Abstract
Macrophages are crucial drivers of tumor-promoting inflammation. Tumor-associated macrophages (TAM) contribute to tumor progression at different levels, including promoting genetic instability, nurturing cancer stem cells, paving the way to metastasis, taming protective adaptive immunity. TAM can exert a dual, yin yang influence on the effectiveness of cytoreductive therapies (chemotherapy and radiotherapy), antagonizing antitumor activity by orchestrating a tumor-promoting, tissue repair response or, instead, contributing to their ultimate antineoplastic efficacy. TAM express triggers of checkpoints of T cell activation and are targets of checkpoint blockade immunotherapy. Macrophage-centered therapeutic approaches include: strategies to block recruitment and survival in tumors; functional reeducation to an antitumor, M1-like mode; tumor-directed monoclonal antibodies which elicit extracellular killing or phagocytosis of cancer cells. We surmise that TAM can provide tools to tailor cytoreductive therapies and immunotherapy and that TAM-centered therapeutic strategies have the potential to complement and synergize with chemotherapy and immunotherapy.
1. Introduction
Inflammatory cells are a key component of the ecological niche of cancer 1–4. The formation of an inflammatory microenvironment in tumors is driven by genetic events which cause cancer (oncogenes and oncosuppressor genes) or by chronic non-resolving inflammatory conditions such as inflammatory bowel disease, which increase the risk of developing cancer 1. In general, cancer-associated inflammation is characterized by being non-resolving 5.
Macrophages are a major component of the leukocyte infiltrate present in widely different amounts in all tumors 6. Tumor-associated macrophages (TAM) have served as a paradigm for leukocytes and inflammatory mediators present in the tumor context and play a dominant role as orchestrators of cancer-related inflammation (CRI). CRI is considerably diverse in tumors arising in different tissues 2, 7. However, though cellular components of CRI differ in quality and quantity and mediators which orchestrate macrophage function can differ considerably in different cancers, TAM represent a final common pathway driving CRI 8.
In the ’70 it was realized that macrophages activated by bacterial products and cytokines acquired the capacity to kill tumor cells 9–11. On the other hand it was soon realized that TAM from malignant metastatic tumors promoted tumor growth and metastasis 12. Thus, early on evidence suggested that macrophages could engage in a dual yin yang relationship with cancer.
Here we review current understanding of the role of TAM in different cancer treatment modalities as well as emerging macrophage targeting therapeutic strategies. As a premise, a concise overview of the role of macrophages in tumor initiation and progression will be provided. Previous reviews on CRI and specifically on myeloid cells in tumors provide the background of the present essay 1–3, 6, 13–15.
2. Role in Tumor Progression
Fig. 1 provides a schematic representation of the origin and function of TAM and a general framework for subsequent sections focused on therapy (see also Box 1). It has long been held that TAM originate from the blood compartment and that chemotactic signals originating from tumor cells or from normal cells present in the cancer microenvironment recruit monocytic precursors at the primary and metastatic tumor sites 11, 15–19. However, recent evidence raises questions as to this long held view. In the mouse, resident macrophages in some tissues (e.g microglia in the brain) originate from precursors seeding there during fetal and embryonal life rather than from circulating monocytes (Box 1) 20,21. In gliomas, tumor-associated macrophages constitute a mixed population that includes resident brain microglia, infiltrating blood monocytes, and macrophages. The relative contribution of these cells has been investigated in a genetically engineered mouse model: accumulation of Ly-6Chi circulating “inflammatory” monocytes into tumor tissue was responsible for the increased tumor incidence and shorter survival times, with no contribution of microglial cells 22. In the perspective of macrophage function in the tumor microenvironment, it is noteworthy that recent results support that in the mouse the ontogenetic origin does not have an appreciable impact on the macrophage phenotype in response to tissue-derived cues 23. Whether embryo-derived tissue macrophages contribute to the number, location and diversity of TAM remains an open question 24. TAM proliferation has been observed in murine and human sarcomas and murine breast carcinomas but this does not appear to be a general mechanism sustaining TAM numbers in the face of growing tumors 25, 26, 27. Circulating precursors that are recruited into tumor tissues and there differentiate into TAM include conventional inflammatory monocytes and Mo-MDSC (see Box 1). Down regulation of the transcription factor STAT3 plays a key role in the differentiation of Mo-MDSCs into mature TAM28. Inflammatory monocytes, defined in the mouse as Ly6C+/CCR2+ cells have been shown to contribute to TAM accumulation and maintenance in a mouse mammary tumor model 27 and pulmonary metastases of murine and human breast cancer cells 19. The process of differentiation of mouse inflammatory monocytes into TAM was dependent on the transcriptional regulator RBPJ and its genetic deletion in TAM resulted into reduced tumor burden, confirming a non-redundant function of monocyte-derived TAM in tumor growth 27. In contrast, protective function has been shown for CX3CR1+/Ly6C- “nonclassical” patrolling mouse monocytes, which depend on the N4a1 transcription factor and patrol the lung microvasculature in steady state conditions. These cells, which rarely extravasate into tissues and differentiate into macrophages, rapidly accumulate into lung metastases and inhibit tumor cell seeding and growth in mouse models 29. Their antitumor function includes scavenging of tumor debris and recruitment and activation of natural killer cells 29.
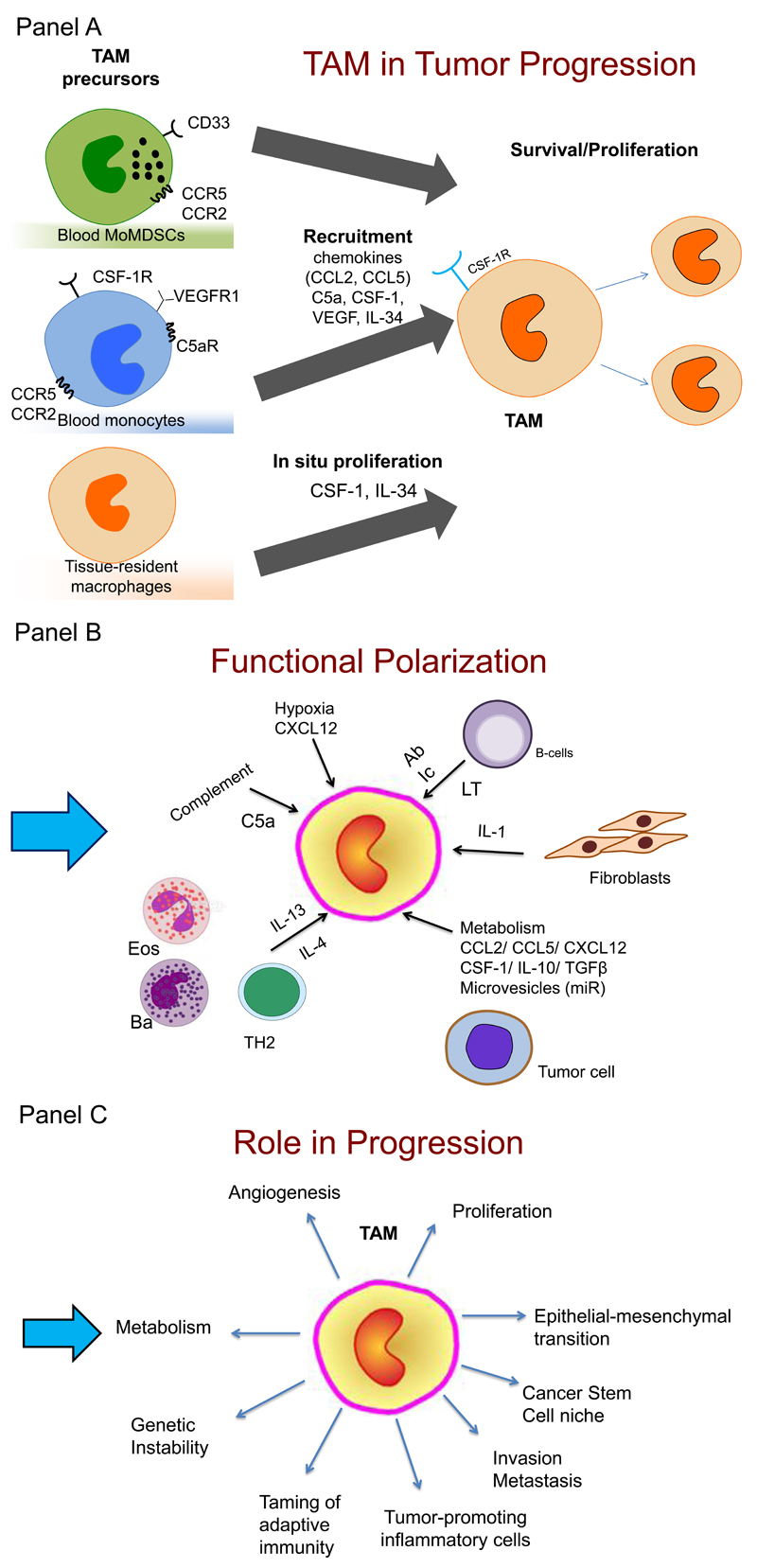
Figure 1. A schematic representation of the role of tumor-associated macrophages in tumor progression.
Panel A. Monocytes and MoMDSC (see Box 1) are recruited in tumors in response to diverse chemoattractants including CSF1, chemokines and complement components. In tumors, monocytes differentiate into macrophages (Tumor-associated macrophages, TAM). In some tumors, in situ proliferation may occur and local tissue resident macrophages of embryonic origin may contribute to TAM. Signals in the tumor microenvironment skew the function of TAM.
Panel B. Pathways and molecules polarizing TAM differ in different tumors. These include: IL-4 and IL-13 derived from TH2 cells, eosinophils (Eos) and basophils (Bas); cytokines and metabolites from tumor cells; antibodies (Ab) from B cells and immune complexes (Ic); stromal cell-derived factors (IL-1, LT).
Panel C. TAM affect virtually all aspects of tumor cell biology, including provision of a niche for cancer stem cells (CSC); angiogenesis; epithelial to mesenchymal transition (EMT); invasion and metastasis; proliferation; genetic instability.
Box 1. Diversity and Nomenclature of myelomonocytic cells.
Diversity and plasticity are hallmarks of cells of the monocyte-macrophage lineage 20,21,185–187. Here we outline key aspects of monocyte-macrophage diversity and nomenclatures. In general there are considerable differences between mouse and man in terms of markers defining monocyte-macrophage diversity. Circulating monocytes originate from bone marrow precursors. In man, two main monocyte subsets have been identified based on expression of CD14 and CD16 (CD14+CD16- and CD14+Cd16+). In the mouse, two monocyte subsets, which have been referred to as “inflammatory” or “patrolling”, based on the expression of Ly6C, CD11b, CCR2 and CX3CR1 20.
During cancer progression, immature bone marrow-derived elements of the myeloid lineage appear in the circulation. These cells are potent suppressors of adaptive immune responses. These immature myeloid cells with immunosuppressive activity have been operationally defined myeloid derived suppressor cells188,52. MDSCs are heterogeneous, being related to monocytes (MoMDSC) or neutrophils (Neu MDSC); they both express the immature myeloid cell marker CD33, but only MoMDSC at high levels. MoMDSCs can differentiate into TAM52.
Tissue macrophages originate either from embryonic precursors which seed peripheral locations and self-sustain or from circulating monocytes 189,21. In tissues, in response to diverse signals, cells of the monocyte-macrophage lineage undergo diverse forms of functional reprogramming. In particular, Interferonγ (IFNγ), produced in type 1 immune responses driven by TH1 cells and type 1 innate lymphoid cells (ILC1), and bacterial products activate the tumor cell killing activity and tissue damaging properties of these cells. Cytokines (IL-4 and IL-13), produced during type 2 immune responses driven by TH2 cells and ILC2 cells, activate an alternative form of macrophage activation oriented to resistance against parasites and to tissue repair and remodeling. Mirroring nomenclatures in current usage (e.g. TH1 and TH2), these two alternative forms of macrophage activation have been frequently referred to as M1 (or classic) and M2 (or alternative). The extremes and continuum between M1 and M2 do not recapitulate the whole spectrum of macrophage plasticity and indeed plasticity and flexibility of phenotypes is now recognized also for T cells and ILC cells. For a discussion of nomenclature issues the reader is referred to ref 34,190. As discussed here and represented in Fig. 1, in neoplastic tissues the signals orchestrating macrophage function are diverse and differ considerably in different tumors or different parts of the same tumor, with different phenotypes which in many cases do not fit the M1/M2 scheme. We and others use M2 to concisely refer to TAM phenotypes driven by IL-4 or IL-13 34 and M2-like to refer to a universe of diverse phenotypes which share, as functional output, tumor promotion and suppression of effective adaptive immunity. The inherent imperfection and utilization value of these oversimplified nomenclatures are discussed elsewhere 190.
Chemoattractants involved in monocyte recruitment include chemokines (e.g. CCL2, CCL5), colony-stimulating factor-1 (CSF-1), and members of the VEGF family. TAM themselves can be a source of CCL2 in cancer. Recently, genetic evidence in the mouse suggested that Complement components, C5a in particular, play an important role in recruitment and functional polarization of TAM 30. Chemotactic factors are more than attractants in that they activate transcriptional programs in macrophages and contribute to functional skewing (e.g. 31). CSF-1 in particular is a monocyte attractant as well as a macrophage survival and polarization signal 6, 32. Unlike M-CSF, GM-CSF activates macrophage functions related to anti-tumor activity 33.
Signals originating from tumor cells, T and B lymphocytes and stroma orchestrate TAM function and their diversity. Classically activated macrophages (M1, Box 1) can kill tumor cells extracellularly and mediate tissue destructive reactions centered on the vessel wall (hemorrhagic necrosis) 15,9,10,11. Accordingly, there is evidence that macrophages contribute to the early elimination phase of nascent tumors orchestrated by T cells and interferons 34 . Tumor progression is associated with skewing and subversion of macrophage function. In established progressing tumors such as murine and human breast and pancreatic cancer, IL-4 and IL-13 derived from TH2 cells 35–37, eosinophils 38 or basophils 39 elicit alternative M2 activation of TAM (Box 1, Fig. 1). In addition, signals originating from tumor cells (e.g. chemokines, CSFs, TGFβ), B cells (immune complexes) and stromal cells (e.g. IL-1) can act as drivers to skew macrophage function to diverse phenotypes (for a recent summary, 8) which do not fit with classic M1/M2 polarized cells (Box 1). In general, in spite of intra and inter-tumor diversity, TAM in progressing neoplasms have surface molecules (e.g. the scavenger receptor CD163; mannose receptor CD206) and properties related to angiogenesis, suppression of specific immunity and promotion of cancer growth and metastasis. For convenience, we and others refer to these diverse populations as M2-like. In line with a consensus recommendation 33 (Box 1) we use M2 when IL-4 or IL-13 are major drivers of polarization.
There is evidence that the relative importance of distinct pathways varies in different tumors, resulting in heterogeneous phenotypes 2, 7. However as diverse as CRI can be in different cancers, TAM polarization appears to represent a final common pathway. Within a given human or mouse tumor, TAM exhibit different phenotypes, influenced for instance by access to oxygen and activation of the HIF pathway 40, 41 42. Dissecting TAM diversity at the single cell level and integrating information represent current challenges.
TAM influence tumor cell intrinsic properties as well as the tumor microenvironment (Fig. 1). TAM can stimulate tumor cell proliferation, migration and genetic instability. Acting at the primary tumor site or at sites of secondary localization they promote invasion and metastasis (Fig. 1). TAM promote angiogenesis and lymphoangiogenesis as well as tissue remodeling with fibrous tissue deposition.
Myelomonocytic cell contribute to suppression of effective adaptive immunity, a key feature of cancer 3, at various levels and through multiple mechanisms. MDSC, and in particular MoDSCs, suppress immunity in lymphoid organs. MoMDSC are recruited in tumors contributing to an immunosuppressive microenvironment and here they undergo differentiation into TAM 28 (Box 1). TAM can promote T regulatory cell (Treg) in a bidirectional interaction 15. Immunosuppressive cytokines (IL-10 and TGFβ) are produced by macrophages in the tumor context. Aminoacid metabolism in polarized macrophages and TAM results in metabolic starving of T cells and production of immunosuppressive metabolites via the indoleamine 2,3 dioxygenase (IDO) pathway 33. Prostaglandins produced from the arachidonic acid metabolism have suppressive functions. Finally, TAM express PD-L1 and PD-L2 which trigger checkpoint blockade in T cells, as well as B7H4 43 and VISTA 44 which may exert similar functions.
Progress has been made defining the molecular pathways responsible for the orchestration of macrophage function in tumors, including members of the STAT and NFkB family 8. Among these, Myc is interesting in that it acts both in cancer cells and macrophages. The Myc oncogene orchestrates approximately 40% of the transcriptional fingerprint of human M2 activation and is overexpressed in human TAM 45. On the cancer cell side, the Myc oncogene was found to induce expression of CD47 and PD-L1 46. CD47 is a “don’t eat me” signal (see below). Thus, the oncogene Myc appears to tame innate immunity in the form of macrophage-mediated phagocytosis through CD47 as well as activation of effective adaptive antitumor immunity through induction of PD-L1.
3. Prognostic or Predictive Biomarkers
Studies on the prognostic significance of TAM have relied on a variety of methodological approaches, ranging from morphological identification in early efforts 11 to gene expression profiling 47. The most extensively used human macrophage marker is CD68, a pan macrophage marker. However, CD68 can occasionally be expressed in stromal cells as well as in cancer cells themselves. Therefore its use requires careful assessment 48. In many studies, CD163, a scavenger receptor associated with M2-like polarization, CD204 and CD206 (the mannose receptor, induced by IL-4) were used, with overall results comparable to usage of CD68. In addition a range of molecules have been used to characterize TAM. These include membrane molecules (e.g. stabilin-1 49, expressed in M2 polarized macrophages and TAM). Chemokines and chemokine receptors (e.g. CCL17), cytokines and cytokine receptors (e.g. IL-10 and IL-12 50). M1-like macrophages polarized with IFNγ and with antitumor activity usually present with high levels of HLA-DR 51, although this marker is widely expressed in other leukocyte populations. Different approaches are used for identification of macrophage precursor monocytes and monocyte-myeloid-derived suppressor cells (MoMDSCs) in cancer, as these circulating cells are commonly investigated by multicolor flow cytometry. Monocytes are referred to as “inflammatory”, when CD14+/CD16- 20, or “patrolling” when CD14dim/CD16+ 20. As to MDSCs, a recent consensus has been reached on MoMSCs as CD11b+/CD14+/HLA-DRlow//CD15-/CD33+, with CD33 being highly expressed only on MoMDSC (compared to NeuMDSC) 52. Macrophages infiltrating mouse and human tumors show considerable diversity within a given cancer depending on their microanatomical localization 41, 49. Hypoxia is a major driver of macrophage diversity within tumors13, 53. Therefore, an inherent limitation of available information is that it does not take into account intratumor diversity of TAM.
It has long been known that in many human solid tumors high macrophage infiltration is associated with poor prognosis 54. These observations represented a pillar for the now largely accepted view that TAM promote tumor progression as discussed above. In breast carcinoma macrophage infiltration was associated with grade, lack of hormone receptors, basal like type and outcome 26. TAM were correlated with more advanced stage in breast and bladder cancer 55, 56 but the reverse was true in ovarian and gastric cancer 57, 58. The negative prognostic significance of TAM infiltration has been confirmed in a recent meta-analysis including all available data 59.
In apparent contrast with the above results in selected human tumors (non-small cell lung cancer, prostate and colorectal cancer, CRC) high macrophage infiltration has been associated with better prognosis. This observation in CRC has stood up in the meta-analysis conducted by Zhang et al 59 and were confirmed in an analysis of 209 CRC patients in our Institution (Malesci et al., unpublished data). Interestingly, in CRC also neutrophil infiltration was found to be associated with better survival 60. As discussed below, the favorable prognostic significance of macrophage infiltration is related to the impact of TAM on response to chemotherapy.
As discussed above, it has long been held that TAM originate from blood monocytes. However, in selected murine tumors 25 TAM proliferation was observed though this was not clearly shown in human tumors. In a recent study, proliferating macrophages were identified as PCNA+ cells in human breast carcinoma. Proliferating TAM were associated with hormone receptor negativity, basal-like phenotype and worse outcome 26. It will be important to assess the presence of PCNA+ TAM in other tumors and assess their significance.
In classic Hodgkin’s lymphoma (CHL), a gene signature of TAM and an increased number of CD68+ cells were associated with shortened survival in patients treated with chemotherapy regimens, so that TAMs have been proposed as a biomarker for risk stratification 61 (Table 1). High CD68 or CD163 expression were later confirmed to be significant independent predictors of worse survival in a multicenter randomized controlled clinical trial, reinforcing the prognostic significance of TAMs in chemotherapy-treated patients with locally extensive and advanced-stage CHL 62 (Table 1).
Table 1. High density of TAMs as an outcome predictor in patients with neoplastic disease receiving chemotherapy.
| Author | Year | Tumor Type | Therapy | Marker | Outcome Prediction | Ref. |
|---|---|---|---|---|---|---|
| Farinha P | 2005 | Follicular lymphoma | BP-VACOP# | CD68 | Positive | 63 |
| Taskinen M | 2007 | Follicular lymphoma | AVP AVP + rituximab |
CD68 CD68 |
Negative Positive |
65 |
| Steidl C | 2010 | Classic Hodgkin Lymphoma | ABVD* | CD68 | Negative | 61 |
| Tan KL | 2012 | Classic Hodgkin Lymphoma§ | ABVD vs Stanford V | CD68 CD163 |
Negative | 62 |
| Kridel R | 2015 | Follicular lymphoma | CHOP + rituximab CVP + rituximab |
CD163 CD163 |
Negative Positive |
66 |
| Algars A | 2012 | Colorectal cancer –stage III | Unspecified° | Clev/Stab | Positive | 49 |
| Di Caro G | 2015 | Pancreatic cancer | No adjuvant Post-surgical adjuvant^ |
CD68 | Negative Positive |
50 |
| Malesci A | submitted | Colorectal cancer –stage III | No adjuvant Post-surgical adjuvant@ |
CD68 | None Positive |
- |
Farinha, multiagent chemotherapy followed by involved region radiation
Steidl, ABVD / ABVD-like / radiation therapy, second line therapy (autologous stem cell transplantation; CVPP; GDP; field radiation)
Tan, locally advanced and advanced stage CHL; E2496 Intergroup trial, a multicenter phase 3 randomized controlled trial
Algars, fluorurail-based adjuvant therapy is the standard regimen for stage III colorectal cancer. CD68, p=0.09 for better survival in this cohort
Di Caro; gemcitabine-based adjuvant treatment
Malesci, 5-flurouracil based adjuvant therapy
Previously, high TAM (CD68+) content had been found associated with unfavorable outcome also in patients with follicular lymphoma (FL) treated with multiagent chemotherapy 63, 64, a prognostic association reversed in CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone)-treated patients receiving an anti-CD20 mAb (rituximab) 65. An independent study confirmed that high TAMs (CD163+) were predictive of a favorable outcome in FL patients receiving rituximab plus CHOP, while being oppositely associated with an adverse outcome in patients treated with rituximab, cyclophosphamide, vincristine, and prednisone (CVP) 66. The latter data not only confirm that TAM predict outcome in FL, but also underscore that their prognostic impact is dependent on treatment. As nowadays neoplastic conditions as a rule receive pharmacological treatment, differently from the past, the prognostic value of a variable is meaningless if not related to the administered treatment (i.e., prognostic vs predictive value). Doxorubicin is the drug differentiating CHOP and CVP, so that a striking parallelism occurs between these data and early pre-clinical studies supporting a role of TAMs in determining the antitumor efficacy of this drug in a mouse lymphoma model 67. At any event, considering that lymphoma patients are heavily treated, it should be concluded that TAMs may serve as predictive biomarker in this neoplastic setting, whose positive or negative value is determined by the type of chemotherapy.
Data in solid tumors on the predictive potential of TAM are limited. Most studies assessing the prognostic impact of TAMs do not report adjuvant therapy regimens, even when these are considered an international standard 49, 68, 69. The only published study comparing TAMs as to the outcome of patients receiving or not chemotherapy after surgery for solid tumor concerns pancreatic cancer, and supports their dual effect depending upon post-surgical chemotherapy 50. High TAMs appear to be critical determinants of responsiveness to gemcitabine following surgery for pancreatic cancer. In parallel, high TAMs density in patients with stage III colorectal cancer were associated with better outcome only in patients receiving post-surgical adjuvant 5-fluorouracil based chemotherapy, but not in untreated patients (Malesci, submitted). These studies point to macrophages as predictive factors of responsiveness to post-surgical chemotherapy, rather than being only prognostic indicators.
4. The Yin Yang of Chemotherapy and Radiotherapy
Chemotherapy can affect macrophage function directly or indirectly, the latter by modulating the function of adaptive T cell mediated immune responses (Fig. 2). Indeed, early on it was found that immunity played a key role in determining the ultimate efficacy of Doxorubicin 67. In this and in subsequent reports it was apparent that chemotherapeutic agents are not born equal in terms of interactions with immunity. In response to selected chemotherapeutic agents, Doxorubicin in particular, tumor cells undergo immunogenic cell death, i.e. express alarm signals which trigger effective adaptive immune responses 70, 71. For instance after exposure to Doxorubicin, in a murine model tumor cells released ATP which caused recruitment of mononuclear phagocytes. Under these conditions myeloid cells differentiated into antigen presenting cells which triggered effective adaptive immune responses 72. Moreover, cooperation between chemotherapeutic agents such as Actinomycin D and human and murine monocytes-macrophages had been observed early on in a phenomenon called drug-dependent cellular cytotoxicity 73.
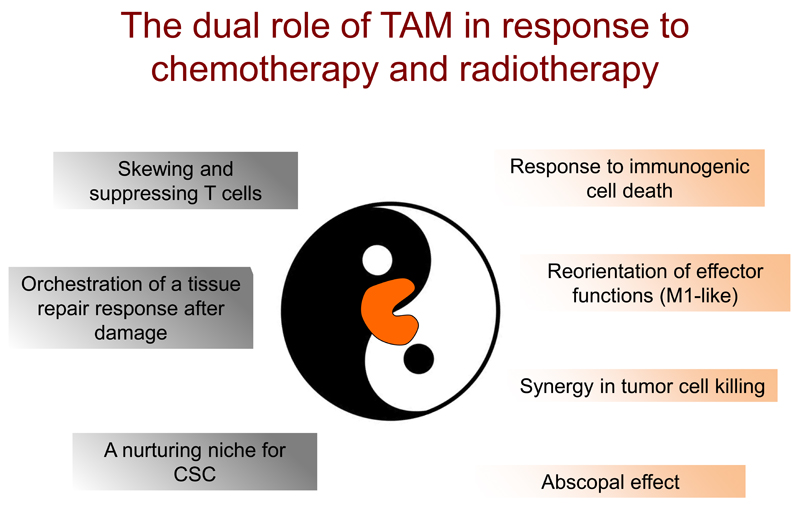
Figure 2. Bimodal function of TAM in response to chemotherapy and radiotherapy.
Macrophages orchestrate immune responses that can either hamper (left) or foster (right) the effectiveness of conventional anticancer strategies.
On the left: cytotoxic agents enhance tumor infiltration by immunosuppressive macrophages, which activate chemoprotective T cells and tame adaptive immune responses; chemotherapy or radiotherapy-induced tissue damage triggers the recruitment of immunosuppressive myeloid cells, which orchestrate a misdirected tissue-repair response, promoting tumor growth and revascularization; macrophages, an essential component of tissue stem cell niches, can protect CSC against cytotoxicity.
On the right: selected chemotherapeutic agents (e.g Doxorubicin) increase the immunogenicity of malignant cells (immunogenic cell death), which stimulate myeloid cells to differentiate into antigen presenting cells and trigger effective adaptive immune responses; anticancer agents like Gemcitabine can directly skew macrophage effector functions towards an antitumor mode and increase their cytotoxicity, resulting into a favorable synergism; neoadjuvants low-dose γ-irradiation set macrophage functions in an antitumor mode, promoting regression at sites distant from irradiated lesions (abscopal effect). CSC: cancer stem cells.
Trabectedin, approved by EMA for soft tissue sarcomas and ovarian cancer, and by FDA for sarcomas, is a DNA binder causing DNA damage and cell cycle arrest in tumor cells, but this “conventional” effect is only part of its complex mechanism of action; by interacting with DNA binding proteins, Trabectedin affects the transcription of selected genes, which include some inflammatory cytokines/chemokines and angiogenic factors 74. Clinical observations were inconsistent with its antitumor activity being accounted for completely by effects on cancer cells (see below), with delayed, prolonged responses. These clinical observations prompted an analysis of the effect of this drug on immunity 75, 76. This compound triggers activation of caspase 8, the key effector molecule of the extrinsic apoptotic pathway, selectively in monocytes, inducing their apoptotic death. In preclinical models, macrophage depletion was demonstrated to be a key mechanism of its antitumor activity. Reduced TAM infiltration and angiogenesis were observed in biopsies from sarcoma patients treated with Trabectedin 75. Thus, preclinical and clinical evidence suggest that reduction in macrophage numbers is a key component of the antitumor activity of Trabectedin.
The microbial context has emerged as an important determinant of the efficacy of chemotherapy and immunotherapy in murine models 77–79. Priming of myeloid cells by microbial components was essential for the antineoplastic efficacy of platinum with a CpG agonist 77. In the same vein, the antineoplastic activity of anthracyclines was compromised in mice with genetic inactivation of the formyl peptide receptor 1 (FPR1), a sensor of microbial components and tissue damage expressed in myeloid cells 80. A loss-of-function FPR1 allele was associated with lower survival in human mammary carcinoma and colorectal cancer receiving adjuvant chemotherapy. Preclinical models suggest that myeloid cells are prime movers of the contribution of immunity to the antitumor activity of selected chemotherapeutic agents 71 and the latter amplify the efficacy of checkpoint blockade therapy 81. In mouse models repolarization of macrophages in the context of targeted therapies has been reported for imatinib in KIT+ gastrointestinal stromal tumors (GISTs) 82 and sorafenib of hepatocellular carcinoma 83.
The apparent discrepancy in the role of TAM in the response to Doxorubicin (e.g. 67, 84–88) is likely to reflect differences in the murine tumor models utilized (for instance immunogenicity). However, as discussed above, high TAM infiltration is associated with better prognosis in patients with lymphomas treated with Doxorubicin-containing regimens mirroring preclinical data 67.
The positive interaction between chemotherapy and macrophage-mediated host defence is reflected by the prognostic/predictive significance of TAM in pancreatic cancer and colorectal cancer discussed above. Mirroring the in vivo association data, in vitro gemcitabine was found to synergize with macrophages in tumor cell killing 50.
M2 and M2-like polarized macrophages orchestrate tissue repair. Consistently with this general property of cells of the monocyte-macrophage lineage, there is evidence that under selected conditions TAM can limit the effectiveness of cytotoxic agents (Fig. 2). These include platinum compounds, paclitaxel and Doxorubicin itself 32, 84–88. In transplanted mouse models, M2-like macrophages were found to accumulate in perivascular areas after chemotherapy 89. Here they promoted tumor revascularization and relapse and their recruitment was CXCR4/CXCL12 dependent.
Two general mechanisms emerge as responsible for the antagonistic action of TAM on chemotherapy. In mouse models, chemotherapy-triggered tissue damage has been linked to triggering recruitment of immunosuppressive myeloid cells 32, 84 or to elicit a skewed Th17 protumor response promoted by IL-1 86. An alternative pathway is centered on cancer stem cells (CSC): TAM have been reported to protect murine CSC against cytotoxicity 87, 88. Indeed, macrophages are an essential component of the stem cell niche in a variety of tissues. Thus, the dark side of the interaction of chemotherapy with TAM is a reflection of fundamental properties of macrophages, i.e. being part of tissue stem cell niches, taming adaptive immunity and orchestrating repair responses.
As for chemotherapy, the impact of radiotherapy on myeloid cells can have dual significance. The influx of monocytes which follows radiotherapy in mouse models drives a profibrotic tissue response and may promote tumor recurrence (e.g. 8, 90. Conversely, tumor regressions observed in patients at sites distant from irradiated lesions, called “abscopal” effect 91 call for activation of host immunity as a plausible explanation. In a mouse model, neoadjuvant low-dose γ-irradiation was found to set macrophage functions in an antitumor mode characterized by lack of immunosuppressive and proangiogenic activity and production of T cell attracting chemokines 92. Therefore as for chemotherapy, TAM can reduce or amplify the antitumor effect of radiotherapy depending on context.
5. Hormonal Therapy
There is evidence suggesting that the two classic pathways of tumor promotion, inflammation and sex steroid hormones are linked 1. In carcinoma of the prostate, IL-1β produced by macrophages converts androgen receptor modulators from being inhibitory to stimulatory 93. The occurrence of TAM was increased in prostate cancer patients who had been treated with androgen blockade therapy 94. TAM frequency was correlated with time to tumor progression. In a preclinical model, androgen blocking therapy induced production of macrophage attracting cytokines, CSF-1 in particular 95. Inhibition of the CSF-1R tyrosine kinase had synergistic antitumor activity with androgen inhibition. Thus, targeting TAM is a candidate approach to amplify susceptibility to hormonal intervention.
6. Anti-Angiogenesis
Strategies targeting VEGF are part of the current therapeutic armamentarium. In addition to eliciting angiogenesis, VEGF is a potent attractant for monocytes 96. It acts on monocytes via the VEGFR1/FLT1 receptor 96 97. Interestingly Qian et al, found that FLT1/VEGFR1 is upregulated in metastasis-associated macrophages in a murine mammary carcinoma model 97. Although VEGF has long been known to be chemotactic for monocytes, in this model VEGF did not drive recruitment. VEGF was upstream of autocrine CSF-1-triggered tumor promoting activity of metastasis-associated macrophages.
Macrophages, including TAM, are a major source of angiogenic growth factors acting on vascular and lymphatic vessels 13. TAM frequency and vascular density are generally associated in human tumors. Resistance to current anti-VEGF therapies is frequently associated with high levels of myeloid cell infiltration 53. Preclinical evidence suggests that hypoxia following the destruction of the vascular bed by anti-angiogenic drugs triggers a compensative recruitment of myeloid cells which promote angiogenesis via alternative routes 8, 13, 98.
Angiopoietin-2 is a regulator of vessel wall function which is functionally linked to angiogenesis and TAM function. In addition to providing an escape pathway to VEGF inhibition, Angiopoietin-2 can trigger a proangiogenic phenotype in macrophages 99. In human glioblastoma macrophage infiltration is correlated with poor prognosis and this tumor is resistant to anti-VEGF treatment. In preclinical models, a dual Angiopoietin-2/VEGF bispecific antibody had appreciable antitumor activity and reprogrammed TAM from an M2 protumor to an M1 antitumor phenotype 100, 101. Thus targeting TAM may complement current anti-angiogenic therapies.
7. Immunotherapy with Checkpoint Blockade Inhibitors
Immunotherapy using checkpoint blockade inhibitors is now part of the therapeutic armamentarium in an increasing number of cancers 102. Clinically validated targets include CTLA4 and PD-1/PD-L1, and more will undergo clinical evaluation. Myelomonocytic cells are a key component of the immunosuppressive pathways targeted by checkpoint blockade inhibitors, and may offer tools to predict or increase their activity.
Macrophages express the ligands for checkpoint molecules, including PD-L1, PD-L2, B7H4 and the CTLA4 ligands B7-1 and B7-2. PD-L1 and PD-L2 are upregulated in response to various stimuli including cytokines and hypoxia 103, 104. TAM express high levels of PD-L1 and/or B7H4 in a variety of human tumor types, such as hepatocellular carcinoma 105, 106, glioblastoma 107 and pancreatic cancer 108. It has not been fully elucidated how and to what extent the expression of inhibitory receptors PDL-1, PDL-2 and B7-H4 on macrophages contributes to their immunosuppressive function. The expression of triggers of checkpoint blockade (e.g. PD-L1) by TAM as a predictor of response needs to be carefully assessed 109, 110.
Analysis of the mode of action of CTLA4 blocking mAb revealed an unexpected role of TAM. In preclinical models, FcγR expressing macrophages eliminated CTLA4 expressing, mAb coated Treg cells by ADCC 111, 112. TAM-mediated elimination of anti-CTLA4 sensitized Treg unleashed effective antitumor immunity. The role of macrophage-mediated ADCC in the activity of anti-CTLA4 (Iplimumab) was examined in 15 responding and 14 non-responding matched melanoma patients 113. Responding patients had higher number of CD16+ monocytes and at the tumor sites a higher CD68+/CD163+ ratio, used as a correlate of M2 skewing. Moreover, response was associated with a decrease of Treg cells at the tumor site 113. These results are consistent with the preclinical data mentioned above and suggest that macrophages contribute to the action of anti-CTLA4.
Macrophage contribute to immunosuppression observed in the tumor microenvironment. Therefore macrophage targeting may complement the action of checkpoint blockade inhibitors. Indeed in a model of pancreatic cancer, CSF-1 inhibition synergized with checkpoint immunotherapy 84, 114. Combination based on this principle are undergoing early clinical evaluation (see Table 2).
Table 2. Clinical trials targeting macrophages in tumors (from http://clinicaltrials.gov).
| COMPOUND | CLINICAL PHASE | SPONSOR | TUMOR TYPE | COMBINATION |
|---|---|---|---|---|
|
PLX3397 (CSF-1R inhibitor) |
Phase 1/2 (O) |
Plexxikon | Sarcoma, Nerve Sheath tumors |
Sirolimus |
| Phase 2 (O) |
Melanoma | |||
| Phase 1 (O) |
Prostate Cancer | Radiation therapy, Anti-androgen Therapy | ||
| Phase 1/2 (O) |
Solid tumors PVNS* and GCT-TS* |
Pembrolizumab | ||
| Phase 3 (O) |
Breast cancer | Eribulin | ||
| Phase 1B/2 (O) |
Leukemia, Sarcoma, Neurofibroma | |||
| Phase 1/2 (O) |
Acute Myeloid Leukemia | |||
| Phase 1/2 (O) |
Glioblastoma | Radiation therapy; Drug: Temozoloide |
||
| Phase 1/2 (O) |
Solid tumors | Paclitaxel | ||
| Phase 1/2 (O) |
Breast cancer | Standard therapy** | ||
|
| ||||
|
PLX7486 (CSF-1R inhibitor) |
Phase 1 (O) |
Pancreatic adenocarcinoma | Gemcitabine; nab-Paclitaxel |
|
|
| ||||
|
LY3022855 (CSF-1R inhibitor) |
Phase 1 (not yet open) |
Eli Lilly | Solid tumors | Durvalumab; Tremelimumab |
| Phase 1 (O) |
Breast and Prostate cancer | |||
|
| ||||
|
IMC-CS4 Anti-CSF-1R Ab |
Phase 1 (O) |
Eli Lilly | Solid tumors | |
|
| ||||
|
RO5509554
(RG7155) Anti-CSF-1R Ab |
Phase 1 (O) |
Hoffmann-La Roche | Solid tumors | MPDL3280A (anti-PD-L1 Ab) |
| Phase 1 (O) |
Solid tumors | Paclitaxel | ||
|
| ||||
|
AMG820 Anti-CSF-1R Ab |
Phase 1/2 (not yet open) |
Amgen | Pancreatic cancer; Colorectal cancer; Non-small cell lung cancer |
Pembrolizumab |
| Phase 1 (C) |
Solid tumors | |||
|
| ||||
|
Hu5F9-G4 Anti-CD47 Ab |
Phase 1 (O) |
Stanford University |
Myeloid leukemia | |
|
| ||||
|
CC-90002 Anti-CD47 Ab |
Phase 1 (O) |
Celgene | Myeloid leukemia Myelodysplastic syndromes |
|
| Phase 1 (O) |
Hematologic malignancies | |||
|
| ||||
|
TTI-621 CD47 Fc fusion Protein |
Phase 1 (O) |
Trillium Ther. | Hematologic malignancies | |
|
| ||||
|
CP-870,893 Agonist CD40 Ab |
Phase 1 (C) |
Pfizer (UPenn) | Melanoma | |
| Phase 1 (C) |
Solid neoplasms | Paclitaxel + Carboplatin | ||
| Phase 1 (C) |
Adenocarcinoma Pancreas |
Gemcitabine | ||
|
| ||||
|
RO7009789 Agonist CD40 Ab |
Phase 1 (O) |
Hoffmann-La Roche | Solid neoplasms | Anti-PD-L1 |
| Phase 1 (O) |
Solid neoplasms | Vanucizumab | ||
| Phase 1 (O) |
Adenocarcinoma Pancreas | Nab-Paclitaxel and Gemcitabine | ||
|
| ||||
|
Xilonix Anti-IL-1a Ab |
Phase III (O) |
XBiotech | Colorectal cancer | |
|
| ||||
|
Carlumab Anti-CCL2 Ab |
Phase 2 (C) | Centocor | Prostate cancer | |
|
| ||||
|
CNTO888 Anti-CCL2 Ab |
Phase 1 (C) |
Centocor | Solid tumors | Gemcitabine + Paclitaxel + Carboplatin |
|
| ||||
|
NCT02052492 EF-022 Vit D Binding Protein Macrophage Activator |
Phase 1 | Efranat | Solid tumors | |
(O) Ongoing; (C) Completed;
Pigmented Villonodular Synovitis (PVNS) or Giant Cell Tumor of the Tendon Sheath (GCT-TS);
Standard therapy
Drug: AMG 386
Drug: AMG 479 (Ganitumab) plus Metformin
Drug: MK-2206 with or without Trastuzumab
Drug: AMG 386 and Trastuzumab
Drug: T-DM1 and Pertuzumab
Drug: Pertuzumab and Trastuzumab
Drug: Ganetespib
Drug: ABT-888
Drug: Neratinib
Drug: Pembrolizumab
8. Antibody-Dependent Cellular Phagocytosis (ADCP) and Antibody-Dependent Cellular Cytotoxicity (ADCC)
Phagocytosis is a fundamental mechanism of innate resistance against microbes and of effete cell disposal. It has long been held by students in the field including one of the authors (AM) that phagocytosis does not represent a significant mechanism in the antitumor activity mediated by activated macrophages and that mechanisms of extracellular killing are the ones relevant for macrophage-mediated killing of cancer cells 10. Evidence has now challenged this long held view and has spurred interest into clinical translation.
The CD47-signal regulatory protein α (SIRP-α) pathway plays an important role in homeostasis and in regulating the engulfment of old erythrocytes. Both molecules are members of the Ig superfamily with SIRP-α and CD47 being expressed on macrophages and candidate target cells, respectively 115–117. SIRP-α is a negative regulator which acts as a docking protein for the SHP-1 and SHP-2 phosphatases which dampen intracellular signaling. Therefore CD47 acts physiologically as a “don’t eat me” signal. CD47 is frequently overexpressed in cancer cells 115, 116, 118, 119. Masking CD47 on cancer cells using mAb or engineered SIRP-a triggers ADCP in vitro and results in antitumor activity in diverse mouse tumor models 115, 116, 118–120. Strategies targeting the CD47/SIRP-a pathway using mAb or SIRP-α Fc fusion have proven to act synergistically with diverse anti-tumor mAb including anti-CD20 and anti-Her2. This result is consistent with the ability of SIRP-α to downregulate FcγR signaling 115, 116. In an interesting twist, anti-CD47 mAb treatment was found to activate adaptive immune responses 121–123 by activating accessory cell function. Moreover, CD47 blockade triggered DC-dependent activation of CD8 anti-tumor responses. Interestingly, CD47 blockade targeted pancreatic cancer stem cells and resulted in synergistic antitumor activity with chemotherapy 124. The ultimate efficacy of CD47 blockade requires activation of an adaptive immune response in the B16F10 murine melanoma model 125.
It has long been known that macrophages can kill tumor cells extracellularly via ADCC 126. ADCC, which can be mediated by NK cells and by macrophages is an essential component of the antitumor activity of anti-cancer mAb, including anti-CD20, anti-Her2 and anti-EGFR 127. Polymorphisms in FcγRIIIa and FcγRIIa are correlated with response in lymphoma (rituximab, anti-CD20), colon cancer (cetuximab, anti-EGFR) and breast carcinoma (trastuzumab, anti-HER2) 128–130. Since FcγRII is only expressed in myeloid cells, these data suggest an important role of macrophages in the clinical activity of mAbs. In a preclinical model the antitumor activity of anti-CD20 was dependent on chemokine-mediated macrophage recruitment and on macrophage effector function 131. Interestingly, in a mouse model signals present in the tumor microenvironment which skew TAM function (IL-10 and CSF-1) were found to increase macrophage effector function in the presence of anti-lymphoma mAb 132. Consistently with this observation, TAM infiltration is associated with worse prognosis in lymphoma 63. However, when treatment with anti-CD20 is taken into consideration, high TAM infiltration was associated with better outcome 65. Similarly, in a breast cancer model TAM promoted tumor growth but were essential for the therapeutic efficacy of mAb 133. Thus, preclinical models, mechanistic analysis and clinical correlative analysis suggest that the functional polarization of TAM may represent an advantage in terms of mAb triggered effector functions.
Maximizing TAM-mediated ADCC and ADCP could be part of combination approaches with chemotherapy. In a B cell leukemia model, cyclophosphamide-mediated tissue damage and elicited macrophage recruitment via chemokines and cytokines. In this setting chemotherapy and mAb (alemtuzumab, anti-CD52) synergistically drove cancer cell death and macrophage-mediated disposal 134.
9. Targeting Macrophage Recruitment, Survival and Polarization
Fig. 3 provides a schematic representation of macrophage targeting strategies. In general, macrophage-centered therapeutic approaches are aimed either at inhibiting functions related to tumor promotion or at activating antitumor activity.
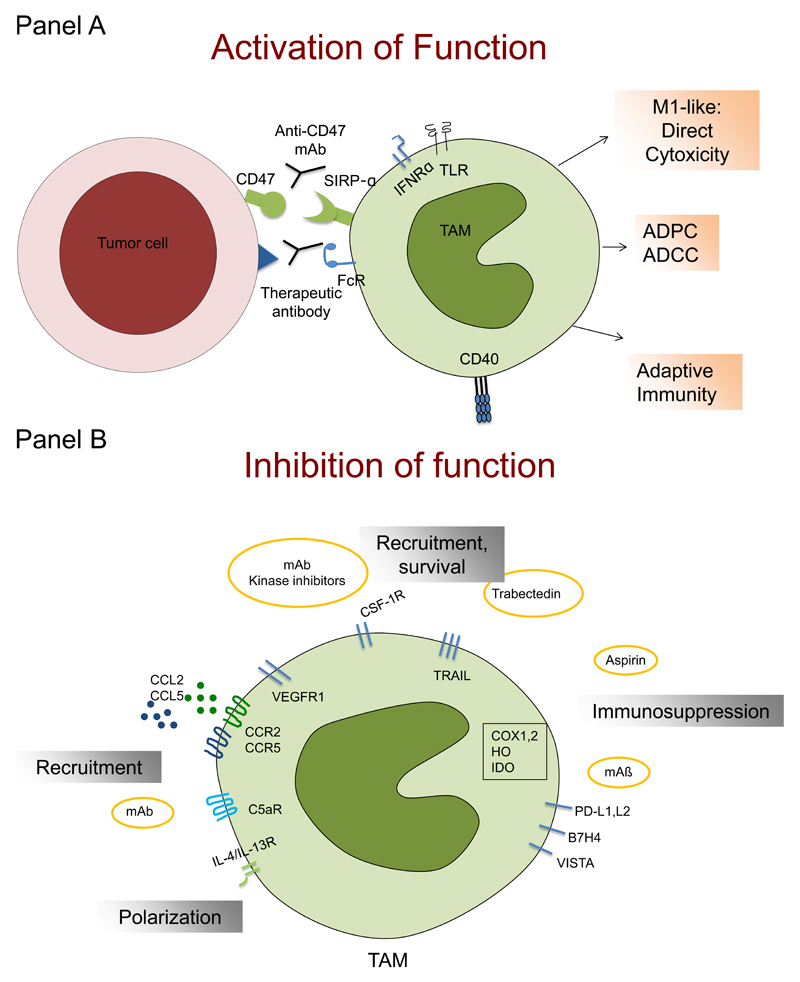
Figure 3. Schematic representation of strategies targeting macrophages in tumor settings.
Macrophage-centered therapeutic approaches are aimed either at activating their antitumor activity (Panel A) or inhibiting their recruitment and functions related to tumor promotion (panel B).
Panel A: the concerted action of microbial moieties (acting via TLRs) and IFNγ induces M1-like functional polarization and can activate macrophage killing of tumor cells; macrophage-mediated antibody-dependent cytotoxicity (ADCC) can mediate the therapeutic effect of therapeutic antibodies; interference with the SIRPα-CD47 pathway activates macrophage-mediated antibody-dependent phagocytosis (ADCP) and results in functional skewing of macrophages in an M1 direction and antitumor activity; an anti-CD40 antibody re-educates M2-like macrophages in the tumor microenvironment, leading to re-establishment of tumor immune surveillance.
Panel B: Inhibition of monocyte-attracting molecules, including chemokines (e.g CCL2, CCL5), VEGF, CSF-1 and complement mediators (C5a) with specific monoclonal antibodies (e.g. carlumab, emactuzumab) or antagonists (e.g. maraviroc) prevent macrophage recruitment to the tumor microenvironment, reducing tumor growth and dissemination; inhibitors of CSF-1 have also the potential to inhibit macrophage survival; Trabectedin activates a caspase-dependent pathway of apoptosis, selectively in cells of the monocyte lineage, causing a partial depletion of circulating monocytes and TAM; the protective function of NAIDS, aspirin in particular, against primary cancer and metastasis relies on the inhibition of prostaglandin production, which have immunosuppressive properties; TAM contribute to suppression of adaptive immunity by expression of immunosuppressive molecules, such as IDO, cyclooxygenases (COX1,2), TGFβ and IL-10. Moreover, TAM express triggers of checkpoint blockade, such PD-L1, PD-L2, B7H4 and VISTA.
TLR: Toll-like receptors; IFNRα: interferon receptor alpha; FcR: Fc receptor, mAb: monoclonal antibody; VEGF: vascular endothelial growth factor; CSF-1: colony-stimulating factor; NAIDS: nonsteroidal anti-inflammatory drugs; IDO: indoleamine 2,3 dioxygenase; TGFβ: transforming growth factor β; IL-10: interleukin 10.
Recruitment and localization
As mentioned above, mediators which have been involved in macrophage recruitment in tumors are diverse and include chemokines, complement components, CSF-1, VEGF. Chemokines have long been involved in macrophage accumulation in tumors 11, 15–17, 19, 27, 135. Stumbling blocks in translating anti-chemokine strategies into chemical benefit in inflammatory and neoplastic diseases include the fact that multiple chemokines and chemokine receptors are involved in phagocyte attraction (“robustness” of the system) and the fact that individual chemokines act on multiple cell types. Inhibition of CCL2 with specific antibodies reduced tumor growth and dissemination in different experimental models such as prostate, melanoma, breast, lung and liver cancer; when administered in combination with chemotherapy, anti-CCL2 antibodies improved the therapeutic efficacy 136–140. However, it has been also shown, in a breast cancer mouse model, that withdrawal of anti-CCL2 treatment increased the mobilization of bone marrow monocyte and infiltration in tumors, accelerating lung metastasis 141.
Selective antibodies to CCL2 have entered phase I and II clinical trials. The antibody CNTO 888 (carlumab) showed preliminary antitumor activity in advanced cancer patients and was well tolerated 142, 143. However, no responses were observed in a phase II study in castration resistant prostate cancer 142. Combinations of carlumab with conventional chemotherapy have been studied in a phase Ib clinical trial. The results indicated that carlumab has a good safety profile but no significant tumor responses were observed 144. A recent study demonstrated the feasibility of combining a novel CCR2 antagonist (PF-04136309) with conventional chemotherapy in locally advanced pancreatic cancer patients not eligible for surgery. In a Phase 1b clinical trial, patients with adenocarcinoma of the pancreas received FOLFIRINOX (oxaliplatin and irinotecan plus leucovorin and fluorouracil) alone, or combined with the oral CCR2 antagonist. Patients in the latter group did not experience worse toxicity than chemotherapy alone and had clinical signs of stable disease or partial tumor response in 49% of 33 imaging-evaluated patients 145.
Analysis of leukocyte migration in CRC metastasis revealed overproduction of CCL5, a monocyte attractant also responsible for functional skewing of TAM 146. Treatment with Maraviroc, an antagonist for the CCR5 cognate receptor of CCR5 approved for clinical use in AIDS, resulted in biological and clinical responses in a small cohort of advanced CRC patients 146. Targeting the CCL5/CCR5 axis deserves further scouting given its long recognized activation for instance in breast cancer 135.
Survival
CSF-1-CSF-1R
The CSF-1 receptor (CSF-1R) is exclusively expressed by the monocytic lineage and represents an obvious target to hit TAM directly or indirectly, acting on precursors. CSF-1 (also known as M-CSF) is the major growth and differentiation factor for cells of the monocyte-macrophage lineages. CSF-1 is abundantly expressed by several tumor types; this ligand-receptor pathways has been extensively investigated in tumor models and constitutes a paradigm of TAM-cancer cell interaction 6, 147. A CSF1 or CSF1R-related signature has been associated with poor survival in different malignancies, such as classical Hodgkin lymphoma, breast cancer and hepatocellular carcinoma 148–150.
CSF-1R is a tyrosine kinase receptor. Antagonists and antibodies to the CSF1R have been developed and tested in preclinical models 84, 151, 152, 32. The humanized monoclonal antibody RG7155 (Emactuzumab ) blocks CSF1R activation. In mouse tumor models and in cancer patients, treatment with RG7155 reduced macrophage infiltration in tumors and increased the CD8/CD4 T cell ratio in tumor biopsies of patients 153 (ClinicalTrials.gov, number NCT01494688) (Table 2). In patients with a rare neoplasia (diffuse-type tenosynovial giant cell tumor), characterized by the over-expression of CSF1R, no dose-limiting toxicities were observed. Common adverse events were facial oedema, asthenia and pruritus, 26/28 patients achieved clinically objective responses 154. The small molecule PLX3397 is a CSF-1R inhibitor and can be administered orally. Also this compound was able to induce clinical regression in patients with tenosynovial giant cell tumors (ClinicalTrials.gov number, NCT01004861) 155.
PLX3397 penetrates the blood-brain barrier and was tested in a phase II study in patients with recurrent glioblastoma. The drug was well tolerated and, as a proof-of-principle of its activity, circulating CD14dim/CD16+ monocytes were reduced after treatment. However, the primary endpoint of 6 months progression free survival was met only in 8% of 37 patients. Clearly, the potential of these inhibitors needs to be maximized with combination therapies 156. In preclinical mouse xenograft models with intracranial human glioblastoma, radiotherapy is known to increase CSF-1 ligand expression and tumor infiltration by myeloid cells. Treatment with PLX3397 potentiated the therapeutic effects of radiotherapy, suggesting that radiotherapy of glioblastoma may be improved by combinations with CSF-1R inhibition 157. In a syngeneic mouse model of BRAFV600-driven melanoma, PLX3397 also improved the anti-tumor efficacy of adoptive cell transfer immunotherapy by inhibiting the intratumoral accumulation of immunosuppressive macrophages 158. The CSF-1 inhibitor BZL945 blocked glioma progression and improved survival in preclinical models. Interestingly CSF-1R blockade did not result in TAM depletion but contributed, together with glioma-supplied factors (i.e. GM-CSF and IFNγ), to “re-educate” macrophages from a pro-tumor phenotype to an anti-tumor effector cell 32. Analysis of glioblastomas recurring after CSF-1R inhibition revealed an interplay between cancer cells and the microenvironment 159. In recurrent tumors, IL-4-driven M2 activation of macrophages drove Stat6 and nuclear factor of activated T cells (NFAT)-mediated induction of insulin-like growth factor 1 (Igf1). Igf1 acted on tumor cells via the PI3K pathway. Blocking Igf1 or PI3K in tumor cells and CSF-1R in macrophages resulted in prolongation of survival in this mouse model.
The CSF-1R inhibitor GW2580 enhanced the activity of gemcitabine in a transgenic pancreatic ductal adenocarcinoma model. Mechanistically, the study demonstrated that macrophages induced the up-regulation of cytidine deaminase causing resistance to gemcitabine 160. This and other studies suggest that macrophage targeting could be a complementary strategy to enhance the efficacy of conventional chemotherapy. Along the same line in a transgenic mouse model of mammary adenocarcinoma treatment with Paclitaxel up-regulated the production of CSF-1, IL-34 (another growth factor using the CSF-1R), and the chemokine CCL8. Blockade of the CSF-1–CSF-1R loop, either by anti-CSF-1 antibodies or a CSF-1R inhibitor, enhanced the therapeutic efficacy of chemotherapy, inhibited metastasis, and increased CD8 T cell infiltration in tumors 84. In a mouse model of ovarian cancer, the administration of the CSF-1R kinase inhibitor GW2580 in the late stages of disease (peritoneal dissemination) resulted in dramatically reduced ascites volume and decreased number of infiltrating macrophages 161. In prostate cancer, androgen blockade therapy induced cancer cells to express CSF-1 and other cytokines that caused increased infiltration of TAM. Combination treatment of androgen blockade with inhibitors of CSF-1R achieved more durable therapeutic responses compared with hormonal therapy alone 95. Collectively, preclinical results strongly suggest that targeting the CSF1-CSF-1R axis has the potential to complement conventional therapeutic strategies.
Recent evidence has been provided of specific factors important for the survival and expansion of myeloid cells in cancer. The retinoic-acid-related orphan receptor (RORC1) expressed in myeloid cells drives cancer-related myelopoiesis in response to colony-stimulating factors. Its ablation in the myeloid compartment impairs tumor development and the generation of suppressive MDSCs, while promoting M1-polarized TAMs with antitumor activity. Thus, RORC1 is a potential molecule to target myeloid cells in cancer 162.
Biphosphonates
Bisphosphonates have cytotoxic potential on myeloid cells and are used for the treatment of osteoporosis and prevention of complications associated with bone metastases. They are inhibitors of the farnesyldiphosphonate synthase, a key enzyme responsible for cholesterol synthesis and protein prenylation, and have high affinity for bone hydroxyapatite where they are internalized by bone macrophages (osteoclasts), causing their apoptosis 163, 164. Tissue macrophages other than the ones in bone, including TAM, have been reported to be affected by bisphosphonates 165, in particular by clodronate in a liposomal formulation 166, 167. The current clinical usage of bisphosphonates in the treatment of solid malignancies is in combination with chemotherapy or hormonal therapy. In postmenopausal women with breast cancer, recurrence and overall mortality were significantly reduced. Moreover, clodronate was reported to reduce the incidence of bone and visceral metastases in human mammary carcinomas, an observation which points to actions unrelated to the bone metastatic niche 168. In patients with bone metastatic hormone-naïve prostate cancer, Zoledronate reduced skeletal–related events and improved progression-free survival time (see for review 169). The relative importance of targeting macrophages in particular in the bone metastatic niche versus modifying bone resistance to osteolysis in the clinical activity of bisphosphonates remains to be assessed.
Trabectedin
As discussed above, Trabectedin, originally developed as an antiproliferative agent, was found to cause a partial depletion of circulating monocytes and TAM 75, 76. These studies stemmed from the clinical observation of delayed, persistent responses in cancer patients (see above). Monocyte depletion includes the monocytic component of MDSCs 75. Trabectedin activates a TRAIL dependent pathway of apoptosis75. Monocytes are exquisitely sensitive to TRAIL triggering of apoptosis because, unlike other leukocytes like neutrophils, they express very low levels of TRAIL decoy receptors 170 . In murine tumors and in human sarcomas Trabectedin-induced TAM reduction was associated with decreased angiogenesis. In murine tumors increase T cell infiltration was also observed. These observations raise the issue of combinations of Trabectedin with inhibitors of angiogenesis and of checkpoint blockade inhibitors.
Functional activation
Microbial preparations and microbe-derived molecules (e.g. MDP) activate macrophages for tumor cytotoxicity and have undergone clinical testing in the ‘70s. The only remainder of the bacterial era of immunotherapy is intravescical BCG in recurrent bladder carcinoma. In addition to or in concert with microbial moieties such as LPS, IFNγ is a classic inducer of macrophage killing of tumor cells and M1 activation 10. With the aim to avoid unwarranted systemic macrophage activation, IFNγ was administered ip first in advanced ovarian cancer patients and then in patients with minimal residual disease 171, 172. I.p. IFNγ resulted in activation of tumor cytotoxicity and clinical responses. It remains unclear whether the potential of IFNγ immunotherapy under these conditions has been fully exploited.
More specific, although unexpected, macrophage targeting came from the administration of an anti-CD40 antibody in a preclinical model of pancreatic cancer 173. Alternatively activated, M2-like macrophages were re-educated in the tumor microenvironment, and acquired antigen-presenting capabilities, leading to re-establishment of tumor immune surveillance and short-term reduction in tumor volume 173. This preclinical evidence spurred a clinical trial with a fully human CD40 agonist antibody in combination with gemcitabine in advanced pancreatic cancer patients, with partial responses 174. Repolarization of proangiogenic macrophages was achieved in mice by expressing the host-produced antiangiogenic and immunomodulatory protein histidine-rich glycoprotein (HRG), which induced downregulation of placental growth factor (PlGF) in macrophages, supporting the evaluation of PlGF-blockade strategies 175. A modified Vitamin D binding protein (EF-022) is undergoing early clinical evaluation based on effects on macrophage activation (Table 2).
ADCC and ADCP are strategies capitalizing on the effector function of TAM (see above). Macrophage ADCP activated by interfering with the SIRPa-CD47 pathway may be more than a mechanism of effector function. In a recent study focused on glioblastoma, where the M1/M2 ratio had prognostic significance, anti-CD47-elicited ADCP resulted in functional skewing of murine macrophages in an M1 direction 125.
In a pancreatic cancer preclinical model 114, administration of the Bruton’s tyrosine kinase (BTK) inhibitor Ibrutinib reset macrophages toward a phenotype that promoted CD8+ T M1-like cell cytotoxicity and curbed PDAC growth 176, a strategy that is currently under evaluation in combination with checkpoint inhibitors clinical trials 176.
Usage of nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin in particular, is associated with protection against occurrence of many tumors and metastasis 177–179. PGE2 is well-known to have immune-suppressive effects, for instance on dendritic cells, and to favour the development of myeloid suppressor cells (MDSC) 180. Intriguingly, in a recent report PGE2 transactivated the CSF-1 receptor 181. Prostaglandins (e.g. PGE2 ) have also been reported to be involved in the M2-like polarization of macrophages in part through activation of the cAMP pathway 182, 183. Thus, it is tempting to speculate that targeting the tumor-associated myeloid cells plays a major role in the protective function of NSAIDs against primary cancer and metastasis.
10. Concluding Remarks and Perspectives
Cells of the monocyte-macrophage lineage are an essential element of the inflammatory component of the ecological niche of cancer and play a key role in progression. Progress has been made in defining the molecular landscapes and mechanisms of macrophage differentiation and diversity in tissues including cancer (e.g. 21, 184 24). Macrophage diversity includes the presence within tumors of TAM with different functional profiles, dictated by hypoxia. Current general paradigms on TAM reflect assessment at a population level. Deconvoluting TAM diversity at a single cell level and integrating information represent a challenge and may provide new vistas on cancer-related inflammation.
Macrophage can exert dual influences on the effect of conventional cytoreductive therapies, radiotherapy and chemotherapy. Moreover, TAM contribute to creating an immunosuppressive environment in tumors through multiple routes including triggers of checkpoint blockade in T cells. It will be important to assess whether TAM can provide predictive biomarkers for cytoreductive therapies and immunotherapy and contribute to personalized patient care.
Macrophage-centered therapeutic approaches are entering the clinical arena. These include blocking TAM-sustained tumor promotion and taking advantage of macrophage antitumor effector potential (ADCC, ADCP, M1-like polarization). While macrophage targeting strategies may per se result in therapeutic benefits, it is out tenet that macrophage therapeutics are borne to complement conventional citoreductive therapies, angiogenesis inhibitors and immunotherapy.
Bullet Points.
Tumor-associated macrophages (TAM) are a key component of the cancer microenvironment.
TAM contribute to tumor growth and progression.
Macrophages can have a dual influence on cancer, depending on stage in progression, tissue and microbiome.
TAM can limit the antitumor activity of conventional chemotherapy and radiotherapy by orchestrating a tumor promoting, tissue repair response to damage and providing a protective niche for cancer stem cells
On the other hand, macrophages contribute to the antitumor activity of selected chemotherapeutic agents such as Doxorubicin, under selected conditions
Macrophage depletion plays a key role in the antitumor activity of a clinically approved agent Trabectedin.
Macrophages mediate antibody-dependent cellular cytotoxicity (ADCC) and phagocytosis (ADCP) and play a key role in the antitumor activity of anticancer monoclonal antibodies.
Therapeutic strategies targeting macrophages as tumor promoters and/or aiming at their activation and reeducation are undergoing clinical assessment.
Macrophage-centered strategies have the potential to synergize with, and complement cytoreductive therapies, anti-angiogenic agents and checkpoint blockade inhibitors.
Acknowledgements
AM, PA and LL are supported by AIRC – Associazione Italiana per la Ricerca sul Cancro. AM is supported by European Commission (ERC Advanced Grant); Fondazione Cariplo; Italian Ministry of Health. PA is supported by a grant from World Wide Cancer Research, UK. We thank Dr. Hridayesh Prakash for stimulating discussions on clodronate and visceral metastasis.
References
- 1.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 2.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–91. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 5.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Noy R, Pollard JW. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–26. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med. 2015;212:435–445. doi: 10.1084/jem.20150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans R, Alexander P. Cooperation of immune lymphoid cells with macrophages in tumour immunity. Nature. 1970;228:620–622. doi: 10.1038/228620a0. [DOI] [PubMed] [Google Scholar]
- 10.Adams DO, Hamilton TA. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- 11.Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunol Today. 1992;13:265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- 12.Mantovani A. Effects on in vitro tumor growth of murine macrophages isolated from sarcoma lines differing in immunogenicity and metastasizing capacity. Int J Cancer. 1978;22:741–6. doi: 10.1002/ijc.2910220617. [DOI] [PubMed] [Google Scholar]
- 13.De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23:277–86. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Biswas SK. Metabolic Reprogramming of Immune Cells in Cancer Progression. Immunity. 2015;43:435–49. doi: 10.1016/j.immuni.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 16.Bottazzi B, et al. Regulation of the macrophage content of neoplasms by chemoattractants. Science. 1983;220:210–2. doi: 10.1126/science.6828888. [DOI] [PubMed] [Google Scholar]
- 17.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19:20–7. doi: 10.1038/nn.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian BZ, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geissmann F, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–61. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ginhoux F, Schultze JL, Murray PJ, Ochando J, Biswas SK. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol. 2016;17:34–40. doi: 10.1038/ni.3324. [DOI] [PubMed] [Google Scholar]
- 22.Feng X, et al. Loss of CX3CR1 increases accumulation of inflammatory monocytes and promotes gliomagenesis. Oncotarget. 2015;6:15077–94. doi: 10.18632/oncotarget.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van de Laar L, et al. Yolk Sac Macrophages, Fetal Liver, and Adult Monocytes Can Colonize an Empty Niche and Develop into Functional Tissue-Resident Macrophages. Immunity. 2016;44:755–68. doi: 10.1016/j.immuni.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Movahedi K, Van Ginderachter JA. The Ontogeny and Microenvironmental Regulation of Tumor-Associated Macrophages. Antioxid Redox Signal. 2016 doi: 10.1089/ars.2016.6704. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Bottazzi B, et al. A paracrine circuit in the regulation of the proliferation of macrophages infiltrating murine sarcomas. J Immunol. 1990;144:2409–12. [PubMed] [Google Scholar]
- 26.Campbell MJ, et al. Proliferating macrophages associated with high grade, hormone receptor negative breast cancer and poor clinical outcome. Breast Cancer Res Treat. 2011;128:703–11. doi: 10.1007/s10549-010-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franklin RA, et al. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–5. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar V, et al. CD45 Phosphatase Inhibits STAT3 Transcription Factor Activity in Myeloid Cells and Promotes Tumor-Associated Macrophage Differentiation. Immunity. 2016;44:303–15. doi: 10.1016/j.immuni.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanna RN, et al. Patrolling monocytes control tumor metastasis to the lung. Science. 2015;350:985–90. doi: 10.1126/science.aac9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonavita E, Galdiero MR, Jaillon S, Mantovani A. Phagocytes as Corrupted Policemen in Cancer-Related Inflammation. Adv Cancer Res. 2015;128:141–71. doi: 10.1016/bs.acr.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 31.Kitamura T, et al. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med. 2015;212:1043–59. doi: 10.1084/jem.20141836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pyonteck SM, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–72. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray PJ, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 35.DeNardo DG, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shiao SL, et al. TH2-Polarized CD4(+) T Cells and Macrophages Limit Efficacy of Radiotherapy. Cancer Immunol Res. 2015;3:518–25. doi: 10.1158/2326-6066.CIR-14-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pedroza-Gonzalez A, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med. 2011;208:479–90. doi: 10.1084/jem.20102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kratochvill F, et al. TNF Counterbalances the Emergence of M2 Tumor Macrophages. Cell Rep. 2015;12:1902–14. doi: 10.1016/j.celrep.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Monte L, et al. Basophil Recruitment into Tumor-Draining Lymph Nodes Correlates with Th2 Inflammation and Reduced Survival in Pancreatic Cancer Patients. Cancer Res. 2016;76:1792–803. doi: 10.1158/0008-5472.CAN-15-1801-T. [DOI] [PubMed] [Google Scholar]
- 40.Laoui D, et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res. 2014;74:24–30. doi: 10.1158/0008-5472.CAN-13-1196. [DOI] [PubMed] [Google Scholar]
- 41.Movahedi K, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–39. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 42.Henze AT, Mazzone M. The impact of hypoxia on tumor-associated macrophages. J Clin Invest. 2016 doi: 10.1172/JCI84427. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kryczek I, et al. Cutting edge: induction of B7-H4 on APCs through IL-10: novel suppressive mode for regulatory T cells. J Immunol. 2006;177:40–4. doi: 10.4049/jimmunol.177.1.40. [DOI] [PubMed] [Google Scholar]
- 44.Wang L, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208:577–92. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pello OM, et al. Role of c-MYC in alternative activation of human macrophages and tumor-associated macrophage biology. Blood. 2012;119:411–21. doi: 10.1182/blood-2011-02-339911. [DOI] [PubMed] [Google Scholar]
- 46.Casey SC, et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352:227–31. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity. 2013;39:11–26. doi: 10.1016/j.immuni.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 48.Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462–72. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Algars A, et al. Type and location of tumor-infiltrating macrophages and lymphatic vessels predict survival of colorectal cancer patients. Int J Cancer. 2012;131:864–73. doi: 10.1002/ijc.26457. [DOI] [PubMed] [Google Scholar]
- 50.Di Caro G, et al. Dual prognostic significance of tumour-associated macrophages in human pancreatic adenocarcinoma treated or untreated with chemotherapy. Gut. 2015 doi: 10.1136/gutjnl-2015-309193. [DOI] [PubMed] [Google Scholar]
- 51.Ino Y, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108:914–23. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bronte V, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Casazza A, et al. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell. 2013;24:695–709. doi: 10.1016/j.ccr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–65. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 55.Leek RD, et al. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–9. [PubMed] [Google Scholar]
- 56.Hanada T, et al. Prognostic value of tumor-associated macrophage count in human bladder cancer. Int J Urol. 2000;7:263–9. doi: 10.1046/j.1442-2042.2000.00190.x. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka Y, Kobayashi H, Suzuki M, Kanayama N, Terao T. Upregulation of bikunin in tumor-infiltrating macrophages as a factor of favorable prognosis in ovarian cancer. Gynecol Oncol. 2004;94:725–34. doi: 10.1016/j.ygyno.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 58.Ishigami S, et al. Tumor-associated macrophage (TAM) infiltration in gastric cancer. Anticancer Res. 2003;23:4079–83. [PubMed] [Google Scholar]
- 59.Zhang QW, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7:e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galdiero MR, et al. Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. Int J Cancer. 2016;139:446–456. doi: 10.1002/ijc.30076. [DOI] [PubMed] [Google Scholar]
- 61.Steidl C, et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med. 2010;362:875–85. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tan KL, et al. Tumor-associated macrophages predict inferior outcomes in classic Hodgkin lymphoma: a correlative study from the E2496 Intergroup trial. Blood. 2012;120:3280–7. doi: 10.1182/blood-2012-04-421057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Farinha P, et al. Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL) Blood. 2005;106:2169–74. doi: 10.1182/blood-2005-04-1565. [DOI] [PubMed] [Google Scholar]
- 64.Alvaro T, et al. The presence of STAT1-positive tumor-associated macrophages and their relation to outcome in patients with follicular lymphoma. Haematologica. 2006;91:1605–12. [PubMed] [Google Scholar]
- 65.Taskinen M, Karjalainen-Lindsberg ML, Nyman H, Eerola LM, Leppa S. A high tumor-associated macrophage content predicts favorable outcome in follicular lymphoma patients treated with rituximab and cyclophosphamide-doxorubicin-vincristine-prednisone. Clin Cancer Res. 2007;13:5784–9. doi: 10.1158/1078-0432.CCR-07-0778. [DOI] [PubMed] [Google Scholar]
- 66.Kridel R, et al. The Prognostic Impact of CD163-Positive Macrophages in Follicular Lymphoma: A Study from the BC Cancer Agency and the Lymphoma Study Association. Clin Cancer Res. 2015;21:3428–35. doi: 10.1158/1078-0432.CCR-14-3253. [DOI] [PubMed] [Google Scholar]
- 67.Mantovani A, Polentarutti N, Luini W, Peri G, Spreafico F. Role of host defense merchanisms in the antitumor activity of adriamycin and daunomycin in mice. J Natl Cancer Inst. 1979;63:61–66. [PubMed] [Google Scholar]
- 68.Kim KJ, Wen XY, Yang HK, Kim WH, Kang GH. Prognostic Implication of M2 Macrophages Are Determined by the Proportional Balance of Tumor Associated Macrophages and Tumor Infiltrating Lymphocytes in Microsatellite-Unstable Gastric Carcinoma. PLoS One. 2015;10:e0144192. doi: 10.1371/journal.pone.0144192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang B, et al. Association of intra-tumoral infiltrating macrophages and regulatory T cells is an independent prognostic factor in gastric cancer after radical resection. Ann Surg Oncol. 2011;18:2585–93. doi: 10.1245/s10434-011-1609-3. [DOI] [PubMed] [Google Scholar]
- 70.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 71.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell. 2015;28:690–714. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 72.Ma Y, Galluzzi L, Zitvogel L, Kroemer G. Autophagy and cellular immune responses. Immunity. 2013;39:211–227. doi: 10.1016/j.immuni.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 73.Colotta F, Peri G, Villa A, Mantovani A. Rapid killing of actinomycin D-treated tumor cells by human mononuclear cells. I. Effectors belong to the monocyte-macrophage lineage. J Immunol. 1984;132:936–44. [PubMed] [Google Scholar]
- 74.D'Incalci M, Badri N, Galmarini CM, Allavena P. Trabectedin, a drug acting on both cancer cells and the tumour microenvironment. Br J Cancer. 2014;111:646–50. doi: 10.1038/bjc.2014.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Germano G, et al. Role of macrophage targeting in the anti-tumor activity of Trabectedin. Cancer Cell. 2013;23:249–262. doi: 10.1016/j.ccr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 76.Germano G, et al. Antitumor and anti-inflammatory effects of trabectedin on human myxoid liposarcoma cells. Cancer Res. 2010;70:2235–44. doi: 10.1158/0008-5472.CAN-09-2335. [DOI] [PubMed] [Google Scholar]
- 77.Iida N, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–70. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Viaud S, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–6. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vetizou M, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–84. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vacchelli E, et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science. 2015;350:972–8. doi: 10.1126/science.aad0779. [DOI] [PubMed] [Google Scholar]
- 81.Pfirschke C, et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity. 2016;44:343–54. doi: 10.1016/j.immuni.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cavnar MJ, et al. KIT oncogene inhibition drives intratumoral macrophage M2 polarization. J Exp Med. 2013;210:2873–86. doi: 10.1084/jem.20130875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sprinzl MF, et al. Sorafenib perpetuates cellular anticancer effector functions by modulating the crosstalk between macrophages and natural killer cells. Hepatology. 2013;57:2358–68. doi: 10.1002/hep.26328. [DOI] [PubMed] [Google Scholar]
- 84.DeNardo DG, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dijkgraaf EM, et al. Chemotherapy alters monocyte differentiation to favor generation of cancer-supporting M2 macrophages in the tumor microenvironment. Cancer Res. 2013;73:2480–2492. doi: 10.1158/0008-5472.CAN-12-3542. [DOI] [PubMed] [Google Scholar]
- 86.Bruchard M, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2013;19:57–64. doi: 10.1038/nm.2999. [DOI] [PubMed] [Google Scholar]
- 87.Jinushi M, et al. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci U S A. 2011;108:12425–30. doi: 10.1073/pnas.1106645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mitchem JB, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hughes R, et al. Perivascular M2 Macrophages Stimulate Tumor Relapse after Chemotherapy. Cancer Res. 2015;75:3479–91. doi: 10.1158/0008-5472.CAN-14-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu J, et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–94. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Durante M, Reppingen N, Held KD. Immunologically augmented cancer treatment using modern radiotherapy. Trends Mol Med. 2013;19:565–82. doi: 10.1016/j.molmed.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 92.Klug F, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 93.Zhu P, et al. Macrophage/cancer cell interactions mediate hormone resistance by a nuclear receptor derepression pathway. Cell. 2006;124:615–29. doi: 10.1016/j.cell.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 94.Nonomura N, et al. Infiltration of tumour-associated macrophages in prostate biopsy specimens is predictive of disease progression after hormonal therapy for prostate cancer. BJU Int. 2011;107:1918–22. doi: 10.1111/j.1464-410X.2010.09804.x. [DOI] [PubMed] [Google Scholar]
- 95.Escamilla J, et al. CSF1 receptor targeting in prostate cancer reverses macrophage-mediated resistance to androgen blockade therapy. Cancer Res. 2015;75:950–62. doi: 10.1158/0008-5472.CAN-14-0992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barleon B, et al. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–43. [PubMed] [Google Scholar]
- 97.Qian BZ, et al. FLT1 signaling in metastasis-associated macrophages activates an inflammatory signature that promotes breast cancer metastasis. J Exp Med. 2015;212:1433–48. doi: 10.1084/jem.20141555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10:505–14. doi: 10.1038/nrc2868. [DOI] [PubMed] [Google Scholar]
- 99.Coffelt SB, et al. Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Res. 2010;70:5270–80. doi: 10.1158/0008-5472.CAN-10-0012. [DOI] [PubMed] [Google Scholar]
- 100.Peterson TE, et al. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc Natl Acad Sci U S A. 2016;113:4470–5. doi: 10.1073/pnas.1525349113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kloepper J, et al. Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proc Natl Acad Sci U S A. 2016;113:4476–81. doi: 10.1073/pnas.1525360113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 103.Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci U S A. 2003;100:5336–41. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Noman MZ, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–90. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kuang DM, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327–37. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kryczek I, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–81. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bloch O, et al. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res. 2013;19:3165–75. doi: 10.1158/1078-0432.CCR-12-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Winograd R, et al. Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer Immunol Res. 2015;3:399–411. doi: 10.1158/2326-6066.CIR-14-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tumeh PC, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Herbst RS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Selby MJ, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1:32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 112.Simpson TR, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Romano E, et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci U S A. 2015;112:6140–5. doi: 10.1073/pnas.1417320112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhu Y, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–69. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Murata Y, Kotani T, Ohnishi H, Matozaki T. The CD47-SIRPalpha signalling system: its physiological roles and therapeutic application. J Biochem. 2014;155:335–44. doi: 10.1093/jb/mvu017. [DOI] [PubMed] [Google Scholar]
- 116.McCracken MN, Cha AC, Weissman IL. Molecular Pathways: Activating T Cells after Cancer Cell Phagocytosis from Blockade of CD47 “Don't Eat Me” Signals. Clin Cancer Res. 2015;21:3597–601. doi: 10.1158/1078-0432.CCR-14-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gul N, van Egmond M. Antibody-Dependent Phagocytosis of Tumor Cells by Macrophages: A Potent Effector Mechanism of Monoclonal Antibody Therapy of Cancer. Cancer Res. 2015;75:5008–13. doi: 10.1158/0008-5472.CAN-15-1330. [DOI] [PubMed] [Google Scholar]
- 118.Chao MP, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Majeti R, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–99. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weiskopf K, et al. Engineered SIRPalpha variants as immunotherapeutic adjuvants to anticancer antibodies. Science. 2013;341:88–91. doi: 10.1126/science.1238856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tseng D, et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci U S A. 2013;110:11103–8. doi: 10.1073/pnas.1305569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Soto-Pantoja DR, et al. CD47 in the tumor microenvironment limits cooperation between antitumor T-cell immunity and radiotherapy. Cancer Res. 2014;74:6771–83. doi: 10.1158/0008-5472.CAN-14-0037-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shi Y, et al. Trastuzumab triggers phagocytic killing of high HER2 cancer cells in vitro and in vivo by interaction with Fcgamma receptors on macrophages. J Immunol. 2015;194:4379–86. doi: 10.4049/jimmunol.1402891. [DOI] [PubMed] [Google Scholar]
- 124.Cioffi M, et al. Inhibition of CD47 Effectively Targets Pancreatic Cancer Stem Cells via Dual Mechanisms. Clin Cancer Res. 2015;21:2325–37. doi: 10.1158/1078-0432.CCR-14-1399. [DOI] [PubMed] [Google Scholar]
- 125.Sockolosky JT, et al. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc Natl Acad Sci U S A. 2016;113:E2646–54. doi: 10.1073/pnas.1604268113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mantovani A, Caprioli V, Gritti P, Spreafico F. Human mature macrophages mediate antibody-dependent cellular cytotoxicity on tumour cells. Transplantation. 1977;24:291–3. doi: 10.1097/00007890-197710000-00010. [DOI] [PubMed] [Google Scholar]
- 127.Weiskopf K, Weissman IL. Macrophages are critical effectors of antibody therapies for cancer. MAbs. 2015;7:303–10. doi: 10.1080/19420862.2015.1011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–7. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 129.Zhang W, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol. 2007;25:3712–8. doi: 10.1200/JCO.2006.08.8021. [DOI] [PubMed] [Google Scholar]
- 130.Tamura K, et al. FcgammaR2A and 3A polymorphisms predict clinical outcome of trastuzumab in both neoadjuvant and metastatic settings in patients with HER2-positive breast cancer. Ann Oncol. 2011;22:1302–7. doi: 10.1093/annonc/mdq585. [DOI] [PubMed] [Google Scholar]
- 131.Cittera E, et al. The CCL3 family of chemokines and innate immunity cooperate in vivo in the eradication of an established lymphoma xenograft by rituximab. J Immunol. 2007;178:6616–23. doi: 10.4049/jimmunol.178.10.6616. [DOI] [PubMed] [Google Scholar]
- 132.Leidi M, et al. M2 macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than m1 cells in vitro. J Immunol. 2009;182:4415–22. doi: 10.4049/jimmunol.0713732. [DOI] [PubMed] [Google Scholar]
- 133.Grugan KD, et al. Tumor-associated macrophages promote invasion while retaining Fc-dependent anti-tumor function. J Immunol. 2012;189:5457–66. doi: 10.4049/jimmunol.1201889. [DOI] [PubMed] [Google Scholar]
- 134.Pallasch CP, et al. Sensitizing protective tumor microenvironments to antibody-mediated therapy. Cell. 2014;156:590–602. doi: 10.1016/j.cell.2013.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Weitzenfeld P, Ben-Baruch A. The chemokine system, and its CCR5 and CXCR4 receptors, as potential targets for personalized therapy in cancer. Cancer Lett. 2014;352:36–53. doi: 10.1016/j.canlet.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 136.Loberg RD, et al. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res. 2007;67:9417–24. doi: 10.1158/0008-5472.CAN-07-1286. [DOI] [PubMed] [Google Scholar]
- 137.Fridlender ZG, et al. Monocyte chemoattractant protein-1 blockade inhibits lung cancer tumor growth by altering macrophage phenotype and activating CD8+ cells. Am J Respir Cell Mol Biol. 2011;44:230–7. doi: 10.1165/rcmb.2010-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lu X, Kang Y. Chemokine (C-C motif) ligand 2 engages CCR2+ stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone. J Biol Chem. 2009;284:29087–96. doi: 10.1074/jbc.M109.035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moisan F, et al. Enhancement of paclitaxel and carboplatin therapies by CCL2 blockade in ovarian cancers. Mol Oncol. 2014;8:1231–9. doi: 10.1016/j.molonc.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li X, et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2015 doi: 10.1136/gutjnl-2015-310514. [DOI] [PubMed] [Google Scholar]
- 141.Bonapace L, et al. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014;515:130–3. doi: 10.1038/nature13862. [DOI] [PubMed] [Google Scholar]
- 142.Pienta KJ, et al. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Invest New Drugs. 2013;31:760–8. doi: 10.1007/s10637-012-9869-8. [DOI] [PubMed] [Google Scholar]
- 143.Sandhu SK, et al. A first-in-human, first-in-class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumors. Cancer Chemother Pharmacol. 2013;71:1041–50. doi: 10.1007/s00280-013-2099-8. [DOI] [PubMed] [Google Scholar]
- 144.Brana I, et al. Carlumab, an anti-C-C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: an open-label, multicenter phase 1b study. Target Oncol. 2015;10:111–123. doi: 10.1007/s11523-014-0320-2. [DOI] [PubMed] [Google Scholar]
- 145.Nywening TM, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 2016 doi: 10.1016/S1470-2045(16)00078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Halama N, et al. Tumoral Immune Cell Exploitation in Colorectal Cancer Metastases Can Be Targeted Effectively by Anti-CCR5 Therapy in Cancer Patients. Cancer Cell. 2016;29:587–601. doi: 10.1016/j.ccell.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 147.Hume DA, MacDonald KP. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood. 2012;119:1810–20. doi: 10.1182/blood-2011-09-379214. [DOI] [PubMed] [Google Scholar]
- 148.Zhu XD, et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26:2707–16. doi: 10.1200/JCO.2007.15.6521. [DOI] [PubMed] [Google Scholar]
- 149.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–40. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Koh YW, Park C, Yoon DH, Suh C, Huh J. CSF-1R expression in tumor-associated macrophages is associated with worse prognosis in classical Hodgkin lymphoma. Am J Clin Pathol. 2014;141:573–83. doi: 10.1309/AJCPR92TDDFARISU. [DOI] [PubMed] [Google Scholar]
- 151.Goswami S, et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65:5278–83. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 152.Manthey CL, et al. JNJ-28312141, a novel orally active colony-stimulating factor-1 receptor/FMS-related receptor tyrosine kinase-3 receptor tyrosine kinase inhibitor with potential utility in solid tumors, bone metastases, and acute myeloid leukemia. Mol Cancer Ther. 2009;8:3151–61. doi: 10.1158/1535-7163.MCT-09-0255. [DOI] [PubMed] [Google Scholar]
- 153.Ries CH, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–59. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 154.Cassier PA, et al. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study. Lancet Oncol. 2015;16:949–56. doi: 10.1016/S1470-2045(15)00132-1. [DOI] [PubMed] [Google Scholar]
- 155.Tap WD, et al. Structure-Guided Blockade of CSF1R Kinase in Tenosynovial Giant-Cell Tumor. N Engl J Med. 2015;373:428–37. doi: 10.1056/NEJMoa1411366. [DOI] [PubMed] [Google Scholar]
- 156.Butowski N, et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro Oncol. 2016;18:557–64. doi: 10.1093/neuonc/nov245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stafford JH, et al. Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro Oncol. 2015 doi: 10.1093/neuonc/nov272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mok S, et al. Inhibition of CSF-1 receptor improves the antitumor efficacy of adoptive cell transfer immunotherapy. Cancer Res. 2014;74:153–61. doi: 10.1158/0008-5472.CAN-13-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Quail DF, et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science. 2016;352 doi: 10.1126/science.aad3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Weizman N, et al. Macrophages mediate gemcitabine resistance of pancreatic adenocarcinoma by upregulating cytidine deaminase. Oncogene. 2014;33:3812–9. doi: 10.1038/onc.2013.357. [DOI] [PubMed] [Google Scholar]
- 161.Moughon DL, et al. Macrophage Blockade Using CSF1R Inhibitors Reverses the Vascular Leakage Underlying Malignant Ascites in Late-Stage Epithelial Ovarian Cancer. Cancer Res. 2015;75:4742–52. doi: 10.1158/0008-5472.CAN-14-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Strauss L, et al. RORC1 Regulates Tumor-Promoting "Emergency" Granulo-Monocytopoiesis. Cancer Cell. 2015;28:253–69. doi: 10.1016/j.ccell.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 163.Russell RG, Rogers MJ. Bisphosphonates: from the laboratory to the clinic and back again. Bone. 1999;25:97–106. doi: 10.1016/s8756-3282(99)00116-7. [DOI] [PubMed] [Google Scholar]
- 164.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–93. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 165.Junankar S, et al. Real-time intravital imaging establishes tumor-associated macrophages as the extraskeletal target of bisphosphonate action in cancer. Cancer Discov. 2015;5:35–42. doi: 10.1158/2159-8290.CD-14-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Miselis NR, Wu ZJ, Van Rooijen N, Kane AB. Targeting tumor-associated macrophages in an orthotopic murine model of diffuse malignant mesothelioma. Mol Cancer Ther. 2008;7:788–99. doi: 10.1158/1535-7163.MCT-07-0579. [DOI] [PubMed] [Google Scholar]
- 167.Fritz JM, et al. Depletion of tumor-associated macrophages slows the growth of chemically induced mouse lung adenocarcinomas. Front Immunol. 2014;5:587. doi: 10.3389/fimmu.2014.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Diel IJ, et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med. 1998;339:357–63. doi: 10.1056/NEJM199808063390601. [DOI] [PubMed] [Google Scholar]
- 169.Van Acker HH, Anguille S, Willemen Y, Smits EL, Van Tendeloo VF. Bisphosphonates for cancer treatment: Mechanisms of action and lessons from clinical trials. Pharmacol Ther. 2016;158:24–40. doi: 10.1016/j.pharmthera.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 170.Liguori M, et al. Functional TRAIL receptors in monocytes and tumor-associated macrophages: a possible targeting pathway in the tumor microenvironment. Oncotarget. 2016 doi: 10.18632/oncotarget.9340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Colombo N, et al. Anti-tumor and immunomodulatory activity of intraperitoneal IFN-gamma in ovarian carcinoma patients with minimal residual tumor after chemotherapy. Int J Cancer. 1992;51:42–6. doi: 10.1002/ijc.2910510109. [DOI] [PubMed] [Google Scholar]
- 172.Pujade-Lauraine E, et al. Intraperitoneal recombinant interferon gamma in ovarian cancer patients with residual disease at second-look laparotomy. J Clin Oncol. 1996;14:343–50. doi: 10.1200/JCO.1996.14.2.343. [DOI] [PubMed] [Google Scholar]
- 173.Beatty GL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Beatty GL, et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013;19:6286–6295. doi: 10.1158/1078-0432.CCR-13-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rolny C, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 176.Gunderson AJ, et al. Bruton Tyrosine Kinase-Dependent Immune Cell Cross-talk Drives Pancreas Cancer. Cancer Discov. 2016;6:270–85. doi: 10.1158/2159-8290.CD-15-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–93. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cuzick J, et al. Estimates of benefits and harms of prophylactic use of aspirin in the general population. Ann Oncol. 2015;26:47–57. doi: 10.1093/annonc/mdu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Trinchieri G. Innate inflammation and cancer: Is it time for cancer prevention? F1000 Med Rep. 2011;3:11. doi: 10.3410/M3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Obermajer N, et al. PGE(2)-driven induction and maintenance of cancer-associated myeloid-derived suppressor cells. Immunol Invest. 2012;41:635–57. doi: 10.3109/08820139.2012.695417. [DOI] [PubMed] [Google Scholar]
- 181.Digiacomo G, Ziche M, Dello Sbarba P, Donnini S, Rovida E. Prostaglandin E2 transactivates the colony-stimulating factor-1 receptor and synergizes with colony-stimulating factor-1 in the induction of macrophage migration via the mitogen-activated protein kinase ERK1/2. FASEB J. 2015;29:2545–54. doi: 10.1096/fj.14-258939. [DOI] [PubMed] [Google Scholar]
- 182.Larsson K, et al. COX/mPGES-1/PGE2 pathway depicts an inflammatory-dependent high-risk neuroblastoma subset. Proc Natl Acad Sci U S A. 2015;112:8070–5. doi: 10.1073/pnas.1424355112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Luan B, et al. CREB pathway links PGE2 signaling with macrophage polarization. Proc Natl Acad Sci U S A. 2015;112:15642–7. doi: 10.1073/pnas.1519644112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Glass CK, Natoli G. Molecular control of activation and priming in macrophages. Nat Immunol. 2016;17:26–33. doi: 10.1038/ni.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 186.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 187.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nat Immunol. 2016;17:2–8. doi: 10.1038/ni.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mantovani A. Reflections on immunological nomenclature: in praise of imperfection. Nat Immunol. 2016;17:215–6. doi: 10.1038/ni.3354. [DOI] [PubMed] [Google Scholar]