Abstract
It has been suggested that accommodation induces increases in axial eye length which could contribute to the development of myopia. However, it is debated whether changes in eye length occur during accommodation as the degree of change varies widely across literature. In this study, an extended-depth optical coherence tomography (OCT) system that provides dynamic whole eye biometry was utilized to assess changes in lens thickness (LT) and axial eye length (AEL) in young subjects responding to step disaccommodation stimuli of amplitude 2D, 4D, and 6D. The decrease in lens thickness with disaccommodation was strongly correlated with stimulus amplitude. No statistically significant changes in AEL during accommodation were observed.
OCIS codes: (170.4500) Optical coherence tomography; (330.7322) Visual optics, accommodation; (330.5370) Physiological optics; (330.7327) Visual optics, ophthalmic instrumentation
1. Introduction
Near work [1, 2] and accommodation response [3–5] have been implicated in the development and progression of myopia but the mechanism underlying physical changes leading from near work to myopia remains unknown. One particular theory has been proposed which suggests the act of accommodation might provide an additional mechanical contribution to the elongation of the eye [6, 7]. According to this theory, the eye elongates during accommodation and this stretching of the eye might not completely reverse after disaccommodation, causing incremental increases in eye length with repeated accommodation.
However, the influence of accommodation on AEL remains inconclusive given the pronounced variability of reported results. Changes in AEL reported in literature range from 1.2 to 9.7 µm/D [6–10]. Further, differences reported between myopes and emmetropes are inconsistent, ranging from greater [8] to lesser [7] to no difference [6, 9] in AEL change between the two groups, rendering association between eye length change during accommodation and myopia equivocal.
This uncertainty in the magnitude and direction of AEL change during accommodation may originate from limitations in current methods of AEL measurement which use optical biometry. In particular, calculation of AEL requires that measurements from optical biometers undergo conversion from optical to geometric path lengths. To perform this conversion, measurements must be scaled by the group refractive indices of the different media of the eye. In practice, an average summary refractive index for the whole eye is often used that approximates the contributions of the different ocular media, but this approach has been demonstrated to introduce errors in AEL measurements [11]. Furthermore, in all prior studies except that of Fan et al. [10], measurements of accommodative changes in AEL have been performed statically, comparing AEL from separate recordings at different accommodative states. The separation between recordings might introduce changes in alignment by which differences in AEL can represent differences in the location or axis of measurements. These changes in alignment may not be readily apparent, especially when using optical biometers which measure from a single A-line. In addition, changes in the position of the pupil center with accommodation might additionally contribute to differences in measured eye-length when measurements are recorded along the visual axis. Finally, prior studies have reported changes in AEL but have not included analyses to determine measurement and experimental variability. The lack of such analyses prevents a definitive assessment of whether accommodative changes in AEL occur or represent operator, subject, or instrument variability.
In order to address these limitations, we employed a recently developed custom extended-depth SD-OCT system [12] to provide more accurate characterization of AEL in accommodation. The custom SD-OCT system produces 2-D whole-eye images, which enable dynamic biometry of ocular structures for calculation of AEL using structure-specific refractive indices and provide a wide field of view around the corneal apex to verify consistent alignment during imaging. In addition, the dynamic nature of measurements allows direct observation of AEL throughout the process of accommodation, minimizing the potential for changes in alignment during imaging and providing a basis for analyses on measurement variability during a constant accommodative state and experimental variability between sessions. By considering AEL values in the context of measurement variability, we can reconcile differences in literature due to non-physiological variability to clarify the current level of understanding of axial eye length in accommodation.
2. Methods
2.1 Study design
This prospective study was carried out at the Bascom Palmer Eye Institute, University of Miami Miller School of Medicine with approval from the Institutional Review Board at the University of Miami while following the tenets of the Declaration of Helsinki. Written informed consent was obtained for all participants. The experiments were performed on the left eyes of 10 young healthy subjects (age: 25.1 ± 3.9 y/o, 20 to 31 y/o) with refractive errors of −1.2 ± 1.2 D (range: 0 to −3 D). The exclusion criteria were presbyopia, history of ocular disease or ocular surgery and high myopia (> 6D).
2.2 OCT imaging system
Ocular dimensions were measured from 2-D OCT images acquired with a custom SD-OCT system previously described [12]. Briefly, the SD-OCT system operates at a central wavelength of 840 nm. The system features an axial resolution of 8 µm (in air) and is operated at an axial scan rate of up to 12,500 A-lines/s. Imaging of the eye ranging from the cornea to the retina was performed by automatically stitching three OCT frames consecutively acquired at 25.9 frames per second at three depths using a high-speed optical switch implemented in the reference arm. Each frame consists of 400 A-lines acquired over the central 8 mm zone with 10.43 mm depth (in air). The final image which comprises the entire anterior segment from anterior cornea and posterior lens, and the posterior vitreous and retina is acquired at a frame rate of 8.7 frames per second.
The SD-OCT system also includes a custom accommodation module for generating monocular step stimuli [12]. The module consists of two optical channels, each based on a variation of the Badal optometer, which provide either a low vergence stimulus (at distance) or high vergence stimulus (at proximity), both consisting of a high contrast cross target. The two channels are optically and co-axially coupled to ensure mutual alignment, eliminating target parallax during accommodation/disaccommodation to enable steady fixation during stimulus presentation. The targets of each channel are alternately retro-illuminated to produce a monocular step stimulus. A timing circuit provides synchronization of the stimulus with OCT acquisition.
2.3 Imaging protocol
Subjects were seated in front of the instrument with their heads stabilized by a chin rest and contour head frame. To correct for the subject's refractive error, the distance channel of the accommodation module was adjusted to the subject’s far point by moving the target until the subject was able to see the target in focus. The near channel of the module was set to produce the desired accommodative effort relative to the distance target. The OCT system was then aligned to the optical axis of the subject’s eye by visualizing the real-time image and finding the corneal reflex. The final adjustment was refined by also using the iris plane to help adjust the angle of the beam to minimize tilt of the lens. In each trial, subjects were asked to fixate on the near target before OCT recording was started. Once recording was started, the target was electronically switched from the near to far channel after 1.50 s, eliciting a disaccommodative response in the subject. Recording continued after the subject completed disaccommodation and reached a stable disaccommodated state resulting in a total scan duration of 6.22 s. A minimum of three trials was performed in succession at each stimulus level without adjusting the location of the near and far targets. Three trials were performed for all but two subjects for whom ten trials were performed for variability analysis. Subjects performed trials at 2, 4, and 6 D in order of increasing stimulus amplitude.
2.4 Image post-processing and data analysis
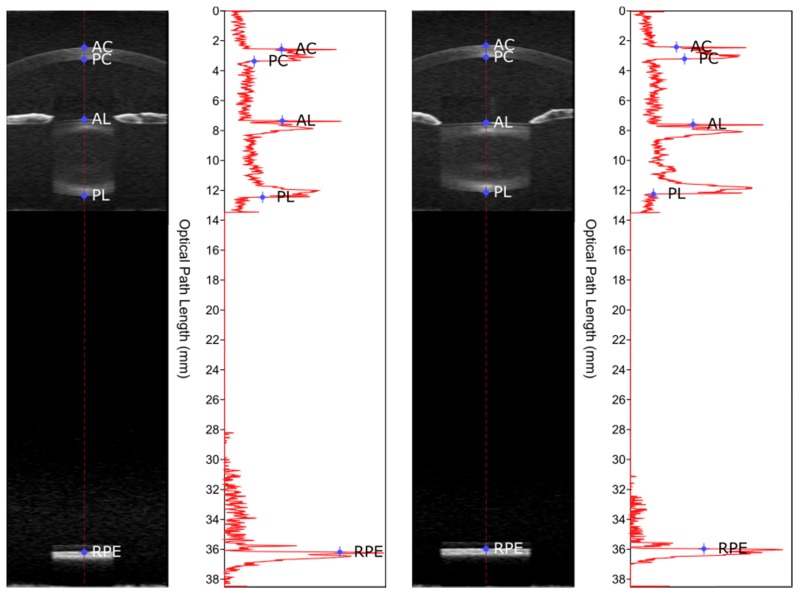
Each dynamic sequence acquired with the SD-OCT system at 840 nm consists of 54 OCT images ranging in depth from the cornea to the retina at an effective frame rate of 8.7 frames per second or 0.11 s per frame, sufficient to avoid significant eye movement, misalignment, or accommodation fluctuations. An automated segmentation algorithm previously reported [13] was applied to each of the 54 whole eye OCT images to detect the boundaries of the cornea, lens, and retinal pigment epithelium. The optical distances between the surface boundaries were then measured along the A-line passing through the apex of the cornea. An example of the determination of optical distances is shown in Fig. 1.
Fig. 1.
Example of measurements derived from SD-OCT images. The figure shows images of the eye and the corresponding intensity profile of the A-line at the corneal apex while accommodated in response to stimulus at 6 D (left) and while relaxed at 0 D (right). Boundaries of ocular structures were determined through automated segmentation and are indicated by points on the A-line of the corneal apex and on the intensity profile (AC = anterior cornea, PC = posterior cornea, AL = anterior lens, PL = posterior lens, RPE = retinal pigment epithelium). A video of an example response to a stimulus at 6 D from the same subject is shown in Visualization 1 (33.9MB, AVI) .
Anterior chamber depth (ACD), lens thickness (LT), and vitreous chamber depth (VCD) were then obtained by dividing the optical distances by the group refractive index of the corresponding ocular media at 840nm (nAQUEOUS = 1.341 [14, 15], nLENS = 1.415 [15, 16], and nVITREOUS = 1.341 [14, 15]). Axial eye length was then calculated as the sum of ACD, LT, and VCD. To avoid reduced measurement precision due to signal saturation at the anterior corneal surface, corneal thickness was not included in calculations. Changes in lens thickness and axial eye length due to accommodation were defined as the difference in lens thickness and axial eye length between the average value in the first 13 images acquired before stimulus presentation (accommodated state), and the last 13 images acquired after stimulus presentation (relaxed state). Values obtained after stimulus presentation were subtracted from those before to show accommodative change. Results were averaged across trials after removing trials with errant subject behavior, excessive noise in biometry, or issues in acquisition (see Data File 1 (1.6KB, CSV) for more details and results from individual trials).
2.5 Repeatability of LT and AEL measurements and repeatability of experiments
To quantify the precision of the measurement, a repeatability study was performed in two randomly chosen subjects out of the total ten. In these two subjects, ten trials were performed at each stimulus level (2, 4, and 6 D). The standard deviation of LT and AEL was determined in the first 13 and in the last 13 images of each imaging session when subjects were at a fixed accommodative state. Repeatability of LT and AEL measurements were reported as the range of standard deviations found across trials, accommodative stimuli, and subjects. Similarly, the standard deviation of accommodative changes (difference between accommodated and relaxed states) in LT and AEL was determined across trials. Repeatability of experiments was reported as the range of the standard deviation of accommodative changes in LT and AEL across accommodative stimuli and subjects (results shown in Tables 2 and 3).
Table 2. Table shows mean and standard deviation of LT accommodative change (difference between accommodated and relaxed states), mean and standard deviation of the standard deviation of LT in first 13 frames (when accommodated), and of the standard deviation of LT in last 13 frames (when unaccommodated) over 10 trials for two subjects at 2, 4, and 6 D.
| Subject A | Subject B | ||
|---|---|---|---|
| 2 to 0 D | Mean Change (µm) | 105.3 ± 24.3 | 119.2 ± 11.2 |
| Mean SD of First 13 (µm) | 12.3 ± 5.0 | 8.3 ± 3.5 | |
| Mean SD of Last 13 (µm) | 4.8 ± 3.6 | 9.3 ± 6.8 | |
|
| |||
| 4 to 0 D | Mean Change (µm) | 211.3 ± 30.7 | 187.2 ± 58.7 |
| Mean SD of First 13 (µm) | 13.5 ± 4.1 | 6.1 ± 2.9 | |
| Mean SD of Last 13 (µm) | 4.5 ± 1.4 | 7.6 ± 6.9 | |
|
| |||
| 6 to 0 D | Mean Change (µm) | 284.9 ± 52.2 | 331.7 ± 14.5 |
| Mean SD of First 13 (µm) | 13.3 ± 4.2 | 11.1 ± 4.9 | |
| Mean SD of Last 13 (µm) | 5.3 ± 1.8 | 15.0 ± 6.4 | |
Table 3. Table shows mean and standard deviation of AEL accommodative change (difference between accommodated and relaxed states), mean and standard deviation of the standard deviation of AEL in first 13 frames (when accommodated), and of the standard deviation of AEL in last 13 frames (when unaccommodated) over 10 trials for two subjects at 2, 4, and 6 D.
| Subject A | Subject B | ||
|---|---|---|---|
| 2 to 0 D | Mean Change (µm) | 7.3 ± 6.8 | −4.7 ± 5.7 |
| Mean SD of First 13 (µm) | 21.8 ± 9.1 | 17.0 ± 4.0 | |
| Mean SD of Last 13 (µm) | 18.1 ± 9.0 | 15.8 ± 4.7 | |
|
| |||
| 4 to 0 D | Mean Change (µm) | 18.3 ± 7.2 | −7.0 ± 5.0 |
| Mean SD of First 13 (µm) | 20.1 ± 7.3 | 20.9 ± 6.5 | |
| Mean SD of Last 13 (µm) | 16.0 ± 7.3 | 21.1 ± 15.0 | |
|
| |||
| 6 to 0 D | Mean Change (µm) | 18.2 ± 5.0 | −3.8 ± 10.0 |
| Mean SD of First 13 (µm) | 22.7 ± 12.1 | 21.8 ± 9.0 | |
| Mean SD of Last 13 (µm) | 22.0 ± 12.0 | 19.8 ± 5.3 | |
3. Results
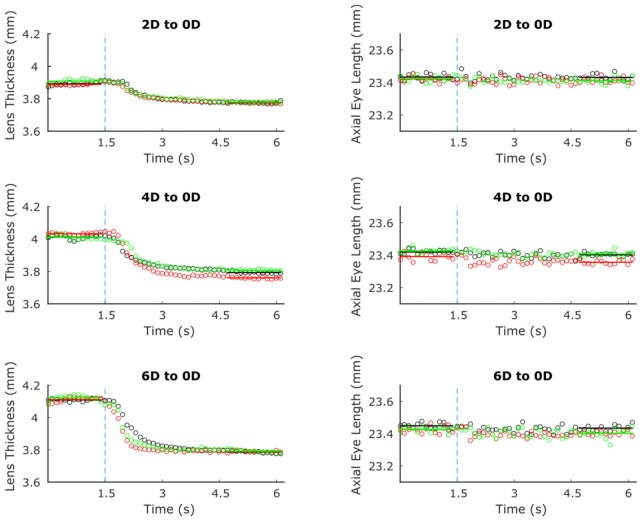
Responses from a representative subject are shown in Fig. 2. Lens thickness started at higher values for larger stimuli but decreased to a similar end value after disaccommodative stimuli were presented. Both LT and AEL demonstrated consistent responses between separate trials.
Fig. 2.
Sample disaccommodative responses from three different trials (indicated by square, circle, and asterisk points) of a subject responding to 2, 4, and 6 D step stimuli to distance (shown from top to bottom, respectively). Lens thickness is shown in plots on the left, whereas axial eye length is shown on the right. Stimulus presentation is indicated by the dotted line.
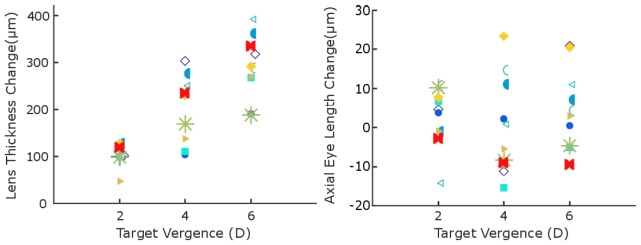
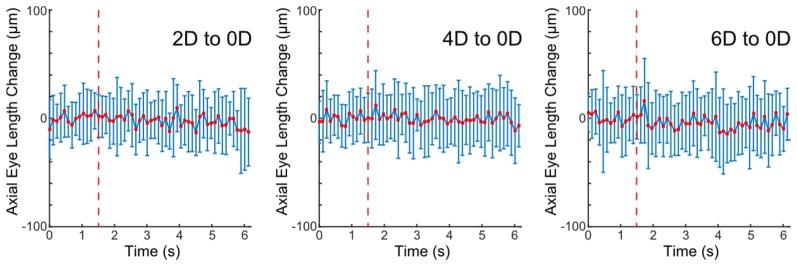
Accommodative changes in LT and AEL for individual subjects after averaging across trials are shown for different stimuli (Fig. 3). Average LT increases across subjects were 104 ± 23, 205 ± 70, and 290 ± 19 µm for 2, 4, and 6 D, respectively, while average AEL increases were 9 ± 15, 2 ± 22, and 2 ± 10 µm for 2, 4, and 6 D, respectively. The change in LT was significantly correlated with increasing stimulus amplitude (p < 0.001); however, AEL change had no correlation with stimulus amplitude (p = 0.62). Notably, responses varied such that AEL did not consistently increase or decrease during accommodation in 6 out of 10 subjects. Average changes in AEL were within a standard deviation of zero despite increases in lens thickness (LT) with accommodation. In addition, as shown in Fig. 4, AEL does not change at any point throughout the duration of subject responses when responses are averaged across subjects.
Fig. 3.
(Left) Lens thickness accommodative change (difference between accommodated and relaxed states) averaged over trials (if more than one trial was performed) for subjects from 2, 4, and 6 D to distance. Different symbols represent results from different subjects. LT change was significantly correlated with stimulus amplitude (p < 0.001). (Right) AEL accommodative change from the same subject in the same session as the recorded lens thickness changes. AEL change was not correlated with stimulus amplitude (p = 0.62). See Data File 1 (1.6KB, CSV) for lens thickness and AEL accommodative change from individual trials for each subject.
Fig. 4.
Average and standard deviation of axial eye length change (change from baseline axial eye length at accommodation) over all subject trials at each timepoint. Individual plots shown from left to right represent responses at 2, 4 and 6 D stimulus conditions, respectively. The timing of stimulus presentation is indicated by a dotted red line.
A further analysis was performed by plotting AEL change against LT change for all subjects, accommodation levels, and trials. Association between AEL change and LT change was tested using a linear mixed model to account for intra-subject correlation in the data. There was no significant relationship between AEL and LT change at a p-value of 0.114 (Fig. 5).
Fig. 5.
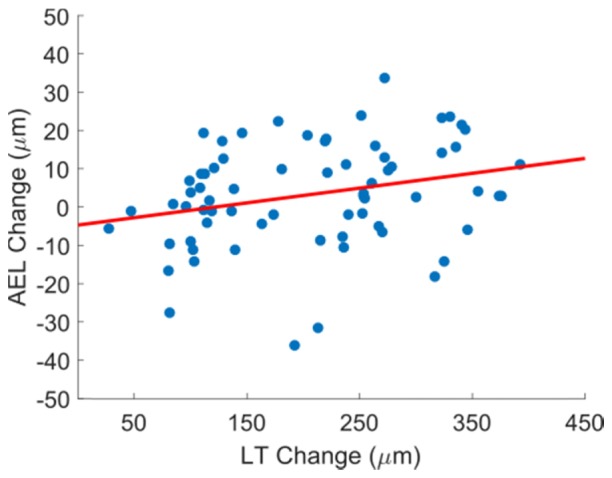
AEL accommodative change (difference between accommodated and relaxed states) versus lens thickness accommodative change from responses for each subject at each stimulus level. A linear mixed model shows no significant relationship (p = 0.114, y = 0.0387 x - 4.7) between LT and AEL change.
4. Discussion
Dynamic axial biometry of the eye using a custom SD-OCT system was used to improve the accuracy of axial eye length measurements in accommodation. Dynamic whole-eye measurements provided direct observation of eye length in accommodation, which enabled calculation of AEL with structure-specific refractive indices and minimized changes in alignment between accommodative states. In contrast to recent studies performed with optical biometry [6–9], no significant change in AEL was found in response to accommodation, suggesting AEL remained constant within the precision of our method.
Differences between the present study and other studies performed with optical biometry bring up possible issues in analysis and methodology. In previous studies [6, 8], the optical path length of the whole eye was converted to distance using an average refractive index of the unaccommodated eye rather than using the individual refractive indices of each element of the eye. Given the increase in lens thickness during accommodation, the relative contribution of the lens to the average refractive index increases, causing an overestimation of AEL in an accommodated state [11, 17]. This effect might explain the relatively large AEL changes seen in Mallen et al. [8] which were approximately twice the changes seen in other studies when accounting for accommodative amplitude. Read et al. [6] reported distances calculated both with an average index for the entire eye and with individual refractive indices, and found lower values of changes in AEL when individual refractive indices were used for correction. The changes in eye length were reduced to within a standard deviation of zero, suggesting results from individual subjects after correction might be mixed, showing increases in AEL in some and decreases in others, although only the average and standard deviation after correction were shown. This overestimation produced by correction with an average refractive index was reproduced in our study. As shown in Table 1, using the average refractive index increased AEL accommodative changes such that average AEL changes were now significantly distributed from zero, possibly explaining the large AEL changes seen by Mallen et al. [8].
Table 1. Accommodative change (difference between accommodated and relaxed states) in axial eye length in response to stimuli at 2, 4, and 6 D upon conversion of axial eye length from optical to geometric path length using an average refractive index of the eye and, alternatively, refractive indices of individual media. Mean and 95% confidence intervals are shown for both cases in each cell (RI = Refractive Index).
| Target Vergence (D) | Change in AEL | |
|---|---|---|
| Average RI (µm) | Individual RI (µm) | |
| 2D | 6.9 ± 13.7 | 2.5 ± 5.4 |
| 4D | 10.7 ± 21.2 | 0.3 ± 9.0 |
| 6D | 19.7 ± 19.7 | 4.8 ± 7.4 |
Correction for individual elements of the accommodative system were performed in other studies [6, 7, 9]. Although an increase in AEL during accommodation was also found, this increase might be caused by changes in alignment or pupil center with accommodation. In prior studies, measurements were performed statically. Accommodative responses to different stimuli were recorded in separate sessions, raising the possibility of subject movement or changes in fixation in between sessions. In particular a change in alignment is evident in representative OCT images provided by Zhong et al. [9] and Fan et al. [10]. In our study using dynamic imaging, the alignment of the subject is preserved due to the short session duration (see Fig. 1 and Data File 1 (1.6KB, CSV) ), short break between different trials, and precise co-alignment of the near and far stimuli.
Alternatively, an explanation posed in Drexler et al. [7] suggests that the observed increase in axial eye length might be an artifact related to changes in the refractive index of the lens during accommodation. Drexler et al. [7] found that an increase in refractive index of 0.3% after accommodation could account for the increase in AEL seen in their study. Changes in the gradient refractive index of the lens in an accommodated state have since been observed by Kasthurirangan et al. [18], which would cause an increase in the average index. If an increase in refractive index of the lens occurs with accommodation, our results, which are based on a constant refractive index of the lens, would suggest a decrease in AEL during accommodation in sharp contrast with the literature discussed so far.
Other possible sources of change in alignment, including those inherent to the mechanism of accommodation such as changes in pupil centration during accommodation [19], might prevent quantification of AEL along the same path before and after accommodation when measurements are acquired along the visual axis (i.e., aligned with the pupil center). The effect of these sources have been minimized in the present study by using the corneal apex, which does not shift during accommodation, as a reference for alignment as well as a landmark for determining a location for AEL measurement.
It should also be noted that AEL was observed during disaccommodation instead of accommodation. Disaccommodation was chosen to be studied during preliminary tests of the imaging protocol which tests indicated that subjects provided a more consistent accommodative response when performing disaccommodation instead of accommodation to the same stimulus level. However, the total change in AEL from accommodation is expected to be the same as the total change in disaccommodation.
Critically, changes in LT and AEL should be considered in light of measurement and experimental variability. Here, we define measurement variability as the variation during subject fixation on a constant accommodation stimulus (e.g. due to subject motion or instrument and segmentation precision). We define experimental variability as trial-to-trial variation in subject responses to a set accommodation stimulus. Both sources of variability might produce uncertainty in measurements unrelated to changes occurring in the eye. To determine the confidence with which changes can be seen in LT and AEL, analyses of variability were performed as shown in Tables 2 and 3. Measurement variability ranged from 5 to 15 µm for LT and 15.8 to 22.7 µm for AEL while experimental variability ranged from 11 to 70 µm for LT and 5 to 10 µm for AEL, suggesting variability greater than accommodative changes in AEL reported in the literature [6–9]. It may be reasonable to assume similar variability in other measurements and experiments performed with optical biometers. Although analyses of variability have been performed on the devices used for studying accommodative changes in AEL in the literature [20–26], these analyses often do not address variability in the conversion from optical lengths to AEL [21–26] or report variability in measurements averaged across a population of subjects rather than variability in individuals [27]. Our reported variability is comparable to that seen in previous studies [20] and indicates measurements of AEL cannot be obtained with a precision below ~25 microns with current optical biometry devices. Changes in AEL reported below this level of precision are likely due to operator, subject, or instrument variability instead of actual elongation of the eye.
In summary, changes in AEL due to accommodation were not observed at the precision of a custom extended-depth OCT system. Changes in AEL might occur below this level of precision, but a conclusive answer as to whether AEL changes during accommodation and consequently whether the act of accommodation has a mechanical contribution to myopia cannot be determined with the current level of variability inherent to optical biometry.
Author disclosure
The University of Miami and authors MR, FM, and JMP stand to benefit from intellectual property in the OCT technology used in this study.
Funding
The study was supported in part by National Eye Institute Grants 2R01EY14225, P30EY14801 (Center Grant); the Florida Lions Eye Bank; the Henri and Flore Lesieur Foundation (JMP); Drs. Raksha Urs and Aaron Furtado; Karl R. Olsen, MD and Martha E. Hildebrandt, PhD; an unrestricted grant from Research to Prevent Blindness; and the Australian Federal Government Cooperative Research Centre Scheme through the Vision Cooperative Research Centre.
References and links
- 1.Saw S. M., Chua W. H., Hong C. Y., Wu H. M., Chan W. Y., Chia K. S., Stone R. A., Tan D., “Nearwork in early-onset myopia,” Invest. Ophthalmol. Vis. Sci. 43(2), 332–339 (2002). [PubMed] [Google Scholar]
- 2.French A. N., Morgan I. G., Mitchell P., Rose K. A., “Risk factors for incident myopia in Australian schoolchildren: the Sydney adolescent vascular and eye study,” Ophthalmology 120(10), 2100–2108 (2013). 10.1016/j.ophtha.2013.02.035 [DOI] [PubMed] [Google Scholar]
- 3.Gwiazda J. E., Hyman L., Norton T. T., Hussein M. E., Marsh-Tootle W., Manny R., Wang Y., Everett D., COMET Grouup , “Accommodation and related risk factors associated with myopia progression and their interaction with treatment in COMET children,” Invest. Ophthalmol. Vis. Sci. 45(7), 2143–2151 (2004). 10.1167/iovs.03-1306 [DOI] [PubMed] [Google Scholar]
- 4.Abbott M. L., Schmid K. L., Strang N. C., “Differences in the accommodation stimulus response curves of adult myopes and emmetropes,” Ophthalmic Physiol. Opt. 18(1), 13–20 (1998). 10.1016/S0275-5408(97)00072-0 [DOI] [PubMed] [Google Scholar]
- 5.Allen P. M., O’Leary D. J., “Accommodation functions: co-dependency and relationship to refractive error,” Vision Res. 46(4), 491–505 (2006). 10.1016/j.visres.2005.05.007 [DOI] [PubMed] [Google Scholar]
- 6.Read S. A., Collins M. J., Woodman E. C., Cheong S. H., “Axial length changes during accommodation in myopes and emmetropes,” Optom. Vis. Sci. 87(9), 656–662 (2010). 10.1097/OPX.0b013e3181e87dd3 [DOI] [PubMed] [Google Scholar]
- 7.Drexler W., Findl O., Schmetterer L., Hitzenberger C. K., Fercher A. F., “Eye elongation during accommodation in humans: differences between emmetropes and myopes,” Invest. Ophthalmol. Vis. Sci. 39(11), 2140–2147 (1998). [PubMed] [Google Scholar]
- 8.Mallen E. A., Kashyap P., Hampson K. M., “Transient axial length change during the accommodation response in young adults,” Invest. Ophthalmol. Vis. Sci. 47(3), 1251–1254 (2006). 10.1167/iovs.05-1086 [DOI] [PubMed] [Google Scholar]
- 9.Zhong J., Tao A., Xu Z., Jiang H., Shao Y., Zhang H., Liu C., Wang J., “Whole eye axial biometry during accommodation using ultra-long scan depth optical coherence tomography,” Am. J. Ophthalmol. 157(5), 1064–1069 (2014). 10.1016/j.ajo.2014.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan S., Li L., Li Q., Dai C., Ren Q., Jiao S., Zhou C., “Dual band dual focus optical coherence tomography for imaging the whole eye segment,” Biomed. Opt. Express 6(7), 2481–2493 (2015). 10.1364/BOE.6.002481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atchison D. A., Smith G., “Possible errors in determining axial length changes during accommodation with the IOLMaster,” Optom. Vis. Sci. 81(4), 283–286 (2004). 10.1097/00006324-200404000-00015 [DOI] [PubMed] [Google Scholar]
- 12.Ruggeri M., Uhlhorn S. R., De Freitas C., Ho A., Manns F., Parel J. M., “Imaging and full-length biometry of the eye during accommodation using spectral domain OCT with an optical switch,” Biomed. Opt. Express 3(7), 1506–1520 (2012). 10.1364/BOE.3.001506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruggeri M., de Freitas C., Williams S., Hernandez V. M., Cabot F., Yesilirmak N., Alawa K., Chang Y.-C., Yoo S. H., Gregori G., Parel J.-M., Manns F., “Quantification of the ciliary muscle and crystalline lens interaction during accommodation with synchronous OCT imaging,” Biomed. Opt. Express 7(4), 1351–1364 (2016). 10.1364/BOE.7.001351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atchison D. A., Smith G., “Chromatic dispersions of the ocular media of human eyes,” J. Opt. Soc. Am. A 22(1), 29–37 (2005). 10.1364/JOSAA.22.000029 [DOI] [PubMed] [Google Scholar]
- 15.Drexler W., Hitzenberger C. K., Baumgartner A., Findl O., Sattmann H., Fercher A. F., “Investigation of dispersion effects in ocular media by multiple wavelength partial coherence interferometry,” Exp. Eye Res. 66(1), 25–33 (1998). 10.1006/exer.1997.0401 [DOI] [PubMed] [Google Scholar]
- 16.Uhlhorn S. R., Borja D., Manns F., Parel J. M., “Refractive index measurement of the isolated crystalline lens using optical coherence tomography,” Vision Res. 48(27), 2732–2738 (2008). 10.1016/j.visres.2008.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faria-Ribeiro M., Lopes-Ferreira D., López-Gil N., Jorge J., González-Méijome J. M., “Errors associated with IOLMaster biometry as a function of internal ocular dimensions,” J. Optom. 7(2), 75–78 (2014). 10.1016/j.optom.2013.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasthurirangan S., Markwell E. L., Atchison D. A., Pope J. M., “In vivo study of changes in refractive index distribution in the human crystalline lens with age and accommodation,” Invest. Ophthalmol. Vis. Sci. 49(6), 2531–2540 (2008). 10.1167/iovs.07-1443 [DOI] [PubMed] [Google Scholar]
- 19.Enright J. T., “Ocular translation and cyclotorsion due to changes in fixation distance,” Vision Res. 20(7), 595–601 (1980). 10.1016/0042-6989(80)90116-9 [DOI] [PubMed] [Google Scholar]
- 20.Hitzenberger C. K., “Optical measurement of the axial eye length by laser Doppler interferometry,” Invest. Ophthalmol. Vis. Sci. 32(3), 616–624 (1991). [PubMed] [Google Scholar]
- 21.Santodomingo-Rubido J., Mallen E. A., Gilmartin B., Wolffsohn J. S., “A new non-contact optical device for ocular biometry,” Br. J. Ophthalmol. 86(4), 458–462 (2002). 10.1136/bjo.86.4.458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam A. K., Chan R., Pang P. C., “The repeatability and accuracy of axial length and anterior chamber depth measurements from the IOLMaster,” Ophthalmic Physiol. Opt. 21(6), 477–483 (2001). 10.1046/j.1475-1313.2001.00611.x [DOI] [PubMed] [Google Scholar]
- 23.Rohrer K., Frueh B. E., Wälti R., Clemetson I. A., Tappeiner C., Goldblum D., “Comparison and evaluation of ocular biometry using a new noncontact optical low-coherence reflectometer,” Ophthalmology 116(11), 2087–2092 (2009). 10.1016/j.ophtha.2009.04.019 [DOI] [PubMed] [Google Scholar]
- 24.Cruysberg L. P., Doors M., Verbakel F., Berendschot T. T., De Brabander J., Nuijts R. M., “Evaluation of the Lenstar LS 900 non-contact biometer,” Br. J. Ophthalmol. 94(1), 106–110 (2010). 10.1136/bjo.2009.161729 [DOI] [PubMed] [Google Scholar]
- 25.Holzer M. P., Mamusa M., Auffarth G. U., “Accuracy of a new partial coherence interferometry analyser for biometric measurements,” Br. J. Ophthalmol. 93(6), 807–810 (2009). 10.1136/bjo.2008.152736 [DOI] [PubMed] [Google Scholar]
- 26.Buckhurst P. J., Wolffsohn J. S., Shah S., Naroo S. A., Davies L. N., Berrow E. J., “A new optical low coherence reflectometry device for ocular biometry in cataract patients,” Br. J. Ophthalmol. 93(7), 949–953 (2009). 10.1136/bjo.2008.156554 [DOI] [PubMed] [Google Scholar]
- 27.Zhong J., Shao Y., Tao A., Jiang H., Liu C., Zhang H., Wang J., “Axial biometry of the entire eye using ultra-long scan depth optical coherence tomography,” Am. J. Ophthalmol. 157, 412–420 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]