Abstract
The ocular damage effects induced by transitional near-infrared (NIR) lasers have been investigated for years. However, no retinal damage thresholds are determined in a wide interval between 0.65 ms and 80 ms, and a definite relationship between corneal damage threshold and spot size cannot be drawn from existing data points. In this paper, the in-vivo corneal damage thresholds (ED50s) were determined in New Zealand white rabbits for a single 5 ms pulse at the wavelength of 1338 nm for spot sizes from 0.28 mm to 3.55 mm. Meanwhile, the retinal damage threshold for this laser was determined in chinchilla grey rabbits under the condition that the beam was collimated, and the incident corneal spot diameter was 5.0 mm. The corneal ED50s given in terms of the corneal radiant exposure for spot diameters of 0.28, 0.94, 1.91, and 3.55 mm were 70.3, 35.6, 29.6 and 30.3 J/cm2, respectively. The retinal ED50 given in terms of total intraocular energy (TIE) was 0.904 J. The most sensitive ocular tissue to this laser changed from the cornea to retina with the increase of spot size.
OCIS codes: (140.3360) Laser safety and eye protection, (350.5340) Photothermal effects
1. Introduction
The near-infrared (NIR) laser radiation region between 1300 nm and 1400 nm is a unique area in the electromagnetic spectrum for laser safety [1]. With the wavelength increase in this region, the most sensitive tissue changes gradually from the retina to the cornea [2]. Developments of lasers in this transitional wavelength region promote increased applications throughout telecommunication, medicine and military. Due to the widespread use of laser systems in this region and the rapid increase of laser power/energy, the risk of ocular damage becomes more serious and thus receives more concern [3]. In the past two decades, ocular damage threshold studies have been conducted at diverse laser radiation parameters to quantify the ocular hazards [1–13]. International safety committees, such as the International Commission of Non-Ionizing Radiation Protection (ICNIRP) and the American National Standards Institute (ANSI), analyze these damage threshold studies and make recommendations for the Maximum Permissible Exposures (MPEs) [14,15].
Ocular bioeffects studies for the transitional NIR lasers have revealed some unique damage characteristics which are obviously different from those of the visible or the far-IR lasers. Firstly, the absorption of an incident laser beam in this region is more evenly distributed across the ocular components and depending on the precise exposure parameters, damage may be induced in one or more of the cornea, lens, iris, and retina/choroid [1–3]. In contrast, the most sensitive tissue is the retina for visible lasers and near-IR wavelengths up to the frequently studied 1064 nm Nd: YAG emission, and the cornea for infrared wavelengths longer than 1400 nm [16–20]. Secondly, a retinal or corneal lesion involves the full thickness of that layer, even at the threshold level. In contrast, the damage site is either at the retinal pigment epithelium for visible and near-IR lasers up to the 1064 nm wavelength, or the anterior corneal surface for far-infrared wavelengths, following the threshold exposure [10,11,20]. Thirdly, for transitional NIR lasers, retinal lesions at threshold level may take 24 hours to become apparent and longer to reach maximum expression. Therefore, retinal ED50 thresholds were invariably lower when determined based on lesion/no lesion readings collected 24-h post-exposure than when using 1-h post-exposure readings [1–3,10,11]. Fourthly, strong spot dependencies exist for this wavelength region. At relatively large beam spot sizes at the cornea, threshold-level damage occurs at the back of the eye, involving the retina and the nerve fiber layers. While for relatively small corneal spot sizes, threshold-level damage occurs at the cornea. Fifthly, the ocular axial length has great, while the retinal pigmentation has only slight influence on retinal damage threshold, contrary to the damage effects by visible lasers [7]. Finally, retinal damage thresholds are determined in rabbit models at exposure durations up to 10 s, but in nonhuman primate models, no damage thresholds are determined for exposure durations longer than 80 ms. Significant presence of thermal lensing effect is believed to explain this variance [12,13].
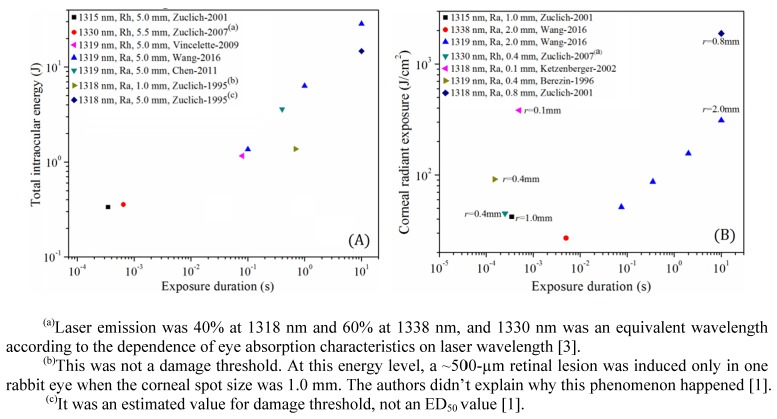
Despite above bioeffects studies in transitional NIR region, some problems still remain unexplored. It is well known that the dependence of damage threshold on exposure duration is a critical issue for the setting of MPEs in laser safety standards [3,16]. For the retina, the dependence from 0.1 s to 10 s has been established in a rabbit model [9]. However, one remarkable insufficiency for the existing retinal damage studies could be noted that no damage thresholds were determined between 0.65 ms and 80 ms, as shown in Fig. 1(A). This deficiency may block the complete clarification of the damage threshold dependence on exposure duration and experiments for the determination of threshold values in such a wide interval from 0.65 ms to 80 ms are needed. For the cornea, spot size is an important influence factor for the determination of damage threshold. Previous studies for 1.54 μm, 2.02 μm and 10.6 μm lasers have shown that corneal damage thresholds increase dramatically as the spot size decreases to a certain value which depends on the exposure duration [17–20]. Existing studies for the transitional NIR region also show the strong dependence of damage threshold on spot size, as can be seen from the left four and right two points in Fig. 1(B). However, due to the diverse experimental conditions, a definite relationship between damage threshold and laser spot size could not be drawn from these data points, and damage thresholds using spot size as a variable need to be determined under the same controlled experimental conditions, just like previous studies for other infrared lasers.
Fig. 1.
(A) Previous reported retinal damage thresholds in terms of TIE (Total Intraocular Energy) for NIR 1300-1400 nm lasers [1–3,6,7,9]. (B) Selected reported corneal damage thresholds in terms of corneal radiant exposure for NIR 1300-1400 nm lasers [2–5,8] (r denotes the corneal spot size). The texts behind the symbols denote the laser wavelength, the animal species (Rh: Rhesus; Ra: Rabbit), the corneal spot size, and the reference.
In this paper, using a 1338 nm laser with the pulse duration of 5 ms, we determined the corneal damage thresholds at 0.28, 0.94, 1.91, and 3.55 mm spot sizes, and the retinal damage threshold at 5.0 mm corneal spot size under the condition that the beam was collimated. The results will contribute to the knowledge base for the setting of laser safety standard in the transitional NIR range.
2. Methods
2.1 Experimental setup
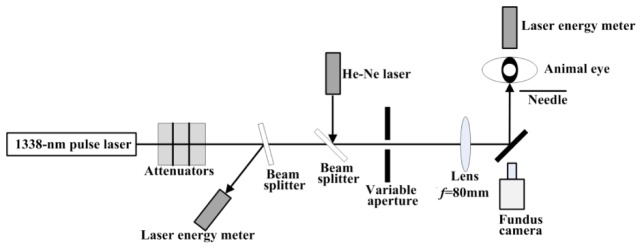
The laser employed for this study was a Xe-lamp pumped pulsed Nd: YAG solid state laser (Beijing Hongxiang Optoelectronic Technology Co., Ltd., Beijing, China), producing 5 ms pulse with a maximum 3 J output at the wavelength of 1338 nm, as shown in the schematic diagram of Fig. 2. The irradiance of the laser spot was nearly “top hat” distributed which was provided by the laser manufactures and verified through the knife edge method. Some neutral density attenuators (Daheng Optics, Beijing, China), with different transmittances, were used to adjust the incident energy incident on the animal cornea. A constant proportion of the pulse energy was reflected onto a calibrated laser energy meter (3A, Ophir, Jerusalem, Israel) using a beam splitter. Before the rabbit was positioned, another calibrated laser energy meter (30A, Ophir, Jerusalem, Israel) was placed to directly receive the energy that would normally enter the eye. The ratio of the pulse energy at this position to the energy at the reference was obtained. Subsequently, when the eye was exposed, the energy incident on the eye for each exposure was determined by multiplying the energy at the reference detector by the ratio previously determined. A low-power 633 nm He-Ne laser, coaxial with the 1338 nm laser, facilitated the targeting of the un-visible 1338 nm laser.
Fig. 2.
Schematic drawing of the laser exposure setup for the determinations of rabbit corneal and retinal damage thresholds at the wavelength of 1338 nm. The nominal beam diameters on the corneal plane were 0.3, 1.0, 2.0 and 3.7 mm for corneal damage, and 5.0 mm for retinal damage. For the determination of retinal damage threshold, the incident laser beam was collimated.
For the corneal damage determination, a 5 mm circular variable aperture and a focusing lens (with the focal length of 80 mm at 587.6 nm) were positioned to select the central portion of the laser beam and control the corneal spot diameter. The method for the determination of corneal spot size was as follows. Firstly, a graphite plate was placed at a fixed position and irradiated by the single 5 ms pulsed 1338 nm laser. Then, the ablation spot was compared with a known scale and the spot size could be determined. Through measuring the spot diameters at different positions, the exact position corresponding to the desired corneal spot diameter could be determined and then located by a needle. Through this method, the nominal beam diameters incident on the animal cornea were selected as 0.3, 1.0, 2.0 and 3.7 mm. However, the radiant exposure distribution, which was very important to analyze the real spot size and the damage threshold, couldn’t be obtained using above method which in practice was super-threshold effect. Therefore, the knife edge method was additionally employed to perform the characterization of the beam profile. A narrow slit having a width of about 0.7 mm was inserted between the aperture and the lens, and a knife-edge mounted on a two-axis translational stage was placed in the corneal plane. The energy of the laser pulse transmitted past the knife-edge was measured by another calibrated laser energy meter (3A, Ophir, Jerusalem, Israel). By this method, we obtained the radiant exposure distributions along the horizontal and vertical center lines with the above nominal corneal spot sizes determined by the ablation of graphite plate. As shown in Fig. 3., for the nominal corneal spot size of 1.0 mm, the exposure radiant profiles of the laser spot along the horizontal and vertical central lines could be reasonably approximated as a trapezoidal shape, although some asymmetry could be noted that the radiant exposures were higher in the “right” parts of the two curves (especially for the vertical direction). Using the 50% radiant exposure point as the assessment criterion, the actual spot diameter was 0.94 mm, slightly less than the nominal value. For the nominal corneal spot diameters of 0.3, 2.0 and 3.7 mm, the actual values were respectively determined as 0.28, 1.91 and 3.55 mm using the same method.
Fig. 3.
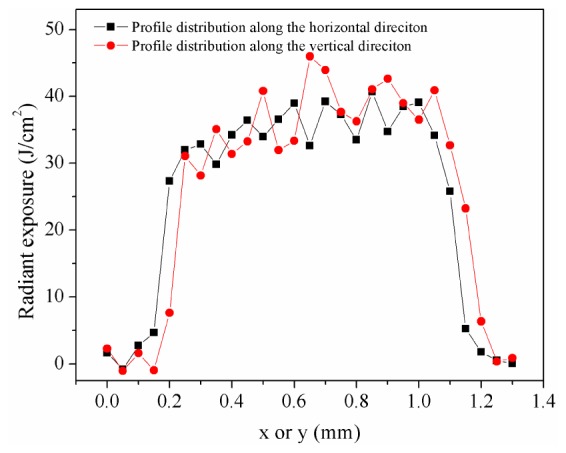
Radiant exposure profile of the laser spot along the horizontal and vertical center lines with the nominal spot size of 1.0 mm when the incident total pulse energy was set as the damage threshold value (0.247 J). The relative radiant exposure profile was firstly measured by the knife edge method. By integrating the measured curve and multiplying a factor with this integral, the total relative energy could be obtained. Then comparing this value against the total measured energy, a “calibration” factor could be obtained. Finally using this factor, the absolute radiant exposure profile could be determined. The step size of the knife-edge was set as 0.05 mm. The use of the slit between the aperture and the lens simplified the subsequent analysis for the radiant exposure profile. It was shown that the curve could be roughly approximated as a trapezoidal shape. Following this assumption and using the 50% radiant exposure point as the assessment criterion, the actual laser spot diameters for both the horizontal and vertical directions were 0.94 mm, slightly less than the nominal value.
For the retinal damage determination, the focusing lens was removed and the collimated beam was used. The spot diameter incident on the cornea was set as 5.0 mm using the variable aperture. In addition, a mirror was mounted on a translation stage so it could be moved to permit observation and accurately repositioned for exposure. A fundus retinal camera (Topcon, Tokyo, Japan) permitted observation of the retina and selection of sites for exposure.
2.2 Animal subjects
New Zealand white rabbits of either sex were selected for corneal damage determination and chinchilla grey rabbits of either sex for retinal damage determination. All animals, weighing 2.4-3.0 kg, were procured and maintained in the Center for Laboratory Animal Medicine and Care, Beijing, China and used in accordance with the institutional guidelines of the Animal Care and Use Committee; and the ARVO Resolution on the Use of Animals in Research. Subjects were pre-exposure examined by a slit-lamp (Topcon, Tokyo, Japan) and a fundus camera to insure clear ocular media and normal ocular tissue. To ensure the subjects did not experience pain and distress, all rabbits were anesthetized with an intramuscular injection of a mixture of ketamine hydrochloride (40 mg/kg) and xylazine (12 mg/kg). Full pupil dilation was performed with two drops of proparacaine hydrochloride 0.5%, phenylephrine hydrochloride 2.5% and tropicamide 1% at a 5-minute interval. The anesthetized animals were placed in a conventional holder where they were positioned with the aid of the low-power He-Ne laser. The eyes were positioned to ensure the incident beam was perpendicular to the central cornea. The cornea was kept hydrated with one drop of physiological saline solution about 10 s prior to exposure and the excess fluid was blotted at the limbus.
2.3 Experimental procedures and data analysis
A pilot study was firstly conducted to generate damages from no visible burn to obvious corneal burn for each spot size incident on the cornea (0.28, 0.94, 1.91, and 3.55 mm). From the post-exposure reading, we estimated the approximate range of the damage threshold for each spot size. Based on the estimation, four different energy levels were chosen in the subsequent experiment to determine the damage threshold. Four to thirteen exposures were delivered to each eye, depending on the spot size. Overlapping of corneal lesions was avoided with the aid of the He-Ne laser pointer. A total of 30 New Zealand white rabbits were involved. Procedures for retina were similar to that for cornea, and the main difference was that 10 chinchilla grey rabbits were involved.
Following each exposure session, lesion/no lesion determinations were made for each exposure site by two experienced investigators. For the corneal damage determination, the lens and retina were also examined with a slit lamp and a fundus camera to determine whether those ocular tissues were damaged, however, at larger corneal spot sizes (1.91 and 3.55 mm), the retina was hard to observe when the corneal damages were induced. For the retinal damage determination, the corneas and lens were also examined with a slit lamp to determine whether the anterior components of the eye were damaged. Examinations were performed at 1 hour, 6 hours, 24 hours and 48 hours post-exposure. The lesion/no lesion data were collected and analyzed using SAS statistical package (version 6.12, SAS Institute, Inc., Cary, NC). Bliss probit analysis was performed to determine the ED50 thresholds, fiducial limits at the 95% confidence level and probit slopes (ED84/ED50).
A slit-lamp and a fundus camera were used to observe the damage features and capture the pictures. Furthermore, qualitative histopathologic studies were also performed. For the corneal damage, some of the rabbits were euthanized at 24 hours after exposure. While for the retinal damage, some of the rabbits were euthanized at 48 or 72 hours after exposure. After the euthanizition, the eyeballs were taken and fixed in Davidson solution for 24 hours, then dehydrated with ethanol, embedded with paraffin, serially sectioned, and the sections stained with hematoxylin and eosin (H&E). A microscope (Model BX43F, Olympus, Tokyo, Japan) was used to observe and capture the damage pictures.
3. Results
3.1 Corneal damage characteristics and damage thresholds at different corneal spot sizes
For the determination of corneal damage threshold, a total of 387 exposures were evaluated for the four corneal spot sizes. All the experimental conditions, the ED50 termed in corneal radiant exposure, the 95% fiducial limits, and the probit slopes, were summarized in Table 1.
Table 1. Corneal damage thresholds induced by 1338 nm laser at different spot sizes for slit lamp imaging at 6 hours post exposure.
| Corneal spot diameter (mm) | Number of eyes | Number of exposures | Damage threshold radiant exposure (95% fiducial limits) (J/cm2) | Probit slope (ED84/ED50) |
|---|---|---|---|---|
| 0.28 | 8 | 90 | 70.3 (68.6, 71.9) | 1.40 |
| 0.94 | 12 | 89 | 35.6 (33.8, 36.3) | 1.28 |
| 1.91 | 24 | 160 | 29.6 (28.3, 30.8) | 1.14 |
| 3.55 | 12 | 48 | 30.3 (28.8, 31.8) | 1.22 |
At threshold level, some of the lesions which couldn’t be observed at immediately post-exposure appeared at 1 hour post-exposure and became more distinctive at 6 hours post-exposure (Fig. 4(A)). At 24 hours post-exposure, the number of lesions kept constant but some lesions became obscured. Therefore, the data at 6 hours post-exposure were chosen for damage threshold analysis. At the condition of full pupil dilation, these threshold lesions could be distinguished by naked eye but difficult to capture pictures using camera. Through a slit-lamp biomicroscopy examination with direct broad-beam illumination, no obvious distortions could be found for these lesions. Under direct slit-beam illumination, highly reflective white straps with a thickness identical to the unexposed region could be seen, indicating that the damage involved the full thickness of the cornea. Another phenomenon we noted was that the size of the damage lesions was nearly equal to the corneal spot size. For example, it was measured that the diameter of the lesions for 1.91 mm corneal spot size was about 1.9 mm. Lens damages were not found at this level.
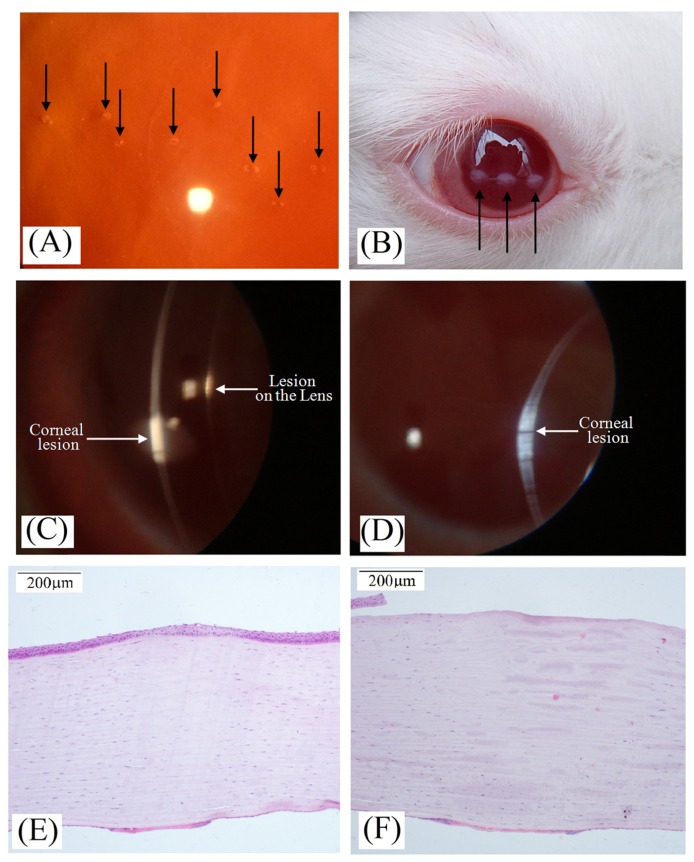
Fig. 4.
Corneal damage observations induced by 1338 nm pulse laser. Arrows in the figure indicated the corneal or lenticular lesions. (A) Lesions with the spot size of 0.28 mm at 6 hours postexposure, and the corneal radiant exposure was 72.1 J/cm2 (about threshold level). (B) Lesions under unaided eye immediately postexposure. The spot size was 1.91 mm and the corneal radiant exposure was 44.4 J/cm2 (about 1.5 times threshold level). (C) Lesions under direct slit-beam illumination immediately postexposure. (D) Lesions under direct slit-beam illumination at 24 hours postexposure. (E) Histological section of corneal tissue at threshold level. The spot size was 0.28 mm and the corneal radiant exposure was 72.1 J/cm2. (F) Histological section of corneal tissue at about 1.5 times threshold level. The spot size was 1.91 mm and the corneal radiant exposure was 44.4 J/cm2.
At about 1.5 times of ED50 damage threshold by 1338 nm laser, the corneal lesions appeared as porcelain white circular spots under unaided eye (Fig. 4(B)), which could be observed immediately following exposure. Through a slit-lamp biomicroscopy examination with direct broad-beam illumination, obvious distortions could be found for these lesions, and the lesion edges were distinct from surrounding normal tissue. The lenticular damage could also be found at a specific observation angle. Highly reflective white strap with thickness identical to the unexposed region were also observed under direct slit-beam illumination, which indicated the damage involved the full thickness of the cornea. At a specific illumination angle, a white strap, which was just directly behind the corneal lesion, could be observed at the anterior lens capsule, indicating that lenticular damage occurred (Fig. 4(C)). At 24 hours post-exposure, the thickness of the corneal damage regions increases remarkably (Fig. 4(D)). For mild damage, the epithelium arranged unorderly, the number of cell nuclei in the stroma layer decreased obviously, cell proliferation could be found in the endothelium layer (Fig. 4(E)). For severe damage, the epithelium layer disappeared (Fig. 4(F)). Another phenomenon could be noted that the damage lesion was not very homogenous, which resulted from the asymmetry and some hot points of the laser profile as shown in Fig. 3.
Retinal damage was not found even at about 1.5 times of corneal ED50 damage threshold, since a focusing laser beam was employed to induce the corneal damage and the beam would significantly expand at the retina with the beam divergence of about 61 mrad.
3.2 Retinal damage characteristics and damage threshold
For the determination of retinal damage threshold, four energy levels were chosen and a total of 160 exposures were evaluated. All the experimental results, including the ED50 thresholds termed in TIE and corneal radiant exposure, the 95% fiducial limits, and the probit slopes, were summarized in Table 2. The ED50 threshold determined at 24-hour check point was lower than that at 1-hour, therefore was chosen as damage threshold. Lens or corneal damage was not observed for any the energy level. The maximal level we chose was 5.73 J/cm2 (1.125 J), obviously smaller than the corneal damage threshold.
Table 2. Ophthalmoscopically visible retinal damage thresholds induced by 1338 nm laser as the corneal spot size was 5.0 mm and the incident beam was collimated (24 eyes were involved and a total of 150 data points were obtained).
| Observation time | Damage threshold energy (95% confidence interval) (J) | Damage threshold radiant exposure (95% confidence interval) (J/cm2) | Slope |
|---|---|---|---|
| 1 h post-exposure | 0.948 (0.903, 0.991) | 4.83 (4.60, 5.05) | 1.18 |
| 24 h post-exposure | 0.904 (0.847, 0.955) | 4.60 (4.31, 4.86) | 1.24 |
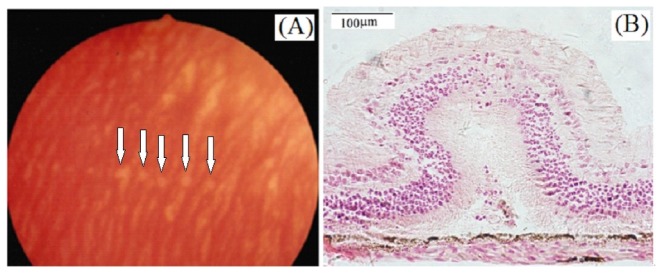
Grossly, retinal lesion induced by 1338 nm laser appeared as circular, well-demarcated, white, opaque lesions. Under threshold radiant exposure condition, some retinal lesions that were indiscernible immediately or within 1-hour post-exposure were readily detected by 24-hour post-exposure, as shown in Fig. 5(A). Figure 5(B) showed the light micrograph of lesion corresponding to the near-threshold damage. The tissue was fixed at 48-h following laser exposures. The damage lesion involved the full thickness of the retinal layer. Slight alteration of the retinal pigment epithelium (RPE) could be observed and tips of some photoreceptor out segments adhered to the apical portions of the RPE. Cells of the outer nuclear layer, the inner nuclear layer and the ganglion cell layer arranged unorderly and some nuclei heavily stained. Inflammatory cells moved to the inner-limiting membrane.
Fig. 5.
(A) Fundus photograph showing rabbit retinal lesions induced by pulsed 1338 nm laser. Photograph was taken at 24 hours post-exposure. The incident energy was 1.0 J. Arrows indicated the retinal lesions. (B) Histological section of retinal tissue at threshold level. Tissue was fixed at 48-h following laser exposures.
4. Discussion
Corneal damage experiments for transitional NIR lasers have been conducted for different wavelengths, exposure durations, and spot sizes during the last two decades [2–5,8]. However, due to the diverse experimental conditions, a definite relationship between damage threshold and laser spot size could not be drawn from existing data points. This study determined corneal damage thresholds for 1338 nm laser with spot sizes from 0.28 mm to 3.55 mm at fixed exposure duration. In a recent report, the retinal damage thresholds were determined in chinchilla grey rabbits for 1319 nm laser radiation for exposure durations from 0.1 s to 10 s [9]. The damage thresholds for exposure durations of 0.35 ms, 0.65 ms, and 80 ms were also determined in a rhesus model [2,3,6]. However, no damage thresholds were determined in such a wide interval between 0.65 ms and 80 ms, which may block the complete clarification of the damage threshold dependence on exposure duration. This study determined the retinal damage threshold for 1338 nm laser with exposure duration of 5 ms. Our results will provide reference to the revision of laser safety standards about the MPEs for transitional NIR lasers.
The transitional NIR region is unique in terms of potential ocular hazards, i.e. multiple ocular tissues are sensitive to laser damage, different from other spectrum region where only one ocular media (cornea or retina) is the most sensitive tissue. Therefore, retinal and corneal injury risks should be simultaneously evaluated. One fact has to be emphasized that the retinal damage threshold determined in this paper is only for a collimated beam. Due to the chromatic aberration, the retinal spot size is not minimal. If the beam is not collimated, the retinal spot size can be small or larger compared to the collimated beam and the damage threshold can thus be smaller or larger due to the damage threshold dependence on the retinal spot size.
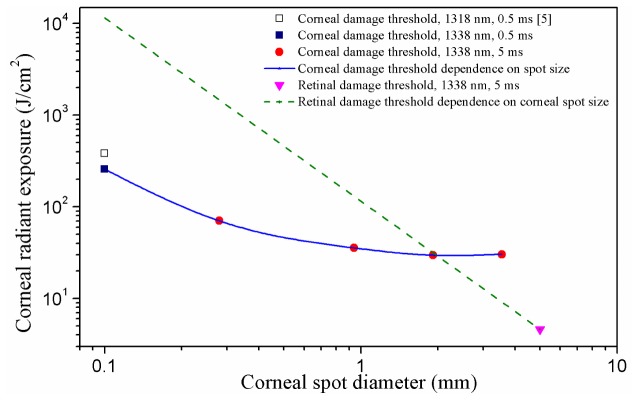
Under the condition that the incident laser beam is collimated and the corneal spot size is smaller than the pupil diameter, the retinal damage threshold expressed in TIE is nearly a constant value and independent of the corneal spot size, as shown by the dash straight line in Fig. 6. In fact, the optical quality of the eye is much better in the central part than in outer parts, so a smaller beam could well be focused better as compared to a larger beam as is used in this paper to determine the threshold, which would result in a lower damage threshold. Therefore, the straight line plotted in Fig. 6 was only an approximate analysis. The corneal damage threshold dependence on the spot size was shown by the blue solid line. These two lines crossed at the spot size of about 2.0 mm. This indicated that for the 5 ms pulsed 1338 nm laser, when the corneal spot size was smaller than 2.0 mm the cornea would be damaged first, and as the spot size increased to larger than 2.0 mm the retina had a higher damage risk. In other words, the most sensitive ocular tissue to laser damage changed from the cornea to retina as the increase of corneal spot size.
Fig. 6.
Corneal and retinal damage thresholds as a function of the incident corneal spot diameter for 5 ms pulsed 1338 nm laser. According to the action spectrum theory [3,8], the data point from Reference [5] could be adjusted to the data for 1338 nm, using the water absorption coefficients at the wavelengths of 1318 nm and 1338 nm (1.76 cm−1 for 1318 nm and 2.62 cm−1 for 1338 nm [23]). This point was plotted to illustrate the rapid change of damage threshold with the decrease of corneal spot size for small corneal spot sizes; for corneal spot diameters of 1 mm and above no significant spot size dependence could be observed. The green dashed line showed how the retinal damage threshold varied as the corneal spot diameter was decreased down from 5 mm, on the condition that the laser beam remained collimated and the damage threshold was expressed in corneal radiant exposure. The straight line was only an approximate analysis because the optical quality of the eye is much better in the central part than in outer parts and thus a smaller beam could well be focused better as compared to a larger beam, resulting in a smaller damage threshold.
It is well known that the spot size is an important factor in determining damage threshold. According to Schulmeister et al. [21,22], for thermally induced ocular damage, there is an inflection point which separates the curve of damage threshold dependence on spot size. For spot sizes smaller than the inflection point, the damage threshold increases rapidly with the decrease of the spot size. While for spot sizes larger than the inflection point, the thresholds are nearly independent of spot size. In this experiment, the inflection point located at about 1.0 mm for 5 ms exposure duration. This phenomenon was determined by the heat accumulation and thermal diffusion in cornea by laser irradiation. As the incident spot size is small, the temperature rise of the irradiated corneal tissue is strongly influenced by the heat diffusion to the surrounding unexposed region. With the increase of the spot size, the influence by the heat diffusion decreases and the absorbed energy is more easily to accumulate in the exposed tissue, so the damage threshold decreases. After the spot size increases to a certain value, the heat diffusion will have little influence to the temperature rise of the exposed tissue region, thus the damage thresholds keep as a constant value.
5. Summary
The rabbit corneal damage thresholds for 5 ms pulsed 1338 nm laser at incident spot sizes of 0.28, 0.94, 1.91, and 3.55 mm were 70.3, 35.6, 29.6 and 30.3 J/cm2 respectively. The rabbit retinal damage threshold for this laser given in terms of TIE was 0.904 J at 5.0 mm corneal spot size when the beam was collimated. The most sensitive ocular tissue to this laser changed from the cornea to retina as the increase of spot size. For rabbit, when the corneal spot size was smaller than about 2.0 mm the cornea would be damaged first, and as the spot size increased to larger than 2.0 mm the retina had a higher damage risk. The obtained results could be used for the refinement of the safety standards for transitional NIR lasers.
Acknowledgments
We thank Jinggeng Yang for the preparation of the animals used in the experiments.
Funding
National Natural Science Foundation of China (NSFC) (61275194, 61575221).
References and links
- 1.Zuclich J. A., Gagliano D. A., Cheney F., Stuck B. E., Zwick H., Edsall P., Lund D. J., “Ocular effects of penetrating IR laser wavelengths,” Proc. SPIE 2391, 112–125 (1995). 10.1117/12.209874 [DOI] [Google Scholar]
- 2.Zuclich J. A., Lund D. J., Edsall P. R., Stuck B. E., Hengst G., “High power lasers in the 1.3-1.4 μm wavelength range: ocular effects and safety standard implications,” Proc. SPIE 4246, 78–88 (2001). 10.1117/12.426706 [DOI] [Google Scholar]
- 3.Zuclich J. A., Lund D. J., Stuck B. E., “Wavelength dependence of ocular damage thresholds in the near-ir to far-ir transition region: proposed revisions to MPES,” Health Phys. 92(1), 15–23 (2007). 10.1097/01.HP.0000232188.69779.69 [DOI] [PubMed] [Google Scholar]
- 4.Berezin Y. D., Boiko E. V., Volkov V. V., Danilichev V. F., Ganin D. V., Gatzu A. F., Smirnov N. N., Lazo V. V., Tkachuk A. M., “Peculiarities of coagulation action of IR lasers (1-3 μm) radiation on cornea,” Proc. SPIE 2769, 9–13 (1996). 10.1117/12.238014 [DOI] [Google Scholar]
- 5.Ketzenberger B., Johnson T. E., Van Gessel Y. A., Wild S. P., Roach W. P., “Study of corneal lesions induced by 1,318-nm laser radiation pulses in Dutch belted rabbits (Oryctolagus cuniculus),” Comp. Med. 52(6), 513–517 (2002). [PubMed] [Google Scholar]
- 6.Vincelette R. L., Rockwell B. A., Oliver J. W., Kumru S. S., Thomas R. J., Schuster K. J., Noojin G. D., Shingledecker A. D., Stolarski D. J., Welch A. J., “Trends in retinal damage thresholds from 100-millisecond near-infrared laser radiation exposures: a study at 1,110, 1,130, 1,150, and 1,319 nm,” Lasers Surg. Med. 41(5), 382–390 (2009). 10.1002/lsm.20772 [DOI] [PubMed] [Google Scholar]
- 7.Chen H., Yang Z., Wang J., Chen P., Qian H., “A comparative study on ocular damage induced by 1319nm laser radiation,” Lasers Surg. Med. 43(4), 306–312 (2011). 10.1002/lsm.21052 [DOI] [PubMed] [Google Scholar]
- 8.Wang J., Jiao L., Chen H., Yang Z., Hu X., “Corneal thermal damage threshold dependence on the exposure duration for near-infrared laser radiation at 1319 nm,” J. Biomed. Opt. 21(1), 015011 (2016). 10.1117/1.JBO.21.1.015011 [DOI] [PubMed] [Google Scholar]
- 9.Wang J., Jiao L., Jing X., Chen H., Hu X., Yang Z., “Retinal thermal damage threshold dependence on exposure duration for the transitional near-infrared laser radiation at 1319 nm,” Biomed. Opt. Express 7(5), 2016–2021 (2016). 10.1364/BOE.7.002016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuclich J. A., Schuschereba S. T., Zwick H., Boppart S. A., Fujimoto J. G., Cheney F. E., Stuck B. E., “A comparison of laser-induced retinal damage from infrared wavelengths from that from visible wavelengths,” Laser Light Ophthalmol. 8(1), 15–29 (1997). [Google Scholar]
- 11.Zuclich J. A., Zwick H., Schuschereba S. T., Stuck B. W., Cheney F. E., “Ophthalmoscopic and pathologic description of ocular damage induced by infrared laser radiation,” J. Laser Appl. 10(3), 114–120 (1998). 10.2351/1.521836 [DOI] [Google Scholar]
- 12.Vincelette R. L., Welch A. J., Thomas R. J., Rockwell B. A., Lund D. J., “Thermal lensing in ocular media exposed to continuous-wave near-infrared radiation: the 1150-1350-nm region,” J. Biomed. Opt. 13(5), 054005 (2008). 10.1117/1.2978066 [DOI] [PubMed] [Google Scholar]
- 13.Pocock G. M., Oliver J. W., Noojin G. D., Schuster K. J., Stolarski D., Shingledecker A., Rockwell B. A., “Follow up study of NIR (1100 to 1319 nm) retinal damage thresholds and trends,” Proc. SPIE 7562, 75620E (2010). 10.1117/12.846987 [DOI] [Google Scholar]
- 14.ANSI, American National Standard for Safety Use of Lasers, Z136.1, Laser Institute of America, Orlando (2014). [Google Scholar]
- 15.ICNIRP , “Guidelines on limits of exposure to laser radiation of wavelength between 180 nm and 1,000 microns,” Health Phys. 105(3), 271–295 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Lund D. J., Edsall P. R., Fuller D. R., Hoxie S. W., “Bioeffects of near-infrared lasers,” J. Laser Appl. 10(3), 140–143 (1998). 10.2351/1.521841 [DOI] [Google Scholar]
- 17.McCally R. L., Bonney-Ray J., Bargeron C. B., “corneal epithelial injury thresholds for exposures to 1.54 microm radiation-dependence on beam diameter,” Health Phys. 87(6), 615–624 (2004). 10.1097/01.HP.0000137181.53428.04 [DOI] [PubMed] [Google Scholar]
- 18.McCally R. L., Farrell R. A., Bargeron C. B., ““Corneal epithelial damage thresholds in rabbits exposed to Tm: YAG laser radiation at 2.02 μm,” Laser in Surg,” Med. 12(2), 598–603 (1992). [DOI] [PubMed] [Google Scholar]
- 19.Zuclich J. A., Blankenstein M. F., Thomas S. J., Harrison R. F., “Corneal damage induced by pulsed CO2 laser radiation,” Health Phys. 47(6), 829–835 (1984). 10.1097/00004032-198412000-00003 [DOI] [PubMed] [Google Scholar]
- 20.Bargeron C. B., Deters O. J., Farrell R. A., McCally R. L., “Epithelial damage in rabbit corneas exposed to CO2 laser radiation,” Health Phys. 56(1), 85–89 (1989). 10.1097/00004032-198901000-00008 [DOI] [PubMed] [Google Scholar]
- 21.Schulmeister K., Husinsky J., Seiser B., Edthofer F., Fekete B., Farmer L., Lund D. J., “Ex vivo and computer model study on retinal thermal laser-induced damage in the visible wavelength range,” J. Biomed. Opt. 13(5), 054038 (2008). 10.1117/1.2982526 [DOI] [PubMed] [Google Scholar]
- 22.Schulmeister K., Ullah R., Jean M., “Near infrared ex-vivo bovine and computer model thresholds for laser-induced retinal damage,” Photonics Lasers Med. 1(2), 123–131 (2012). 10.1515/plm-2012-0007 [DOI] [Google Scholar]
- 23.Kou L., Labrie D., Chylek P., “Refractive indices of water and ice in the 0.65- to 2.5-µm spectral range,” Appl. Opt. 32(19), 3531–3540 (1993). 10.1364/AO.32.003531 [DOI] [PubMed] [Google Scholar]