Abstract
Lakes in the Alps represent a considerable fraction of nutrient-poor lakes in Central Europe, with unique biodiversity and ecosystem properties. Although some individual lakes are well-studied, less knowledge is available on large-scale patterns essential to generalise the understanding of their functioning. Here, we aimed to describe crustacean zooplankton communities (Cladocera, Copepoda) and identify their environmental drivers in the pelagic zone of 54 oligotrophic lakes in the montane region of the Alps (400–1200 m) in Austria, Germany, and Switzerland, covering a spatial scale of 650 km. Moreover, we aimed to provide data on the distribution and ecological requirements of the North American invader Bytotrephes longimanus in its Central European native range. Communities were mainly dominated by widespread species typical of lowland habitats, and only a few true specialists of oligotrophic alpine lakes were present. The most frequent taxa were the Daphnia longispina complex and Eudiaptomus gracilis, with 48 and 45 occurrences, respectively. Species richness decreased with altitude and increased with lake area. The main structuring factors of community composition were chlorophyll a concentration and depth, which drove an apparent separation of mesotrophic and oligotrophic communities. Bytotrephes had 13 occurrences, showing a preference for deep oligotrophic lakes. Its presence was not coupled with lower crustacean species richness as it was repeatedly observed in North America. Additionally, it frequently co-occurred with the other large predatory cladoceran, Leptodora kindtii. B. longimanus might be considered a truly montane species in Central Europe, given its absence in lowland and alpine lakes.
Keywords: Alps, Bythotrephes, elevation, montane, oligotrophic, zooplankton
Introduction
In oligotrophic lakes, nutrients are the primary limiting factor for biomass production. Such nutrient-poor lakes can be numerous in the boreal and mountainous regions. In these lakes, plankton communities are relatively simple and species-poor, yet zooplankton–phytoplankton interactions are stronger than in eutrophic lakes (McQueen et al. 1986), and plankton in general plays a central role in ecosystem functioning (Straškrabová et al. 1999, Callieri et al. 2002, Sarnelle and Knapp 2005).
Compared to their boreal counterparts (Rühland et al. 2003, Lepistö et al. 2004, Hessen et al. 2006, Walseng et al. 2006, Ptacnik et al. 2008, 2010), little is known about regional patterns of plankton diversity in alpine lakes (Anderson 1971, Knapp et al. 2001, Reche et al. 2005, Tolotti et al. 2006), mostly due to the remoteness and low accessibility of lakes in mountainous areas (Straškrabová et al. 1999, Sommaruga 2001). Studies of alpine lakes more often emphasise localised patterns of diversity and environmental change, for example in paleolimnological studies (Wolfe et al. 2001, Nevalainen et al. 2014) or long-term monitoring programs of a few chosen lakes (e.g., Ruggiu et al. 1998, Straile 2000, Anneville et al. 2004, Pomati et al. 2015). A regional-scale perspective on the diversity of alpine lakes is greatly needed, especially for monitoring and predicting the large-scale effects of environmental changes, particularly climate change, to which diversity and functioning of these ecosystems are highly sensitive (Anneville et al. 2004, Holzapfel and Vinebrooke 2005, Blenckner et al. 2007, Manca et al. 2007, Parker et al. 2008). Aquatic habitats in mountainous areas are especially perceptive to changes in both precipitation and temperature regimes (Sommaruga-Wögrath et al. 1997, Sommaruga et al. 1999), which can induce significant changes in the biomass and composition of plankton communities of these lakes (e.g., Sommaruga et al. 1999, Holzapfel and Vinebrooke 2005, Shatwell et al. 2008).
Within the plankton communities, crustacean zooplankton is a key component of lake ecosystems, linking energy flow from bacterioplankton and phytoplankton to higher trophic levels, such as fish. Zooplankton communities are strongly influenced by environmental conditions within a lake (such as temperature, trophic state and fish stocks; e.g., Hessen et al. 1995), with the additional influence of dispersal effects and lake history (e.g., Shurin 2001, Forrest and Arnott 2007).
Predatory cladocerans occupy a special position among crustacean zooplankton because they are both driven by environmental factors similar to those that drive other zooplankters (e.g., temperature, acidity, fish predation; Brooks and Dodson 1965, Garton et al. 1990, Sarvala and Halsinaho 1990, Herzig 1995, Vogt et al. 2013) and yet can play a significant structuring role to communities of their zooplankton prey (Herzig and Auer 1990, Yan et al. 2002, Barbiero and Tuchman 2004). Among them, Bythotrephes gained considerable attention after invading lakes in North America, first spreading rapidly throughout the Laurentian Great Lakes in the late 1970s and 1980s (Sprules et al. 1990), and soon afterward to surrounding inland lakes (Yan et al. 1992), and are currently established in ~150 lakes in Canada and the United States (Kerfoot et al. 2016). This invasion has, in some instances, caused lasting changes in the zooplankton communities of the affected lakes (e.g., Yan et al. 1992, Barbiero and Tuchman 2004). The invasion has initiated numerous studies on the ecology of this species in North America, whereas we know far less about the species in its native range.
Bythotrephes is native to northern Eurasia and has a disjunctive distribution, occurring in northern Europe (British Isles, Denmark, Scandinavia, northern Germany, Poland, Baltic countries, Belorussia, and Russia) and in the Alps in Central Europe (Flössner 1972, Ketelaars and Gille 1994). Such an arctic–alpine distribution is typical for ice age relicts; the Alps today provide a cold interglacial refugium for these species in Central Europe (Stewart et al. 2010). Although recent morphological studies suggest that Bythotrephes consists of at least 5 closely related and relatively young species (Korovchinsky 2015, Litvinchuk and Litvinchuk 2016), molecular research showed that genetically they are all a single polymorphic species, B. longimanus (Therriault et al. 2002, Colautti et al. 2005). Most of these studies agree, nevertheless, that populations in the alpine region certainly belong to B. longimanus s. str.
Other than a review from Gaviria-Melo et al. (2005), mostly based on old data from Austria, little is known about the current distribution of B. longimanus within the Alps. Some data are available from lakes with long-term monitoring programs (e.g., Lake Constance, Mondsee, Laggo Maggiore, and some Swiss lakes; Dokulil et al. 1990, Enz et al. 2001, Palmer et al. 2001, Manca et al. 2007), but it is unclear how widespread the species actually is in this area or whether any changes have occurred in response to ongoing environmental changes. Although Bythotrephes in North America seems to be successful at spreading to new localities, within Europe, a new occurrence and permanent establishment outside its native range has to date been documented only in artificial lakes in the Netherlands and Belgium in the late 1980s (Ketelaars and Breemen 1993).
Furthermore, for the alpine region, no systematic studies have been conducted on the environmental preferences and co-occurrence of this species with other zooplankton across a range of lakes, unlike for other areas of Bythotrephes occurrence, such as Norway (Hessen et al. 2011), the Commonwealth of Independent States (Grigorovich et al. 1998), Canadian Shield Lakes (Boudreau and Yan 2003, Weisz and Yan 2010), or selected European and North American lakes (MacIsaac et al. 2000).
Our aims were to describe the crustacean zooplankton communities of lakes in the montane zone of the northern fringe of the Alps and identify the main environmental drivers of species richness and community composition. In addition, we aimed to update the view on the current distribution of Bythotrephes in relation to the local environment and investigate its possible effect on the zooplankton communities in these lakes with its native occurrence.
Methods
Sample collection and analysis
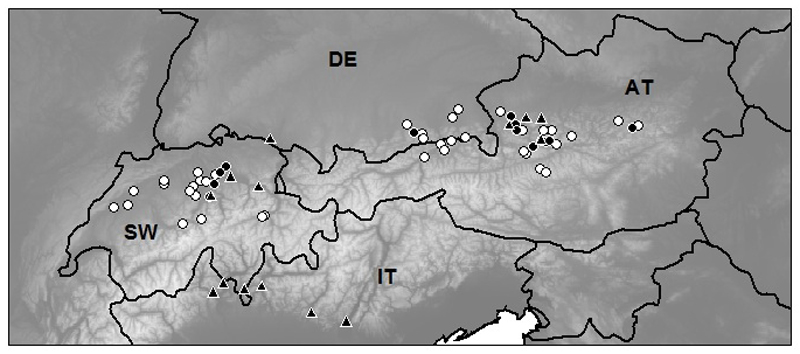
We sampled 54 lakes in Switzerland (n = 20; EAWAG sampling, summer and autumn 2011), Germany, and Austria (WasserCluster sampling, summer and autumn 2012; n = 8 and 26, respectively), with most of the lakes situated in the montane region of the Alps (400–1200 m; Table 1, Fig. 1).
Table 1.
Environmental parameters of the 54 lakes in the Alps.
| Abbrev. | N | Min | Max | Mean | SD | |
|---|---|---|---|---|---|---|
| Altitude (m) | Alt | 50 | 406 | 1891 | 695.4 | 300.2 |
| Chlorophyll a (μg L−1) | Chl-a | 50 | 0.8 | 37.5 | 5.9 | 7.3 |
| Mean temperature in the upper 3 m (°C) | T | 41 | 9.1 | 23.1 | 17.0 | 3.3 |
| Conductivity (μS cm−1) | Cond | 49 | 16.7 | 443.4 | 236.4 | 85.6 |
| Total phosphorus concentration (μg L−1) | TP | 31 | 1 | 16.2 | 5.3 | 3.4 |
| Total nitrogen concentration (μg L−1) | TN | 31 | 204 | 2741 | 678.8 | 406.1 |
| Secchi disk transparency (m) | ZS | 50 | 0.6 | 16.3 | 5.4 | 3.5 |
| Soluble reactive phosphorus concentration (μg L−1) | PO4 | 50 | 0.5 | 6 | 2.3 | 1.2 |
| Lake area (ha) | lake_area | 48 | 1 | 50 | 25.6 | 14.5 |
| Lake volume (1000 m3) | lake_vol | 43 | 0.01 | 667 070 | 58 296 | 149 969 |
| Max depth (m) | Zmax | 47 | 2 | 261 | 54.9 | 55.1 |
Figure 1.
Distribution of lakes in our 2011–2012 sampling campaign (circles, n = 54). ● = presence of Bythotrephes longimanus; ▲ = occurrence in other lakes, based on published data from the last 30 years (data sources in Appendix 7). Country identification: SW = Switzerland, DE = Germany, AT = Austria, IT = Italy. Background greyscale shows elevation, with white as high and darker grey as low elevations.
Sampling was carried out at the deepest spot of small, shallow lakes (based on a bathymetric map or Google satellite picture) and at a location with a depth of >15 m in large, deep lakes.
During sampling of German and Austrian lakes, a depth-integrated water sample was taken with a 1 m tube sampler covering the whole epilimnion (surface to metalimnion, based on the temperature profile). The mixed sample was used for measuring the concentrations of chlorophyll a (Chl-a), total phosphorus (TP), soluble reactive phosphorus (PO4), and total nitrogen (TN). For Chl-a, we used fluorometry with aceton extraction (Arar and Collins 1997), without correcting for phaeophytin. TP was measured by using persulfate digestion (Clesceri et al. 1999) followed by the ascorbic acid colorimetric method (Hansen and Koroleff 1999). For PO4, the same ascorbic acid method was used for water samples filtered on muffled and acid-washed GF/F filters. TN was digested according to Clesceri et al. (1999), and afterward the automated hydrazine reduction method was used with the continuous flow analyser (Clesceri et al. 1999). In the Swiss lakes, Chl-a was extracted with ethanol and measured with spectrophotometry, whereas the ascorbic acid method was used for PO4 (Hansen et al. 1999).
Zooplankton was sampled with a 100 µm mesh size (diameter: 40 cm) plankton net in the German and Austrian lakes. For most lakes, zooplankton were sampled by taking a vertical net haul of the upper 15 m water layer. In shallow lakes, the net was towed from a few meters above the sediment, and in deep lakes (epilimnion thickness >15 m), the net was hauled from 25 m depth. In cases of low zooplankton abundance, an additional net haul was performed. All samples were fixed with ethanol (70%). In the Swiss lakes, zooplankton were analysed from samples collected for molecular analyses (not part of the present study) with a 100 µm mesh sized net (diameter: 50 cm) through the entire water column (maximum 140 m in Lake Zurich); zooplankton were then concentrated and stored frozen until analysis, when they were transferred to 70% ethanol.
All crustacean zooplankters (Cladocera, Copepoda) were identified to species (apart from the Daphnia longispina complex, which was treated as one species, D. longispina) based on the keys of Flössner (1972, 2000), Einsle (1993), Gulyás and Forró (1999), Hołyńska and Dahms (2004), and Petrusek et al. (2005). In the Austrian and German samples, density was enumerated by subsampling (10% of the total concentrated sample), after which the whole sample was checked for rare species. In the Swiss zooplankton samples, only presence–absence was recorded by screening the entire sample because of the differences in methods of collection.
Statistical analyses
For the statistical analyses, altitude (Alt), conductivity (Cond), Secchi disk transparency (ZS), lake volume (lake_vol), and concentrations of Chl-a, TP, and TN were ln transformed, whereas maximum depth (Zmax) was square root transformed to minimise residuals. We focused on lakes situated in the calcareous northern fringe of the Alps. There were 2 non-alkaline lakes with low Cond (<50 μS cm−1; Riesachsee and Schwarzensee Sölk), 1 lake with high TP (>15 μg L−1, Spitzingsee), and 1 lake situated at high altitude (>1200 m; Melchsee; Appendix 1); these 4 lakes were excluded from all analyses involving environmental predictors.
Among trophic predictors (TP, TN, PO4, Chl-a), TP and TN data were not available from the Swiss lakes; therefore, for the analysis of the predictors of species number (carried out for the whole dataset, n = 50), we included only PO4 and Chl-a concentrations, which were not correlated (r = −0.06, p = 0.70). We also excluded ZS of the analyses because of its high correlation with Chl-a (r = −0.72, p < 0.001; Appendix 2). We also reduced the correlated lake size parameters (lake area, lake volume, Zmax) to 2 uncorrelated predictors, lake area and Zmax, a priori (Appendix 2 and 3) to avoid possible overfitting.
We used multiple linear regression models to test which environmental parameters affect species richness (n = 50). We built 3 separate models for total zooplankton, cladoceran, and copepod species numbers. We performed stepwise model selection by Akaike information criterion (AIC) with 1000 permutation steps, using ‘both’ as the mode of stepwise search.
To study the effect of environmental predictors on the zooplankton community (density data), we applied a canonical correspondence analysis (CCA). Here we analysed Austrian and German lakes only (without the outliers previously listed, 3 of which were Austrian or German, resulting in n = 31) because more environmental predictors were measured in this dataset than in the Swiss dataset (Appendix 4). Because of the large variation in zooplankton densities, data were square root transformed prior to analysis. In the null model, we included Alt, lake area, Zmax, TP, TN, ZS, Chl-a, and Cond as environmental predictors. Significant predictors were selected with the ‘ordistep’ function (direction: both; number of permutations: 1000) of the R package ‘vegan’ (Oksanen et al. 2012). We carried out the analysis both with and without singletons (11 of the 30 species), but significant predictors were the same in both cases. We present here the ordination without singletons; including them would make the centroid of the ordination plot less readable, and, moreover, the species-specific results are more reliable for species with multiple occurrences.
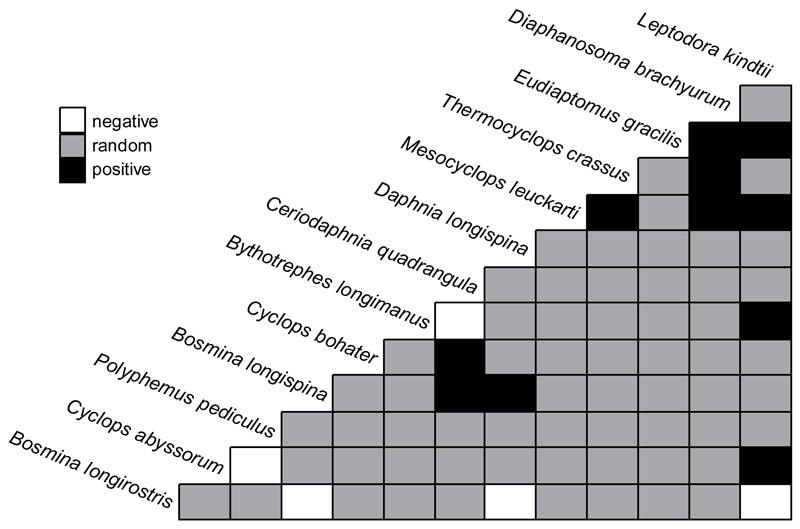
Co-occurrence of species with >5 occurrences was analysed with a probabilistic model by Veech (2013; ‘cooccur’ function of ‘cooccur’ package in R) using the presence–absence matrix of 54 lakes. The algorithm calculates the observed and expected frequencies of co-occurrence between each pair of species and classifies all possible species pairs as positively, negatively, or randomly associated.
We studied the effect of local environment on the abundance of Bythotrephes in Austrian and German lakes (n = 31) based on stepwise model selection by AIC of multiple linear regressions (1000 permutation steps, with ‘both’ as the mode of stepwise search). Apart from the environmental predictors previously used for the CCA, we also included here the total density of other zooplankters (square root transformed) to represent the potential prey abundance. We made a model prediction based on the 2 most important environmental predictors. We compared models with all possible combinations of linear and generalised additive model (GAM) fitting of these 2 significant predictors based on AIC and found linear fitting to be best for both predictors. Hence, we used this model in a graphical representation of the abundance distribution of the species along the 2 most important environmental gradients, based on a surface fitting. Here the surface is represented by a contour map, whereas the empirical abundance data can be illustrated with coloured symbols.
The test was repeated on the presence–absence data of Bythotrephes for the entire dataset (n = 50) to confirm the results on the entire spatial scale of our study. We used a general linear model (GLM) with a logistic function and a stepwise model selection by AIC (1000 permutation steps, with ‘both’ as the mode of stepwise search). Zmax, Alt, lake area, ZS, Chl-a, and Cond were included in the null model.
We ran a variation partitioning model to compare the role of local environmental factors and the possible effect of Bythotrephes predation on zooplankton communities. We first carried out a redundancy analysis (RDA) on the Austrian and German lakes (n = 31, including all species except Bythotrephes), in which significant predictors were selected with the ‘ordistep’ function (direction: both, number of permutations: 1000). We chose RDA over CCA because the subsequent variation partitioning is based on RDA. We carried out a variation partitioning analysis on the zooplankton community, with the significant local predictors grouped together and Bythotrephes density as the other explanatory variable.
Additionally, we tested whether the presence of Bythotrephes affected species richness in the lakes. We included all lakes except the 4 excluded lakes discussed earlier (n = 50) and ran a Welch’s 2 sample t-test (unequal variances were found in the a priori F-test), with the number of zooplankton species (excluding Bythotrephes) as the explained variable and the presence or absence of Bythotrephes as the grouping factor.
Finally, we reviewed the current occurrence of Bythotrephes in the Alps, including data reported in the last 30 years. All analyses were carried out with packages ‘MASS’ (Venables and Ripley 2002), ‘vegan’ (Oksanen et al. 2012), ‘coocur’ (Veech 2013), and ‘mgcv’ (Wood 2011) in R (R Development Core Team 2012).
Results
We found 34 microcrustacean species in the 54 alpine lakes, of which 17 were copepods (4 Calanoida and 13 Cyclopoida) and 17 belonged to cladocerans. D. longispina (found in 48 lakes) and Eudiaptomus gracilis (45 lakes) were the most frequent species. Seven other species occurred in >10 lakes (Cyclops abyssorum, Bosmina longispina, Mesocyclops leuckarti, Diaphanosoma brachyurum, Leptodora kindtii, Bythotrephes longimanus, Ceriodaphnia quadrangula), whereas the other 25 species were less frequent (Appendix 5). Bythotrephes was present in all 3 countries in 13 lakes (Fig. 1).
Lakes hosted 1–12 species (mean 6.2, SD 2.1). According to the multiple linear regression models, larger lakes at lower altitudes held more species. Altitude was a significant (negative) predictor of total zooplankton, copepod, and cladoceran richness. Additionally, lake area had a positive effect on both total zooplankton and cladoceran richness. For cladoceran richness, Chl-a also proved to have a significant negative effect (Table 2).
Table 2.
Significant predictors of zooplankton species richness based on stepwise model selection of multiple linear regressions.
| Model | Predictor | Estimate | Std. Error | t value | p value |
|---|---|---|---|---|---|
| Total zooplankton | Alt | −2.97 | 0.96 | −3.10 | 0.003 |
| lake_area | 0.03 | 0.02 | 1.84 | 0.07 | |
| Cladocera | Alt | −1.38 | 0.60 | −2.28 | 0.03 |
| lake_area | 0.03 | 0.01 | 2.69 | 0.01 | |
| Chl-a | −0.45 | 0.18 | −2.47 | 0.02 | |
| Copepoda | Alt | −2.06 | 0.62 | −3.32 | 0.002 |
The 2 large predatory cladocerans, L. kindtii and B. longimanus, showed a positive co-occurrence (Fig. 2). The congeneric Bosmina species (B. longirostris and B. longispina) clearly separated in the lakes, with B. longirostris negatively and B. longispina positively associated with D. longispina. The 2 mesotrophic cyclopoids, Thermocyclops crassus and M. leuckarti, also showed a positive co-occurrence.
Figure 2.
Co-occurrence matrix of species with >5 occurrences in the 54 lakes. Relationships presented as positive or negative were significant at p < 0.05, and random species-pair associations (p > 0.05) are in grey.
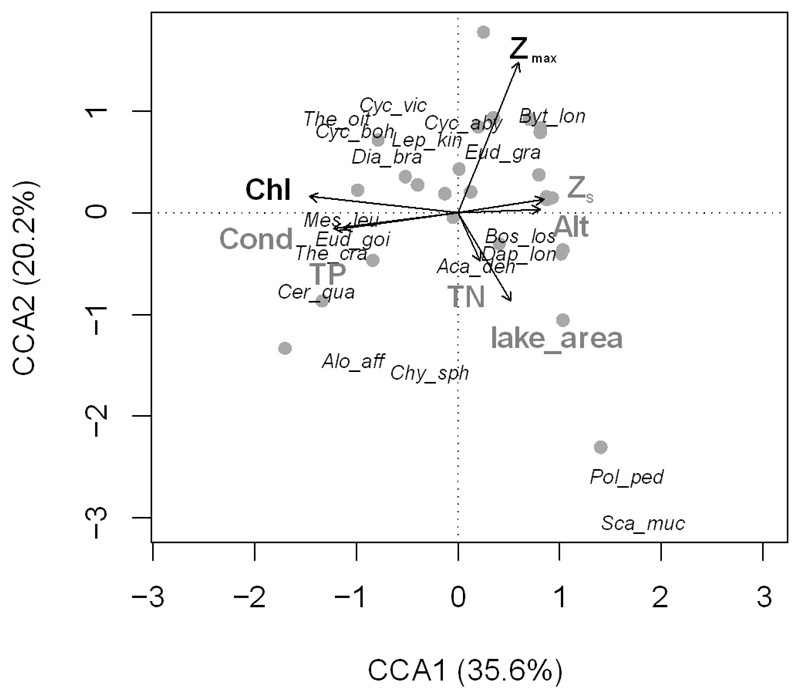
In the Austrian and German lakes dataset, we found total zooplankton densities between 1.4 and 45.4 ind L−1, with most (90%) of the data <20 ind L−1. Chl-a and Zmax were identified as significant environmental predictors of the zooplankton communities (Fig. 3). The first CCA axis was strongly related to the trophic state of lakes, whereas the second axis explained variation in size (depth and area).
Figure 3.
CCA ordination plot of Austrian and German lakes (significant environmental predictors in black; abbreviations of predictors in Table 1). Species abbreviations are based on the first 3 letters of genus and species names (see Appendix 5), apart from Eudiaptomus gracilis and E. graciloides, which are differentiated as Eud_gra and Eud_goi).
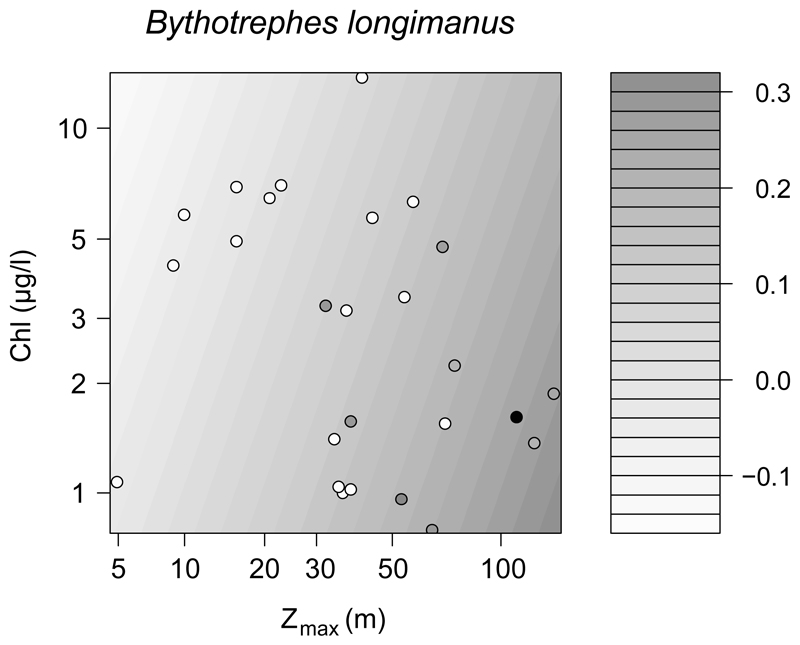
In our separate analysis carried out for Bythotrephes, Zmax and Chl-a were the strongest predictors of its abundance in the Austrian and German lakes (Table 3, Fig. 4). According to the model, deep lakes with low Chl-a hosted this species in the highest abundances. For presence–absence data in the entire dataset (n = 50; results not shown), the repeated test with logistic GLM confirmed the positive effect of Zmax because it was the only significant predictor for the presence of Bythotrephes (p = 0.005).
Table 3.
Predictors of the abundance of Bythotrephes longimanus in Austrian and German lakes based on stepwise model selection of multiple linear regressions (multiple R2: 0.43).
| Estimate | Std. Error | t value | p value | |
|---|---|---|---|---|
| (Intercept) | −0.15 | 0.06 | −2.30 | 0.03 |
| Zmax | 0.07 | 0.02 | 3.30 | 0.003 |
| Chl-a | −0.04 | 0.02 | −1.89 | 0.07 |
| TP | 0.04 | 0.03 | 1.41 | 0.17 |
Figure 4.
Empirical linear regression model (R2 = 0.37) predicting the abundance (ind L−1) of Bythotrephes longimanus in the Austrian and German lakes from lake depth (Zmax; p = 0.007) and chlorophyll a concentrations (Chl-a; p = 0.22), which were the strongest predictors of its occurrence (see Table 3). Points show the original empirical data, and surface is the fitted model, with darker grey as higher abundance.
In the RDA carried out for the communities without Bythotrephes, the same environmental predictors proved significant, as in the CCA model built for the whole communities (Chl-a and Zmax; ordination plot not presented). According to the results of the variation partitioning, Bythotrephes had no individual effect on the communities (0%, p = 0.6), and only the local environmental predictors had a significant individual effect (16%, p = 0.001), with a shared effect of 3%. Similarly, Bythotrephes had no significant effect on zooplankton species richness (Welch 2 sample t-test, t = −0.84, df = 41.42, p = 0.40).
In addition to the lakes visited during our survey, we found 14 instances in the literature where Bythotrephes has been reported in the Alps within the last 30 years (Fig. 1; Appendix 7), mostly in the vicinity of Austrian or Swiss lakes involved in our analyses, with an additional group of lakes in the Italian Alps.
Discussion
Zooplankton communities
The lakes varied widely in TP and environmental conditions. Trophic state, in general, had a strong effect on the zooplankton communities and was a primary structuring factor for the communities and a significant predictor of cladoceran species richness. Lake trophic state is overall an important shaping factor for zooplankton (Tallberg et al. 1999, Tolotti et al. 2006, Jeppesen et al. 2011, Jensen et al. 2012). In lakes at higher altitudes than lakes in our study (in the alpine and subalpine regions of the Alps, 1800–2800 m), Tolotti et al. (2006) found the primary role of catchment characteristics, lake depth, and trophic state in shaping zooplankton communities. Trophic state indicated by Chl-a and TP decreased with increasing elevation in our dataset, although these relationships were not significant, neither in the whole dataset (Appendix 2) nor in the subset of Austrian and German lakes (Appendix 6).
The drivers of species richness in our dataset were similar to those in boreal lakes. In our study, altitude was the most important driver, with fewer species at higher altitudes. In boreal lakes, species richness showed a similar negative relationship with altitude (Hessen et al. 2006), although its effect on species richness is weaker than it was in our alpine lake dataset, with a stronger primary role of trophic state (Hessen et al. 2006). Furthermore, lake area was also found to be more important for alpine than for boreal lake zooplankton richness. We did not have data on the fish communities of the lakes, which could have also contributed to community variation, as it does in boreal lakes (Hessen et al. 2006, 2011).
The most apparent separation in our community analysis was between oligotrophic and mesotrophic species. Lakes with higher Chl-a and TP were characterised by Thermocyclops oithonoides, T. crassus, M. leuckarti, Cyclops bohater, and C. vicinus among cyclopoids, species widespread in lowland mesotrophic and eutrophic lakes (except for C. bohater), expanding also to lower-montane regions (Maier 1996, Nilssen and Wærvågen 2000, Jersabek et al. 2001). This finding was partly also apparent in the co-occurrence matrix, with T. crassus and M. leuckarti occurring together. Less productive lakes at higher altitude usually hosted only C. abyssorum among cyclopoids, which is widespread in high altitude lakes of Europe (Jersabek et al. 2001, Tolotti et al. 2006, Kernan et al. 2009).
E. gracilis was the most frequent copepod in the lakes, regardless of their trophic state (occurring in 45 of the 54 lakes) and has a broad geographic distribution (Riccardi and Rossetti 2007) reported from oligotrophic (Straile and Geller 1998) to hypertrophic conditions (Ponyi and Zánkai 1982). Another calanoid copepod, Acanthodiaptomus denticornis, was present in shallower lakes than E. gracilis. In Central Europe, this species is typical in both permanent and temporary habitats in the montane and alpine regions (Einsle 1993), where it occurs most frequently in upper-montane and subalpine waters between 1500 and 2000 m (Jersabek et al. 2001). This preference is the most likely explanation for the low number of encounters (n = 3) in our dataset.
Lower altitude, more productive lakes hosted several cladoceran species, whereas at higher elevation, only B. longspina and D. longispina were typical. Among the 3 congeneric Bosmina species we found, B. coregoni was the rarest (n = 3), followed by B. longirostris (n = 8), and the most frequent, B. longispina (n = 33). The latter 2 species are frequently used in paleolimnology to track changes in trophic conditions because B. longispina is a typical species of oligotrophic waters and B. longirostris is a eutrophic species (Frey 1976, Gannon and Stemberger 1978, Boucherle and Züllig 1983), which can explain the rareness of the 2 species in these oligotrophic lakes.
Regional plankton biodiversity in the Alps is, in general, much less studied compared to northern European boreal lakes. Within the Alps, most of the available regional-scale knowledge on the diversity and its environmental constraints is from high-altitude alpine lakes above the treeline (Jersabek et al. 2001, Tolotti 2001, Tolotti et al. 2003, 2006), and the montane region receives even less attention. In our large-scale study covering a 650 km scale, we found that the main drivers of community composition and species richness in oligotrophic lakes in the montane zone were similar to those reported for lakes in the boreal zone (Hessen et al. 2006). The lakes were characterised mainly by widespread microcrustacean species known also from mesotrophic or eutrophic lowland habitats (e.g., E. gracilis, C. vicinus, Thermocyclops spp., D. longispina, L. kindtii), together with a few alpine species (e.g., A. denticornis) or specialists of oligotrophic lakes (e.g., C. abyssorum, B. longispina). Therefore, regarding zooplankton communities, the montane region of the Alps represents a transitional zone between lowland lakes and the alpine lakes at higher elevations. B. longimanus seems to represent the only exception and might be considered as a truly montane species; it is rarely found in lakes >1200 m and is not present in lowland lakes around the Alps.
Bythotrephes longimanus in the Alps
Interestingly, the 2 frequent predatory cladocerans in the lakes, B. longimanus and L. kindtii showed a positive co-occurrence pattern not found previously. In Scandinavian lakes, these 2 species show a negative co-occurrence (Hessen et al. 2011), an observation based on lakes covering a much wider climatic gradient compared to our study, which might invoke differences. In our dataset, we found a positive co-occurrence covered habitats solely within the montane zone. Moreover, in Austria, both species prefer elevations <1600 m (Gaviria-Melo et al. 2005), suggesting that the co-occurrence pattern would be similar even if we considered higher elevation zones in the Alps.
Although it might seem unexpected that oligotrophic lakes can sustain populations of 2 large-bodied predators simultaneously, there is evidence for the effective spatial niche separation within lakes where these species co-occur, especially in oligotrophic lakes (Enz et al. 2001). In its native range, Bythotrephes was found to inhabit greater depths than Leptodora when co-occurring in the same lake (Enz et al. 2001, Palmer et al. 2001). In one of the invaded Great Lakes, Lake Michigan, a similar pattern was found (Cavaletto et al. 2010). Because our samples were long vertical net hauls (sampling a water column of 15–140 m), we cannot assess their vertical separation in the investigated lakes.
Gaviria-Melo et al. (2005) report that the highest altitude ever recorded for Bythotrephes is 1555 m. In our dataset, Bythotrephes was present between 406–835 m, but few lakes in our dataset were situated >1000 m. According to the observation of Gaviria-Melo et al. (2005) and Therriault et al. (2002), the species prefers deep lakes in the alpine region, a finding also supported by our analysis. It has also been reported previously that Bythotrephes might be sensitive to anthropogenic eutrophication, which was repeatedly implied by case studies of individual lakes (reviewed in Therriault et al. 2002). Our data show that altitude effects on Bythotrephes were overall weak compared to the effect of productivity, even though our data spanned an altitudinal gradient of 1000 m.
In addition to trophic state, we found that lake depth has the highest importance for the species in its native habitats. A possible explanation for this preference (apart from the previously mentioned spatial separation from L. kindtii) can be avoiding fish predation. Although the spiny Bytotrephes is protected against predation by juvenile fish (Barnhisel 1991, Barnhisel and Kerfoot 2004), it is positively selected by larger fish (Fitzmaurice 1979, Coulas et al. 1998). Bythotrephes was shown to exhibit diel vertical migration to avoid predation (Straile and Hälbich 2000, Young and Yan 2008), and in Lago Maggiore, its presence is enhanced by the increasing duration and thickness of the predation refuge with long-term warming (Manca and DeMott 2009).
There was only one lake (Brienzersee) in which we did not find Bythotrephes, although its occurrence was reported in the last 30 years (Müller et al. 2007). Nine of the 13 lakes with Bythotrephes in 2011–2012 represented new data on its occurrence, and we confirmed its presence in 4 lakes in which it was reported previously (Hallstättersee, Mondsee, Toplitzsee in Austria: Gaviria-Melo et al. 2005; Zürichsee in Switzerland: Enz et al. 2001).
Bythotrephes has been shown to have strong multiple effects on the zooplankton communities in invaded North American lakes. Previous works found that the appearance of Bythotrephes decreased species richness (Yan et al. 2002, Barbiero and Tuchman 2004, Kelly et al. 2013) and biomass of zooplankton (Kerfoot et al. 2016), reduced the biomass of the native predator Leptodora (Lehman and Cáceres 1993), altered the daytime vertical distributions of native Daphnia (Lehman and Cáceres 1993), and even its kairomones have nonlethal adverse effects on zooplankton (Bourdeau et al. 2016). Contrary to these effects seen in invaded lakes, we found no pronounced effects of Bythotrephes on the zooplankton communities in our survey. In northern Europe, Walseng et al. (2015) report a positive effect of Bythotrephes on crustacean zooplankton; however, their study represents a special case because it is embedded in a spatial diversity gradient of phytoplankton and zooplankton (Hessen et al. 2006, Ptacnik et al. 2010). Likewise, Kelly et al. (2013) found that the community-shaping effect of Bythotrephes was much stronger in invaded Canadian lakes than in Norwegian lakes. This information collectively suggests a possibly general difference among communities co-evolved with Bythotrephes and communities of lakes only recently invaded by the species, which should receive more focus in future research.
Supplementary Material
Acknowledgements
Zsófia Horváth and Robert Ptacnik acknowledge support by the Austrian Research Foundation (FWF, P 26119) and the office of the Regional Government of Lower Austria (starting grant to Robert Ptacnik). Additional financial support was provided by the SNF grant 31003A-125006 to Blake Matthews. The authors thank the private owners of involved lakes for granting access by car or allowing sampling from a boat, Christina Klonner and Daniel Steiner for practical help during field work, and 2 anonymous reviewers for their helpful comments.
References
- Anderson RS. Crustacean plankton of 146 alpine and subalpine lakes and ponds in western Canada. J Fish Res Board Can. 1971;28:311–321. [Google Scholar]
- Anneville O, Souissi S, Gammeter S, Straile D. Seasonal and inter-annual scales of variability in phytoplankton assemblages: comparison of phytoplankton dynamics in three peri-alpine lakes over a period of 28 years. Freshwater Biol. 2004;49:98–115. [Google Scholar]
- Arar EJ, Collins GB. In-vitro determination of chlorophyll a and pheophytin a in marine and freshwater algae by fluorescence. Vol. 445. Cincinnati (OH): US Environmental Protection Agency, National Exposure Research Laboratory, Office of Research and Development; 1997. EPA Method. [Google Scholar]
- Barbiero RP, Tuchman ML. Changes in the crustacean communities of Lakes Michigan, Huron, and Erie following the invasion of the predatory cladoceran Bythotrephes longimanus. Can J Fish Aquat Sci. 2004;61:2111–2125. [Google Scholar]
- Barnhisel DR. Zooplankton spine induces aversion in small fish predators. Oecologia. 1991;88:444–450. doi: 10.1007/BF00317591. [DOI] [PubMed] [Google Scholar]
- Barnhisel DR, Kerfoot WC. Fitting into food webs: behavioral and functional response of young lake trout (Salvelinus namaycush) to an introduced prey, the spiny cladoceran (Bythotrephes cederstroemi) J Great Lakes Res. 2004;30:300–314. [Google Scholar]
- Blenckner T, Adrian R, Livingstone DM, Jennings E, Weyhenmeyer GA, George D, Jankowski T, Järvinen M, Aonghusa CN, Nõges T, et al. Large-scale climatic signatures in lakes across Europe: a meta-analysis. Glob Change Biol. 2007;13:1314–1326. [Google Scholar]
- Boucherle MM, Züllig H. Cladoceran remains as evidence of change in trophic state in three Swiss lakes. In: Meriläinen J, Huttunen P, Battarbee RW, editors. Paleolimnology. Springer; Netherlands: 1983. pp. 141–146. [Google Scholar]
- Boudreau SA, Yan ND. The differing crustacean zooplankton communities of Canadian Shield lakes with and without the nonindigenous zooplanktivore Bythotrephes longimanus. Can J Fish Aquat Sci. 2003;60:1307–1313. [Google Scholar]
- Bourdeau PE, Bach MT, Peacor SD. Predator presence dramatically reduces copepod abundance through condition-mediated non-consumptive effects. Freshwater Biol. 2016;61:1020–1031. [Google Scholar]
- Brooks JL, Dodson SI. Predation, body size, and composition of plankton. Science. 1965;150:28–35. doi: 10.1126/science.150.3692.28. [DOI] [PubMed] [Google Scholar]
- Callieri C, Bertoni R, Corno G. Dynamics of bacteria and mixotrophic flagellates in an Alpine lake in relation to Daphnia population development. J Limnol. 2002;61:177–182. [Google Scholar]
- Cavaletto JF, Vanderploeg HA, Pichlová-Ptáčníková R, Pothoven SA, Liebig JR, Fahnenstiel GL. Temporal and spatial separation allow coexistence of predatory cladocerans: Leptodora kindtii, Bythotrephes longimanus, and Cercopagis pengoi, in southeastern Lake Michigan. J Great Lakes Res. 2010;36:65–73. [Google Scholar]
- Clesceri LS, Greenberg AE, Eaton AD. Standard methods for examination of water and wastewater. 1999 Available from: http://ipkosar.ir/jspui/handle/961944/280820.
- Colautti RI, Manca M, Viljanen M, Ketelaars HAM, Buergi H, Macisaac HJ, Heath DD. Invasion genetics of the Eurasian spiny waterflea: evidence for bottlenecks and gene flow using microsatellites. Mol Ecol. 2005;14:1869–1879. doi: 10.1111/j.1365-294X.2005.02565.x. [DOI] [PubMed] [Google Scholar]
- Coulas RA, Macisaac HJ, Dunlop W. Selective predation on an introduced zooplankter (Bythotrephes cederstroemi) by lake herring (Coregonus artedii) in Harp Lake, Ontario. Freshwater Biol. 1998;40:343–355. [Google Scholar]
- Dokulil M, Herzig A, Jagsch A. Trophic relationships in the pelagic zone of Mondsee, Austria. In: Biró P, Talling JF, editors. Trophic Relationships of Inland Waters. Springer; 1990. pp. 199–212. Proceedings of an International Symposium in Tihany (Hungary), 1–4, Sep 1987. [Google Scholar]
- Einsle U. Crustacea: Copepoda: Calanoida und Cyclopoida. In: Schwoerbel J, Zwick P, editors. Süßwasserfauna von Mitteleuropa. 8/4. Stuttgart (Germany): Gustav Fischer Verlag; 1993. p. 1. [Google Scholar]
- Enz CA, Heller C, Müller R, Bürgi H-R. Investigations on fecundity of Bythotrephes longimanus in Lake Lucerne (Switzerland) and on niche segregation of Leptodora kindti and Bythotrephes longimanus in Swiss lakes. Hydrobiologia. 2001;464:143–151. [Google Scholar]
- Flössner D. Krebstiere, Crustacea. Kiemen-und Blattfüsser, Branchiopoda. Fischläuse, Branchiura. Jena (Germany): Veb Gustav Fischer Verlag; 1972. [Google Scholar]
- Flössner D. Die Haplopoda und Cladocera (ohne Bosminidae) Mitteleuropas. Leiden (Netherlands): Backhuys Publishers; 2000. [Google Scholar]
- Fitzmaurice P. Selective predation on Cladocera by brown trout Salmo trutta L. J Fish Biol. 1979;15:521–525. [Google Scholar]
- Forrest J, Arnott SE. Variability and predictability in a zooplankton community: the roles of disturbance and dispersal. Ecoscience. 2007;14:137–145. [Google Scholar]
- Frey DG. Interpretation of Quaternary paleoecology from Cladocera and midges, and prognosis regarding usability of other organisms. Can J Zool. 1976;54:2208–2226. [Google Scholar]
- Gannon JE, Stemberger RS. Zooplankton (especially crustaceans and rotifers) as indicators of water quality. T Am Microsc Soc. 1978;97:16–35. [Google Scholar]
- Garton DW, Berg DJ, Fletcher RJ. Thermal tolerances of the predatory cladocerans Bythotrephes cederstroemi and Leptodora kindti: relationship to seasonal abundance in western Lake Erie. Can J Fish Aquat Sci. 1990;47:731–738. [Google Scholar]
- Gaviria-Melo S, Forró L, Jersabek CD, Schabetsberger R. Checklist and distribution of cladocerans and leptodorans (Crustacea: Branchiopoda) from Austria. Ann Naturhist Mus Wien. 2005;106:145–216. [Google Scholar]
- Grigorovich IA, Pashkova OV, Gromova YF, Van Overdijk CD. Bythotrephes longimanus in the Commonwealth of Independent States: variability, distribution and ecology. Hydrobiologia. 1998;379:183–198. [Google Scholar]
- Gulyás P, Forró L. Az ágascsápú rákok (Cladocera) kishatározója [A guide for the identification of Cladocera occurring in Hungary. Vízi Termész- És Környvéd. 1999;9:237. Hungarian (English abstract) [Google Scholar]
- Hansen HP, Koroleff F. Determination of nutrients. In: Grasshoff K, Kremling K, Ehrhardt M, editors. Methods of seawater analysis. Wiley-VCH Ger; 1999. pp. 170–174. [Google Scholar]
- Herzig A. Leptodora kindti: efficient predator and preferred prey item in Neusiedler See, Austria. Hydrobiologia. 1995;307:273–282. [Google Scholar]
- Herzig A, Auer B. The feeding behaviour of Leptodora kindti and its impact on the zooplankton community of Neusiedler See (Austria) In: Dumont HJ, Tundisi JG, Roche K, editors. Intrazooplankton Predation. Springer; Netherlands: 1990. pp. 107–117. [Google Scholar]
- Hessen DO, Andersen T, Faafeng BA. Replacement of herbivore zooplankton species along gradients of ecosystem productivity and fish predation pressure. Can J Fish Aquat Sci. 1995;52:733–742. [Google Scholar]
- Hessen DO, Bakkestuen V, Walseng B. The ecological niches of Bythotrephes and Leptodora: lessons for predicting long-term effects of invasion. Biol Invasions. 2011;13:2561–2572. [Google Scholar]
- Hessen DO, Faafeng BA, Smith VH, Bakkestuen V, Walseng B, Walsdeng B. Extrinsic and intrinsic controls of zooplankton diversity in lakes. Ecology. 2006;87:433–443. doi: 10.1890/05-0352. [DOI] [PubMed] [Google Scholar]
- Hołyńska M, Dahms H-U. New diagnostic microcharacters of the cephalothoracic appendages in Cyclops OF Müller, 1776 (Crustacea, Copepoda, Cyclopoida) Zoosystema. 2004;26:175–198. [Google Scholar]
- Holzapfel AM, Vinebrooke RD. Environmental warming increases invasion potential of alpine lake communities by imported species. Glob Change Biol. 2005;11:2009–2015. [Google Scholar]
- Jensen TC, Dimante-Deimantovica I, Schartau AK, Walseng B. Cladocerans respond to differences in trophic state in deeper nutrient poor lakes from southern Norway. Hydrobiologia. 2012;715:101–112. [Google Scholar]
- Jeppesen E, Nõges P, Davidson TA, Haberman J, Nõges T, Blank K, Lauridsen TL, Søndergaard M, Sayer C, Laugaste R, et al. Zooplankton as indicators in lakes: a scientific-based plea for including zooplankton in the ecological quality assessment of lakes according to the European Water Framework Directive (WFD) Hydrobiologia. 2011;676:279–297. [Google Scholar]
- Jersabek CD, Brancelj A, Stoch F, Schabetsberger R. Distribution and ecology of copepods in mountainous regions of the eastern Alps. Hydrobiologia. 2001;453:309–324. [Google Scholar]
- Kelly NE, Yan ND, Walseng B, Hessen DO. Differential short-and long-term effects of an invertebrate predator on zooplankton communities in invaded and native lakes. Divers Distrib. 2013;19:396–410. [Google Scholar]
- Kerfoot WC, Hobmeier MM, Yousef F, Lafrancois BM, Maki RP, Hirsch JK. A plague of waterfleas (Bythotrephes): impacts on microcrustacean community structure, seasonal biomass, and secondary production in a large inland-lake complex. Biol Invasions. 2016;18:1121–1145. [Google Scholar]
- Kernan M, Ventura M, Bitušík P, Brancelj A, Clarke G, Velle G, Raddum GG, Stuchlík E, Catalan J. Regionalisation of remote European mountain lake ecosystems according to their biota: environmental versus geographical patterns. Freshwater Biol. 2009;54:2470–2493. [Google Scholar]
- Ketelaars HAM, Breemen LV. The invasion of the predatory cladoceran Bythotrephes longimanus Leydig and its influence on the plankton communities in the Biesbosch reservoirs. Internat Verein Theor Angew Limnol Verh. 1993;25:1168–1175. [Google Scholar]
- Ketelaars HAM, Gille L. Range extension of the predatory cladoceran Bythotrephes longimanus Leydig 1860 (Crustacea, Onychopoda) in Western Europe. Neth J Aquat Ecol. 1994;28:175–180. [Google Scholar]
- Knapp RA, Matthews KR, Sarnelle O. Resistance and resilience of alpine lake fauna to fish introductions. Ecol Monogr. 2001;71:401–421. [Google Scholar]
- Korovchinsky NM. Redescription of Bythotrephes longimanus Leydig, 1860 and B. cederströmii Schödler, 1877 (Crustacea: Cladocera: Onychopoda), with notes on the morphology and systematics of the genus Bythotrephes Leydig, 1860. Zootaxa. 2015;3955:1–44. doi: 10.11646/zootaxa.3955.1.1. [DOI] [PubMed] [Google Scholar]
- Lehman JT, Cáceres CE. Food-web responses to species invasion by a predatory invertebrate: Bythotrephes in Lake Michigan. Limnol Oceanogr. 1993;38:879–891. [Google Scholar]
- Lepistö L, Holopainen A-L, Vuoristo H. Type-specific and indicator taxa of phytoplankton as a quality criterion for assessing the ecological status of Finnish boreal lakes. Limnologica. 2004;34:236–248. [Google Scholar]
- Litvinchuk LF, Litvinchuk SN. Morphological diversity and widespread hybridization in the genus Bythotrephes Leydig, 1860 (Branchiopoda, Onychopoda, Cercopagidae) Arch Biol Sci Belgrade. 2016;68:67–79. [Google Scholar]
- MacIsaac HJ, Ketelaars HAM, Grigorovich IA, Ramcharan CW, Yan ND. Modeling Bythotrephes longimanus invasions in the Great Lakes basin based on its European distribution. Arch Hydrobiol. 2000;149:1–21. [Google Scholar]
- Maier G. Copepod communities in lakes of varying trophic degree. Arch Hydrobiol. 1996;136:455–465. [Google Scholar]
- Manca M, DeMott WR. Response of the invertebrate predator Bythotrephes to a climate-linked increase in the duration of a refuge from fish predation. Limnol Oceanogr. 2009;54:2506–2512. [Google Scholar]
- Manca M, Portogallo M, Brown ME. Shifts in phenology of Bythotrephes longimanus and its modern success in Lake Maggiore as a result of changes in climate and trophy. J Plankton Res. 2007;29:515–525. [Google Scholar]
- McQueen DJ, Post JR, Mills EL. Trophic relationships in freshwater pelagic ecosystems. Can J Fish Aquat Sci. 1986;43:1571–1581. [Google Scholar]
- Müller R, Breitenstein M, Bia MM, Rellstab C, Kirchhofer A. Bottom-up control of whitefish populations in ultra-oligotrophic Lake Brienz. Aquat Sci. 2007;69:271–288. [Google Scholar]
- Nevalainen L, Ketola M, Korosi JB, Manca M, Kurmayer R, Koinig KA, Psenner R, Luoto TP. Zooplankton (Cladocera) species turnover and long-term decline of Daphnia in two high mountain lakes in the Austrian Alps. Hydrobiologia. 2014;722:75–91. [Google Scholar]
- Nilssen JP, Wærvågen SB. Superficial ecosystem similarities vs. autecological stripping: the“ twin species” Mesocyclops leuckarti (Claus) and Thermocyclops oithonoides (Sars) - seasonal habitat utilisation and life history traits. J Limnol. 2000;59:79–102. [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevenes MHH, Wagner H. Package “vegan”: community ecology package. R package version. 2012 2.0-4. [Google Scholar]
- Palmer A, Stich H-B, Maier G. Distribution patterns and predation risk of the coexisting cladocerans Bythotrephes longimanus and Leptodora kindtii in a large lake–Lake Constance. Hydrobiologia. 2001;442:301–307. [Google Scholar]
- Parker BR, Vinebrooke RD, Schindler DW. Recent climate extremes alter alpine lake ecosystems. P Natl Acad Sci USA. 2008;105:12927–12931. doi: 10.1073/pnas.0806481105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrusek A, Bastiansen F, Schwenk K. European Daphnia species (EDS) - taxonomic and genetic keys. [Build 2006-01-12 beta] Department of Ecology and Evolution, J.W. Goethe-University, Frankfurt am Main, Germany and Department of Ecology; Charles University, Prague, Czechia: 2005. [Google Scholar]
- Pomati F, Tellenbach C, Matthews B, Venail P, Ibelings BW, Ptacnik R. Challenges and prospects for interpreting long-term phytoplankton diversity changes in Lake Zurich (Switzerland) Freshwater Biol. 2015;60:1052–1059. [Google Scholar]
- Ponyi JE, Zánkai NP. Population dynamics, biomass and biomass production of Eudiaptomus gracilis (G. O. SARS) in two water areas of differing trophic state of Lake Balaton (Hungary) Acta Hydrochim Hydrobiol. 1982;10:597–610. [Google Scholar]
- Ptacnik R, Andersen T, Brettum P, Lepistö L, Willén E. Regional species pools control community saturation in lake phytoplankton. P Roy Soc B-Biol Sci. 2010;277:3755–3764. doi: 10.1098/rspb.2010.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptacnik R, Solimini AG, Andersen T, Tamminen T, Brettum P, Lepisto L, Willen E, Rekolainen S. Diversity predicts stability and resource use efficiency in natural phytoplankton communities. P Natl Acad Sci USA. 2008;105:5134–5138. doi: 10.1073/pnas.0708328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. Vienna (Austria): R Foundation for Statistical Computing; 2012. R: a language and environment for statistical computing. ISBN 3-900051-07-0. [Google Scholar]
- Reche I, Pulido-Villena E, Morales-Baquero R, Casamayor EO. Does ecosystem size determine aquatic bacterial richness? Ecology. 2005;86:1715–1722. [Google Scholar]
- Riccardi N, Rossetti G. Eudiaptomus gracilis in Italy: how, where and why. J Limnol. 2007;66:64–69. [Google Scholar]
- Ruggiu D, Morabito G, Panzani P, Pugnetti A. Trends and relations among basic phytoplankton characteristics in the course of the long-term oligotrophication of Lake Maggiore (Italy) In: Alvarez-Cobelas M, Reynolds CS, Sanchez-Castillo P, Kristiansen J, editors. Phytoplankton trophic gradients. Springer; 1998. pp. 243–257. [Google Scholar]
- Rühland K, Priesnitz A, Smol JP. Paleolimnological evidence from diatoms for recent environmental changes in 50 lakes across Canadian arctic treeline. Arct Antarct Alp Res. 2003;35:110–123. [Google Scholar]
- Sarnelle O, Knapp RA. Nutrient recycling by fish versus zooplankton grazing as drivers of the trophic cascade in alpine lakes. Limnol Oceanogr. 2005;50:2032–2042. [Google Scholar]
- Sarvala J, Halsinaho S. Crustacean zooplankton of finnish forest lakes in relation to acidity and other environmental factors. In: Kauppi P, Anttila P, Kenttämies K, editors. Berlin, Heidelberg (Germany): Springer; 1990. pp. 1009–1027. [Google Scholar]
- Shatwell T, Koehler J, Nicklisch A. Warming promotes cold-adapted phytoplankton in temperate lakes and opens a loophole for Oscillatoriales in spring. Glob Change Biol. 2008;14:2194–2200. [Google Scholar]
- Shurin JB. Interactive effects of predation and dispersal on zooplankton communities. Ecology. 2001;82:3404–3416. [Google Scholar]
- Sommaruga R. The role of solar UV radiation in the ecology of alpine lakes. J Photochem Photobiol B. 2001;62:35–42. doi: 10.1016/s1011-1344(01)00154-3. [DOI] [PubMed] [Google Scholar]
- Sommaruga R, Psenner R, Schafferer E, Koinig KA, Sommaruga-Wögrath S. Dissolved organic carbon concentration and phytoplankton biomass in high-mountain lakes of the Austrian Alps: potential effect of climatic warming on UV underwater attenuation. Arct Antarct Alp Res. 1999;31:247–253. [Google Scholar]
- Sommaruga-Wögrath S, Koinig KA, Schmidt R, Sommaruga R, Tessadri R, Psenner R. Temperature effects on the acidity of remote alpine lakes. Nature. 1997;387:64–67. [Google Scholar]
- Sprules WG, Riessen HP, Jin EH. Dynamics of the Bythotrephes invasion of the St. Lawrence great lakes. J Great Lakes Res. 1990;16:346–351. [Google Scholar]
- Stewart JR, Lister AM, Barnes I, Dalén L. Refugia revisited: individualistic responses of species in space and time. P Roy Soc B-Biol Sci. 2010;277:661–671. doi: 10.1098/rspb.2009.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straile D. Meteorological forcing of plankton dynamics in a large and deep continental European lake. Oecologia. 2000;122:44–50. doi: 10.1007/PL00008834. [DOI] [PubMed] [Google Scholar]
- Straile D, Geller W. Crustacean zooplankton in Lake Constance from 1920 to 1995: Response to eutrophication and re-oligotrophication. Adv Limnol. 1998;53:255–274. [Google Scholar]
- Straile D, Hälbich A. Life history and multiple antipredator defenses of an invertebrate pelagic predator, Bythotrephes longimanus. Ecology. 2000;81:150–163. [Google Scholar]
- Straškrabová V, Callieri C, Carillo P, Luis L, Fott J, Hartman P, Macek M, Medina-Sánchez JM, Nedoma J, Šimek K. Investigations on pelagic food webs in mountain lakes–aims and methods. J Limnol. 1999;58:77–87. [Google Scholar]
- Tallberg P, Horppila J, Väisänen A, Nurminen L. Seasonal succession of phytoplankton and zooplankton along a trophic gradient in a eutrophic lake–implications for food web management. Hydrobiologia. 1999;412:81–94. [Google Scholar]
- Therriault TW, Grigorovich IA, Cristescu ME, Ketelaars HAM, Viljanen M, Heath DD, Macisaac HJ. Taxonomic resolution of the genus Bythotrephes Leydig using molecular markers and re-evaluation of its global distribution. Divers Distrib. 2002;8:67–84. [Google Scholar]
- Tolotti M. Phytoplankton and littoral epilithic diatoms in high mountain lakes of the Adamello-Brenta Regional Park (Trentino, Italy) and their relation to trophic status and acidification risk. J Limnol. 2001;60:171–188. [Google Scholar]
- Tolotti M, Manca M, Angeli N, Morabito G, Thaler B, Rott E, Stuchlik E. Phytoplankton and zooplankton associations in a set of Alpine high altitude lakes: geographic distribution and ecology. Hydrobiologia. 2006;562:99–122. [Google Scholar]
- Tolotti M, Thies H, Cantonati M, Hansen CM, Thaler B. Flagellate algae (Chrysophyceae, Dinophyceae, Cryptophyceae) in 48 high mountain lakes of the northern and southern slope of the eastern Alps: biodiversity, taxa distribution and their driving variables. Hydrobiologia. 2003;502:331–348. [Google Scholar]
- Veech JA. A probabilistic model for analysing species co-occurrence. Global Ecol Biogeogr. 2013;22:252–260. [Google Scholar]
- Venables W, Ripley B. Modern applied statistics using S. New York (N): Springer; 2002. [Google Scholar]
- Vogt RJ, Matthews B, Cobb TP, Graham MD, Leavitt PR. Food web consequences of size-based predation and vertical migration of an invertebrate predator (Leptodora kindtii) Limnol Oceanogr. 2013;58:1790–1801. [Google Scholar]
- Walseng B, Andersen T, Hessen DO. Higher zooplankton species richness associated with an invertebrate top predator. Freshwater Biol. 2015;60:903–910. [Google Scholar]
- Walseng B, Hessen DO, Halvorsen G, Schartau AK. Major contribution from littoral crustaceans to zooplankton species richness in lakes. Limnol Oceanogr. 2006;51:2600–2606. [Google Scholar]
- Weisz EJ, Yan ND. Relative value of limnological, geographic, and human use variables as predictors of the presence of Bythotrephes longimanus in Canadian Shield lakes. Can J Fish Aquat Sci. 2010;67:462–472. [Google Scholar]
- Wolfe AP, Baron JS, Cornett RJ. Anthropogenic nitrogen deposition induces rapid ecological changes in alpine lakes of the Colorado Front Range (USA) J Paleolimnol. 2001;25:1–7. [Google Scholar]
- Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc B Meth. 2011;73:3–36. [Google Scholar]
- Yan ND, Dunlop WI, Pawson TW, MacKay LE. Bythotrephes cederstroemi (Schoedler) in Muskoka lakes: first records of the European invader in inland lakes in Canada. Can J Fish Aquat Sci. 1992;49:422–426. [Google Scholar]
- Yan ND, Girard R, Boudreau S. An introduced invertebrate predator (Bythotrephes) reduces zooplankton species richness. Ecol Lett. 2002;5:481–485. [Google Scholar]
- Young JD, Yan ND. Modification of the diel vertical migration of Bythotrephes longimanus by the cold-water planktivore, Coregonus artedi. Freshwater Biol. 2008;53:981–995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.