Abstract
Thermotherapy, as a method of treating cancer, has recently attracted considerable attention from basic and clinical investigators. A number of studies and clinical trials have shown that thermotherapy can be successfully used as a therapeutic approach for various cancers. However, the effects of temperature on cancer bioenergetics have not been studied in detail with a real time, in a microplate, label-free detection approach.
This study investigate how changes in temperature affect the bioenergetics characteristics (mitochondrial function and glycolysis) of three colorectal cancer (CRC) cell lines utilizing the Seahorse XF96 technology. Experiments were performed at 32°C, 37°C and 42°C using assay medium conditions and equipment settings adjusted to produce equal oxygen and pH levels ubiquitously at the beginning of all experiments. The results suggest that temperature significantly changes multiple components of glycolytic and mitochondrial function of all cell lines tested. Under hypothermia conditions (32°C), the extracellular acidification rates (ECAR) of CRC cells were significantly lower compared to the same basal ECAR levels measured at 37°C. Mitochondrial stress test for SW480 cells at 37°C vs 42°C demonstrated increased proton leak while all other OCR components remained unchanged (similar results were detected also for the patient-derived xenograft cells Pt.93). Interestingly, the FCCP dose response at 37°C vs 42°C show significant shifts in profiles, suggesting that single dose FCCP experiments might not be sufficient to characterize the mitochondrial metabolic potential when comparing groups, conditions or treatments.
These findings provide valuable insights for the metabolic and bioenergetic changes of CRC cells under hypo- and hyperthermia conditions that could potentially lead to development of better targeted and personalized strategies for patients undergoing combined thermotherapy with chemotherapy.
Keywords: Cancer bioenergetics, Hyperthermia, Seahorse XF Phenograms, Hypothermia, FCCP dose response
1. INTRODUCTION
Thermotherapy (hyper- and hypothermia) has been utilized for the treatment of various diseases since ancient times. One of the oldest reports describing the use of hyperthermia for cancer dates to about 3000 BC and is found in the Egyptian Edwin Smith surgical papyrus1. Despite centuries of research on thermotherapy, the mechanism by which this modality produces its clinical effectiveness noted in multiple randomized clinical trials across a spectrum of malignancies is yet unknown2. Currently, one of the main theories in the field of temperature treatment for malignancy is that exposure to hyperthermia leads to disruption of the mitotic cycle (G1 arrest) and apoptosis. Most experimental evidence underlying this theory is from in vitro studies in which cell cultures were exposed to prolonged periods (24–72 h) of hyperthermia (defined as exposure to 39° – 42°C). In a recent study, Zhu et al., (2015) reported that various cancer cells maintained at 39°C for 72 h demonstrated mild inhibition of cell growth by arresting the cells at the G1 phase of the cell cycle which also resulted in hyperthermia-enhanced efficacy of several chemotherapeutic agents3. In contrast to the in vitro experiments, the clinical in vivo protocols typically utilize only short-term exposure to hyperthermia. For example, several human breast cancer protocols incorporate radiation therapy hyperthermia treatments (41–42°C) for ~60 min with at least 72 h between individual treatment sessions4–6.
Since the first cancer-related Seahorse XF paper7 was published in 2007, this technology has been exponentially utilized to study cancer cell metabolism. Currently, there are more than 500 cancer related-publications on various topics but few of them focus on the use of thermotherapy to modify cancer cell bioenergetics, metabolism and drug resistance. There is only one previous study using Seahorse technology to investigate effects of hyperthermia on cancer cell bioenergetics, which is focused only on basal levels of OCR. Curley et al., (2014) used human pancreatic carcinoma cells to investigate how short term hyperthermia (cell heated up to 46°C for 5 min) affects the base line oxygen consumption rates (OCR) when mitochondrial function is assessed 24 hours post hyperthermia treatment on a Seahorse XF Extracellular Flux Analyzer8. The results suggest that the 5min hyperthermia treatment reduced OCR by approximately 50%. Total OCR levels reported represent a sum of both non-mitochondrial respiration and base level respiration and were not normalized to cell count or protein levels.
The goal of our study was to perform a detailed analysis of the changes in cancer cell bioenergetics (mitochondrial and glycolytic cellular functions and their components) that occur in real time when cells are incubated for 1 hour at temperatures equivalent to those commonly used in clinical practice for hypo- or hyperthermia. We chose to use colorectal cancer (CRC) cells for this study because CRC have been previously reported to the final slope of the cell survival curve where the curve approximates a straight line (aka final slope or D0) at 43°C applied for 1.5hr and when hyperthermia is combined with mitomycin C and cis-dichlorodiammineplatinum(II) treatment9
The results from our study indicate that changes in temperature (shifting from 37°C to 32°C or 42°C for period of 90–120 min) significantly alters glycolysis and, to a less extent, modifies various components of mitochondrial function. Similar, findings has been previously reported for yeast studies, where analysis of the high temperature-induced glycolytic flux increasde suggested (without the use of Seahorse technology) that hyperthermia leads to a rapid increase in glycolytic flux, which is not accompanied by an increase in respiration10. Secondly, the study demonstrates that the Seahorse XF technology can be successfully utilized to measure changes in cellular bioenergetics in conditions different from normal physiological temperature of 37°C which is the standard condition for most cancer studies. Present findings might be of significance not only for cancer research, but also for other areas of biomedical research such as immunology, stem cells research and many others where potentially temperature could be used to modify cellular bioenergetics.
In summary, this study provides important insights into the nature of cancer cell response to thermotherapy, such as changes in metabolic potentials which can be potentially translated and utilized for the development of better clinical thermotherapy protocols for cancer treatment.
2. MATERIALS AND METHODS
2.1 Instrumentation, protocols and settings
All experiments were performed with the Seahorse XF 96 and XF software version 1.4. This system allows instrument temperature settings to be lowered (31–32°C) or increased (41–42°C) for the standard duration of typical mitochondrial and glycolysis stress tests. The maximum number of experiments completed in one day was three with increasing temperature (the first experiment was performed at 32°C, the second at 37°C, and the final experiment at 42°C). In a separate set of experiments, the order of temperature testing (from 42° to 37°C) was reversed to determine whether the order had any impact on the observed results. All OCR and ECAR analyses were performed at least two times with a minimal of 6–8 technical replicates (up to 80 replicates in some cases) for each treatment or assay. All cells were incubated for 60 min in a non-CO2 incubator before plate calibration was performed and mitochondria and glycolysis stress test experiments were initiated at corresponding temperature conditions.
2.2 Cell lines
The human colon CRC cell lines, SW480 and HCT116, were obtained from ATCC (Manassas, Virginia) and authenticated in February 2016 (Genetica DNA Laboratories, Cincinnati, OH). All cell lines were grown in DMEM media containing 10% fetal bovine serum (FBS), 25mM Glucose and 1mM Pyruvate. At the time of experimentation, cells were in a passage range of 15–20 and cell seeding was 30,000 cells per well.
Following appropriate approval by the Institutional Review Board (IRB) at the University of Kentucky, CRC tissues were collected after surgical resection and implanted into NSG™, NOD scid gamma mice (The Jacksons laboratory) to established the patient-derived xenograft (PDX). The resultant primary CRC cell line (Pt. 93) was established after three sequential generations in NSG mice and authenticated as a unique human cell line in February 2016 (Genetica DNA Laboratories, Cincinnati, OH). For all of the Seahorse experiments, Pt.93 cells were seeded at 30,000 per well in DMEM media containing 10% FBS.
2.3 Mitochondrial stress tests
The calibration step for all assays was completed within 15 min of loading each plate and no additional equilibrations steps were used to minimize chances for prolonged exposure to hypo- or hyperthermia. The instrument was programmed for 3 cycles of drug injection followed be 3 mixing and 3 measuring steps (3 min each). Total experimental time was ~110 min. The assay media for oxygen consumption rate (OCR) experiments was DMEM-based XF modified media containing 25mM glucose and 1mM pyruvate (pH was adjusted to 7.40 ± 0.05 at 32°, 37° and 42°C accordingly).
Three drugs were injected during the course of the assays as shown on Fig 4A: 1) Port A – 1.00 μM oligomycin A, an inhibitor of mitochondrial membrane adenosine triphosphate (ATP) synthase (i.e., F1F0 ATP synthase or Complex V); 2) Port B – 0.3 μM FCCP - Carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone, an ionophore uncoupler of oxidative phosphorylation in mitochondria that disrupts ATP synthesis by transporting protons across cell membranes; and 3) Port C – 1.0 μM antimycin A, which inhibits the mitochondrial electron transport chain from cytochrome b to cytochrome C1 and 1.0 μM Rotenone, an inhibitor of mitochondrial electron transport at nicotinamide adenine dinucleotide: ubiquinone oxidoreductase in Complex I.
Figure 4. Mitochondria stress test for SW480 at normal and high temperatures and FCCP dose response.
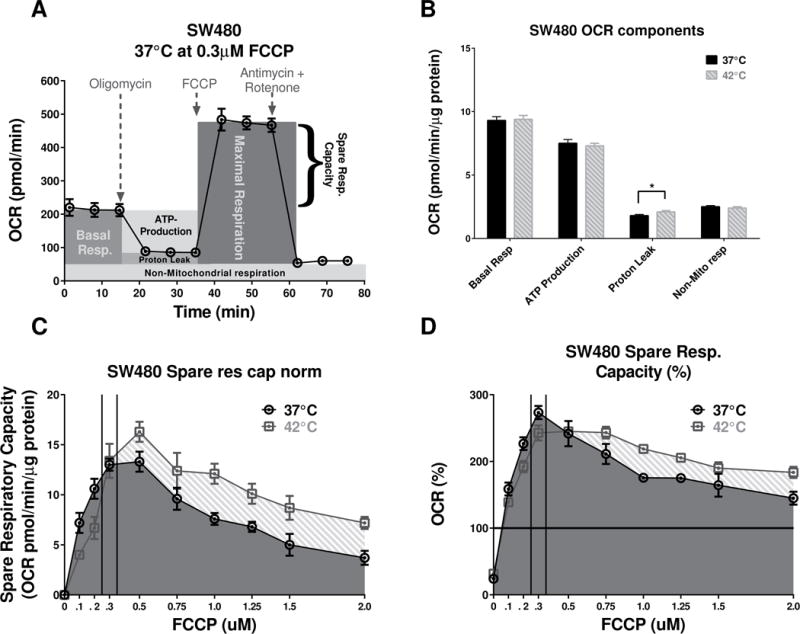
(A) The “raw” (not normalized to protein) 37°C SW480 OCR profile at 0.3 μM FCCP was used to illustrate the various components of the mitochondrial function. (B) Quantification of the temperature difference in SW480 OCR components that are independent of FCCP concentrations. Proton leak was significantly increased (P= 0.0359, at the higher unpaired t-test with Welch’s correction, n=60–66) at the higher temperature, while all other components remained without significant changes. (C) The changes in the SRC, due to various FCCP doses at 37°C, were less prominent compared to the 42°C. (AUC analysis shows a 32% increase of the “overall” respiratory capacity at 42°C vs the 37°C condition.) (D) The analysis of AUC for %SRC was limited to the baseline, and at 42°C the cells had ~21% higher “overall” metabolic potential.
2.4 Glycolysis stress tests
The glycolysis stress test was performed under similar conditions as the mitochondrial stress test but with few important differences. The assay media contained no glucose or pyruvate (2mM GlutaMAX Supplement was present as part of the stock XF media ingredients). The protocol included 4 measurements for each portion of the experiments (base line and data points between drug injections); the duration of the experiment was approximately 110 min (Fig 5A) and total time for the experiment ~135 min. The additional measurements were required to reach the maximum response signal after drug injection. The drug concentrations used during glycolysis stress tests were: 1) Port A – 10.0 μM glucose; 2) Port B – 1.0 μM oligomycin A; and 3) Port C – 100 μM 2-deoxy-D-glucose (2-DG), a non-metabolizable glucose analog that inhibits glycolysis.
Figure 5. Glycolysis stress test for SW480 cell at normal and high temperatures.
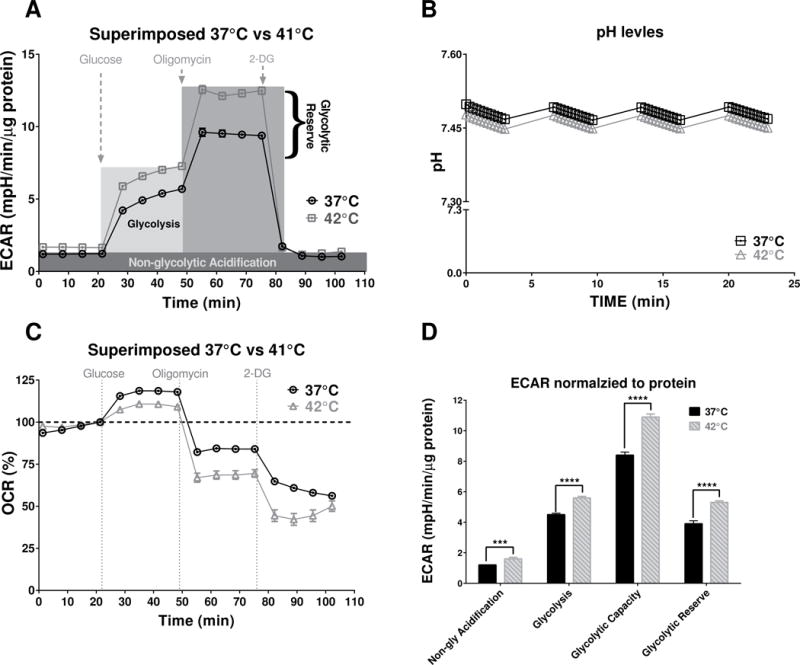
(A) SW480 ECAR profile components presented over the experimental record from 42°C vs. 37°C. (B) There was no statistically difference in pH levels in background wells during the measurement of the baseline ECAR signals. (C) The percent change of oxygen consumption rates during the glycolysis stress test indicated that after adding 10μM Glucose the cells at 37°C consumed more oxygen compare to the cells at 42°C. (D) The unpaired t-test with Welch’s correction of ECAR signal components suggests that all aspects of glycolysis were significantly increased at the higher temperature: Non-glycolytic Acidification (P=0.0002, n=69–77); Glycolysis (P<0.0001, n=69–77); Glycolytic Capacity (P<0.0001, n=69–77) and Glycolytic Reserve (P<0.0001, n=69–77).
2.5 Plate group design, background wells and protein level detection
The first and last columns of each plate (a total of 16 wells) were cell-free with 175μL of the assay media. The empty wells were used to control for any change from baseline level in oxygen and pH during OCR and ECAR measurements, and were also used for the determination of protein level.
When data collection was complete, the media from all wells was carefully removed and replaced with 25 μL of cell lysis buffer containing 0.32 M sucrose, 2 mM EDTA, 2 mM EGTA, 20 mM HEPES, 0.2 mM PMSF, 4 μg/ml leupeptin, 4 μg/ml pepstatin and 5 μg/ml aprotinin. The plates were incubated in a −20°C freezer overnight then brought to room temperature on an orbital shaker for further processing. Increasing volumes (1.25 to 12.5 μL) of known protein standard (2 μg/ μL BSA) were added to the background lanes to create a standard curve for the protein levels (ranging from 2.5 to 25 μg). Thermo Scientific Pierce BCA Protein Assay Kit was used for preparing the developing solution (mixing 4.9ml of Reagent A + 0.1ml of Reagent B) and end volumes were total to 125 μL (25uL of sample containing protein + 100uL of BCA Protein Assay mix. The XF Plates with protein standard and samples were incubated at room temperature for 15 min. Seahorse XF cell plates are not compatible with many plate readers; therefore, the content of each well was moved to a regular-sized 96 well plate for detection of optical densities. An iMark Microplate Absorbance Reader (BioRad) was used to detect absorbance for both standard and samples according to the recommended detection settings for the BCA assay kit.
2.6 Data analysis and statistics
The Seahorse XF 96 instrument was controlled by Seahorse software (version 1.4.2.3) .XFD data files. Seahorse Wave software (version 2.2.0) was used for further processing, including detection of outliers and creation of base line phenograms. Automated data analysis for the OCR and ECAR signals were performed with the help of XF Stress Test Report Generators V2. Figures and statistical analysis were generated with GraphPad Prism version 6.05. Statistical analysis included Welch’s t-test (or unequal variances t-test) and one-way ANOVA followed by Dunnett’s multiple comparisons test when statistically appropriate. Area under the curve (AUC) analysis was performed using standard buit-in analysis tool of Prism 6.0 software (XY analysis AUC) with settings for baseline to be equal to zero for normalized to protein SRC and baseline set up to equal to 100 for the %SRC analysis.
3. RESULTS
3.1 Base line energy phenotypes of SW480 CRC cells at 3 different temperatures
The initial experiments were performed using SW480 CRC cells incubated in a non-CO2 incubator set at 32°C, 37°C and 41°C for 60 min within 175uL per well XF modified DMEM media containing 25mM glucose and 1mM pyruvate. After the incubation the base line OCR and ECAR levels were measured using the Seahorse and no statistical differences was found between OCR normalized to protein levels for the three different temperature conditions. However, the normalized to protein ECAR levels were significantly different for the 37°C vs. 32°C (P=0.0093, one-way ANOVA, Holm-Sidak’s multiple comparisons test) and for the 37°C vs. 42°C conditions (P < 0.0001, one-way ANOVA, Holm-Sidak’s multiple comparisons test) (Fig. 1A). One possible explanation for such temperature-dependent shift in the ECAR signal could be related to the oxygen levels in the media. However, analysis of the oxygen levels (O2 mmHg) in the media from background wells (Fig. 1B) demonstrated that there were no differences in oxygenation during the basal level measurements (one-way ANOVA with Bonferroni’s multiple comparisons test P=0.5606 for the 37°C vs. 32°C and P> 0.9999 for the 37°C vs. 41°C). The average protein levels were measured at the end of these initial Seahorse experiments (Fig. 1C) and levels were highest in the 37°C group while both high and low temprture treatment had lower levels of proten.
Figure 1. Baseline energy profiles and oxygen levels.
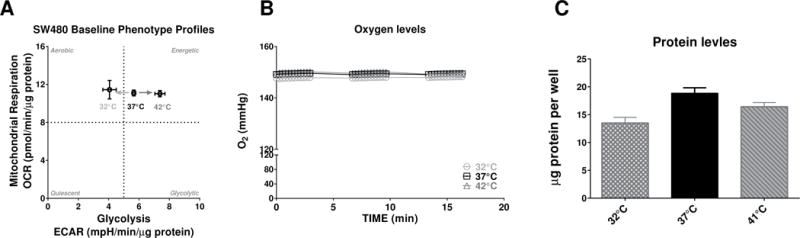
(A) OCR and ECAR levels of SW480 cell line incubated for approximately 60 min in XF modified media containing 25mM glucose and 1mM pyruvate. No statistical differences were found between OCR normalized to protein levels for the three different temperature conditions. The normalized to protein ECAR levels were significantly different for the 37°C vs. 32°C (P=0.0093, one-way ANOVA, Holm-Sidak’s multiple comparisons test, n=14–16) and for the 37°C vs. 42°C conditions (P < 0.0001, one-way ANOVA, Holm-Sidak’s multiple comparisons test, n=14–16); (B) Oxygen levels (mmHg) at the base-line time were not statistically different between temperature conditions. Oxygen levels shown represent control wells containing media alone without cells; (C) Protein levels were determined at the end of the experiments with BCA assay (n=8–16). One-way ANOVA with Bonferroni’s multiple comparisons test show no significant differences between 37°C and 41°C (P=0.2828) and decreased in protein levels for the 32°C group compared to 37°C (P=0.0020).
3.2 Base line energy phenotypes and OXPHOS potentials in simulated hypothermia conditions
Next, we investigated whether the reduction of ECAR base levels at lower temperature was unique for SW480 CRC cells or if other CRC cell lines have a similar response to changes temperature for 1 hour. Two additional CRC cell lines (HCT116 and pt.93) were subjected to standard mitochondrial stress tests with FCCP concentrations of 0.3μM for all tested cell lines. The results suggested a similar shift in the baseline phenograms (Fig. 2A, B and C) as initially observed in the SW480 cell line (Fig 1A). All baseline bioenergetic phenotypes were calculated from the third baseline measurement (before Oligomycin was injected to produce 1μM final concentration in each well) and results were normalized to protein levels for each plate to reduce variability between conditions that might be due to increased/reduced growth rates. All tested CRC cell lines had significantly lower ECAR levels at hypothermia level temperatures compared to 37°C (unpaired t test with Welch’s correction SW480 P< 0.0001, pt.93 P=0.0042 and HCT116 P=0.0076). There were no significant changes in %OCR profiles (Fig. 3A, B and C), with exception to the magnitude of response to injected 0.3 μM FCCP which could be used to calculate the spare respiratory capacity also as percent change from base line or %SRC (Fig. 3D) the is presented as percent change of baseline.
Figure 2. Raw and normalized to protein base line phenograms for 3 different colon cancer cell lines.
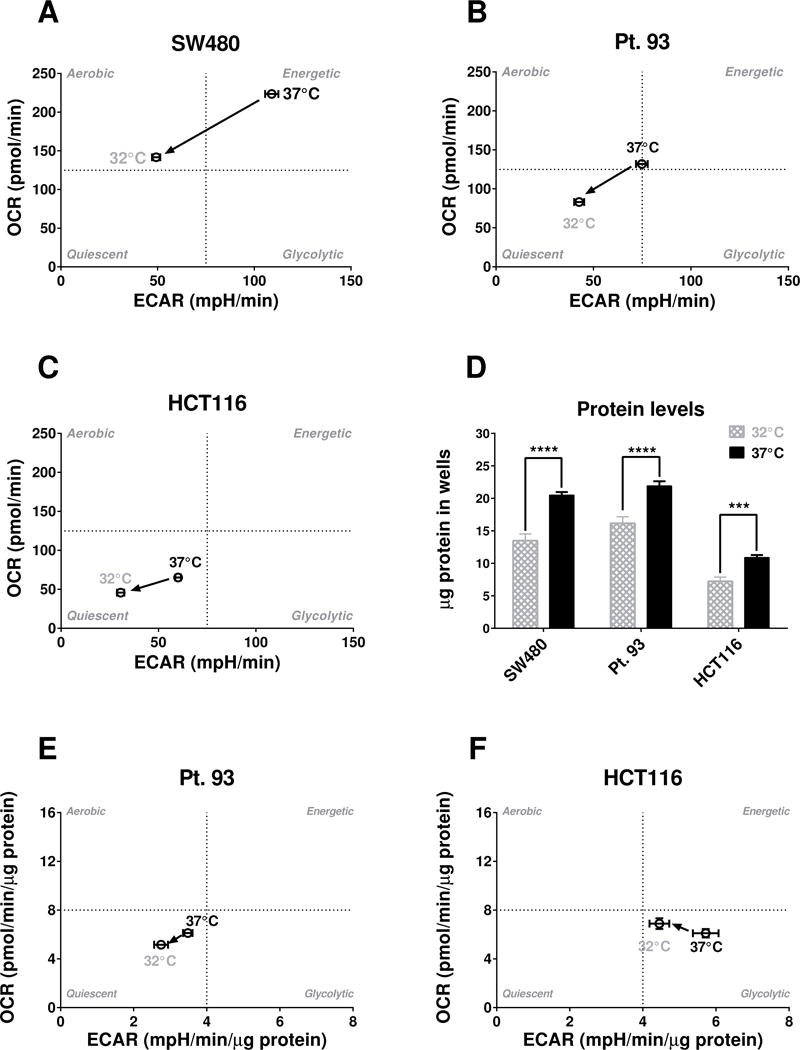
(A) Base line phenograms for SW480 show significant differences in both Glycolysis (ECAR) and Mitochondrial Respiration (OCR) at the third base line data point while bioenergetics data have not been normalized to protein (n=8–16). Please note: The quadrants have been set at arbitrary values only to indicate direction of bioenergetic changes for cells and treatments; (B) Base line phenograms for Pt. 93 show significant differences in both Glycolysis (ECAR) and Mitochondrial Respiration (OCR) when data is not normalized to protein (n=8–16); (C) Base line phenograms for HCT116 show significant differences in both Glycolysis (ECAR) and Mitochondrial Respiration (OCR) when data is not normalized to protein (n=8–16). Also the 37°C group of HCT116 seems to be more quiescent compared to the other two cell lines at 37°C; (C) Protein levels were significantly lower at 32°C compared to 37°C (P values were ≤0.0001 for all cells, one-way ANOVA, Holm-Sidak’s multiple comparisons test, n=13–16); (E) Pt. 93 cells data was normalized to protein level and ECAR and OCR were both significantly lower at 32°C (P=0.0042 and P=0.0047, respectively, unpaired t-test with Welch’s correction, n=14–15); (F) HCT 116 cell line also had a lower baseline ECAR phenotype for the 32°C vs 37°C conditions (P=0.0076, unpaired t-test with Welch’s correction, n=16) and no statistical difference for the OCR.
Figure 3. Mitochondria stress test profiles and SRC as percent change of base line for 3 different colon cancer cell lines at hypothermia condtions.
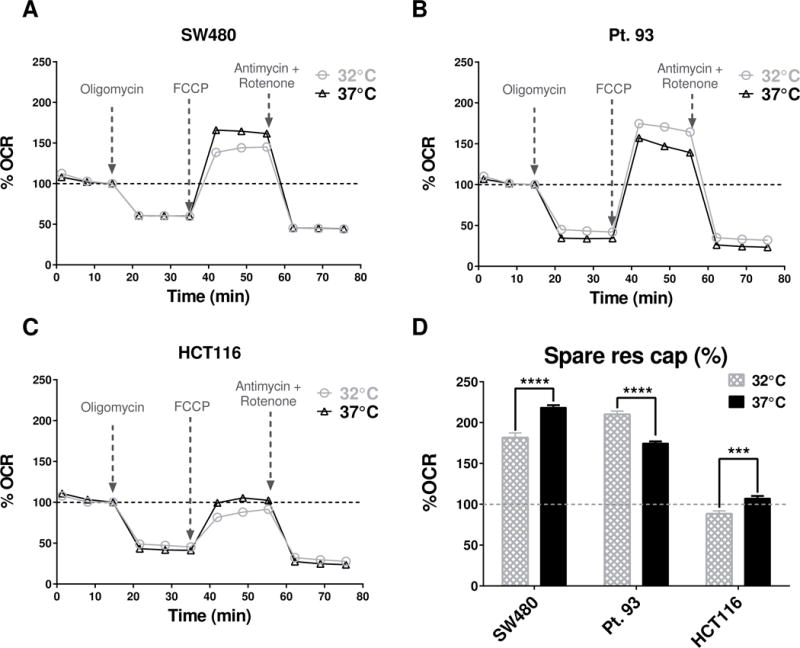
(A) SW480 cell profile (n=8–16), OCR profiles were converted to % change OCR from of third base-line data point (which was set to be 100% for all wells); (B) Pt. 93 cells (n=8–16) %OCR profiles; (C) HCT116 cells (n=8–16) %OCR profiles; (D) Analysis of percent change of spare respiratory capacity (%SRC) in response to hypothermia show that two of the cell lines (SW480 and HCT116) had a statistically significant decrease (P<0.0001, unpaired t-test with Welch’s correction, n=8–16) at lower temperature and one cell line (pt.93) had significant increase (P=0.003, unpaired t-test with Welch’s correction, n=8–16).
Interestingly, two cell lines (SW480 and HCT116) had a decrease in %SRC while the pt.93 cell line had statistically significant increased metabolic potential (P=0.003, unpaired t-test with Welch’s correction, n= 8–16). All mitochondrial stress test experiments for this section were performed under the same protocol, including injection of 0.3 μM FCCP, therefore the dose of the mitochondrial uncoupler FCCP was not optimized for individual cells shown in Fig. 3D.
3.3 Hyperthermia effects on FCCP dose response profiles of SW480 CRC cells
Based on the different trends observed in Fig. 3D, we hypothesized that changes in temperature may induce changes in the dose-response profiles for FCCP. To test this hypothesis, a full FCCP dose-response study was performed with the SW480 cell line at normal temperatures and hyperthermia. The results (Fig. 4C & D) confirmed that hyperthermia caused shifts in the FCCP dose-response spare respiratory capacity (SRC) profiles. It has appeared as an hyperthermia-related decrease in SRC at 0.3 μM FCCP was only a trend at the narrow peak (marked with two vertical lines on Fig. 4C & D) of maximum values, while the area under the entire FCCP dose range indicated an increase of SRC with hyperthermia. Analysis of area under the curves (AUC) shows that at 42°C the cells have almost a 32% increase of their “overall” respiratory capacity vs the 37°C condition. Such an increase in %SRC over the range of different doses of FCCP indicated an increased capacity of cells to meet energy demands in stress conditions.
The proton leak was the only component of the OCR signal that was statistically different (P=0.0359, unpaired t-test with Welch’s correction, n=60–66) at higher temperatures (Fig. 4B) suggesting that temperature might have an effect on the mitochondrial inner membrane integrity independent of ATP synthase activity which was unchanged by temperature.
3.4 Hyperthermia effects on Glycolysis and it components of SW480 CRC cells
The SW480 cell line, used for the first round of experiments detecting base level phenotypes and FCCP dose response curves, was also subjected to a standard glycolysis stress test under normal and hyperthermia conditions (Fig. 5). The results show that all components of glycolysis (Fig. 5A, D) were significant increased (P≤0.0001, unpaired t-test with Welch’s correction, n=69–77), suggesting that glycolytic aspect of cancer bioenergetics is much more affected under hyperthermia conditions than mitochondrial respiration components. These biological changes were confirmed to be independent from the pH (Fig. 5B).
Additionally, the results of glycolysis stress tests, in which cells are deprived of glucose and pyruvate for 1h, indicated that hyperthermia-treated cells did not consume as much oxygen as those maintained at 37°C when 10 mM glucose was added to the media (Fig. 5C). This finding suggests that hyperthermia does not simply cause an increased total energy metabolism, but rather a more specific shift in cancer bioenergetics and substrate preference that requires further investigation.
The order by which the experiments were performed (starting at 37°C or 42°C) did not affect the results due to cell growth since OCR and ECAR values were normalized to the total protein level (μg of protein per well).
3.5 Hyperthermia effects on FCCP dose response profiles of Pt. 93 CRC cells
Based on the different trends observed in Fig. 3D, we hypothesized that changes in temperature may induce opposite changes in the dose-response profiles for FCCP for the Pt. 93 cell line. To test this hypothesis, a full FCCP dose-response study was performed with the Pt. 93 cell line following same protocol as for the SW480 cells. The results (Fig. 6C & D) confirmed that hyperthermia caused shifts in the FCCP dose-response spare respiratory capacity (SRC) profiles but in different direction of changes observed for SW480.
Figure 6. Mitochondria stress test for Pt. 93 at normal and high temperatures and FCCP dose response.
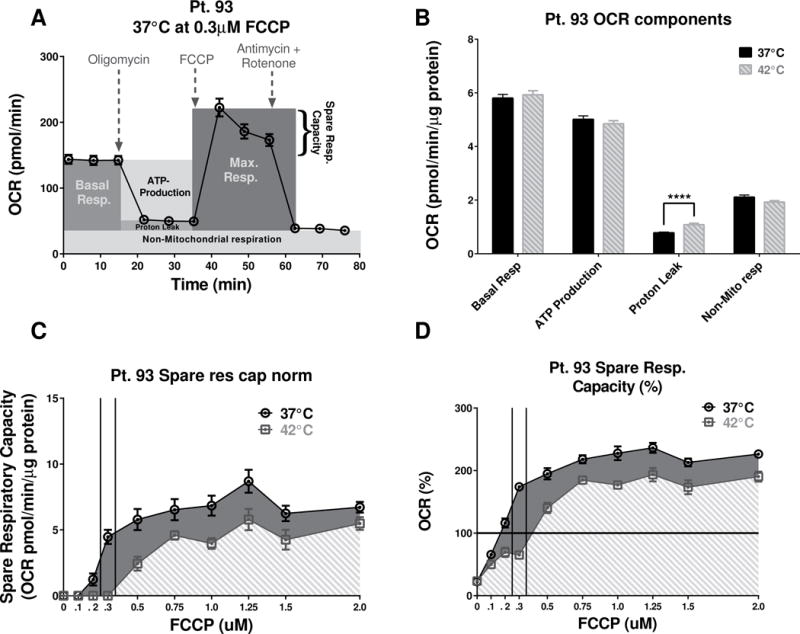
(A) The “raw” (not normalized to protein) 37°C Pt. 93 OCR profile at 0.3 μM FCCP was used to illustrate the various components of the Pt. 93 mitochondrial function. (B) Quantification of the temperature difference in Pt. 93 OCR components that are independent of FCCP concentrations. Proton leak was significantly increased (P<0.0001, unpaired t-test with Welch’s correction, n=65–66) at the higher temperature, while all other components remained without significant changes. (C) The changes in the SRC, due to various FCCP doses at 37°C, were more prominent (unlike the SW480) compared to the 42°C. (AUC analysis shows a 39% increase of the “overall” respiratory capacity at 37°C vs the 42°C condition.) (D) The analysis of AUC for the %SRC was limited to the baseline, and at 42°C the cells had ~40% lower “overall” metabolic potential.
Analysis of area under the curves (AUC) shows that at 42°C the Pt. 93 cells had almost a 40% decrease of their “overall” respiratory capacity vs the 37°C condition.
Another interesting observation was that similarly to the SW480 cells, the Pt. 93 had no significant changes in any of the mitochondrial components (Fig 6B). Again the only component of Mitochondrial respiration that was affected was proton leaks and it was significantly increased at the higher temperature (P<0.0001, unpaired t-test with Welch’s correction, n=65–66).
4. DISCUSSION
In this study, we investigated changes in the bioenergetics of colorectal cancer (CRC) cell lines as a result of temperature exposures similar to current clinical protocols. This is the first study utilizing the Seahorse XF technology to investigate temperature-induced changes in cancer cell bioenergetics in which cancer cells were exposed to 32°C, 37°C and 42°C for 60 min and mitochondrial and glycolysis stress tests performed. However, it is important to note that despite of advantages in new technology it has been long ago suspected and shown that temperature could have significant impact on the cellular metabolism11.
The rationale for using Seahorse XF approach is based on the fact that currently there is a gap between the clinical duration of the hyperthermia (typically limited only 1–2 h exposure12) and the in vitro cell line studies where hyperthermia periods are much longer or variable. The cancer literature reports use of different protocols involving exposure of cell lines to hyperthermia from 1 hour13 up to 3 days3, and some cases up for 2 weeks14 (in most cases 12–36 h) which makes difficult to draw direct parallels between possible molecular mechanisms underlying the in vitro results and the outcomes of the human hyperthermia clinical studies. The Seahorse extracellular flux analyzer has been reported to produce valuable data measuring cancer metabolism under different experimental conditions including, but not limited to the following: testing effects on novel therapies15–17, changes in cancer cell substrate preferences18–20, effects of gene overexpression or knockdown in cancer cells21–23, effects of hypoxia20, 24 and cell environmental factors25–27.
This is the probably the first report documenting that changes in the temperature of CRC cellular environment have a significant impact on cancer glycolysis even if oxygen levels are unchanged. Notably, while hyperthermia significantly affects all aspects of glycolysis (Fig. 5D) it seems to have a little effect on oxidative phosphorylation (Fig. 4B). These results suggest that hypo- or hyperthermia treatments may potentially be used to modify the glycolytic metabolism of cancer cells while components of mitochondrial function such as basal level respiration and ATP production remain unaffected by temperature.
The results of the mitochondrial and glycolysis stress tests under hyperthermia conditions show an increase of both SRC profiles (Fig. 4C) and glycolytic reserve (Fig. 5D) in SW480 CRC cells. In general, SRC is an indicator of metabolic plasticity or the ability of a cell to meet an increased energy demand28. A recent study in cardiac myocytes demonstrated that the main source of the spare/reserve capacity is the mitochondrial complex II, which, by activation of metabolic sensors including pyruvate dehydrogenase and AMP-dependent kinase, increases the respiratory capacity via a Sirtuin 3 (Sirt3)-dependent mechanism29. A similar pathway for modification of SRC may also exist in cancer cells, but it is yet unclear how temperature alters the metabolic enzymes toward an enhanced SRC.
One potential mechanism utilized by cancer cells and observed in this study may be the change in their dose-response profiles with an increased production of exosomes. A recent study showed that when cancer cells are treated with anticancer drugs such as Doxorubicin (Dox), the cells use exosomes to remove Dox from cytosol and when heat stress is applied the process of drug removal by exosomes intensifies30. However, if this exosome theory is true for FCCP it is logical to suspect that the other drugs (i.e. Oligomycin, Rotenone and Antimycin) used in a standard Seahorse mitochondrial stress test also might be subjected to the action of exosomes.
Another possible mechanism for changes observed in the differential sensitivity to FCCP between SW480 and Pt. 93 cells could be based on temperature effects on membrane potential. Several studies in the past have shown that temperature could impact membrane barrier function in in isolated heart and liver mitochondria31, and in intact breast cancer cells32 which also might explain the proton leak results. Furthermore, as explanation for the proton leak results could be find in potental increased expression of UCP2, which is often found characteristic feature of various tumors33, 34.
The experimental results (Fig. 4D) suggest there is no difference in the outcome OCR signal if the same doses (1.0 μM final in the wells) are applied at different temperatures. This result could be due to cells being less sensitive to the doses of Oligomycin, Rotenone and Antimycin compared to the FCCP. In fact, it is highly recommended that an FCCP dose-response is performed for every Seahorse experiment to optimize uncoupling results. The suggested concentrations for all other drugs (used for mitochondria and glycolysis stress test) rarely need any titration or dose optimization under most experimental conditions and cell lines. In most cases when researchers report experimental results for mitochondrial maximum respiration and SRC the results are represented as an outcome of a single FCCP dose study. Single dose FCCP studies may actually represent a narrow snapshot compared to the larger range of FCCP doses. For example, Fig. 4D shows that the maximum FCCP response is at a concentration of 0.3μM. In addition, Fig. 4D shows, on ascending and descending sides, a higher %SRC value for 37°C initially and a higher %SRC for 42°C with an increasing dose of FCCP. When the differences in FCCP dose responses were evaluated as an area under the curve calculation, the range of doses for the 42°C condition clearly indicated better metabolic plasticity, or the ability to meet an increased energy demand.
Another theory that may explain the outcomes of hyperthermia in cancer therapy is chromosomal fragmentation during hyperthermia treatment. Such fragmentations have been reported to cause mitotic cell death, distinct from apoptosis and other mitotic arrests or catastrophes; however, this process seems to occur most in cell cultures grown at high temperatures for an extended period of time (42°C for days or weeks)14. An additional hypothesis more relevant to clinical protocols is that a short term hyperthermia treatment is effective as an anti-cancer treatment due to a temperature-dependent activation of the immune system involving mechanisms that include increasing vascular perfusion, lymphocyte trafficking, inflammatory cytokine expression, tumor cell metabolism, and innate and adaptive immune functions35. Additionally, exosomes are known as structures that also mediate cancer cell resistance to drugs36; however, little is known about the effects of hyperthermia on cancer exosomes.
Increases (or decreases/exhaustion) of the spare respiratory and glycolytic capacities are known to play an important role in a variety of pathologies including heart diseases 37, neurodegenerative disorders38, 39, immunity40, 41 and muscle function42, 43. The results of this study show that thermotherapy can be used to modify the metabolic capacity of cancer cells and this finding opens several new avenues for future research in cellular bioenergetics based on temperature interventions. The main limitation of this study is that it provides only a functional description of changes in cancer bioenergetics without investigating the mechanistic details for the molecular components or cell signaling pathways involved in the bioenergetic changes observed at high and low temperatures. The scope of this study was to provide functional information about cell bioenergetics under thermal stress and lay the groundwork for future studies in the mechanisms underlying these observations.
The glycolysis tests determined that hyperthermia causes a significant increase in glycolytic reserve (Fig. 5D). There are two accepted directions in the search of novel agents and oncologic therapeutic regimens: 1) to modify the cancer cells’ characteristics and phenotype to shift toward a less glycolytic profile and move cell metabolism toward a normal non-cancer cell bioenergetic profile and 2) to push the cells toward greater glycolysis, and find targets and drugs that will inhibit the glycolysis, which on a systemic level will have less impact for normal cells. However, the second modification will have more injurious effect for cells that are highly dependent on glycolysis. It is known that in normal cells activation of the oncogenic signaling pathways such as PI3K/Akt/mTOR, c-Myc, and Ras leads to an increased glucose uptake and a high glycolytic activity similar to the Warburg effect in cancer cells44. It has been reported that these characteristics of cancer cells can be successfully exploited to bypass the problem of systemic toxicity of antiglycolytic agents45.
5. CONCLUSIONS
This study introduces new concepts that extend clinical and translational interests in the search of better multimodal oncologic therapies. Most importantly, hyperthermia results show increases in all aspects of glycolytic cellular function and less significant changes in OXPHOS. Short-term hypothermia increases both glycolic capacity and mitochondrial spare respiratory capacity in the tested cancer cells, which theoretically should have impact also on cellular resistance to chemotherapy. Clinical studies indicate that hyperthermia could successfully enhance chemotherapy are likely due to immune activation effects of hyperthermia. The present study also provides evidence that temperature changes can modulate colorectal cancer cells metabolism, thus potentially affecting treatment outcome. Moreover, we demonstrate that a real time, in a microplate, label-free detection technology can be effectively used to study complex aspects of oncologic bioenergetics in the future studies.
Acknowledgments
The research was supported by NCI-T32CA165990 (to JWH) and the Redox Metabolism Shared Resource Facility of the University of Kentucky Markey Cancer Center (P30CA177558). We also thank Heather N. Russell-Simmons and Cathy Anthony of the Markey Cancer Center Research Communications Office for editorial assistance.
ABBREVIATIONS
- ATP
Adenosine triphosphate
- AUC
Area under the curve
- CRC
Colorectal cancer
- DOX
Doxorubicin
- ECAR
Extracellular acidification rate
- FCCP
Carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone
- OCR
Oxygen consumption rate
- OXPHOS
Oxidative phosphorylation
- PDX
Patient-derived xenograft
- SCID
Severe combined immunodeficiency
- SRC
Spare respiratory capacity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van der Zee J. Heating the patient: a promising approach? Annals of Oncology. 2002;13:1173–84. doi: 10.1093/annonc/mdf280. [DOI] [PubMed] [Google Scholar]
- 2.Mallory M, Gogineni E, Jones GC, Greer L, Simone CB., Ii Therapeutic hyperthermia: The old, the new, and the upcoming. Critical Reviews in Oncology/Hematology. 2016;97:56–64. doi: 10.1016/j.critrevonc.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Zhu S, Wang J, Xie B, Luo Z, Lin X, Liao DJ. Culture at a Higher Temperature Mildly Inhibits Cancer Cell Growth but Enhances Chemotherapeutic Effects by Inhibiting Cell-Cell Collaboration. PLoS ONE. 2015;10:e0137042. doi: 10.1371/journal.pone.0137042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linthorst M, van Geel AN, Baaijens M, Ameziane A, Ghidey W, van Rhoon GC, van der Zee J. Re-irradiation and hyperthermia after surgery for recurrent breast cancer. Radiotherapy and Oncology. 2013;109:188–93. doi: 10.1016/j.radonc.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 5.de Bruijne M, Van der zee J, Ameziane A, Van Rhoon GC. Quality control of superficial hyperthermia by treatment evaluation. International Journal of Hyperthermia. 2011;27:199–213. doi: 10.3109/02656736.2010.525226. [DOI] [PubMed] [Google Scholar]
- 6.Van Der Zee J, De Bruijne M, Mens JWM, Ameziane A, Broekmeyer-Reurink MP, Drizdal T, Linthorst M, Van Rhoon GC. Reirradiation combined with hyperthermia in breast cancer recurrences: Overview of experience in Erasmus MC. International Journal of Hyperthermia. 2010;26:638–48. doi: 10.3109/02656736.2010.495104. [DOI] [PubMed] [Google Scholar]
- 7.Wu M, Neilson A, Swift AL, Moran R, Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J, Chomicz S, Ferrick DA. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. American Journal of Physiology - Cell Physiology. 2007;292:C125–C36. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]
- 8.Curley SA, Palalon F, Sanders KE, Koshkina NV. The Effects of Non-Invasive Radiofrequency Treatment and Hyperthermia on Malignant and Nonmalignant Cells. International Journal of Environmental Research and Public Health. 2014;11:9142–53. doi: 10.3390/ijerph110909142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barlogie B, Corry PM, Drewinko B. In vitro thermochemotherapy of human colon cancer cells with cis-dichlorodiammineplatinum(II) and mitomycin C. Cancer Res. 1980;40:1165–8. [PubMed] [Google Scholar]
- 10.Postmus J, Canelas AB, Bouwman J, Bakker BM, van Gulik W, de Mattos MJT, Brul S, Smits GJ. Quantitative Analysis of the High Temperature-induced Glycolytic Flux Increase in Saccharomyces cerevisiae Reveals Dominant Metabolic Regulation. Journal of Biological Chemistry. 2008;283:23524–32. doi: 10.1074/jbc.M802908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarke A, Fraser KPP. Why does metabolism scale with temperature? Functional Ecology. 2004;18:243–51. [Google Scholar]
- 12.Dewhirst MW, Vujaskovic Z, Jones E, Thrall D. Re-setting the biologic rationale for thermal therapy. International Journal of Hyperthermia. 2005;21:779–90. doi: 10.1080/02656730500271668. [DOI] [PubMed] [Google Scholar]
- 13.Pelz JW, Vetterlein M, Grimmig T, Kerscher A, Moll E, Lazariotou M, Matthes N, Faber M, Germer C-T, Waaga-Gasser A, Gasser M. Hyperthermic Intraperitoneal Chemotherapy in Patients with Peritoneal Carcinomatosis: Role of Heat Shock Proteins and Dissecting Effects of Hyperthermia. Ann Surg Oncol. 2013;20:1105–13. doi: 10.1245/s10434-012-2784-6. [DOI] [PubMed] [Google Scholar]
- 14.Stevens JB, Abdallah BY, Liu G, Ye CJ, Horne SD, Wang G, Savasan S, Shekhar M, Krawetz SA, Huttemann M, Tainsky MA, Wu GS, et al. Diverse system stresses: common mechanisms of chromosome fragmentation. Cell Death and Dis. 2011;2:e178. doi: 10.1038/cddis.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pardee TS, Lee K, Luddy J, Maturo C, Rodriguez R, Isom S, Miller LD, Stadelman KM, Levitan D, Hurd D, Ellis LR, Harrelson R, et al. A Phase I Study of the First-in-Class Antimitochondrial Metabolism Agent, CPI-613, in Patients with Advanced Hematologic Malignancies. Clinical Cancer Research. 2014;20:5255–64. doi: 10.1158/1078-0432.CCR-14-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S-Y, Wei Y-H, Shieh D-B, Lin L-L, Cheng S-P, Wang P-W, Chuang J-H. 2-Deoxy-<sc>d</sc>-Glucose Can Complement Doxorubicin and Sorafenib to Suppress the Growth of Papillary Thyroid Carcinoma Cells. PLoS ONE. 2015;10:e0130959. doi: 10.1371/journal.pone.0130959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Figarola JL, Singhal J, Tompkins JD, Rogers GW, Warden C, Horne D, Riggs AD, Awasthi S, Singhal SS. SR4 Uncouples Mitochondrial Oxidative Phosphorylation, Modulates AMPK-mTOR Signaling, and Inhibits Proliferation of HepG2 Hepatocarcinoma Cells. Journal of Biological Chemistry. 2015 doi: 10.1074/jbc.M115.686352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy KM, Scarbrough PM, Ribeiro A, Richardson R, Yuan H, Sonveaux P, Landon CD, Chi J-T, Pizzo S, Schroeder T, Dewhirst MW. Catabolism of Exogenous Lactate Reveals It as a Legitimate Metabolic Substrate in Breast Cancer. PLoS ONE. 2013;8:e75154. doi: 10.1371/journal.pone.0075154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fletcher M, Ramirez ME, Sierra RA, Raber P, Thevenot P, Al-Khami AA, Sanchez-Pino D, Hernandez C, Wyczechowska DD, Ochoa AC, Rodriguez PC. l-Arginine Depletion Blunts Antitumor T-cell Responses by Inducing Myeloid-Derived Suppressor Cells. Cancer Research. 2015;75:275–83. doi: 10.1158/0008-5472.CAN-14-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takakusagi Y, Matsumoto S, Saito K, Matsuo M, Kishimoto S, Wojtkowiak JW, DeGraff W, Kesarwala AH, Choudhuri R, Devasahayam N, Subramanian S, Munasinghe JP, et al. Pyruvate Induces Transient Tumor Hypoxia by Enhancing Mitochondrial Oxygen Consumption and Potentiates the Anti-Tumor Effect of a Hypoxia-Activated Prodrug TH-302. PLoS ONE. 2014;9:e107995. doi: 10.1371/journal.pone.0107995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaytseva YY, Harris JW, Mitov MI, Kim JT, Butterfield DA, Lee EY, Weiss HL, Gao T, Evers BM. Increased expression of fatty acid synthase provides a survival advantage to colorectal cancer cells via upregulation of cellular respiration. 2015 doi: 10.18632/oncotarget.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brahimi-Horn MC, Giuliano S, Saland E, Lacas-Gervais S, Sheiko T, Pelletier J, Bourget I, Bost F, Féral C, Boulter E, Tauc M, Ivan M, et al. Knockout of Vdac1 activates hypoxia-inducible factor through reactive oxygen species generation and induces tumor growth by promoting metabolic reprogramming and inflammation. Cancer & Metabolism. 2015;3:8. doi: 10.1186/s40170-015-0133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Y, Miriyala S, Fang F, Bakthavatchalu V, Noel T, Schell DM, Wang C, St Clair WH, St Clair DK. Manganese superoxide dismutase deficiency triggers mitochondrial uncoupling and the Warburg effect. Oncogene. 2015;34:4229–37. doi: 10.1038/onc.2014.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reitman ZJ, Duncan CG, Poteet E, Winters A, Yan L-J, Gooden DM, Spasojevic I, Boros LG, Yang S-H, Yan H. Cancer-associated Isocitrate Dehydrogenase 1 (IDH1) R132H Mutation and d-2-Hydroxyglutarate Stimulate Glutamine Metabolism under Hypoxia. Journal of Biological Chemistry. 2014;289:23318–28. doi: 10.1074/jbc.M114.575183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Estrella V, Chen T, Lloyd M, Wojtkowiak J, Cornnell HH, Ibrahim-Hashim A, Bailey K, Balagurunathan Y, Rothberg JM, Sloane BF, Johnson J, Gatenby RA, et al. Acidity Generated by the Tumor Microenvironment Drives Local Invasion. Cancer Research. 2013;73:1524–35. doi: 10.1158/0008-5472.CAN-12-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu K, Boley KM, Moraes R, Barsky SH, Robertson FM. The Paradox of E-Cadherin: Role in response to hypoxia in the tumor microenvironment and regulation of energy metabolism. 2013;4 doi: 10.18632/oncotarget.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang C-H, Qiu J, O’Sullivan D, Buck Michael D, Noguchi T, Curtis Jonathan D, Chen Q, Gindin M, Gubin Matthew M, van der Windt Gerritje JW, Tonc E, Schreiber Robert D, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–41. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Divakaruni AS, Paradyse A, Ferrick DA, Murphy AN, Jastroch M. Chapter Sixteen - Analysis and Interpretation of Microplate-Based Oxygen Consumption and pH Data. In: Anne NM, David CC, editors. Methods in Enzymology. Vol. 547. Academic Press; 2014. pp. 309–54. [DOI] [PubMed] [Google Scholar]
- 29.Pfleger J, He M, Abdellatif M. Mitochondrial complex II is a source of the reserve respiratory capacity that is regulated by metabolic sensors and promotes cell survival. Cell Death Dis. 2015;6:e1835. doi: 10.1038/cddis.2015.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Chen Y, Zhang F, Zhao Q, Zhong H. Increased anti-tumour activity by exosomes derived from doxorubicin-treated tumour cells via heat stress. International Journal of Hyperthermia. 2015;31:498–506. doi: 10.3109/02656736.2015.1036384. [DOI] [PubMed] [Google Scholar]
- 31.Nauciene Z, Zukiene R, Degutyte-Fomins L, Mildaziene V. Mitochondrial membrane barrier function as a target of hyperthermia. Medicina (Kaunas, Lithuania) 2012;48:249–55. [PubMed] [Google Scholar]
- 32.Dressler C, Beuthan J, Mueller G, Zabarylo U, Minet O. Fluorescence imaging of heat-stress induced mitochondrial long-term depolarization in breast cancer cells. Journal of fluorescence. 2006;16:689–95. doi: 10.1007/s10895-006-0110-z. [DOI] [PubMed] [Google Scholar]
- 33.Pitt MA. Overexpression of uncoupling protein-2 in cancer: metabolic and heat changes, inhibition and effects on drug resistance. Inflammopharmacology. 2015;23:365–9. doi: 10.1007/s10787-015-0250-3. [DOI] [PubMed] [Google Scholar]
- 34.Pecqueur C, Alves-Guerra C, Ricquier D, Bouillaud F. UCP2, a metabolic sensor coupling glucose oxidation to mitochondrial metabolism? IUBMB life. 2009;61:762–7. doi: 10.1002/iub.188. [DOI] [PubMed] [Google Scholar]
- 35.Repasky EA, Evans SS, Dewhirst MW. Temperature Matters! And Why It Should Matter to Tumor Immunologists. Cancer Immunology Research. 2013;1:210–6. doi: 10.1158/2326-6066.CIR-13-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azmi AS, Bao B, Sarkar FH. Exosomes in Cancer Development, Metastasis and Drug Resistance: A Comprehensive Review. Cancer metastasis reviews. 2013;32 doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sansbury BE, Jones SP, Riggs DW, Darley-Usmar VM, Hill BG. Bioenergetic function in cardiovascular cells: The importance of the reserve capacity and its biological regulation. Chemico-Biological Interactions. 2011;191:288–95. doi: 10.1016/j.cbi.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yadava N, Nicholls DG. Spare Respiratory Capacity Rather Than Oxidative Stress Regulates Glutamate Excitotoxicity after Partial Respiratory Inhibition of Mitochondrial Complex I with Rotenone. The Journal of Neuroscience. 2007;27:7310–7. doi: 10.1523/JNEUROSCI.0212-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholls DG. Oxidative Stress and Energy Crises in Neuronal Dysfunction. Annals of the New York Academy of Sciences. 2008;1147:53–60. doi: 10.1196/annals.1427.002. [DOI] [PubMed] [Google Scholar]
- 40.van der Windt GJW, Everts B, Chang C-H, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial Respiratory Capacity Is A Critical Regulator Of CD8(+) T Cell Memory Development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Sullivan D, Pearce EL. Targeting T cell metabolism for therapy. Trends in Immunology. 36:71–80. doi: 10.1016/j.it.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hill BG, Higdon AN, Dranka BP, Darley-Usmar VM. REGULATION OF VASCULAR SMOOTH MUSCLE CELL BIOENERGETIC FUNCTION BY PROTEIN GLUTATHIOLATION. Biochimica et biophysica acta. 2010;1797:285–95. doi: 10.1016/j.bbabio.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tyrrell DJ, Bharadwaj MS, Van Horn CG, Kritchevsky SB, Nicklas BJ, Molina AJA. Respirometric Profiling of Muscle Mitochondria and Blood Cells Are Associated With Differences in Gait Speed Among Community-Dwelling Older Adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2015;70:1394–9. doi: 10.1093/gerona/glu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan Y, Dickman KG, Zong W-X. Akt and c-Myc Differentially Activate Cellular Metabolic Programs and Prime Cells to Bioenergetic Inhibition. Journal of Biological Chemistry. 2010;285:7324–33. doi: 10.1074/jbc.M109.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng G, Zielonka J, Dranka BP, McAllister D, Mackinnon AC, Joseph J, Kalyanaraman B. Mitochondria-Targeted Drugs Synergize with 2-Deoxyglucose to Trigger Breast Cancer Cell Death. Cancer Research. 2012;72:2634–44. doi: 10.1158/0008-5472.CAN-11-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]


