Abstract
We report the case of a patient hospitalized with endocarditis. The etiological diagnosis of Bartonella was suggested by detection of high titers of antibodies by immunofluorescence and Western blotting. Two different nested PCRs performed on sera identified Bartonella vinsonii subsp. arupensis by sequencing.
There are now 19 species within the genus Bartonella (11). Eight have been implicated in human diseases. Among these, four have been recognized as agents of infective endocarditis (IE): Bartonella henselae, Bartonella quintana, and, in one case each, Bartonella elizabethae and Bartonella vinsonii subsp. berkhoffii (1, 8, 9). Bartonella endocarditis represents 3% of all cases of endocarditis in France. Serology, mainly the indirect immunofluorescence antibody (IFA) assay, is an excellent tool for its diagnosis (7), but cross-reactivity among Bartonella spp. has been observed (4). Western blotting allows a specific diagnosis when performed with adsorbed sera (4). Recently, a nested PCR was proposed as a diagnostic tool (11). We routinely perform Western blotting and PCR on sera from patients with suspected Bartonella endocarditis. Using these techniques, we have diagnosed a case of endocarditis due to Bartonella vinsonii subsp. arupensis.
A 79-year-old male was admitted to the hospital in June 2003 after having felt unwell for a month. He had received an aortic valve bioprosthesis in 1999. The retired man lived in an urban setting and had no history of exposure to animals. At admission, he presented with a fever of 38°C. Auscultation of the heart revealed a murmur consistent with mitral valve insufficiency. The white blood cell count was 4 × 109/liter (2.9 × 109 polymorphonuclear leukocytes/liter). Anemia was observed at 65 g of hemoglobin/liter. The C-reactive protein concentration had increased to 35 mg/ml. The patient had renal insufficiency with an elevated creatinine level (400 μmol/liter) and proteinuria of 2 g/liter. The antibody nuclear test and the rheumatoid factor test were positive (160 and 128 U, respectively). After the completion of three sets of blood cultures, treatment with amoxicillin (12 g per day) was started. Transesophageal echocardiography showed a new regurgitation of the mitral valve. Because vascularitis was initially suspected, a kidney biopsy was performed, revealing postinfectious glomerulonephritis. Blood cultures were negative, but the length of incubation was only 10 days, because the diagnosis of Bartonella endocarditis was not suspected. Q fever serology was negative. Results of Chlamydia sp. and Bartonella sp. serology by use of microimmunofluorescence were positive, with immunoglobulin G (IgG) titers of 1:128 and 1:512, respectively. Antibiotic therapy based on doxycycline (200 mg per day) and ofloxacin (200 mg per day) was started and produced an improvement in the patient's state.
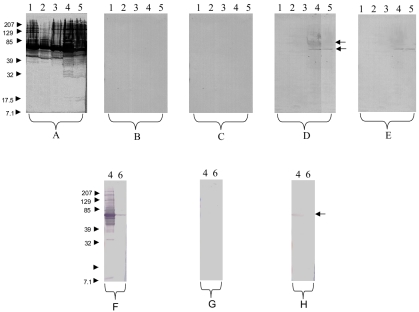
To explore the hypothesis of Bartonella endocarditis, serum samples collected from the first visit through convalescence were sent to the Unité des Rickettsies, Marseille, France. IFA assays were performed on all the serum samples, PCR was performed on the first two, and Western blotting was performed on the first, as previously described (3, 4, 11). Results are presented in Table 1 and Fig. 1. The serology showed an IgG titer of 1:400 against all the Bartonella antigens tested. Western blotting performed with nonadsorbed serum samples showed a profile similar to those observed for B. henselae and B. quintana endocarditis, with numerous reactive protein bands against Bartonella antigens, whereas a weak reaction to Chlamydia pneumoniae was observed (Fig. 1). Therefore, we speculate that this profile corresponds to those of Bartonella endocarditis. Western blotting performed using serum samples adsorbed with B. vinsonii subsp. arupensis or B. vinsonii subsp. berkhoffii showed the disappearance of all cross-reactive antibodies, whereas adsorption with B. quintana, B. henselae, or C. pneumoniae (Fig. 1) showed the persistence of reactive bands of approximately 60 and 83 kDa against B. vinsonii antigens.
TABLE 1.
Results of serological analysis performed on five serum samples and PCR assays performed on the first two serum samples of the patient
| Serum sampling date | IgG titer against antigens of:
|
PCR result | ||||
|---|---|---|---|---|---|---|
| B. henselae | B. quintana | B. elizabethae | B. vinsonii subsp. berkhoffii | B. vinsonii subsp. arupensis | ||
| 5 Junea | 1:400 | 1:400 | 1:100 | 1:400 | 1:400 | +b |
| 25 Junec | 1:400 | 1:200 | 1:100 | 1:400 | 1:400 | − |
| 30 Junec | 1:200 | 1:100 | 1:100 | 1:200 | 1:200 | NDd |
| 31 Julyc | 1:100 | 1:50 | 1:50 | 1:100 | 1:100 | ND |
| 2 Septembere | 1:50 | 1:50 | 1:50 | 1:50 | 1:50 | ND |
Sample was taken before therapy.
PCRs targeting the 23S rRNA and GroEL DNA sequences were positive and allowed the identification of B. vinsonii subsp. arupensis.
Sample was taken during therapy.
ND, not done.
Sample was taken after therapy.
FIG. 1.
Western blotting performed with the first serum sample from 5 June at a 1:200 dilution. Molecular masses (in kilodaltons) are given on the left. (A through E) Serum was analyzed by using B. quintana (lane 1), B. henselae (lane 2), B. elizabethae (lane 3), B. vinsonii subsp. arupensis (lane 4), and B. vinsonii subsp. berkhoffii (lane 5) antigens. (A) Untreated serum. (B) B. vinsonii subsp. arupensis-adsorbed serum. All antibodies were removed. (C) B. vinsonii subsp. berkhoffii-adsorbed serum. All antibodies were removed. (D) B. quintana-adsorbed serum. Antibodies to the two subspecies of B. vinsonii remained. (E) B. henselae-adsorbed serum. Antibodies to the two subspecies of B. vinsonii remained. (F through H) Serum was analyzed by using B. vinsonii subsp. arupensis (lane 4) and C. pneumoniae (lane 6) antigens. (F) Untreated serum. Note the lower reaction to C. pneumoniae. (G) B. vinsonii subsp. arupensis-adsorbed serum. All antibodies were removed. (H) C. pneumoniae-adsorbed serum. Antibodies to B. vinsonii subsp. arupensis remained.
Endocarditis due to B. vinsonii was suspected, but the causative subspecies was not identified. Nested PCR with the LightCycler, targeting the ribC gene, was performed on the sera and gave a negative result. We performed a nested PCR with primers targeting a 302-bp portion from the 23S rRNA sequence, never before used in our laboratory, without including any positive control to avoid contamination (11). The PCR was performed using B23SF1 (5′-GGGTTCCTGCTTAAAGTT-3′) and B23SR1 (5′-CGCAGAGCCCTGTGTTT-3′) as external primers and B23Srseq2 (5′-CACGCTTCTTCCGAAGTT-3′) and B23Sfseq2 (5′-TATTCTGAGCAGGGTGA-3′) as internal primers. A PCR product was detected and sequenced, as previously described (11). The sequence obtained was 100% similar to that of B. vinsonii subsp. arupensis (GenBank accession no. AF410937). To confirm this finding, we performed a second nested PCR targeting a 50-bp GroEL sequence using GroEL-1F (5′-CACAAATGCTGAGAAAATGG-3′) and GroEL-1R (5′-CATATCCAAAGTGACATTTC-3′) as external primers and GroEL-2F (5′-CCACTTCTTATTATCGC-3′) and GroEL-2R (5′-ATTTTCAAACCACCACG-3′) as internal primers. A PCR product was obtained and sequenced. The sequence obtained was 100% similar to that of B. vinsonii subsp. arupensis (GenBank accession no. AF304016.1). For both nested PCRs, all the negative controls, which included a mixture of all reagents used for DNA extraction and DNA extracted from normal heart tissue, were PCR negative.
IE is a life-threatening disease. It is critical that a diagnosis be made as early as possible. Here, we confirm that IFA serology is a useful tool for the diagnosis of Bartonella endocarditis when high titers (≥1:400) are found (3, 7). A positive diagnosis of a Bartonella sp. can be made even when the Bartonella sp. involved is not tested in the assay due to antigen cross-reactivity. Western blotting performed after adsorption allowed a specific diagnosis of the species B. vinsonii but did not distinguish among subspecies. The results obtained by Western blotting were confirmed by those obtained by using two nested PCRs. The first nested PCR was highly specific, because the targeted sequence was used for the first time in the laboratory, with no possible risk of contamination with previous amplified DNA (we call it “suicide PCR”) (6).
Based on our findings, B. vinsonii subsp. arupensis should be added to the list of pathogens capable of causing endocarditis in humans. In 1998, the DNA of this bacterium was detected incidentally for the first time during the course of studies on the reservoirs of tick-borne pathogens in Minnesota and Wisconsin when amplification of Bartonella-like 16S rRNA segments was observed in the blood of mice (10). One year later, this bacterium was isolated in Wyoming from the blood culture of a cattle rancher with valvulopathy, but the exact contribution of this Bartonella isolate to the patient's illness was not clear (10). The role of Bartonella in patients with blood culture-negative endocarditis could be supported by a combination of other factors, such as contact with body lice and unknown valvulopathy for B. quintana, or contact with cats and known valvulopathy for B. henselae. For B. vinsonii subsp. arupensis, a rodent reservoir host has been suggested. Our patient could not remember being in contact with rodents, and he presented with known valvular damage.
Finally, our data also underline the fact that cross-reactions between Bartonella and Chlamydia spp. occur (2, 5) and can lead to misdiagnoses of Chlamydia endocarditis. Indeed, it has been shown that almost all cases of Chlamydia endocarditis were in fact Bartonella endocarditis (5). Here, we show that B. vinsonii subsp. arupensis is a human pathogen responsible for endocarditis.
Acknowledgments
We thank Kelly Johnston for reviewing the manuscript, Nathalie Wilhelm and Alain Le Coustumier for providing the patient samples, and Bernard Amphoux for technical assistance.
REFERENCES
- 1.Daly, J. S., M. G. Worthington, D. J. Brenner, C. W. Moss, D. G. Hollis, R. S. Weyant, A. G. Steigerwalt, R. E. Weaver, M. I. Daneshvar, and S. P. O'Connor. 1993. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J. Clin. Microbiol. 31:872-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drancourt, M., J. L. Mainardi, P. Brouqui, F. Vandenesch, A. Carta, F. Lehnert, J. Etienne, F. Goldstein, J. Acar, and D. Raoult. 1995. Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N. Engl. J. Med. 332:419-423. [DOI] [PubMed] [Google Scholar]
- 3.Fournier, P., J. Mainardi, and D. Raoult. 2002. Value of microimmunofluorescence for the diagnosis and follow-up of Bartonella endocarditis. Clin. Diagn. Lab. Immunol. 9:795-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houpikian, P., and D. Raoult. 2003. Western immunoblotting for Bartonella endocarditis. Clin. Diagn. Lab. Immunol. 10:95-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maurin, M., F. Eb, J. Etienne, and D. Raoult. 1997. Serological cross-reactions between Bartonella and Chlamydia species: implications for diagnosis. J. Clin. Microbiol. 35:2283-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raoult, D., G. Aboudharam, E. Crubezy, G. Larrouy, B. Ludes, and M. Drancourt. 2000. Molecular identification by “suicide PCR” of Yersinia pestis as the agent of medieval Black Death. Proc. Natl. Acad. Sci. USA 97:12800-12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rolain, J., C. Lecam, and D. Raoult. 2003. Simplified serological diagnosis of endocarditis due to Coxiella burnetii and Bartonella. Clin. Diagn. Lab. Immunol. 10:1147-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roux, V., S. Eykyn, S. Wyllie, and D. Raoult. 2000. Bartonella vinsonii subsp. berkhoffii as an agent of afebrile blood culture-negative endocarditis in a human. J. Clin. Microbiol. 38:1698-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spach, D. H., and J. E. Koehler. 1998. Bartonella-associated infections. Infect. Dis. Clin N. Am. 12:137-155. [DOI] [PubMed] [Google Scholar]
- 10.Welch, D., K. Carroll, E. Hofmeister, D. Persing, D. Robinson, A. Steigerwalt, and D. Brenner. 1999. Isolation of a new subspecies, Bartonella vinsonii subsp. arupensis, from a cattle rancher: identity with isolates found in conjunction with Borrelia burgdorferi and Babesia microti among naturally infected mice. J. Clin. Microbiol. 37:2598-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeaiter, Z., P. Fournier, G. Greub, and D. Raoult. 2003. Diagnosis of Bartonella endocarditis by a real-time nested-PCR assay using serum. J. Clin. Microbiol. 41:919-925. [DOI] [PMC free article] [PubMed] [Google Scholar]