-vitro activity of voriconazole (UK-109,496) and six other antifungal agents against clinical isolates of Scedosporium prolificans and Scedosporium apiospermum. J. Antimicrob. Chemother. 43:149-151. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham, R., and D. C. Mitchell. 1996. Amphotericin B responsive Scedosporium apiospermum infection in a patient with acute myeloid leukaemia. J. Clin. Pathol. 49:93-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denning, D. W., P. Ribaud, N. Milpied, D. Caillot, R. Herbrecht, E. Thiel, A. Haas, M. Ruhnke, and H. Lode. 2002. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin. Infect. Dis. 34:563-571. [DOI] [PubMed] [Google Scholar]
- 11.Ellis, D. 2002. Amphotericin B: spectrum and resistance. J. Antimicrob. Chemother. 49(Suppl. 1):7-10. [DOI] [PubMed] [Google Scholar]
- 12.Espinel-Ingroff, A. 2001. In vitro fungicidal activities of voriconazole, itraconazole, and amphotericin B against opportunistic moniliaceous and dematiaceous fungi. J. Clin. Microbiol. 39:954-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ginter, G., B. Petutschnig, G. Pierer, H. P. Soyer, S. Reischle, T. Kern, and S. de Hoog. 1999. Case report. Atypical cutaneous pseudallescheriosis refractory to antifungal agents. Mycoses 42:507-511. [DOI] [PubMed] [Google Scholar]
- 14.Girmenia, C., G. Luzi, M. Monaco, and P. Martino. 1998. Use of voriconazole in treatment of Scedosporium apiospermum infection: case report. J. Clin. Microbiol. 36:1436-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heller, H. M., and M. N. Swartz. 1994. Nodular lymphangitis: clinical features, differential diagnosis and management. Curr. Clin. Top. Infect. Dis. 14:142-158. [PubMed] [Google Scholar]
- 16.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J. W. Oestmann, W. V. Kern, K. A. Marr, P. Ribaud, O. Lortholary, R. Sylvester, R. H. Rubin, J. R. Wingard, P. Stark, C. Durand, D. Caillot, E. Thiel, P. H. Chandrasekar, M. R. Hodges, H. T. Schlamm, P. F. Troke, and B. de Pauw. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408-415. [DOI] [PubMed] [Google Scholar]
- 17.Ichikawa, T., M. Saiki, S. Tokunaga, and T. Saida. 1997. Scedosporium apiospermum skin infection in a patient with nephrotic syndrome. Acta Derm. Venereol. 77:172-173. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, L. B., and C. A. Kauffman. 2003. Voriconazole: a new triazole antifungal agent. Clin. Infect. Dis. 36:630-637. [DOI] [PubMed] [Google Scholar]
- 19.Kappe, R. 1999. Antifungal activity of the new azole UK-109,496 (voriconazole). Mycoses 42:83-86. [PubMed] [Google Scholar]
- 20.Karaarslan, A., S. Arikan, F. Karaarslan, and E. S. Cetin. 2003. Skin infection caused by Scedosporium apiospermum. Mycoses 46:524-526. [DOI] [PubMed] [Google Scholar]
- 21.Kim, H. U., S. C. Kim, and H. S. Lee. 1999. Localized skin infection due to Scedosporium apiospermum: report of two cases. Br. J. Dermatol. 141:605-606. [DOI] [PubMed] [Google Scholar]
- 22.Klopfenstein, K. J., R. Rosselet, A. Termuhlen, and D. Powell. 2003. Successful treatment of Scedosporium pneumonia with voriconazole during AML therapy and bone marrow transplantation. Med. Pediatr. Oncol. 41:494-495. [DOI] [PubMed] [Google Scholar]
- 23.Kusuhara, M., and H. Hachisuka. 1997. Lymphocutaneous infection due to Scedosporium apiospermum. Int. J. Dermatol. 36:684-688. [DOI] [PubMed] [Google Scholar]
- 24.Kwon-Chung, K., and J. Bennett. 1992. Pseudallescheriasis and Scedosporium infection, p. 678-694. In Medical mycology. Lea & Febiger, Philadelphia, Pa.
- 25.Lavigne, C., F. Maillot, A. de Muret, M. Therizol-Ferly, F. Lamisse, and L. Machet. 1999. Cutaneous infection with Scedosporium apiospermum in a patient treated with corticosteroids. Acta Dermatol. Venereol. 79:402-403. [DOI] [PubMed] [Google Scholar]
- 26.Lazarus, H. S., J. P. Myers, and R. J. Brocker. 1986. Post-craniotomy wound infection caused by Pseudallescheria boydii: case report. J. Neurosurg. 64:153-154. [DOI] [PubMed] [Google Scholar]
- 27.Liu, Y. F., X. D. Zhao, C. L. Ma, C. X. Li, T. S. Zhang, and W. J. Liao. 1997. Cutaneous infection by Scedosporium apiospermum and its successful treatment with itraconazole. Clin. Exp. Dermatol. 22:198-200. [PubMed] [Google Scholar]
- 28.Lopes, J. O., S. H. Alves, J. P. Benevenga, A. Salla, C. Khmohan, and C. B. Silva. 1994. Subcutaneous pseudallescheriasis in a renal transplant recipient. Mycopathologia 125:153-156. [DOI] [PubMed] [Google Scholar]
- 29.Lopez, F. A., R. S. Crowley, L. Wastila, H. A. Valantine, and J. S. Remington. 1998. Scedosporium apiospermum (Pseudallescheria boydii) infection in a heart transplant recipient: a case of mistaken identity. J. Heart Lung Transplant. 17:321-324. [PubMed] [Google Scholar]
- 30.Miele, P. S., C. S. Levy, M. A. Smith, E. M. Dugan, R. H. Cooke, J. A. Light, and D. R. Lucey. 2002. Primary cutaneous fungal infections in solid organ transplantation: a case series. Am. J. Transplant. 2:678-683. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto, T., R. Sasaoka, M. Kawaguchi, S. Ishioka, T. Inoue, N. Yamada, and M. Mihara. 1998. Scedosporium apiospermum skin infection: a case report and review of the literature. J. Am. Acad. Dermatol. 39:498-500. [DOI] [PubMed] [Google Scholar]
- 32.Montejo, M., M. L. Muniz, S. Zarraga, K. Aguirrebengoa, J. J. Amenabar, L. Lopez-Soria, and R. Gonzalez. 2002. Infection due to Scedosporium apiospermum in renal transplant recipients: a report of two cases and literature review of central nervous system and cutaneous infections by Pseudallescheria boydii/Sc. apiospermum. Mycoses 45:418-427. [DOI] [PubMed] [Google Scholar]
- 33.Munoz, P., M. Marin, P. Tornero, P. Martin Rabadan, M. Rodriguez-Creixems, and E. Bouza. 2000. Successful outcome of Scedosporium apiospermum disseminated infection treated with voriconazole in a patient receiving corticosteroid therapy. Clin. Infect. Dis. 31:1499-1501. [DOI] [PubMed] [Google Scholar]
- 34.Nesky, M. A., E. C. McDougal, and J. E. Peacock, Jr. 2000. Pseudallescheria boydii brain abscess successfully treated with voriconazole and surgical drainage: case report and literature review of central nervous system pseudallescheriasis. Clin. Infect. Dis. 31:673-677. [DOI] [PubMed] [Google Scholar]
- 35.Nonaka, D., H. Yfantis, P. Southall, and C. C. Sun. 2002. Pseudallescheriasis as an aggressive opportunistic infection in a bone marrow transplant recipient. Arch. Pathol. Lab. Med. 126:207-209. [DOI] [PubMed] [Google Scholar]
- 36.Nulens, E., C. Eggink, A. J. Rijs, P. Wesseling, and P. E. Verweij. 2003. Keratitis caused by Scedosporium apiospermum successfully treated with a cornea transplant and voriconazole. J. Clin. Microbiol. 41:2261-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ochiai, N., C. Shimazaki, R. Uchida, S. Fuchida, A. Okano, E. Ashihara, T. Inaba, N. Fujita, and M. Nakagawa. 2003. Disseminated infection due to Scedosporium apiospermum in a patient with acute myelogenous leukemia. Leuk. Lymphoma 44:369-372. [DOI] [PubMed] [Google Scholar]
- 38.Patterson, T. F., V. T. Andriole, M. J. Zervos, D. Therasse, and C. A. Kauffman. 1990. The epidemiology of pseudallescheriasis complicating transplantation: nosocomial and community-acquired infection. Mycoses 33:297-302. [PubMed] [Google Scholar]
- 39.Perfect, J. R., K. A. Marr, T. J. Walsh, R. N. Greenberg, B. DuPont, J. de la Torre-Cisneros, G. Just-Nubling, H. T. Schlamm, I. Lutsar, A. Espinel-Ingroff, and E. Johnson. 2003. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin. Infect. Dis. 36:1122-1131. [DOI] [PubMed] [Google Scholar]
- 40.Pether, J. V., W. Jones, F. B. Greatorex, and W. Bunting. 1992. Acute pyogenic Pseudallescheria boydii foot infection sequentially treated with miconazole and itraconazole. J. Infect. 25:335-336. [DOI] [PubMed] [Google Scholar]
- 41.Reimann, D., E. Bussemaker, and P. Gross. 2004. Successful treatment due to vacuum seal technique of a severe Scedosporium apiospermum skin infection in a renal transplant recipient. Nephrol. Dial. Transplant. 19:245-248. [DOI] [PubMed] [Google Scholar]
- 42.Ruxin, T. A., W. D. Steck, T. N. Helm, W. F. Bergfeld, and B. J. Bolwell. 1996. Pseudallescheria boydii in an immunocompromised host. Successful treatment with debridement and itraconazole. Arch. Dermatol. 132:382-384. [PubMed] [Google Scholar]
- 43.Severo, L. C., M. Oliveira Fde, and A. T. Londero. 1997. Subcutaneous scedosporiosis: report of two cases and review of the literature. Rev. Inst. Med. Trop. Sao Paulo 39:227-230. [DOI] [PubMed] [Google Scholar]
- 44.Sheftel, T. G., J. T. Mader, and G. Cierny. 1987. Pseudoallescheria boydii soft tissue abscess. Clin. Orthop. 215:212-216. [PubMed] [Google Scholar]
- 45.Steinbach, W. J., and J. R. Perfect. 2003. Scedosporium species infections and treatments. J. Chemother. 15(Suppl. 2):16-27. [DOI] [PubMed] [Google Scholar]
- 46.Torok, L., G. Simon, A. Csornai, M. Tapai, and I. Torok. 1995. Scedosporium apiospermum infection imitating lymphocutaneous sporotrichosis in a patient with myeloblastic-monocytic leukaemia. Br. J. Dermatol. 133:805-809. [DOI] [PubMed] [Google Scholar]
- 47.Walsh, T. J., I. Lutsar, T. Driscoll, B. Dupont, M. Roden, P. Ghahramani, M. Hodges, A. H. Groll, and J. R. Perfect. 2002. Voriconazole in the treatment of aspergillosis, scedosporiosis, and other invasive fungal infections in children. Pediatr. Infect. Dis. J. 21:240-248. [DOI] [PubMed] [Google Scholar]