Abstract
Normal tissue radiation toxicities are evaluated subjectively and cannot predict the development of severe side-effects. Using a hand-held diffuse reflectance optical spectroscopy probe, we measured optical parameters in mouse skin 1-4 days after irradiation. Using a radiation toxicity model and a therapeutic mitigator described previously [BMC Cancer 14, 614 (2014)], we found that hemoglobin (Hb) levels increased sharply 24 h after irradiation only in the irradiated group without the mitigator. This group also had the largest peak wound areas after 14 days. We conclude that increased Hb one day after skin irradiation predicts the severity of the subsequent irradiation-induced wound.
OCIS codes: (170.4580) Optical diagnostics for medicine; (170.6510) Spectroscopy, tissue diagnostics
1. Introduction
External beam radiation therapy (RT) employs precisely targeted radical doses of ionizing radiation to destroy or control cancerous lesions. Despite modern improvements in radiation planning and delivery, normal tissue radiation toxicity remains a significant concern. A common side-effect for breast and head and neck patients undergoing radical treatment is radiation-induced skin reactions, which impact up to 95% of patients receiving treatment [1]. Skin damage can lead to fibrosis and wound healing issues, which interfere with patient comfort and compliance. Interventional therapies such as biologically-targeted pre-clinical agents [2–4], biologically-targeted clinically tested agents [5,6], clinically tested gels [7] and creams [8,9] have recently been developed to alleviate RT-induced skin toxicities; these may be economically impractical to administer to all patients [10]. Yet, clinically-assessed erythema does not manifest until well into the RT regimen and is largely irreversible following onset. Prediction of radiation skin reaction severity at an early point in treatment would allow for appropriate interventional therapy use while minimizing healthcare costs [11].
Diffuse optical spectroscopy (DOS) employs non-ionizing, visible light to extract functional parameters such as hemoglobin concentration, oxygen saturation and optical scattering that has shown promise as a quantitative imaging tool for characterizing skin irritation [12–14] and irradiation-induced skin erythema [15,16]. Previously, we investigated the potential of optical biomarkers to quantitatively detect radiation-induced skin reactions as the overt damage was manifesting [16]. We also showed that five days post irradiation exposure, concentrations of oxygenated hemoglobin could differentiate mice treated with and without a radiation skin toxicity mitigator (an endothelial-targeting agent) [2]. However, this stratification was measured over a time when the radiation skin damage was already perceptible. Further, in studies where optical biomarkers have been shown to demonstrate early (< day five) temporal and dose-based patterns following irradiation before overt toxicity [15,17], their relationship with the subsequent outcome (acute wound severity) and a toxicity mitigator have not been explored.
In this work, we hypothesize that early (< day five after irradiation) optical parameters can stratify the severity of subsequent acute radiation toxicity in the context of a pre-clinical radiation toxicity mitigator. We now provide evidence that optical biomarkers measured from DOS show statistically significant differences as early as one day following irradiation, which may be indicative of final wound severity two weeks after treatment. The results show the potential of DOS biomarkers for predicting future RT-induced normal tissue toxicity. This application may lead to improved personalized RT by enabling immediate administration of interventional therapies, or alteration of treatment regimens.
2. Methods
2.1 Animal irradiation
Animals were handled in accordance with Sunnybrook Research Institute review board approved protocols. Seven week old female athymic nude mice (Charles River Canada) were irradiated as described before [18]. Briefly, 35 Gy was delivered to 5 cm2 of the flank skin using a 160 kVp small animal irradiator (CP 160, Faxitron X-Ray Corp, Wheeling, IL, USA) previously commissioned by our group [19]. Animals were distributed evenly by weight into the following groups: saline (phosphate-buffered saline, PBS) and non-irradiation (n = 3), PBS and irradiation (n = 9), and Angiopoietin-1 mimic (Vasculotide, VT) and irradiation (n = 8). VT opposes microvascular perturbations in endothelial cells and was shown previously by our group to reduce overall radiation skin toxicity [2]. Injections of PBS or VT were performed by the intra-peritoneal route every two days.
2.2 Diffuse optical spectroscopy
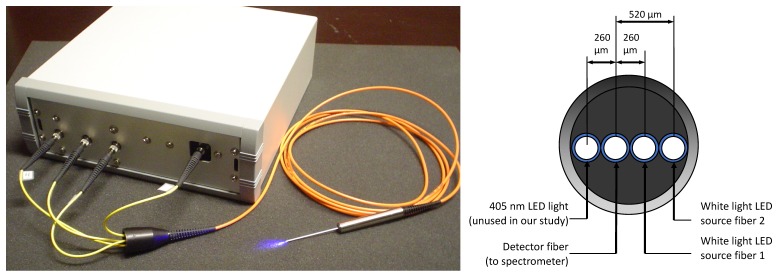
DOS measurements were performed using a previously reported reflectance system [18,20,21] optimized for skin depth quantification (~1-2 mm) and is described here for completeness (Fig. 1). The portable DOS system is composed of a fiber-optic probe connected to an acquisition box that houses two broadband light emitting diodes (LEDs) and an optical spectrometer. The fiber optic probe is a linear array of 200 μm core optical fibers spaced 260 μm apart and enclosed in an 18-gauge stainless steel needle. An in-house data acquisition program was used to acquire sequential reflectance spectra (within ~1-3 s) that include background readings. The probe was calibrated for absolute reflectance [20,21]. All raw measurements were background and noise floor subtracted and post-processed to remove variations in LED output. The processed reflectance measurements were fit using a diffusion theory model of light transport that was previously well validated for the described probe geometry and expected optical properties of biological tissues with our wavelength analysis range (450-650 nm) [20,21]. The fitting algorithm was spectrally constrained for the absorption spectrum using known chromophore spectra of oxy-, , and deoxy- hemoglobin, : where Hb is the total hemoglobin concentration and StO2 is the oxygen saturation. A power law dependence was used to approximate the reduced scattering coefficient spectrum where A is the value of μs’ at λo and b is a medium dependent power factor [22]. This approach was previously shown to return unique and accurate values of Hb concentration (g/L), StO2 (0–1 unitless) and power law scattering parameters A (cm−1) and b (unitless). Melanin was excluded since nude albino mice were employed. Water was negligible within the analyzed wavelength range. Inverse fitting was performed using Matlab’s lsqcurvefit function (Mathworks Inc., Natrick MA).
Fig. 1.
DOS imaging equipment (left) and diagram of the probe source-detector configuration (right).
2.3 Correlation of DOS with skin damage
On each day and for each mouse, five scans were performed over the irradiated area by gently placing the probe on the skin surface [18]. Mice were measured on day 0 (baseline, before irradiation), days 1-4, and then sacrificed using cervical dislocation on day 14. Each of the four variables measured (Hb, StO2, A and b) from all five scans were averaged for each individual mouse on each day, and was divided by the baseline value for normalization. Radiation skin toxicity scores, desquamated wound area and body weights were evaluated daily. Median radiation skin toxicity scores were assigned using a qualitative scoring system (outlined in [2], adapted from [3,23]): 0 = normal, 0.5 = slight reddening, 1 = severe reddening, 1.5 = moist desquamation of one very small area, 2 = moist desquamation of a small fraction (~10%) of the irradiated area, 3 = desquamation of most of the irradiated area with moist exudates. Wounds were photographed using a digital camera (Olympus TG-820); the wound surface areas were outlined using ImageJ (NIH, Bethesda, MD, USA). Each wound area was divided by the total irradiated area to determine the fraction of the wound size relative to the area exposed. Data normality was tested using the Shapiro-Wilk test. Welch’s t-test was used to assess differences in means instead of the Student’s t-test when variances between groups were unequal. Differences between medians were assessed with Mann-Whitney tests. To reduce the risk of false positives, the Holm-Bonferroni method was applied to post-hoc comparisons where p < 0.05. After any post-hoc tests, statistical significance was denoted by *. Means or medians were plotted with measures of variability (i.e. standard deviation, SD, or interquartile range, IQR).
3. Results
3.1 Optical biomarker changes days 0-4 post-irradiation
Experimental spectra and associated fits were similar to our previously reported DOS studies (data not shown, see references [16,18] for a complete discussion). Figure 2 shows the post-irradiation changes relative to baseline on days 1-4 in Hb, StO2 and b.
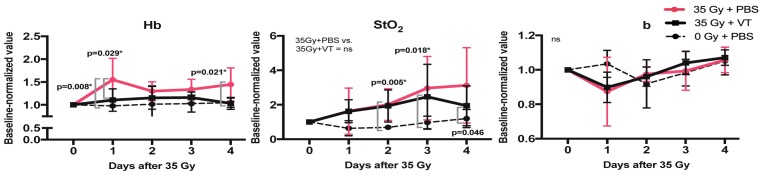
Fig. 2.
Mean Hb, StO2, and b ( ± SD) normalized to baseline over 4 days post irradiation. (Left) Values rise sharply in the irradiated group treated with PBS (compared to the non-irradiated group). Hb levels of the irradiated group treated with VT remain comparable to the non-irradiated group. (Middle) StO2 increases in both irradiated groups vs. the non-irradiated group. (Right) No statistical significance is reached in differences between groups for b.
As shown in Fig. 2, Hb concentration remains virtually constant for the non-irradiated group (0 Gy + PBS) and VT-treated, irradiated (35 Gy + VT) groups. In contrast, a significant increase in Hb was observed on day 1 for the PBS irradiated (35 Gy + PBS) group. Hb concentration subsequently decreases on day 2 and rises slightly again from days 2-4. The difference in Hb on day 1 is statistically significant compared to the non-irradiated (p < 0.01) and VT irradiated (p < 0.05) groups. While closer in trend to the non-irradiated group, the VT irradiated group also exhibited a small but statistically insignificant increase on day 1 that approached baseline values by day 4.
StO2 values for both irradiated groups (VT and PBS) showed a steady increase relative to baseline values. A slight (statistically insignificant) decrease for the VT group was observed between days 3 and 4. The non-irradiated group exhibited a small statistically insignificant decrease that returned to baseline values by day 3.
Finally, the power factor b exhibited a noticeable qualitative decrease on day 1 post irradiation that returned to baseline by day 4 for both VT and PBS irradiated groups. The non-irradiated group showed slight statistically insignificant fluctuations between days 0-4. While a clear difference is observed between the irradiated and non-irradiated groups for b on day 1, none of the groups showed statistically significant differences compared to the non-irradiated group. No significant trends were found for the other power law constant, A (data not shown).
3.2 Skin erythema area and radiation skin toxicity score on day 14
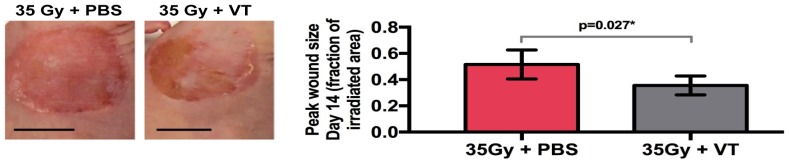
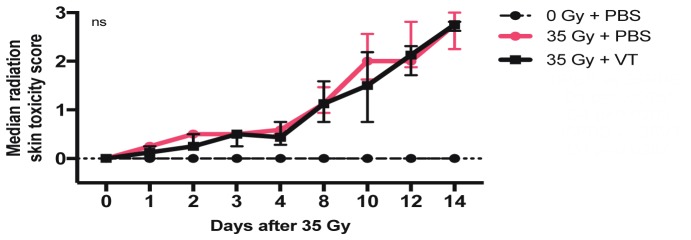
Figure 3 shows representative photographs of the resulting dermatitis for irradiated PBS and VT groups on day 14 (this day was previously found to correspond to the time when skin damage severity peaked [2]). A statistically significant difference in peak mean wound area (p = 0.027) was observed. The PBS group developed a larger overall wound area (0.52 ± 0.12, the fraction of the irradiated area) compared to the VT group (0.36 ± 0.07, the fraction of the irradiated area). Median radiation skin toxicity scores remained very similar in trend between the two groups. Both groups exhibited the same score of 2.7 on day 14 (Fig. 4).
Fig. 3.
Disparate day 14 peak damage radiation toxicity wounds for the PBS-treated and VT-treated, irradiated skin. (Left) Photographs of wounds, scale bars = 1 cm. (Right) Day 14 mean wound sizes ± SD (as a fraction of the total irradiated surface area) between the two irradiated groups. The VT group has smaller wounds, p = 0.027.
Fig. 4.
Median radiation skin toxicity scores ± IQR between the two irradiated groups. No differences reached statistical significance.
4. Discussion and conclusions
The severity of the normal tissue response to radiation varies from patient to patient [24]. Therefore, a rapid, quantitative, and accurate method of predicting radiation effects would be useful for guiding interventional therapies. In particular, early assessment of future damage prior to full manifestation could allow optimal patient triage and minimize the risk of late consequential effects.
In this work, we have investigated the utility of measuring optical biomarkers by DOS in irradiated mouse skin before any overt radiation skin toxicity manifests (days 1-4 post irradiation in our preclinical model), and associated these with peak radiation damage to skin (day 14 in our preclinical model). We performed our investigation using mice injected with either PBS (control) or a validated agent (VT) capable of mitigating radiation skin damage, a set-up which has previously resulted in clear differences in peak wound size [2]. The current work reveals that while optical parameters, such as StO2 and b, show similar trends between the two groups, Hb concentration measured on day 1 post irradiation exhibits a distinctive and statistically significant increase in the irradiated PBS group; this group went on to develop the largest final wound sizes two weeks post irradiation.
There is evidence in the literature of predictive functional DOS-detectable changes amidst cancer therapies that correlate with final outcome or toxicity. Robyler and colleagues (2011) reported that an oxyhemoglobin flare on day 1 correlated with chemotherapy response in patients [25]. However, it is unknown whether the same functional response would be seen with other treatment modalities (i.e. RT) and normal tissue applications. With regards to external beam radiation, Chin et al (2012) previously reported spikes on day 1 and 10 in both Hb concentration and StO2 (as measured by hyperspectral imaging) from beta particle irradiation of mouse skin [17], and Jang et al (2016) confirmed that early cellular changes begin to occur in the first six days post irradiation [26]. These findings agree overall with the trends in our current and previous studies [16]. While Chin et al (2015) correlated early trends in optical biomarker parameters with subsequent overall late microvascular damage, differential damage and patterns were due to different administered radiation doses [17]. To our knowledge, we are the first to provide evidence that Hb changes measured on day 1 associate with the severity of radiation induced wounds in connection with treatment intervention.
We note that the qualitative radiation skin toxicity scoring approach used here, which is similar to clinical scoring, did not provide the necessary sensitivity for differentiating predictive response on day 1. Qualitative approaches are well known to be limited by inter-observer variability, lack of sensitivity and lack of broad expertise for routine observation [27]. In contrast, Hb values measured using a turnkey DOS system provides reproducible, quantitative metrics that exhibit the sensitivity to distinguish between intermediate and severe wound groups as early as day 1.
While the results of this work are promising, some issues will need to be addressed to translate the DOS system to standard clinical practice. In this work, we used a large single radiation dose typical of preclinical mouse models of radiation skin damage [2–4,15–17,26]; it is equivalent to eliciting the pathophysiology of severe, accelerated skin reactions in humans [28]. However, conventional external beam RT employs smaller fractionated dose regimens that may result in smaller day-to-day biomarker responses. In the clinical setting, early Hb differences that may precede the visual damage might not occur until a large cumulative dose of fractionated radiation has accumulated. Yet, if the putative Hb changes at this point of accumulation still precede overt damage manifestation, clinicians may be able to intervene with biological mitigators or modify the radiotherapy treatment plan, thereby optimizing the therapeutic ratio for patients. We believe that our study establishes a proof-of-principle for the potential utility of DOS in early prediction of radiation skin toxicity. Future experiments will address the single fraction limitation by measuring DOS parameters during multiple fractions of radiation. Despite this difference, modern hypofractionated stereotactic body radiation treatments (SBRT) employ dose fractions as much as 10 times greater than conventional fractionation, and may mimic the high dose employed in this work.
In summary, we provide evidence that differential Hb concentrations measured 24 h post radiation exposure associate with mouse cohorts of two different final wound sizes. As such, DOS could be used in pre-clinical studies for quantitatively assessing and optimizing the efficacy of radiation damage mitigating drugs. Clinically, DOS measurement may be employed in the future for triaging these same interventional drugs for patients with enhanced sensitivity to radiation skin toxicity, thereby improving patient compliance and overall cosmesis. Finally, if similar biological pathways for tumor response are present, the DOS system may provide a new means of predicting responders from non-responders during RT.
Acknowledgments
We thank Drs. Dan Dumont and Paul Van Slyke for providing us with the VT compound.
Funding
Abbott CARO Uro-Oncologic Radiation Awards; Allan E. Tiffin Trust.
References and links
- 1.Ryan J. L., “Ionizing radiation: the good, the bad, and the ugly,” J. Invest. Dermatol. 132(3), 985–993 (2012). 10.1038/jid.2011.411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korpela E., Yohan D., Chin L. C., Kim A., Huang X., Sade S., Van Slyke P., Dumont D. J., Liu S. K., “Vasculotide, an Angiopoietin-1 mimetic, reduces acute skin ionizing radiation damage in a preclinical mouse model,” BMC Cancer 14(1), 614 (2014). 10.1186/1471-2407-14-614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holler V., Buard V., Gaugler M. H., Guipaud O., Baudelin C., Sache A., Perez M. R., Squiban C., Tamarat R., Milliat F., Benderitter M., “Pravastatin limits radiation-induced vascular dysfunction in the skin,” J. Invest. Dermatol. 129(5), 1280–1291 (2009). 10.1038/jid.2008.360 [DOI] [PubMed] [Google Scholar]
- 4.Maxhimer J. B., Soto-Pantoja D. R., Ridnour L. A., Shih H. B., Degraff W. G., Tsokos M., Wink D. A., Isenberg J. S., Roberts D. D., “Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling,” Sci. Transl. Med. 1(3), 3ra7 (2009). 10.1126/scitranslmed.3000139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan J. L., Heckler C. E., Ling M., Katz A., Williams J. P., Pentland A. P., Morrow G. R., “Curcumin for radiation dermatitis: a randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients,” Radiat. Res. 180(1), 34–43 (2013). 10.1667/RR3255.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonadou D., Pepelassi M., Synodinou M., Puglisi M., Throuvalas N., “Prophylactic use of amifostine to prevent radiochemotherapy-induced mucositis and xerostomia in head-and-neck cancer,” Int. J. Radiat. Oncol. Biol. Phys. 52(3), 739–747 (2002). 10.1016/S0360-3016(01)02683-9 [DOI] [PubMed] [Google Scholar]
- 7.Herst P. M., Bennett N. C., Sutherland A. E., Peszynski R. I., Paterson D. B., Jasperse M. L., “Prophylactic use of Mepitel Film prevents radiation-induced moist desquamation in an intra-patient randomised controlled clinical trial of 78 breast cancer patients,” Radiother. Oncol. 110(1), 137–143 (2014). 10.1016/j.radonc.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 8.Wells M., Macmillan M., Raab G., MacBride S., Bell N., MacKinnon K., MacDougall H., Samuel L., Munro A., “Does aqueous or sucralfate cream affect the severity of erythematous radiation skin reactions? A randomised controlled trial,” Radiother. Oncol. 73(2), 153–162 (2004). 10.1016/j.radonc.2004.07.032 [DOI] [PubMed] [Google Scholar]
- 9.Salvo N., Barnes E., van Draanen J., Stacey E., Mitera G., Breen D., Giotis A., Czarnota G., Pang J., De Angelis C., “Prophylaxis and management of acute radiation-induced skin reactions: a systematic review of the literature,” Curr. Oncol. 17(4), 94–112 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan R. J., Webster J., Chung B., Marquart L., Ahmed M., Garantziotis S., “Prevention and treatment of acute radiation-induced skin reactions: a systematic review and meta-analysis of randomized controlled trials,” BMC Cancer 14(1), 53 (2014). 10.1186/1471-2407-14-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porock D., Kristjanson L., Nikoletti S., Cameron F., Pedler P., “Predicting the severity of radiation skin reactions in women with breast cancer,” Oncol. Nurs. Forum 25(6), 1019–1029 (1998). [PubMed] [Google Scholar]
- 12.Stamatas G. N., Kollias N., “In vivo documentation of cutaneous inflammation using spectral imaging,” J. Biomed. Opt. 12(5), 051603 (2007). 10.1117/1.2798704 [DOI] [PubMed] [Google Scholar]
- 13.Kollias N., Gillies R., Muccini J. A., Uyeyama R. K., Phillips S. B., Drake L. A., “A single parameter, oxygenated hemoglobin, can be used to quantify experimental irritant-induced inflammation,” J. Invest. Dermatol. 104(3), 421–424 (1995). 10.1111/1523-1747.ep12666001 [DOI] [PubMed] [Google Scholar]
- 14.Smesny S., Riemann S., Riehemann S., Bellemann M. E., Sauer H., “Quantitative measurement of induced skin reddening using optical reflection spectroscopy--methodology and clinical application,” Biomed. Tech. (Berl.) 46(10), 280–286 (2001). 10.1515/bmte.2001.46.10.280 [DOI] [PubMed] [Google Scholar]
- 15.Chin M. S., Freniere B. B., Lo Y. C., Saleeby J. H., Baker S. P., Strom H. M., Ignotz R. A., Lalikos J. F., Fitzgerald T. J., “Hyperspectral imaging for early detection of oxygenation and perfusion changes in irradiated skin,” J. Biomed. Opt. 17(2), 026010 (2012). 10.1117/1.JBO.17.2.026010 [DOI] [PubMed] [Google Scholar]
- 16.Yohan D., Kim A., Korpela E., Liu S., Niu C., Wilson B. C., Chin L. C., “Quantitative monitoring of radiation induced skin toxicities in nude mice using optical biomarkers measured from diffuse optical reflectance spectroscopy,” Biomed. Opt. Express 5(5), 1309–1320 (2014). 10.1364/BOE.5.001309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chin M. S., Freniere B. B., Lancerotto L., Lujan-Hernandez J., Saleeby J. H., Lo Y. C., Orgill D. P., Lalikos J. F., Fitzgerald T. J., “Hyperspectral Imaging as an Early Biomarker for Radiation Exposure and Microcirculatory Damage,” Front. Oncol. 5, 232 (2015). 10.3389/fonc.2015.00232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chin L., Korpela E., Kim A., Yohan D., Niu C., Wilson B. C., Liu S. K., “Diffuse Optical Spectroscopy for the Quantitative Assessment of Acute Ionizing Radiation Induced Skin Toxicity Using a Mouse Model,” J. Vis. Exp. 111, 53573 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woo M., Nordal R., “Commissioning and evaluation of a new commercial small rodent x-ray irradiator,” Biomed Imaging Interv J 2(1), e10 (2006). 10.2349/biij.2.1.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim A., Khurana M., Moriyama Y., Wilson B. C., “Quantification of in vivo fluorescence decoupled from the effects of tissue optical properties using fiber-optic spectroscopy measurements,” J. Biomed. Opt. 15(6), 067006 (2010). 10.1117/1.3523616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim A., Roy M., Dadani F., Wilson B. C., “A fiberoptic reflectance probe with multiple source-collector separations to increase the dynamic range of derived tissue optical absorption and scattering coefficients,” Opt. Express 18(6), 5580–5594 (2010). 10.1364/OE.18.005580 [DOI] [PubMed] [Google Scholar]
- 22.Corlu A., Durduran T., Choe R., Schweiger M., Hillman E. M., Arridge S. R., Yodh A. G., “Uniqueness and wavelength optimization in continuous-wave multispectral diffuse optical tomography,” Opt. Lett. 28(23), 2339–2341 (2003). 10.1364/OL.28.002339 [DOI] [PubMed] [Google Scholar]
- 23.Douglas B. G., Fowler J. F., “The effect of multiple small doses of X rays on skin reactions in the mouse and a basic interpretation. 1976,” Radiat. Res. 178(2), AV125–AV138 (2012). 10.1667/RRAV10.1 [DOI] [PubMed] [Google Scholar]
- 24.Barnett G. C., West C. M., Dunning A. M., Elliott R. M., Coles C. E., Pharoah P. D., Burnet N. G., “Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype,” Nat. Rev. Cancer 9(2), 134–142 (2009). 10.1038/nrc2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roblyer D., Ueda S., Cerussi A., Tanamai W., Durkin A., Mehta R., Hsiang D., Butler J. A., McLaren C., Chen W. P., Tromberg B., “Optical imaging of breast cancer oxyhemoglobin flare correlates with neoadjuvant chemotherapy response one day after starting treatment,” Proc. Natl. Acad. Sci. U.S.A. 108(35), 14626–14631 (2011). 10.1073/pnas.1013103108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jang W. H., Shim S., Wang T., Yoon Y., Jang W. S., Myung J. K., Park S., Kim K. H., “In vivo characterization of early-stage radiation skin injury in a mouse model by two-photon microscopy,” Sci. Rep. 6, 19216 (2016). 10.1038/srep19216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noble-Adams R., “Radiation-induced skin reactions. 3: Evaluating the RISRAS,” Br. J. Nurs. 8(19), 1305–1312 (1999). 10.12968/bjon.1999.8.19.1305 [DOI] [PubMed] [Google Scholar]
- 28.Denham J. W., Hamilton C. S., Simpson S. A., Ostwald P. M., O’Brien M., Kron T., Joseph D. J., Dear K. B., “Factors influencing the degree of erythematous skin reactions in humans,” Radiother. Oncol. 36(2), 107–120 (1995). 10.1016/0167-8140(95)01599-C [DOI] [PubMed] [Google Scholar]